Abstract
Campylobacter spp. are important causes of bacterial gastroenteritis in humans in developed countries. Among Campylobacter spp. Campylobacter jejuni (C. jejuni) and C. coli are the most common causes of human infection. In this study, a multiplex PCR (mPCR) and high resolution melt (HRM) curve analysis were optimized for simultaneous detection and differentiation of C. jejuni and C. coli isolates. A segment of the hippuricase gene (hipO) of C. jejuni and putative aspartokinase (asp) gene of C. coli were amplified from 26 Campylobacter isolates and amplicons were subjected to HRM curve analysis. The mPCR-HRM was able to differentiate between C. jejuni and C. coli species. All DNA amplicons generated by mPCR were sequenced. Analysis of the nucleotide sequences from each isolate revealed that the HRM curves were correlated with the nucleotide sequences of the amplicons. Minor variation in melting point temperatures of C. coli or C. jejuni isolates was also observed and enabled some intraspecies differentiation between C. coli and/or C. jejuni isolates. The potential of PCR-HRM curve analysis for the detection and speciation of Campylobacter in additional human clinical specimens and chicken swab samples was also confirmed. The sensitivity and specificity of the test were found to be 100% and 92%, respectively. The results indicated that mPCR followed by HRM curve analysis provides a rapid (8 hours) technique for differentiation between C. jejuni and C. coli isolates.
Introduction
Thermophilic Campylobacters, C. jejuni and C. coli, are the leading causes of human foodborne bacterial gastroenteritis worldwide, and are of major public health significance [1].Campylobacter has been identified as the major source of food poisoning in the United States [2], Europe [1, 3] and Australia [4].
The prevalence of Campylobacter has been studied in a number of farm animals including cattle, sheep, pigs, and chickens [5, 6]. However, exposure to contaminated food of poultry origin has been considered to be the main risk factor for Campylobacter infection in humans [4, 7, 8].
The routine testing of foodborne pathogens, such as Campylobacter spp., in animal production is an essential component of integrated food safety management systems [9]. Surveillance data, describing the on-farm prevalence and contamination levels of Campylobacter spp. in the slaughterhouse, can be used for the implementation of food safety policies and the development and evaluation of intervention strategies to eliminate or mitigate the risk to the consumer. With innovation being the driving force, there is a constant need to improve diagnostic techniques that can rapidly and accurately detect and identify the foodborne pathogens such as Campylobacter. Molecular techniques with high sensitivity and specificity are now considered a gold standard test for some pathogens [10, 11], however, it is important to understand the limitations of these techniques in the detection of enteric pathogens from faecal samples [12].
Compared to classical phenotypic techniques for the subtyping of Campylobacter spp., genotyping methods are rapid, cost-effective and have been proven to be useful in epidemiological investigations [13, 14]. Various genotyping techniques have been used for differentiation of Campylobacter spp. [15]. Pulsed-field gel electrophoresis (PFGE) has a high discriminatory power and has been extensively used as a gold standard method [16, 17]. However, it is labour-intensive and difficult to standardise between different laboratories [18, 19]. Other molecular methods have been used such as Multilocus Sequence Typing (MLST) [20], triplex Polymerase Chain Reaction (PCR)[21], PCR and restriction fragment length polymorphism (RFLP)[22, 23], real-time PCR [24, 25], multiplex PCR (mPCR) [26–28], ribotyping, flagellin (fla) typing [29], and amplified fragment length polymorphism (AFLP) [30]. The flaA gene is a common feature of C. jejuni and C. coli and has been widely used for genotyping of the species using PCR followed by RFLP and short variable region (SVR) sequencing [29, 31]. Despite extensive use of flaA-based typing techniques, this method may not be reliable since flaA alleles are unstable due to recombination [32] and intra-species exogenous DNA uptake [33].
It has been established that co-colonisation of host animals with more than one bacterial species can occur [34, 35] and this has also been observed in human clinical cases [36]. Therefore, molecular tests such as mPCR that have the potential to simultaneously detect multiple genotypes would be valuable in these circumstances. The development of fluorescent DNA binding dyes with enhanced saturation properties has permitted a more accurate evaluation of nucleotide sequence variation based on the analysis of DNA melting curves. The technique used in this study, which is referred to as high resolution melt (HRM) curve analysis, has been used for genotyping of C. jejuni [37, 38], and later C. jejuni and C. coli [39]. However, in previous studies, the detection and differentiation between Campylobacter species was made based on visual interpretation of differences in melt curves.
The aim of the current study was to optimize a mPCR-HRM curve analysis technique using non-subjective interpretation of the data derived from HRM curve analysis and to evaluate its discriminatory power for the differentiation of C. jejuni, C. coli and intraspecies without requiring enrichment prior testing.
Materials and Methods
Campylobacter Strains
Twenty-six Campylobacter isolates were tested in this study and are shown in Table 1. Campylobacter ATCC29428 and ATCC33559 strains were used as controls for C. jejuni and C. coli, respectively. All C. jejuni and C. coli isolates were provided by Birling Avian Laboratories, New South Wales, Australia. The nine C. jejuni isolates of chicken droppings were previously tested by different techniques [18, 39]. Campylobacter isolates of broiler chicken carcass origin were cultured by Birling Avian Laboratories using standard microbiological methods. All isolates were sub-cultured on sheep blood agar (ThermoFisher Scientific, Australia) and incubated at 42°C under microaerophilc conditions (83% N2, 4% H2, 8% O2 and 5% CO2). After 72 hours, all cultured plates were observed for purity. The suspected Campylobacter colonies were confirmed by phase contrast microscopy for characteristic corkscrew-like motility and spiral shaped cells. A single representative colony from each culture was used for DNA extraction. Pure cultures of the isolates were stored at -70°C using a cryovial (MicrobankTM, Pro-Lab Diagnostics, Australia) for further use. In addition, clinical samples including nine human faecal samples previously confirmed positive for Campylobacter were collected from Westmead Hospital (Sydney, Australia) and 25 swab samples from chicken carcases were used to evaluate the developed PCR-HRM technique for its potential to differentiate C. jejuni and C. coli.
Table 1. Identification, species, source and mean±SD of the melting points and GCP for C. jejuni and C. coli isolates when using ATCC29428 and ATCC33559 as reference strains, respectively.
| Isolate ID. | flA types a | Species | Source | No. of times tested | Peak 1 (°C) | Peak 2 (°C) | GCP±SD (%) | GenBank Acc. No. |
|---|---|---|---|---|---|---|---|---|
| C669 | NT b | C. coli | Chicken dropping | 17 | 79.2 ±0.3 | 82.9±0.3 | 63.5±6.5 | KF830146 |
| C1280 | NT | C. coli | Chicken dropping | 19 | 80.0±0.4 | 83.3±0.3 | 74.5±3.8 | KF830147 |
| C326 | NT | C. coli | Chicken dropping | 22 | 79.4±0.4 | 83.5±0.3 | 96.2±2.0 | KF830145 |
| C286 | NT | C. coli | Chicken dropping | 19 | 79.2±0.4 | 83.3±0.4 | 90.5±3.4 | KF830152 |
| D912 | NT | C. coli | Chicken dropping | 19 | 79.5±0.4 | 83.6±0.4 | 94.7±3.2 | KF830153 |
| BAL172668 | NT | C. coli | Broiler chicken carcass | 21 | 79.9±0.4 | 83.7±0.3 | 82.4±7.7 | KF830150 |
| BAL172832 | NT | C. coli | Broiler chicken carcass | 19 | 79.3±0.4 | 83.5±0.3 | 97.3±1.9 | KF830151 |
| BAL172104 | NT | C. coli | Broiler chicken carcass | 19 | 79.7±0.4 | 83.4±0.4 | 90.6±2.8 | KF830149 |
| ATCC33559 | NT | C. coli | Pig feces | 27 | 79.4±0.4 | 83.5±0.3 | 99.5±2.7 | KF830148 |
| C350 | XV | C. jejuni | Chicken dropping | 19 | 81.2±0.4 | 65.0±6.0 | KF830154 | |
| C1270 | XXIII | C. jejuni | Chicken dropping | 16 | 80.8±0.4 | 82.6±0.4 | 91.5±6.3 | KF830155 |
| C660 | XI | C. jejuni | Chicken dropping | 19 | 81.1±0.4 | 63.6±8.0 | KF830164 | |
| L131 | VIII | C. jejuni | Chicken dropping | 16 | 81.1±0.3 | 83.4±0.3 | 93.5±1.2 | KF830167 |
| M2 | I | C. jejuni | Chicken dropping | 16 | 81.0±0.4 | 73.9±7.6 | KF830168 | |
| C358 | NT | C. jejuni | Chicken dropping | 16 | 81.0±0.3 | 63.8±8.2 | KF830163 | |
| C1212 | V | C. jejuni | Chicken dropping | 16 | 81.1±0.3 | 64.1±7.6 | KF830165 | |
| D190 | NT | C. jejuni | Poultry farm environment | 4 | 80.6±0.4 | 58.8±1.7 | KF830166 | |
| N15 | LIII (XXIV) | C. jejuni | Chicken dropping | 7 | 80.5±0.3 | 82.5±0.4 | 95.9±0.5 | KF830169 |
| N70 | XXVI | C. jejuni | Chicken dropping | 7 | 80.7±0.5 | 85.3±3.1 | KF830170 | |
| A529 | I | C. jejuni | Chicken dropping | 15 | 80.6±0.9 | 88.1±5.4 | KF830162 | |
| BAL172630 | NT | C. jejuni | Broiler chicken carcass | 13 | 80.7±0.5 | 75.8±8.6 | KF830161 | |
| BAL172236 | NT | C. jejuni | Broiler chicken carcass | 19 | 81.0±0.3 | 83.3±0.4 | 83.3±11.6 | KF830159 |
| BAL172643 | NT | C. jejuni | Broiler chicken carcass | 9 | 80.7±0.4 | 82.5±0.3 | 92.9±4.9 | KF830160 |
| BAL172084 | NT | C. jejuni | Broiler chicken carcass | 12 | 80.8±0.4 | 76.9±6.0 | KF830158 | |
| ATCC29428 | NT | C. jejuni | Human feces | 27 | 80.6±1.2 | 83.0±0.5 | 98.2±0.7 | KF830157 |
| NCTC11351 | NT | C. jejuni | Bovine feces | 21 | 81.1±0.4 | 72.5±4.1 | KF830156 |
a flA types were determined by Merchant-Patel, 2008
b Not tested
Ethics Statement
Human faecal samples from unidentifiable patients were provided for research purposes by Westmead hospital (Sydney, Australia). The study was reviewed and approved by Human Research Ethics Committee (HREC) at Charles Sturt University (permit No. 2012/125). The opportunistic chicken swab samples were collected from chicken carcases in abattoir (Poultry Processing Plant, NSW, Australia) where chickens were slathered for meat consumption under the Food Act 2003 (NSW) and Food Regulation 2010. Collected samples from chicken carcase were also used for research study.
