Summary
Circadian clocks orchestrate periods of rest or activity and feeding or fasting over the course of a 24 h day and maintain homeostasis. To assess whether a consolidated 24 h cycle of feeding and fasting can sustain health, we explored the effect of time-restricted feeding (TRF; food access limited to daytime 12 h every day) on neural, peripheral and cardiovascular physiology in Drosophila melanogaster. We detected improved sleep, prevention of body weight gain and deceleration of cardiac aging under TRF, even when caloric intake and expenditure were unchanged. We used temporal gene expression profiling and validation through classical genetics to identify the TCP-1 Ring Complex (TRiC) chaperonin, the mitochondrial electron transport chain complexes and the circadian clock as pathways mediating the benefits of TRF.
To test whether a daily rhythm of feeding and fasting without reducing caloric intake can improve health metrics, we subjected 2 week old wild type (WT) Oregon-R strain (table S1) of Drosophila melanogaster adults to ad libitum feeding (ALF) or 12-hour time-restricted feeding (TRF) of a standard cornmeal diet exclusively during daytime. At nighttime the TRF cohorts were placed in vials with 1.1% agar to prevent desiccation (fig S1A). The daily food intake was equivalent in both groups, although ALF flies consumed some of their food during nighttime (Fig. 1A). Unlike ALF flies, the TRF group did not gain body weight at 5 and 7 weeks of age (Fig. 1B). The ability to fly (flight index) was slightly improved in TRF group (Fig. 1C). Although the total daily activity was equivalent between both groups of flies (Fig. 1D), the TRF flies were more active during daytime. Sleep (defined as 5 consecutive minutes of inactivity) (1) assessment revealed that flies on TRF had less daytime sleep, but more nighttime and more total sleep, than the ALF flies (Fig. 1E and fig. S1).
Fig. 1. TRF improves sleep and prevents body weight gain without reducing caloric intake.
(a) Food consumption (CAFÉ assay) over a 24h period in 5 week old wild type (WT) Oregon-R flies. (b) Body weight of 3, 5 and 7 week old flies. (c) Flight index of 5 week old flies (n>30 flies). (d) Activity counts and (e) sleep duration of 5 week old flies averaged from at least 7 days of recording. (values are average + s.e.m, *: p<0.05, n.s.: p>0.05 Student’s t-test). Error bars: s.e.m.
Increase in sleep duration correlates with improved cardiac function (2). Therefore, by high speed video imaging of ex vivo denervated hearts bathed in artificial hemolymph (3) we measured the diameter of beating Drosophila heart at full relaxation and contraction and time interval between successive contractions to calculate cardiac function parameters (Fig. 2A). At 3 weeks of age, the performance of both ALF and TRF hearts was indistinguishable with equivalent heart period (HP), systolic diameter (SD), systolic interval (SI), diastolic diameter (DD), and diastolic interval (DI), arrhythmia index (AI), and heart contractility, measured as fractional shortening (FS) (Fig. 2, B–F, fig. S2 and movie S1). In the next two weeks, the cardiac performance in ALF flies exhibited characteristic age-dependent deterioration (4) with increased SI, DI, HP, AI, and reduced DD, SD, and FS. TRF flies showed smaller changes in these cardiac performance parameters in both genders (fig. S2).
Fig. 2. TRF protects against age- and diet-induced decline in cardiac function.
(a) M-mode (mechanical mode) traces showing the movement of the heart tube edge (y-axis) over time (x-axis) were generated from videos of the heart beneath the 3rd thoracic segment by digitally excising and aligning 1 pixel width vertical strip spanning the heart tube from a fixed location in successive frames. From the M-mode, cardiac parameters are calculated. Red arrowheads indicate the direction of age- or high-fat diet-induced changes. (b) Example 20 sec M-mode traces of ALF and TRF flies with superimposed orange (ALF) or blue (TRF) bar indicating diastolic intervals. Average (c) DI (d) HP, (e) AI and (f) FS show protection from age dependent deterioration in the TRF flies. (g) Feeding regimens employed to test the effect of TRF at an early or late age revealed improvement in (h) AI. (i) Representative M-modes of 5 week old flies subject to fat diet ALF or TRF. Average (j) DI, (k) AI, and (l) HP improved under TRF. Average values for ALF and TRF flies fed normal cornmeal diets are shown as broken lines for reference. Averages (n>30) are shown. *;p<0.05, Mann Whitney test. Error bars: s.e.m.