DNA Extraction
Total genomic DNA was extracted from Campylobacter cultures using Wizard® SV Genomic DNA Purification kit (Promega, cat no. A2360, VIC, Australia) according to the manufacturer’s instructions. Briefly, 0.5 ml of Campylobacter culture was pelleted by centrifugation at 14, 000 x g for 2 min. The pellet was resuspended in lysis/RNase solution and incubated at 80°C for 10 min. The bacterial cell lysate was transferred into the Wizard® SV mini-column assembly and centrifuged at 13,000 × g for 3 min. The column was washed with 650 μl of wash buffer three times and each time was subjected to centrifugation at 13, 000 g for 2 min. The DNA was eluted from the matrix using 50 μl distilled PCR-grade water. The extraction of DNA from clinical faecal samples and chicken carcase swab samples was performed using QIAmp DNA stool Mini Kit (Qiagen, Australia) and Wizard® SV Genomic DNA Purification kit (Promega, cat no. A2360, VIC, Australia) according to the manufacturer’s instructions. All extracted DNA were quantified using the Nanodrop2000 (ThermoFisher Scientific, Australia) and the concentration of each DNA sample adjusted to 5 ng/μl for subsequent mPCR amplification or stored at -20°C for future use.
mPCR Amplification
The N-benzoylglycine amidohydrolase or hippuricase (hipO) and putative aspartokinase (asp) genes were selected for identification of the species Campylobacter jejuni and coli, respectively. The primer sequences for the gene targets were selected from published literature [40–43]. The mPCR was optimized using two primer sets (HIP400F) 5’-GAAGAGGGTTTGGGTGGTG-3’ and (HIP1134R) 5’-AGCTAGCTTCGCATAATAACTTG-3’ and (CC18F) 5’-GGTATGATTTCTACAAAGCGA-3’ and (CC519R) 5’-ATAAAAGACTATCGTCGCGTG-3’ for amplification of 735 and 500 bp fragments of C. jejuni and C. coli, respectively. BLAST results of primer sequences showed specific identity to C. jejuni and C. coli.
The mPCR amplification was performed in 25 μl reaction volumes on an I-Cycler (Bio-Rad Laboratories Pty., Ltd. Gladesville, Australia). The reaction mixture contained 1 μl extracted genomic DNA, 25 μM of each primer, 1.5 mM MgCl2, 1250 μM of each dNTP, 5 μM SYTO® 9 green fluorescent nucleic acid stain (Life Technologies Australia Pty Ltd., Mulgrave, Australia), 1× GoTaq® Green Flexi Reaction Buffer (Promega) and 1 U of Go Taq DNA polymerase (Promega Corporation, USA). The optimal mPCR conditions were initial denaturation at 96°C for 3 min, then 35 cycles of 96°C for 30 s, 55°C for 30 s and 72°C for 30 s, and a final extension of 72°C for 5 min.
Sequencing and Nucleotide Sequence Analysis of mPCR Amplicons
The mPCR amplicons of all tested samples were purified using the QIAquick® PCR Purification Kit (Qiagen, cat no. 28104, Chadstone, Australia) following the manufacturer’s instructions. Purified amplicons were subjected to automated sequencing (BigDye® Terminator v3.1, Applied Biosystems, Life Technologies Australia Pty Ltd., Mulgrave, Australia) in both directions, using the same primers for each species as used for mPCR. The nucleotide sequences were analysed using ClustalW [44] and BioEdit Sequence Alignment Editor (version 6.0.9.0).
High-Resolution Melt Curve Analysis
HRM curve analysis was performed in a Rotor-Gene™ 6000 thermal cycler (Qiagen, Chadstone, Australia). The mPCR products were subjected to 0.5°C/s ramping between 70°C and 90°C. All specimens were tested in triplicates and their melting profiles were analysed using Rotor Gene 1.7.27 software and the HRM algorithm provided. The normalisation regions of 77–78°C and 85–86°C were used for analysis of melt curves. A reference strain for each target gene (hipO and asp gene) was set as ‘genotype’ (ATCC29428 for C. jejuni and ATCC33559 for C. coli) and the average HRM genotype confidence percentage (GCP) (the value attributed to each strain being compared to the genotype with a value of 100% indicating an exact match) for the replicates was predicted by the software.
The GCPs of all C. jejuni and all C. coli specimens were averaged separately and the standard deviation (SD) calculated for each and used to establish the GCP range for C. jejuni and C. coli strains cut off point. The values above and below cut off points were then used for identification of Campylobacter species.
To evaluate the intraspecies differentiation power of mPCR-HRM and to detect minor differences between C. jejuni or C. coli isolates, the mean GCPs±SD of ATCC29428 and ATCC33559 were calculated and used as cut off value to evaluate the differences between isolates within each Campylobacter species.
Results
Amplified mPCR products from different Campylobacter isolates were analysed by gel electrophoresis (S1 Fig). Each C. jejuni and C. coli isolate generated only one single amplicon approximately 735 and 500 bp, respectively and non-specific amplification was not observed. The primers used in this study were not able to amplify DNA fragments when the test was performed on (Campylobacter negative) stool samples.
The sensitivity of the mPCR-HRM in detecting Campylobacter species was determined by testing serial 10 fold dilutions of DNA from reference strains (ATCC33559 and ATCC29428). The first PCR dilution received 2 ng DNA template. Results showed that the sensitivity of the test was 2x10-5 and 2x10-4 ng DNA for C. jejuni and C. coli respectively.
The sensitivity and specificity of the test for differentiation of Campylobacter species in clinical samples were also determined using the receiver operating characteristic (ROC) analysis at three different cut off points (≥10, ≥60 and ≥90). The sequencing results were used as gold standard. The sensitivity and specificity of the test were 100% and 92% respectively, when cut off point was set ≥60 and were superior compared to those of cut off points ≥10 and ≥90.
Differentiation of C. jejuni and C. coli Strains by Conventional and Normalised HRM Curve Analysis
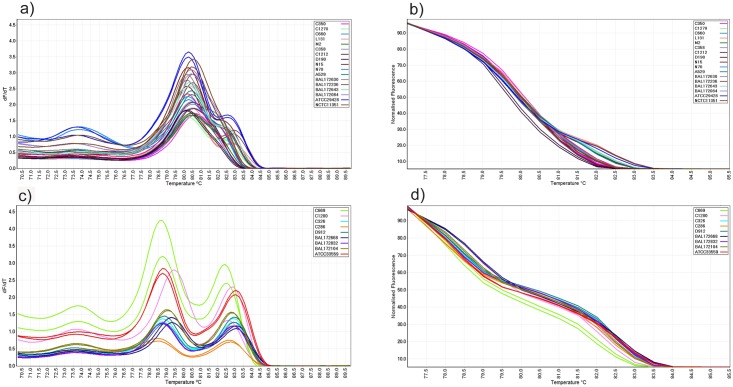
The mPCR amplicons from 26 Campylobacter isolates were subjected to HRM curve analysis (Fig 1). Visual examination of the conventional melt curves at different ramp temperatures revealed that 0.5°C/s resulted in most strains showing distinct profiles. Overall, two major conventional and normalized melt curve profiles were detected (Fig 1a and 1b).
Fig 1. Conventional and normalized melt curve analysis of Campylobacter strains.
(a) Conventional and (b) normalized HRM curve analysis of mPCR amplicons for C. jejuni (blue colour) and C. coli (red colour) isolates.
All C. jejuni isolates generated one peak in the range of 80.5°C–83.4°C. However, nine out of 17 C. jejuni isolates (C1270, L131, N15, N70, A529, BAL172630, BAL172236, BAL172643 and ATCC29428) also generated a shoulder peak at a higher temperature. Therefore, among C. jejuni strains two distinct conventional and normalized melting patterns could also be identified (Fig 2a and 2b). All nine C. coli strains produced two peaks in the range between 79.2°C–83.7°C in the conventional curve (Fig 2c).
Fig 2. Conventional and normalized melt curve analysis of Campylobacter species.
(a) Conventional and (b) normalized melt curve analysis of mPCR amplicons from C. jejuni isolates. All isolates produced a single peak while 9 isolates generated an additional shoulder peak at higher temperatures. (c) Conventional and (d) normalized melt curve analysis of mPCR amplicons from C. coli isolates. All isolates produced 2 peaks in conventional melt curves.
Campylobacter coli specimens, C326 and D912, both produced two peaks, one peak at 78.75°C and one at 82.90°C and had similar conventional and normalized curves (Fig 2d). The C669 C. coli isolate produced two peaks, one peak at 78.75°C and one at 82.5°C and generated conventional and normalized melt curves which were different from the rest of the C. coli isolates.
Non-Subjective Differentiation of Campylobacter Species Using GCPs
High resolution melt curve analysis for mPCR amplicons using templates from DNA extractions and mPCRs run on different days showed slight shifts in melting temperatures, but the shape and the relative position of the conventional and normalized melt curves remained unchanged.
The mean and SD of melting points for the nine C. coli and the 17 C. jejuni isolates and the mean GCP resulting from different runs of mPCR-HRM curve analysis are shown in Table 1.
Using GCPs of all C. coli isolates, a cut-off value was generated as a mathematical model to assess the relationship of the isolates without visual interpretation by the operator. The mean GCP of C. coli specimens was 87.4 and the mean SD was 12.8. The value of 2SD was subtracted from the average GCP to determine a cut off point. A cut off point value of 61.8 was determined for C. coli genotypes. Thus the GCP range of the C. coli isolates was determined to be 61.8–100 and was used for detection of all Campylobacter isolates.
Similarly, GCPs of all C. jejuni isolates were used to determine the cut off point for C. jejuni isolates. The mean GCP of C. jejuni genotypes was 78.9 and the mean SD was 12.6. The value of 2SD was subtracted from the average GCP to determine the cut off point for C. jejuni. A cut off point value of 53.7 was determined for C. jejuni genotypes. Thus the GCP ranges for the C. jejuni samples were determined to be 53.7–100 and this cut-off value was used for detection of all Campylobacter isolates.
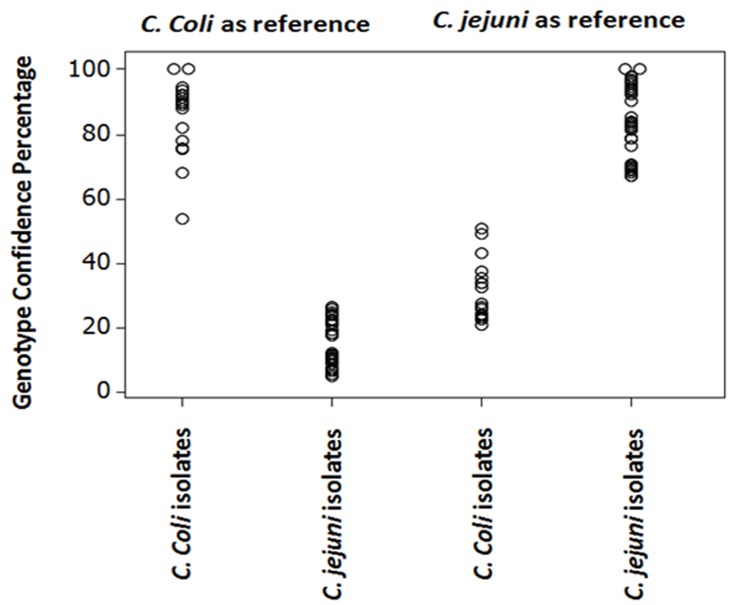
To assess the discriminatory power of the mPCR-HRM technique (i.e. the ability of the method to differentiate between C. jejuni and C. coli isolates) ATCC29428 and ATCC33559 were used as reference strains for C. jejuni and C. coli, respectively, and cut off values were applied for each species.
When ATCC33559 was used as reference genotype with a cut off value of 61.8, all C. coli isolates produced a GCP ≥61.8 and genotyped as C. coli. All C. jejuni isolates also generated GCPs between 6–24 which were <61.8, and therefore were automatically genotyped as ‘variation’.
When ATCC29428 was used as reference genotype using a cut off point of 53.7, all C. jejuni isolates were genotyped as C. jejuni with a GCP ≥53.7 and all C. coli isolates produced GCPs between 19–48 which were <53.7, and therefore automatically identified as variation. Thus gap between the highest and lowest GCP between C. coli and C. jejuni isolates were 27 and 15 when ATCC33559 or ATCC29428 were used as reference genotypes (Fig 3).
Fig 3. Comparison of the distribution of GCPs from C. jejuni and C. coli isolates by individual value plot when ATCC29428 and ATCC33559 were used as reference genotypes, respectively.
Evaluation of Discriminatory Power of mPCR-HRM in Differentiation of Intraspecies within C. jejuni and C. coli Isolates
To assess the differentiation power of newly developed mPCR-HRM technique in detection of minor differences among C. jejuni or C. coli isolates, the mean GCPs±SD of C. jejuni and C. coli reference strains (ATCC29428 and ATCC33559, respectively) were calculated. The GCP±SD for ATCC29428 and ATCC33559 were 98.2±0.7 and 99.5±2.7, respectively. The value of 2SD was subtracted from the mean GCP and the cut off points of 96.8 and 94.1 were calculated for ATCC29428 and ATCC33559, respectively.
When ATCC33559 was used as reference genotype with a cut off point of 94.1, all C. coli isolates produced a GCP≤94.1 and genotyped as variation (Table 2). When ATCC29428 was used as reference genotype with a cut off point of 96.8, all C. jejuni isolates produced a GCP≤96.8 and therefore were automatically genotyped as variation. The only exception was BAL172643 isolate that produced a GCP of 97.9 and could not be differentiated from ATCC29428 by this method (Table 3). However, BAL172643 and ATCC29428 were differentiable by visual examination of their conventional and normalized curves (S2 Fig). The nucleotide sequences of BAL172643 and ATCC29428 were 98.1% identical.
Table 2. Intraspecies differentiation (genotypes) within C. coli isolates when ATCC33559 was used as reference genotype with a cut off point of 94.1.
| Isolate ID. | Species | GCP±SD (%) | Genotype |
|---|---|---|---|
| C669 | C. coli | 58.6±3.8 | Variation |
| C1280 | C. coli | 70.7±2.6 | Variation |
| C326 | C. coli | 89.8±0.8 | Variation |
| C286 | C. coli | 91.4±0.8 | Variation |
| D912 | C. coli | 88.5±3.7 | Variation |
| BAL172668 | C. coli | 76.5±3.0 | Variation |
| BAL172832 | C. coli | 91.4±0.3 | Variation |
| BAL172104 | C. coli | 87.3±1.2 | Variation |
| ATCC33559 | C. coli | 99.9±0.0 | ATCC33559 |
Table 3. Intraspecies differentiation (genotypes) within C. jejuni isolates when ATCC29428 was used as reference genotype with a cut off point of 96.8.
| Isolate ID. | Species | GCP±SD (%) | Genotype |
|---|---|---|---|
| C350 | C. jejuni | 67.2±0.8 | Variation |
| C1270 | C. jejuni | 93.3±0.2 | Variation |
| C660 | C. jejuni | 67.6±0.4 | Variation |
| L131 | C. jejuni | 91.4±0.3 | Variation |
| M2 | C. jejuni | 77.3±0.6 | Variation |
| C358 | C. jejuni | 67.3±0.6 | Variation |
| C1212 | C. jejuni | 67.8±1.3 | Variation |
| D190 | C. jejuni | 56.5±1.5 | Variation |
| N15 | C. jejuni | 95.1±0.5 | Variation |
| N70 | C. jejuni | 82.0±1.1 | Variation |
| A529 | C. jejuni | 92.1±0.6 | Variation |
| BAL172630 | C. jejuni | 82.7±0.6 | Variation |
| BAL172236 | C. jejuni | 93.6±0.6 | Variation |
| BAL172643 | C. jejuni | 97.9±0.1 | ATCC29428 |
| BAL172084 | C. jejuni | 80.1±0.2 | Variation |
| ATCC29428 | C. jejuni | 99.9±0.1 | ATCC29428 |
| NCTC11351 | C. jejuni | 76.0±1.5 | Variation |
Campylobacter HRM Curve Profiles Correlated with Nucleotide Sequence Variation of Tested Specimens
To evaluate the HRM results in detection of similarities or variations within each Campylobacter species and whether these differences are correlated with the nucleotide sequences of amplicons, all C. jejuni and C. coli amplicons were sequenced and sequence analysis was performed.