We tested whether a limited period of TRF during early or late in life could attenuate age-dependent decline in cardiac performance. Flies on ALF or TRF at 5 weeks of age were switched to TRF or ALF condition, respectively (Fig. 2G). In both groups, 7 week-old flies showed improvement in some (not all, see fig. S3) parameters including reduced HP and AI as well as increased FS relative to that of flies maintained in ALF for 7 weeks (Fig. 2H).
Because fat- containing diets deteriorate cardiac performance (5, 6), we tested the effect of TRF on flies fed a standard cornmeal diet supplemented with 2% w/v coconut oil. Flies on this fat diet ad libitum (FA) for 3 weeks showed severe deterioration of cardiac performance relative to standard cornmeal fed counterparts, including long HP, increased AI and reduced FS. Yet flies fed the same fat-supplemented diet under TRF condition (FT; access to fat diet for 12 h of daytime) showed smaller declines in cardiac performance (Fig. 2, I–L, fig.S4, and movie S2) relative to the FA cohort.
The improved cardiac function under TRF could result from systemic changes, local changes in the heart, or both. We measured RNA concentrations in the head and periphery (i.e., entire fly except the head) of 3-, 5- and 7- week old ALF and TRF (standard diet) flies collected every 6 h over 24 h (ZT or zeitgeber time 0, 6, 12, and 18). In TRF flies, the gene expression signature had no resemblance to that of flies exposed to starvation (7) or dietary restriction (DR) (8) (figs. S5 and S6, tables S2 and S3). No transcript showed a large change (fold change>2 between ALF and TRF group, p<0.05) at both 5 and 7 weeks age, indicating that either the diurnal expression pattern or a small but concerted change in the expression level of multiple, functionally related genes might account for the health benefits of TRF.
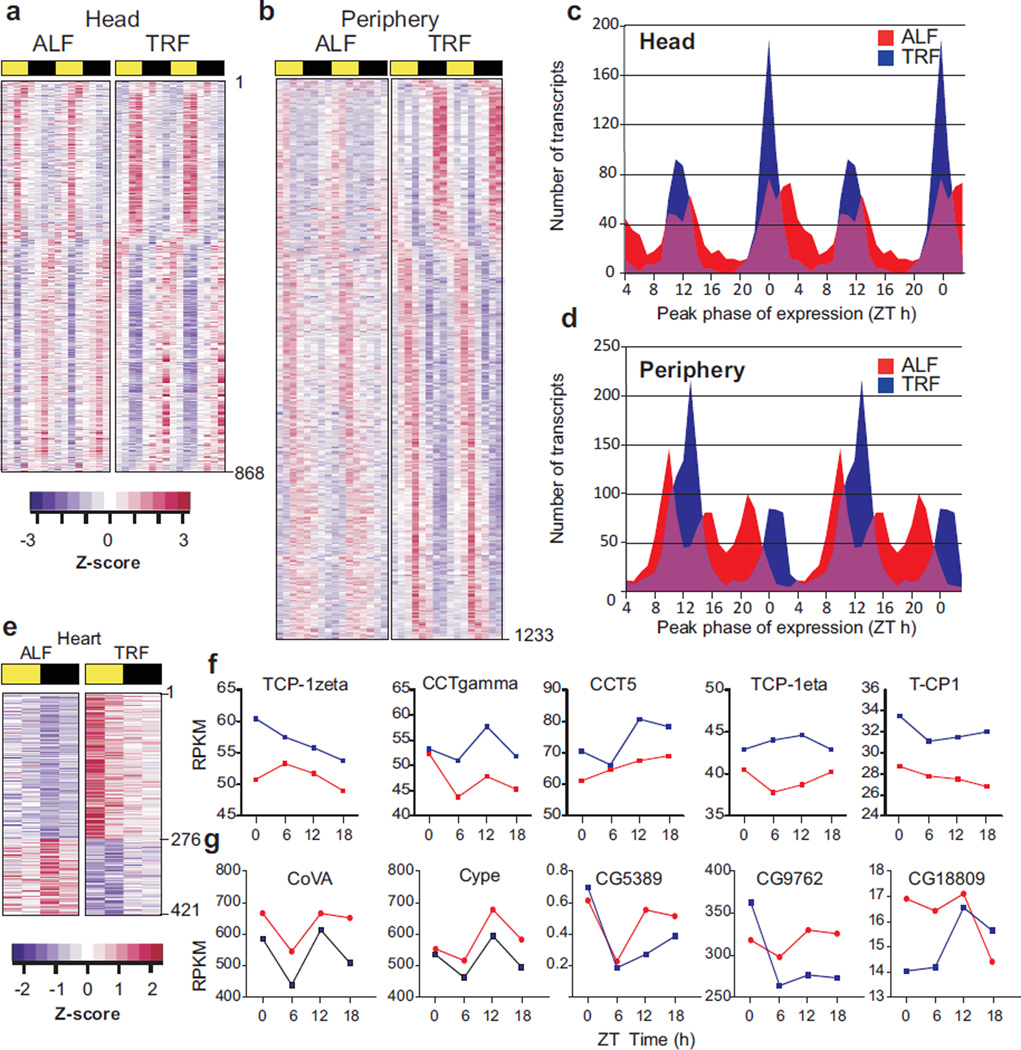
To assess diurnal gene expression, we examined transcripts from head and periphery of 5 week-old flies (on normal diet; ALF or TRF) at 8 different time points spanning 24 h. 868 transcripts in the head and 1233 transcripts in periphery were defined rhythmic under both ALF and TRF conditions (p<0.05, 22 h < period <26 h) (Fig. 3, A and B; tables S4 and S5). There were differences in the amplitude and synchrony of these oscillating transcripts in ALF and TRF flies. The amplitude (peak to trough difference) of oscillation of 876 transcripts (71%) from periphery and 516 transcripts from head (59%) increased under TRF condition. Furthermore, the peak phases of expression of rhythmic transcripts in ALF flies were