The C. jejuni and C. coli isolates with distinct HRM curve profiles within each species, showed nucleotide sequence variation to each other consisting of nucleotide deletion, insertion and/or substitution in the hipO and asp gene, respectively (results not shown).
The highest sequence identity between C. coli strains was 99.2% between D912 and C326, which produced similar conventional and normalized melt curves (S1 Table). The lowest sequence identity was 93.5% between C669 and BAL172668, which produced two distinct conventional and normalized melt curves.
The highest and lowest sequence identity between C. jejuni strains was 99.6% between C358 and C1212 and 95.3% between BAL172084 and ATCC29428, respectively (S2 Table). The C358 and C1212 generated similar conventional and normalized melt curves while BAL172084 and ATCC29428 produced two distinct curves in HRM analysis. The correlation of sequence identities and GCP values was calculated to be 0.691 for C. coli isolates and 0.490 for C. jejuni isolates using Pearson correlation analysis.
The amplicon size plays a crucial role in HRM curve analysis [45]. Amplicon sizes of 200–400 bp (or less) can increase the detection sensitivity of sequence diversity in tested specimens [46, 47]. However, HRM analysis depends on the number of base pair changes in target DNA segment. If there is large number of nucleotide variations, then larger segments can be targeted. Larger segments of amplicons have been successfully used in PCR-HRM analysis in previous studies [17, 35, 48, 49].
Phylogenetic trees were generated for each Campylobacter species based on the sequence alignments (S3 Fig). Campylobacter isolates with high sequence identity such as C326 and D912 (C. coli) or M2 and N70 (C. jejuni) formed a clade, while isolates with sequence variation formed a sister clade (C1280) or a separate clade (C669) based on the level of sequence diversity.
Assessment of mPCR-HRM Technique for Its Potential in Detection and Differentiation of C. jejuni and C. coli in Clinical Specimens
The mPCR amplicons from faecal samples were subjected to HRM curve analysis and conventional and normalized curves were compared with C. jejuni and C. coli reference genotypes. The shape and number of curves and melting point of the peaks were considered in the initial screening. However, genotyping of samples was carried out using Rotor Gene 1.7.27 software. Amplicons of all tested samples were also sequenced for further analysis.
Examination of conventional HRM curves from human faecal samples revealed that samples 5, 10, 50, 55 and 56 produced a peak between 79.9–80.1°C. Sample 50 also produced a shoulder peak at higher temperature (82.2°C) (Table 4). These curves were comparable with that of ATCC29428 (C. jejuni) and therefore, were automatically genotyped as C. jejuni when the cut off point was applied and ATCC29428 was used as reference genotype (Fig 4).
Table 4. Clinical human faecal samples tested with mPCR-HRM.
Mean±SD of the melting points and GCP produced by isolates when ATCC29428 and ATCC33559 were used as reference strains.
| Isolate ID. | Peak 1 (°C) | Peak 2 (°C) | Peak 3 (°C) | GCP±SD (%) | Genotype | GenBank Acc. No. |
|---|---|---|---|---|---|---|
| 5 | 79.9±0.2 | 80.5±0.5 | C. jejuni | KP164637 | ||
| 9 | 77.3±0.5 | 82.6±0.4 | 96.4±0.4 | C. coli | KP164643 | |
| 10 | 80.1±0.8 | 72.5±1.7 | C. jejuni | KP164638 | ||
| 11 | 77.8±0.5 | 80.1±0.3 | 82.0±0.2 | 48.2±0.1 a 39.0±0.6 b | Variation | KP164639 and KP164644 |
| 12 | 77.3±0.5 | 82.6±0.2 | 85.0±0.3 | C. coli | KP164645 | |
| 50 | 80.0±0.1 | 82.2±0.1 | 77.4±0.8 | C. jejuni | KP164640 | |
| 53 | 77.3±0.5 | 82.6±0.1 | 85.2±0.3 | C. coli | KP164646 | |
| 55 | 79.9±0.2 | 81.0±0.8 | C. jejuni | KP164641 | ||
| 56 | 80.0±0.3 | 73.5±1.8 | C. jejuni | KP164642 | ||
| ATCC33559 | 77.3±0.2 | 82.6±0.3 | 99.8±0.1 | C. coli | KF830148 | |
| ATCC29428 | 79.9±0.8 | 82.4±0.5 | 99.9±0.0 | C. jejuni | KF830157 |
a, when ATCC29428 was used as reference strain
b, when ATCC33559 was used as reference strain
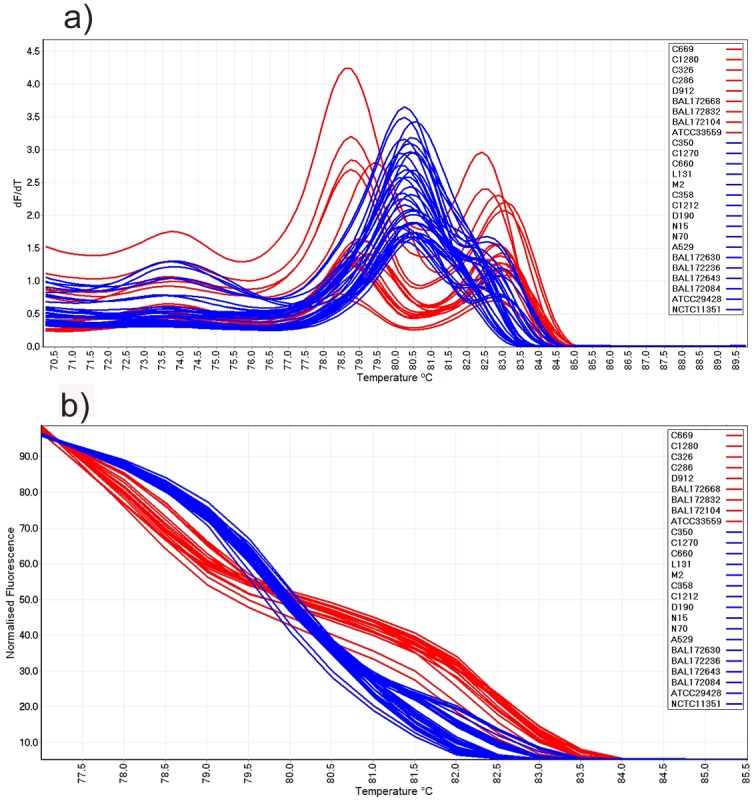
Fig 4. Conventional and normalized melt curve analysis of human clinical samples.
(a) Conventional melt curve and (b) normalized HRM curve analysis of mPCR amplicons of human faecal samples.
Clinical specimens 9, 12 and 53 each generated a conventional curve with two peaks at 77.3 and 82.6°C which were similar to C. coli reference strain ATCC33559 (Fig 4a). The normalized curves of these specimens were also similar and all were genotyped as C. coli when the cut off point was applied and ATCC33559 was used as reference genotype (Fig 4b).
However, specimen 11 produced a conventional curve consisting of one peak at 80°C and two shoulder peaks at 77.8°C and 82.0°C which was different from both reference genotypes (ATCC29428 and ATCC33559) and other clinical specimens (Fig 4a). The normalized curve of sample 11 was also distinct from the rest of samples and therefore, automatically was genotyped as variation when ATCC29428 or ATCC33559 were used as reference genotype (Fig 4b). Results indicated that the test has the capacity to differentiate C. coli and C. jejuni in clinical samples without requiring Campylobacter isolation in culture or enrichment step prior testing.
Agarose gel analysis of these samples revealed that all specimens identified as C. jejuni produced a single DNA fragment about 735 bp while all samples identified as C coli generated a single fragment about 500 bp. Sample 11 produced two DNA fragments of 735 and 500 bp similar to C. jejuni and C. coli, respectively (data not shown). All amplicons including both DNA fragments of sample 11 were sequenced. All sequences were subjected to a BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine their identities. Sample 11 contained a mixed infection of C. jejuni and C. coli. Results of BLAST search confirmed HRM genotyping. The phylogenetic tree generated based on multiple sequence alignment of clinical samples generated two separate clades for C. jejuni and C. coli species (S4 Fig).
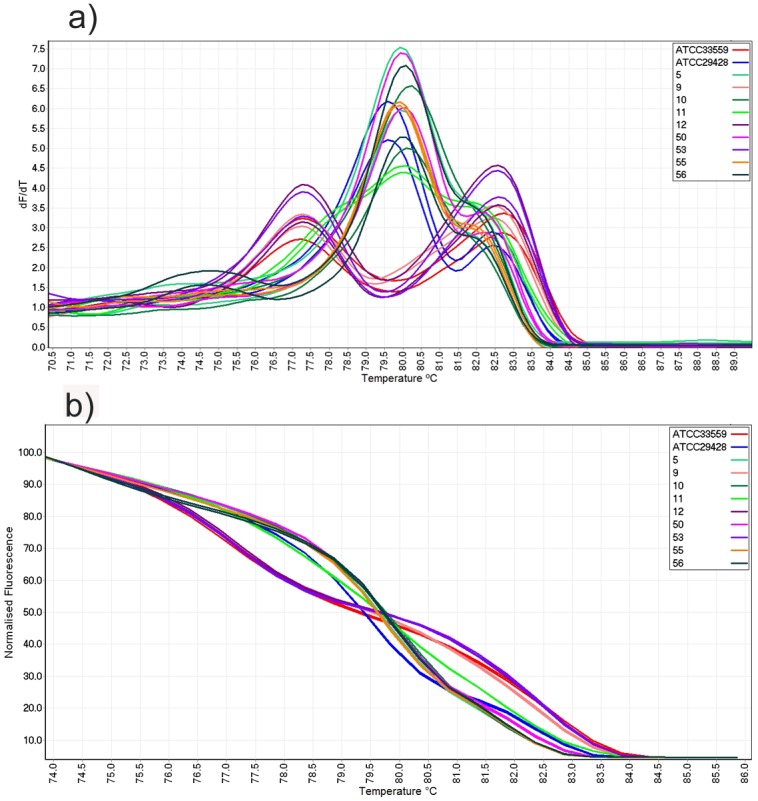
The mPCR amplicons from 25 chicken carcase swab samples were subjected to HRM curve analysis and were genotyped using Rotor Gene software when cut off points of reference strains were applied (Table 5). Five swab samples were genotyped as C. coli and generated a peak between 76.60–81.60°C while 15 samples genotyped as C. jejuni and produced one peak at 79.60–79.65°C or one peak at 78.75–79.65°C and a shoulder peak at higher temperature (81.10–81.40°C) (Fig 5). Three swab samples (212262, 212266 and 212516) did not produce HRM curve or DNA fragment on agarose gel and two samples (212252 and 212255) produced one peak at 79.25°C and two shoulder peak at 76.5°C and 81.25°C which were different to those of C. coli and C. jejuni specimens and also produced two DNA fragments of about 735 and 500 bp in agarose gel indicating of mixed infection with the two species.
Table 5. Chicken carcase swab samples tested with mPCR-HRM.
Mean±SD of the melting points and GCP produced by isolates when ATCC29428 or ATCC33559 were used as reference strains.
| Culture | mPCR-HRM | |||||||
|---|---|---|---|---|---|---|---|---|
| Melting points | Mean GCP±SD (%) | |||||||
| Isolate ID. | culture | species | Peak 1 (°C) | Peak 2 (°C) | Peak 3 (°C) | ATCC33559 used as reference strain | ATCC29428 used as reference strain | Genotype |
| ATCC33559 | Positive | C. coli | 76.1±0.1 | 82.1±0.1 | 99.9±0.1 | 7.7±0.1 | C. coli | |
| ATCC29428 | Positive | C. jejuni | 78.5±0.0 | 81.8±0.0 | 7.7±0.3 | 99.9±0.1 | C. jejuni | |
| 212250 | Negative | NA a | 76.2±0.1 | 81.9±0.3 | 88.0±0.5 | 17.5±0.2 | C. coli | |
| 212251 | Negative | NA | 76.4±0.1 | 81.9±0.2 | 90.3±0.3 | 16.3±0.2 | C. coli | |
| 212252 | Negative | NA | 77.5±1.3 | 80.2±2.4 | 81.9±0.1 | 32.4±1.1 | 49.1±1.1 | Variation |
| 212253 | Negative | NA | 78.5±0.1 | 81.6±0.1 | 4.6±0.4 | 96.3±1.4 | C. jejuni | |
| 212254 | Negative | NA | 76.4±0.0 | 81.9±0.0 | 92.2±0.2 | 11.9±0.2 | C. coli | |
| 212255 | Negative | NA | 76.0±0.1 | 78.3±0.1 | 82.0±0.0 | 49.3±2.1 | 50.3±2.5 | Variation |
| 212256 | Negative | NA | 78.8±0.1 | 81.2±0.1 | 2.5±0.2 | 71.1±0.9 | C. jejuni | |
| 212257 | Negative | NA | 76.3±0.1 | 81.8±0.1 | 81.7±3.2 | 11.9±0.4 | C. coli | |
| 212258 | Negative | NA | 78.8±0.1 | 80.9±0.0 | 1.9±0.2 | 58.3±2.5 | C. jejuni | |
| 212259 | Negative | NA | 76.2±0.1 | 82.1±0.1 | 95.9±2.5 | 9.0±0.5 | C. coli | |
| 212262 | Negative | NA | NA | NA | NA | NA | NA | Negative |
| 212263 | Negative | NA | 78.8±0.0 | 81.5±0.0 | 5.4±0.2 | 71.1±1.8 | C. jejuni | |
| 212264 | Negative | NA | 78.9±0.1 | 81.4±0.0 | 4.4±0.1 | 58.8±0.5 | C. jejuni | |
| 212265 | Negative | NA | 78.3±0.0 | 81.5±0.0 | 6.8±0.2 | 92.2±0.7 | C. jejuni | |
| 212266 | Negative | NA | NA | NA | NA | NA | NA | Negative |
| 212514 | Positive | C. jejuni | 78.5±0.0 | 81.9±0.1 | 5.5±0.1 | 91.8±0.6 | C. jejuni | |
| 212515 | Positive | C. jejuni | 78.5±0.0 | 81.6±0.0 | 5.4±0.2 | 94.5±0.5 | C. jejuni | |
| 212516 | Negative | NA | NA | NA | NA | NA | NA | Negative |
| 212517 | Positive | C. jejuni | 78.5±0.0 | 81.8±0.0 | 3.8±0.3 | 92.4±1.4 | C. jejuni | |
| 212518 | Positive | C. jejuni | 78.5±0.0 | 81.8±0.0 | 7.6±0.1 | 90.3±2.0 | C. jejuni | |
| 212519 | Positive | C. jejuni | 78.5±0.0 | 81.7±0.1 | 4.9±0.2 | 94.4±0.4 | C. jejuni | |
| 212520 | Positive | C. jejuni | 78.5±0.0 | 81.7±0.1 | 6.6±0.1 | 91.9±1.8 | C. jejuni | |
| 212521 | Positive | C. jejuni | 78.5±0.0 | 81.5±0.1 | 4.8±0.5 | 94.4±0.8 | C. jejuni | |
| 212522 | Positive | C. jejuni | 78.7±0.0 | 80.9±0.1 | 5.9±0.7 | 89.8±4.1 | C. jejuni | |
| 212523 | Negative | NA | 78.7±0.0 | 80.8±0.0 | 0.9±0.2 | 56.8±0.8 | C. jejuni | |
aNA, Not applicable
Fig 5. Conventional and normalized melt curve analysis of chicken clinical samples.