distributed over 24 h, whereas TRF consolidated the peak phases to two principal times of the day corresponding to the ends of the feeding and fasting periods (Fig. 3, C and D). These synchronous transcript oscillations may coordinate fasting- or feeding-related metabolism to the appropriate time. As seen in mice (9), the combined action of the molecular circadian clock and the imposed rhythm of feeding and fasting may improve gene expression rhythms under TRF and offer systemic metabolic benefit.
Fig. 3. Transcriptional correlates of improved health in TRF.
Heat map representation of transcripts scored rhythmic under both ALF and TRF conditions in (a) head and (b) periphery of 5 week old flies. Normalized and color coded transcript levels at 8 different Zeitgeber times (ZT) spanning day (yellow bar) and night (dark bar) are shown. Area plots showing the peak phase of expression of rhythmic transcripts in ALF (red) or TRF (blue) fly (c) head or (d) periphery binned into 1 h intervals. For clarity, data in a–d are double plotted over two 24 h periods. (e) Heat map representation of transcripts that are up (purple) or down (blue) regulated in TRF flies. Transcript levels in fly hearts collected at 6 h interval over 24 h are shown. (f) Expression level (RPKMs) of example TRiC chaperonin subunits and (g) ETC components in ALF and TRF hearts at 5 weeks age.
To identify the transcriptomic correlates of improved cardiac physiology, we measured cardiac gene expression in 5 week-old ALF and TRF flies every 6 hours over a 24 hour period. Heart-enriched transcripts including Hand, Tinman, He, and H15 (10) were confirmed to be more abundant in the heart than the head and the periphery. Comparisons between ALF and TRF yielded 145 and 276 transcripts that showed decreased or increased expression respectively at all four timepoints in the TRF hearts (Fig. 3, E–G, table S6). Functional annotation of these transcripts identified the ATP-dependent TCP-1 Ring Complex (TRiC) (also known as chaperonin containing TCP-1;CCT) chaperonin (11) and mitochondrial electron transport chain (ETC) as the top functional clusters with increased or decreased expression in the TRF heart, respectively. 7 out of 8 TRiC subunit RNAs were more abundant at all four timepoints (fig. S7; table S8). Concurrently, mRNAs encoding 52 components of the ETC were decreased in abundance at 3 out of 4 timepoints and 27 at all 4 timepoints in TRF hearts (fig. S8, table S7). Thus we considered the circadian clock, TRiC chaperonin and mitochondrial ETC as potentially mediating the beneficial cardioprotective effects of TRF.