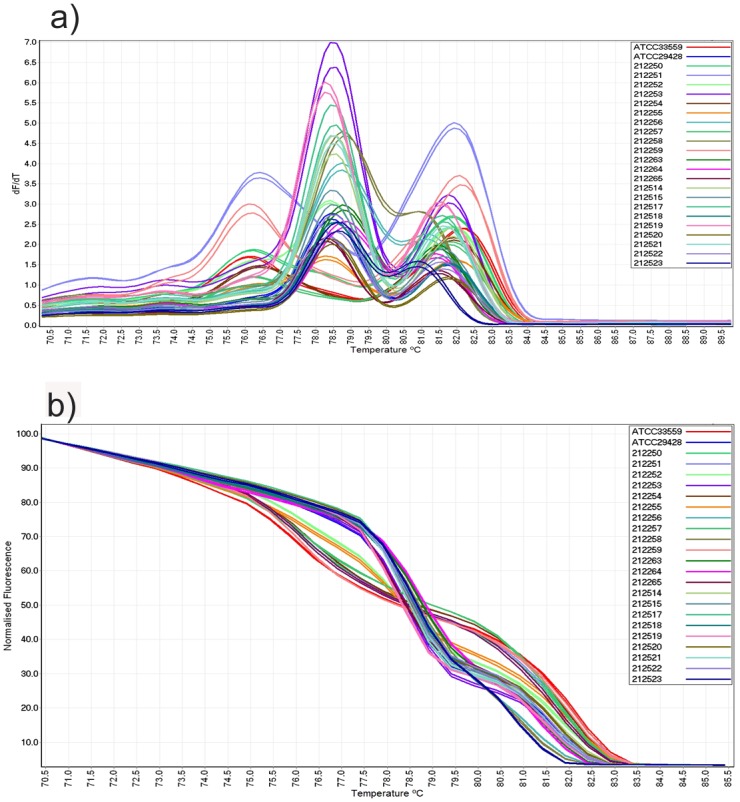
(a) Conventional melt curve and (b) normalized HRM curve analysis of mPCR amplicons from chicken swab samples.
Discussion
This study describes a rapid and reliable mPCR-HRM technique for the differentiation of C. jejuni and C. coli isolates. The melting analysis of Campylobacter DNA, amplified with two sets of primers in a mixture containing a DNA intercalating dye (SYTO9) as a double-stranded DNA binding dye, allowed rapid detection of Campylobacter species C. jejuni and C. coli.
Although C. jejuni and C. coli are very close in their phenotypic and genotypic characteristics, the present study shows that the mPCR-HRM technique was able to differentiate between C. jejuni and C. coli using hipO and asp genes, respectively. Moreover, mPCR-HRM demonstrated some capability in the detection of minor variations within C. jejuni or C. coli species. Subtle differences in conventional and normalized curves within C. jejuni have been reported in the flaA gene [39]. The mPCR-HRM developed in this study, produced two distinct curve profiles for C. jejuni and C. coli species and could automatically differentiate the two species based on their GCPs without visual interpretation.
PCR-HRM curve analysis is a powerful and valuable tool to study the nucleotide diversity of amplicons between tested specimens. Results from this study indicate that mPCR-HRM has the potential to detect Campylobacter species and differentiate isolates based on their sequence variation of targeted gene. In HRM curve analysis, all samples are compared with a given arbitrary reference strain, and those that generate GCPs ≥ cut off point are considered similar to the reference strain while those that produce GCPs ≤ cut off point are considered as “variation”. Therefore samples genotyped as variation could well be different to the others. To study the possible difference between samples recognized as “variation” using a given arbitrary reference, another sample could be set up as reference genotype and samples can be reanalysed for their relationship with the reference. These analyses are conducted readily through the software provided with additional testing.
However, the PCR procedure could be susceptible to several factors such as quality and quantity of DNA template, annealing temperature between primers and DNA templates, self-annealing between PCR products and different copy numbers of the targeted genes. These factors may affect PCR results and subsequently HRM curve analysis. Therefore, optimization of the test including different quantities of DNA template and concentrations of PCR reagents were performed (data not shown).
Experience with HRM curve analysis in our laboratory has shown that the quantification of genomic DNA and use of an equal concentration of DNA for all tested specimens is useful for reliable amplification in PCR (unpublished data). However, equal concentrations of DNA may not be guaranteed in clinical samples, therefore whenever possible, adjustment of DNA to equal concentration would be beneficial for improving the consistency and reproducibility of HRM curve profiles to attain the least variation in curve shape [50]. The differences in amplicon sizes between C. jejuni and C. coli may also have contributed to the differentiation power of this technique due to the variations in nucleotide sequences and length.
In this study, comparable HRM curve profiles generated from three different sources, pure cultures of Campylobacter isolates, human faecal specimens and chicken carcase swab samples, demonstrated the consistency of the results.
Using equal quantity of template DNA, all faecal specimens containing C. coli produced two peaks in the conventional melt curve and all specimens containing C. jejuni generated only one peak (with or without shoulder peak) which were similar to the conventional melt curves produced from pure Campylobacter cultures. Therefore, when equal concentrations of template DNA were used, the quality of DNA did not have a significant effect on the consistency of the melting patterns. In addition, each sample has been tested in different runs/days and in triplicate. The Rotor Gene 1.7.27 software can automatically genotype the samples based on the provided cut off points and therefore, does not necessarily require skilful interpretation by the operator. This feature facilitates the application of the test in the routine diagnostic or research laboratories that the instrument is available. Similar tests are now being used in the routine diagnosis of Mycoplasma gallisepticum [51], infectious bursal disease virus [48], fowl adenovirus [35] and beak and feather disease virus [48] in our laboratory.
The melting profile of amplicons is based on the length and GC content of nucleotide sequence. As the amplicon begins to melt, DNA regions that contain more G/C compared to A/T, are more stable and do not melt immediately, instead, maintain their dsDNA configuration until the temperature is adequately high to cause it to melt. This phenomenon results in tow peaks in conventional melt curve. The first peak of C. coli and some of C. jejuni isolates is likely to be due to A-T rich region which melts at lower temperature and the second peak is likely to be due to melting G-C rich region at a higher temperature.
By using HRM in this study the risk of cross-contamination was reduced as PCR-HRM is a closed-tube technique. Other benefits of this technique include rapid testing and the opportunity to detect some intraspecies variations. All C. jejuni and C. coli isolates generated distinct conventional and normalized melt curves when compared with reference strains ATCC29428 and ATCC33559, respectively. The differences in HRM curves were a reflection of nucleotide sequence variation of the targeted gene in each isolate. Multiple sequence alignments of sequenced amplicons reaffirmed the HRM results (data not shown).
The HRM method may not replace sequence-based differential techniques and any new HRM curve profiles need to be confirmed by sequencing. However, once a new curve profile has been confirmed, it can be used in subsequent HRM analysis [37].
The melt curve profiles generated in this study were consistent and the melt curve profiles of each isolate obtained from different runs were similar with regard to the number, height, and temperature of the peaks. A DNA melting simulation program referred to as POLAND (Heinrich-Heine University in Dusseldorf, Germany, Institute of Biophysics [http://www.biophys.uni-duesseldorf.de/local/POLAND/poland.html]) [52] was also used to confirm whether mPCR-HRM curve analysis could potentially differentiate additional C. jejuni and C. coli strains that were unavailable in our laboratory. This program generates theoretical melting curve patterns according to the nucleotide sequence of a DNA fragment. The melting pattern of the C. jejuni asp gene and C. coli hipO gene (flanked by the primers used in this study) from selected number of Campylobacter strains were assessed in POLAND using sequences available in the GenBank database. The melt curve profiles predicted by POLAND from additional Campylobacter strains were in agreement with those generated in this study (data not shown). This also confirmed that the melting profile of C. jejuni and C. coli amplicons was as a result of DNA dissociation in mPCR-HRM.
The ability of mPCR-HRM curve analysis in detection and differentiation of Campylobacter species was further evaluated by testing additional nine human faecal samples from a different geographical location and 25 chicken carcase swab samples. All clinical specimens were genotyped as C. jejuni or C. coli except human faecal sample 11 and chicken swab samples 212252 and 212255 which contained both C. jejuni and C. coli species and generated different melt curves. The presence of more than one Campylobacter species in host animal [35] or human sample [36] has been reported. Although samples 5, 10, 50, 55 and 56 were genotyped as C. jejuni, their normalized curves were slightly different from ATCC29428 which was a reflection of slight variation in their nucleotide sequences (data not shown). Out of 25 chicken carcase swab samples, only eight were positive in culture while in mPCR-HRM, 22 samples were positive for Campylobacter. The higher sensitivity of mPCR-HRM compared with culture in detecting Campylobacter in swab samples could be due to the detection of genomic DNA by PCR from viable and nonviable bacteria. The clinical chicken samples that were genotyped as C. jejuni produced GCP values in a range of 56.8–96.3. Among all culture negative specimens, three samples (212258, 212264 and 212523) produced lower GCP values (<70) when compared with C. jejuni culture positive samples (Table 5). The lower GCP values in these samples could be due to lower number of organism present in the sample. Comparison of equal volumes of amplicons on agarose gel showed relatively lower concentration of amplicon in these three specimens (data not shown). Samples with variable concentrations of starting DNA template produce different amount of fluorescence in HRM [50]. This along with lower concentration of target sequence in the amplicon could have contributed to the lower GCP values. However, by comparing the peak melting points and normalized melt curves (Fig 5b), similar Campylobacter species were genotyped within the same cluster.
The mPCR-HRM developed in this study represents a relatively simple method for discrimination between C.jejuni and C. coli species and also has the potential to detect differences between isolates. This could be most useful for screening of clinical specimens as these samples may hypothetically contain C. coli, C. jejuni, both species or an unknown field strain. This however should be readily detectable given that the melting curve profile of C. coli and C. jejuni are distinct and already characterized. Where “variation” is detected in clinical specimens from such specimens, the presence of the isolate is best to be confirmed using additional tests such as PCR followed by nucleotide sequencing of the amplicons.
The PFGE still could be considered as a method which has a high discriminatory power in differentiating Campylobacter species. However, it is a time consuming method and requires standard protocol and skilled technician and relatedness among samples are used as a guide not true phylogenetic measure [53].
The newly developed mPCR-HRM is faster and more cost-effective than the other laboratory methods such as Taqman PCR for differentiating these species [54, 55]. Nucleotide sequencing is believed to be the gold standard for detection of sequence variations, however, in a meta analysis, the HRM method was considered as one of the preferred methods in detection of sequence variation among current available techniques and a high sensitive modality when compared with DNA sequencing [56]. The significant advantage of HRM curve analysis relies on differentiation of isolates based on variation of their nucleotide sequences without requiring nucleotide sequencing [38, 57]. The multiplex-PCR HRM curve analysis described in this study can differentiate C. jejuni and C. coli without requiring enrichment or isolating bacteria prior to testing as well as discriminating the intraspecies within each species. In addition, mPCR-HRM curve analysis proved to be rapid, inexpensive, requires minimum requirement for interpretation, and is amenable to automation for screening of a large number of specimens.
Supporting Information
MW, molecular weight marker (PCR Marker, Sigma).
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank Birling Avian Laboratories (NSW) and Westmead Hospital (Sydney, NSW) for providing clinical samples. We also thank Prof. Amir Noormohammadi for providing scientific advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by School of Animal and Veterinary Science under Grant number 40702, the Graham Centre for Agricultural Innovation under Grant number 40830, and Faculty of Science under Grant number 40797 at Charles Sturt University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Birling Avian Laboratories provided support in the form of a salary for JC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.ECDC. European centre for disease prevention and control (ECDC). 2013.
- 2. Zhao S, Young SR, Tong E, Abbott JW, Womack N, Friedman SL, et al. Antimicrobial resistance of Campylobacter isolates from retail meat in the United States between 2002 and 2007. Appl Environ Microbiol. 2010;76(24):7949–56. Epub 2010/10/26. 10.1128/AEM.01297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eurosurveillance editorial team. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011 has been published. Euro Surveill. 2013;18(15):20449 Epub 2013/04/19. . [PubMed] [Google Scholar]
- 4. Whiley H, van den Akker B, Giglio S, Bentham R. The role of environmental reservoirs in human campylobacteriosis. Int J Environ Res Public Health. 2013;10(11):5886–907. Epub 2013/11/13. 10.3390/ijerph10115886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noormohamed A, Fakhr MK. A higher prevalence rate of Campylobacter in retail beef livers compared to other beef and pork meat cuts. Int J Environ Res Public Health. 2013;10(5):2058–68. Epub 2013/05/24. 10.3390/ijerph10052058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strachan NJ, MacRae M, Thomson A, Rotariu O, Ogden ID, Forbes KJ. Source attribution, prevalence and enumeration of Campylobacter spp. from retail liver. Int J Food Microbiol. 2012;153(1–2):234–6. Epub 2011/12/03. 10.1016/j.ijfoodmicro.2011.10.033 . [DOI] [PubMed] [Google Scholar]
- 7. Wingstrand A, Neimann J, Engberg J, Nielsen EM, Gerner-Smidt P, Wegener HC, et al. Fresh chicken as main risk factor for campylobacteriosis, Denmark. Emerg Infect Dis. 2006;12(2):280–5. Epub 2006/02/24. 10.3201/eid1202.050936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenquist H, Boysen L, Krogh AL, Jensen AN, Nauta M. Campylobacter contamination and the relative risk of illness from organic broiler meat in comparison with conventional broiler meat. Int J Food Microbiol. 2013;162(3):226–30. Epub 2013/03/05. 10.1016/j.ijfoodmicro.2013.01.022 . [DOI] [PubMed] [Google Scholar]
- 9. Havelaar AH, Braunig J, Christiansen K, Cornu M, Hald T, Mangen MJ, et al. Towards an integrated approach in supporting microbiological food safety decisions. Zoonoses Public Health. 2007;54(3–4):103–17. Epub 2007/04/26. 10.1111/j.1863-2378.2007.01036.x . [DOI] [PubMed] [Google Scholar]
- 10. Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur J Clin Microbiol Infect Dis. 2007;26(5):311–23. Epub 2007/04/21. . [DOI] [PubMed] [Google Scholar]
- 11. Quinn TC. DNA amplification assays: a new standard for diagnosis of Chlamydia trachomatis infections. Ann Acad Med Singapore. 1995;24(4):627–33. Epub 1995/07/01. . [PubMed] [Google Scholar]
- 12. Gray J, Coupland LJ. The increasing application of multiplex nucleic acid detection tests to the diagnosis of syndromic infections. Epidemiol Infect. 2014;142(1):1–11. Epub 2013/10/08. 10.1017/S0950268813002367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fussing V, Moller Nielsen E, Neimann J, Engberg J. Systematic serotyping and riboprinting of Campylobacter spp. improves surveillance: experiences from two Danish counties. Clinical Microbiology and Infection. 2007;13(6):635–42. Epub 2007/05/11. 10.1111/j.1469-0691.2007.01689.x . [DOI] [PubMed] [Google Scholar]
- 14. Taylor EV, Herman KM, Ailes EC, Fitzgerald C, Yoder JS, Mahon BE, et al. Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiol Infect. 2013;141(5):987–96. Epub 2012/08/16. 10.1017/S0950268812001744 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wassenaar TM, Newell DG. Genotyping of Campylobacter spp . Appl Environ Microbiol. 2000;66(1):1–9. Epub 2000/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerner-Smidt P, Hise K, Kincaid J, Hunter S, Rolando S, Hyytia-Trees E, et al. PulseNet USA: a five-year update. Foodborne Pathog Dis. 2006;3(1):9–19. Epub 2006/04/11. 10.1089/fpd.2006.3.9 . [DOI] [PubMed] [Google Scholar]
- 17. Ghorashi SA, Bradbury JM, Ferguson-Noel NM, Noormohammadi AH. Comparison of multiple genes and 16S-23S rRNA intergenic space region for their capacity in high resolution melt curve analysis to differentiate Mycoplasma gallisepticum vaccine strain ts-11 from field strains. Vet Microbiol. 2013;167(3–4):440–7. Epub 2013/11/19. 10.1016/j.vetmic.2013.09.032 . [DOI] [PubMed] [Google Scholar]
- 18. Merchant-Patel S, Blackall P. J., Templeton J., Price E. P., Tong S. Y., Huygens F., Giffard P. M. Characterisation of chicken Campylobacter jejuni isolates using resolution optimised single nucleotide polymorphisms and binary gene markers. Int J Food Microbiol. 2008;128(2):304–8. Epub 2008/10/07. 10.1016/j.ijfoodmicro.2008.09.002 . [DOI] [PubMed] [Google Scholar]
- 19. Hedberg CW, Smith KE, Besser JM, Boxrud DJ, Hennessy TW, Bender JB, et al. Limitations of pulsed-field gel electrophoresis for the routine surveillance of Campylobacter infections. J Infect Dis. 2001;184(2):242–4. Epub 2001/06/26. 10.1086/322005 . [DOI] [PubMed] [Google Scholar]
- 20. Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, Bolton FE, et al. Multilocus sequence typing system for Campylobacter jejuni . J Clin Microbiol. 2001;39(1):14–23. Epub 2001/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan IU, Edge TA. Development of a novel triplex PCR assay for the detection and differentiation of thermophilic species of Campylobacter using 16S-23S rDNA internal transcribed spacer (ITS) region. J Appl Microbiol. 2007;103(6):2561–9. Epub 2007/11/30. . [DOI] [PubMed] [Google Scholar]
- 22. Owen RJ, Fayos A, Hernandez J, Lastovica A. PCR-based restriction fragment length polymorphism analysis of DNA sequence diversity of flagellin genes of Campylobacter jejuni and allied species. Mol Cell Probes. 1993;7(6):471–80. Epub 1993/12/01. 10.1006/mcpr.1993.1070 . [DOI] [PubMed] [Google Scholar]
- 23. Tsai HJ, Huang HC, Tsai HL, Chang CC. PCR-based restriction fragment length polymorphism (RFLP) analysis of Campylobacter jejuni isolates from humans, chickens and dogs in northern Taiwan. J Vet Med Sci. 2006;68(8):815–9. Epub 2006/09/06. . [DOI] [PubMed] [Google Scholar]