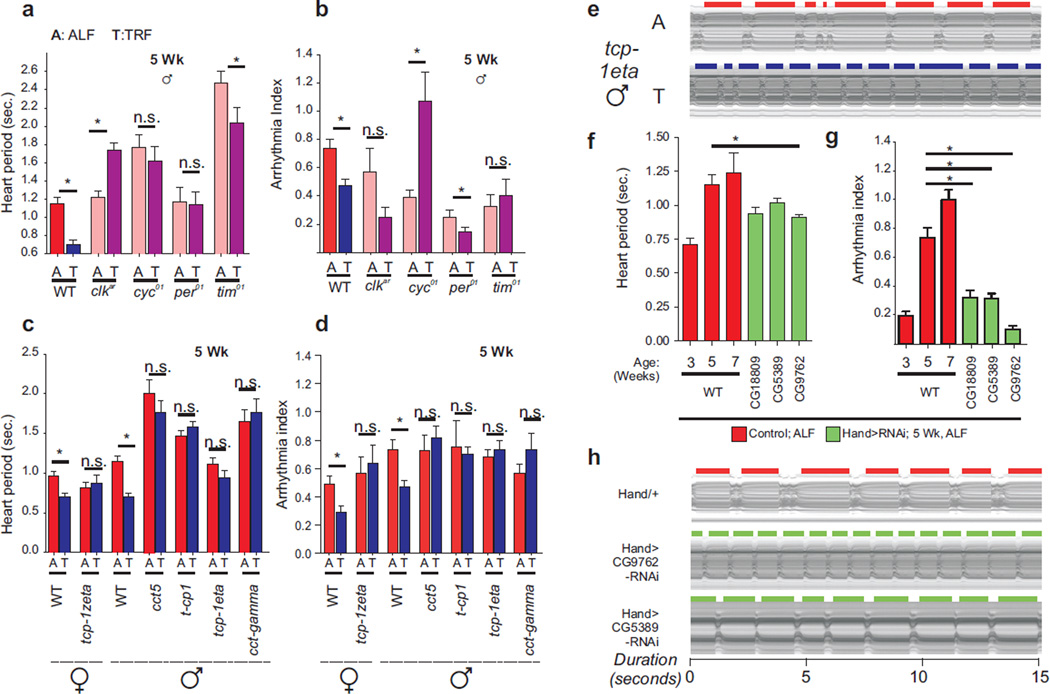
The Drosophila circadian oscillator is based on a transcriptional negative feedback loop generated by the activators Clock (CLK) and Cycle (CYC) and the repressors Period (PER) and Timeless (TIM) (12). To test the role of the molecular clock in TRF-dependent improvement of cardiac physiology, we examined flies carrying loss of function mutations in oscillator components: clk, cyc, per, or tim. These fly strains lacking both molecular and behavioral circadian rhythms are born without major heart morphological defects. Although these mutants all lacked a functional circadian oscillator, cardiac performance in 5 week-old flies was variably affected under ALF (Fig 4A, fig. S8). For example, the heart period of per01 and clkar mutants was comparable to that of WT, whereas cyc01 and tim01 mutants showed slower heart rate (Fig. 4A). The WT flies on TRF showed improved relaxation-contraction function relative to their ALF counterparts as reflected in decreased heart period and arrhythmicity. However, the heart period increased in clkar mutant flies and did not change significantly in per01 and cyc01 mutants. TRF increased arrhythmia in cyc01 flies and had smaller or no significant effect in per01, tim01 or clkar flies (Fig. 4 A and B, movie S3). Thus, imposition of a diurnal feeding rhythm was insufficient for protecting against cardiac aging unless endogenous circadian oscillations were intact (figs. S9 and S10).
Fig. 4. Genetic basis for the beneficial effects of TRF.
5 week old flies carrying loss of function mutations in (a,b) clock components or heterozygous for P-element insertions in (c,d) TRiC chaperonin components fail to improve (a,c) heart period and (b,d) fractional shortening under TRF (n ≥12 circadian mutants, n≥17 TRiC mutants). Wildtype (Oregon-R) data are included for reference. (e) Representative M-modes of Tcp-1eta mutant flies exhibiting lack of TRF-driven cardioprotection. (f) Heart period and (g) arrhythmia index, and (h) representative M-modes show improved cardiac function in 5-week old ALF flies with heart specific knockdown of mitochondrial ETC genes relative to 5 week old male WT flies (n≥24). 3 and 7-week old male WT data are included for reference. *;p<0.05, Mann Whitney test. Error bars: s.e.m.
We tested whether the TRiC chaperonin complex contributes to the beneficial cardioprotective effect of TRF. We investigated heterozygous P-element insertional mutants for 5 TRiC chaperonin subunits (cct5, cct-gamma, tcp1, tcp-1eta, tcp-1zeta) under ALF and TRF. No gross morphological heart defect was seen in ALF TRiC flies (fig. S10). For the TRiC mutants, TRF failed to improve cardiac contractility relative to genotype-, and age-matched ALF flies as measured by heart period and arrhythmicity (Fig. 4, C–E; fig. S11 and S12; movie S4). Potential dominant negative effect of the P-element insertion or reduced expression of some TRiC components (fig. S11) might affect normal function of the TRiC complex in these mutants. The lack of cardioprotective benefits of TRF by multiple mutants for different TRiC subunits provides genetic evidence that the integrity of the entire TRiC complex supports TRF-driven deceleration of cardiac aging.
To test whether the cardiac tissue-restricted reduction of mitochondrial ETC transcripts contributes to TRF-dependent cardioprotection, we tested flies with heart-specific RNAi-mediated reduction of ETC complex components. Heart-specific RNAi of complex I component CG9762 led to improved cardiac physiology in 5 weeks old ALF flies (Fig. 4, F–H; movie S5), reminiscent of TRF benefits in wildtype flies. Heart -specific RNAi of two additional components CG5389 and CG18809 also led to reduced arrhythmia in 5w old ALF flies although improvement in HP was not significant (Fig. 4, F–H; fig. S13). Thus lowering of ETC function may account for at least a part of the beneficial effect of TRF.
Genetic, dietary, and lifestyle (shiftwork) perturbation of circadian rhythms predisposes organisms to chronic diseases including cardiovascular diseases. In rodents, the daily cycle of feeding-fasting under TRF reinforces diurnal rhythms in multiple organs and prevents metabolic diseases when challenged with a high fat diet (13). Here we show that TRF protects against cardiac tissue aging in flies on either a normal or a fat-supplemented diet. This benefit appears to be mediated by the circadian clock, the TRiC chaperonin and mitochondrial ETC components.
Supplementary Material
Acknowledgements
We thank VDRC, Bloomington Stock Center, and Dr. P. Hardin for fly strains; Drs. R. Bodmer and K. Ocorr for use of Drosophila heart analysis setup; Dr. S. Bernstein for use of lab facility, Dr. M. Ku and Dr. C. Benner for RNA sequencing and bioinformatics. This work was partially supported by NIH grants DK091618, EY016807, NS066457 and AFAR grant #M14322 to S.P., NIH RR032100 and an American Heart Association (13BGIA17260057) to G.C.M. S.G. was supported by H.A. and Mary K. Chapman Trust, an Aginsky Research Scholar Award and Leona M. and Harry B. Helmsley Charitable Trust’s grant #2012-PG-MED002. Additional support came from NIH P30 CA014195, P30 EY019005, and the Glenn Center for Aging.
Footnotes
We declare no conflicting interests.
List of Supplementary Content
Materials and methods
Figures S1–S14
Supplementary Figure captions
Tables S1–S9
Supplementary Table captions
Movies S1–S5
Supplementary movie captions
References (14–23) are called out in the SM.
References
- 1.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal. 2011;32:1484. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 3.Fink M, et al. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009 Feb;46:101. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 5.Birse RT, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell metabolism. 2010;12:533. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birse RT, Bodmer R. Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Critical reviews in biochemistry and molecular biology. 2011;46:376. doi: 10.3109/10409238.2011.599830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M, Vosshall LB. Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol Behav. 2012;105:544. doi: 10.1016/j.physbeh.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antosh M, et al. Comparative transcriptional pathway bioinformatic analysis of dietary restriction, Sir2, p53 and resveratrol life span extension in Drosophila. Cell cycle (Georgetown, Tex. 2011;10:904. doi: 10.4161/cc.10.6.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmers C, et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ocorr K, et al. Genetic control of heart function and aging in Drosophila. Trends in cardiovascular medicine. 2007;17:177. doi: 10.1016/j.tcm.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn AY, Melville MW, Frydman J. Review: cellular substrates of the eukaryotic chaperonin TRiC/CCT. Journal of structural biology. 2001;135:176. doi: 10.1006/jsbi.2001.4380. [DOI] [PubMed] [Google Scholar]
- 12.Hardin PE, Panda S. Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol. 2013;23:724. doi: 10.1016/j.conb.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatori M, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell metabolism. 2012;15:848. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, DiAngelo JR, Hughes ME, Hogenesch JB, Sehgal A. The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell metabolism. 2011;13:639. doi: 10.1016/j.cmet.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ocorr K, Akasaka T, Bodmer R. Age-related cardiac disease model of Drosophila. Mech Ageing Dev. 2007;128:112. doi: 10.1016/j.mad.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melkani GC, et al. Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Drosophila Heart. PLoS Genet. 2013;9:e1004024. doi: 10.1371/journal.pgen.1004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melkani GC, Bodmer R, Ocorr K, Bernstein SI. The UNC-45 chaperone is critical for establishing myosin-based myofibrillar organization and cardiac contractility in the Drosophila heart model. PLoS ONE. 2011;6:e22579. doi: 10.1371/journal.pone.0022579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 19.Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straume M. DNA microarray time series analysis: automated statistical assessment of circadian rhythms in gene expression patterning. Methods Enzymol. 2004;383:149. doi: 10.1016/S0076-6879(04)83007-6. [DOI] [PubMed] [Google Scholar]
- 23.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome biology. 2003;4:P3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.