- 24. Ridley AM, Allen VM, Sharma M, Harris JA, Newell DG. Real-time PCR approach for detection of environmental sources of Campylobacter strains colonizing broiler flocks. Appl Environ Microbiol. 2008;74(8):2492–504. Epub 2008/01/22. 10.1128/AEM.01242-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang C, Jiang Y, Huang K, Zhu C, Yin Y. Application of real-time PCR for quantitative detection of Campylobacter jejuni in poultry, milk and environmental water. FEMS Immunol Med Microbiol. 2003;38(3):265–71. Epub 2003/10/03. . [DOI] [PubMed] [Google Scholar]
- 26. Poly F, Serichatalergs O, Schulman M, Ju J, Cates CN, Kanipes M, et al. Discrimination of major capsular types of Campylobacter jejuni by multiplex PCR. J Clin Microbiol. 2011;49(5):1750–7. Epub 2011/03/18. 10.1128/JCM.02348-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiemer D, Loderstaedt U, von Wulffen H, Priesnitz S, Fischer M, Tannich E, et al. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. International Journal of Medical Microbiology. 2011;301(7):577–84. Epub 2011/08/23. 10.1016/j.ijmm.2011.06.001 . [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki-Matsune W, Taguchi M, Seto K, Kawahara R, Kawatsu K, Kumeda Y, et al. Development of a multiplex PCR assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis . J Med Microbiol. 2007;56(Pt 11):1467–73. Epub 2007/10/30. . [DOI] [PubMed] [Google Scholar]
- 29. Meinersmann RJ, Helsel LO, Fields PI, Hiett KL. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J Clin Microbiol. 1997;35(11):2810–4. Epub 1997/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schouls LM, Reulen S, Duim B, Wagenaar JA, Willems RJ, Dingle KE, et al. Comparative genotyping of Campylobacter jejuni by amplified fragment length polymorphism, multilocus sequence typing, and short repeat sequencing: strain diversity, host range, and recombination. J Clin Microbiol. 2003;41(1):15–26. Epub 2003/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nachamkin I, Bohachick K, Patton CM. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31(6):1531–6. Epub 1993/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dingle KE, Colles FM, Falush D, Maiden MC. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni . J Clin Microbiol. 2005;43(1):340–7. Epub 2005/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Taylor DE. Natural transformation in Campylobacter species. J Bacteriol. 1990;172(2):949–55. Epub 1990/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kramer JM, Frost JA, Bolton FJ, Wareing DR. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. J Food Prot. 2000;63(12):1654–9. Epub 2000/01/11. . [DOI] [PubMed] [Google Scholar]
- 35. Steer PA, O'Rourke D, Ghorashi SA, Noormohammadi AH. Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Aust Vet J. 2011;89(5):184–92. 10.1111/j.1751-0813.2011.00695.x ISI:000289641200020. [DOI] [PubMed] [Google Scholar]
- 36. Gilpin B, Robson B, Lin S, Scholes P, On S. Pulsed-field gel electrophoresis analysis of more than one clinical isolate of Campylobacter spp. from each of 49 patients in New Zealand. J Clin Microbiol. 2012;50(2):457–9. Epub 2011/11/26. 10.1128/JCM.05928-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levesque S, Michaud S., Arbeit R. D., Frost E. H.,. High-resolution melting system to perform multilocus sequence typing of Campylobacter jejuni . PLoS One. 2011;6(1):e16167 Epub 2011/02/08. 10.1371/journal.pone.0016167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price EP, Smith H, Huygens F, Giffard PM. High-resolution DNA melt curve analysis of the clustered, regularly interspaced short-palindromic-repeat locus of Campylobacter jejuni . Appl Environ Microbiol. 2007;73(10):3431–6. Epub 2007/04/03. 10.1128/AEM.02702-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merchant-Patel S, Blackall P. J., Templeton J., Price E. P., Tong S. Y., Huygens F., Giffard P. M. Campylobacter jejuni and Campylobacter coli genotyping by high-resolution melting analysis of a flaA fragment. Appl Environ Microbiol. 2010;76(2):493–9. Epub 2009/11/26. 10.1128/AEM.01164-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Debruyne L, Samyn E, De Brandt E, Vandenberg O, Heyndrickx M, Vandamme P. Comparative performance of different PCR assays for the identification of Campylobacter jejuni and Campylobacter coli . Res Microbiol. 2008;159(2):88–93. Epub 2008/02/15. 10.1016/j.resmic.2007.11.020 . [DOI] [PubMed] [Google Scholar]
- 41. Lawson AJ, Logan JM, O'Neill G L, Desai M, Stanley J. Large-scale survey of Campylobacter species in human gastroenteritis by PCR and PCR-enzyme-linked immunosorbent assay. J Clin Microbiol. 1999;37(12):3860–4. Epub 1999/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawson AJ, Shafi MS, Pathak K, Stanley J. Detection of campylobacter in gastroenteritis: comparison of direct PCR assay of faecal samples with selective culture. Epidemiol Infect. 1998;121(3):547–53. Epub 1999/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linton D, Lawson AJ, Owen RJ, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J Clin Microbiol. 1997;35(10):2568–72. Epub 1997/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–80. Epub 1994/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8(6):597–608. Epub 2007/06/15. . [DOI] [PubMed] [Google Scholar]
- 46. Chou LS, Lyon E, Wittwer CT. A comparison of high-resolution melting analysis with denaturing high-performance liquid chromatography for mutation scanning: cystic fibrosis transmembrane conductance regulator gene as a model. Am J Clin Pathol. 2005;124(3):330–8. Epub 2005/09/30. . [DOI] [PubMed] [Google Scholar]
- 47. van der Stoep N, van Paridon CD, Janssens T, Krenkova P, Stambergova A, Macek M, et al. Diagnostic guidelines for high-resolution melting curve (HRM) analysis: an interlaboratory validation of BRCA1 mutation scanning using the 96-well LightScanner. Hum Mutat. 2009;30(6):899–909. Epub 2009/04/17. 10.1002/humu.21004 . [DOI] [PubMed] [Google Scholar]
- 48. Ghorashi SA, O'Rourke D, Ignjatovic J, Noormohammadi AH. Differentiation of infectious bursal disease virus strains using real-time RT-PCR and high resolution melt curve analysis. J Virol Methods. 2011;171(1):264–71. Epub 2010/11/30. 10.1016/j.jviromet.2010.11.013 . [DOI] [PubMed] [Google Scholar]
- 49. Jeffery N, Gasser RB, Steer PA, Noormohammadi AH. Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region. Microbiology. 2007;153(Pt 8):2679–88. Epub 2007/07/31. 10.1099/mic.0.2006/005140-0 . [DOI] [PubMed] [Google Scholar]
- 50. Toi CS, Dwyer DE. Differentiation between vaccine and wild-type varicella-zoster virus genotypes by high-resolution melt analysis of single nucleotide polymorphisms. J Clin Virol. 2008;43(1):18–24. 10.1016/j.jcv.2008.03.027 ISI:000259757700004. [DOI] [PubMed] [Google Scholar]
- 51. Ghorashi SA, Kanci A, Noormohammadi AH. Evaluation of the Capacity of PCR and High-Resolution Melt Curve Analysis for Identification of Mixed Infection with Mycoplasma gallisepticum Strains. PLoS One. 2015;10(5):e0126824 Epub 2015/05/15. 10.1371/journal.pone.0126824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steger G. Thermal denaturation of double-stranded nucleic acids: prediction of temperatures critical for gradient gel electrophoresis and polymerase chain reaction. Nucleic Acids Res. 1994;22(14):2760–8. Epub 1994/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CDC. Pulsed-field Gel Electrophoresis (PFGE); Limitations of PFGE.: Centers for Disease Control and Prevention, USA Government (http://www.cdc.gov/pulsenet/pathogens/pfge.html); 2013 [cited 2015]. Available: http://www.cdc.gov/pulsenet/pathogens/pfge.html.
- 54. Wittwer CT. High-resolution DNA melting analysis: advancements and limitations. Hum Mutat. 2009;30(6):857–9. Epub 2009/05/30. 10.1002/humu.20951 . [DOI] [PubMed] [Google Scholar]
- 55. Goldschmidt P, Degorge S, Che Sarria P, Benallaoua D, Semoun O, Borderie V, et al. New strategy for rapid diagnosis and characterization of fungal infections: the example of corneal scrapings. PLoS One. 2012;7(7):e37660 Epub 2012/07/07. 10.1371/journal.pone.0037660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li BS, Wang XY, Ma FL, Jiang B, Song XX, Xu AG. Is high resolution melting analysis (HRMA) accurate for detection of human disease-associated mutations? A meta analysis. PLoS One. 2011;6(12):e28078 Epub 2011/12/24. 10.1371/journal.pone.0028078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ihle MA, Fassunke J, Konig K, Grunewald I, Schlaak M, Kreuzberg N, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer. 2014;14:13 Epub 2014/01/15. 10.1186/1471-2407-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MW, molecular weight marker (PCR Marker, Sigma).
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.