Abstract
Despite well-documented disparities in cancer pain outcomes among African Americans, surprisingly little research exists on adherence to analgesia for cancer pain in this group. We compared analgesic adherence for cancer-related pain over a 3-month period between African Americans and Whites using Medication Event Monitoring System [MEMS]. Patients (n=207) were recruited from outpatient medical oncology clinics of an academic medical center in Philadelphia [≥18 years of age, diagnosed with solid tumors or multiple myeloma, with cancer-related pain, and at least one prescription of oral around-the-clock analgesic (ATC)]. African Americans reported significantly greater cancer pain (P<.001), were less likely than Whites to have a prescription of long acting opioids (P<.001), and more likely to have a negative pain management index (P<.001). There were considerable differences between African Americans and Whites in the overall MEMS dose adherence, i.e., percentage of the total number of prescribed doses that were actually taken (53% vs. 74%, P<.001). On sub-analysis, analgesic adherence rates for African Americans ranged from 34% (for weak opioids) to 63% (for long acting opioids). Unique predictors of analgesic adherence varied by race; income levels, analgesic side-effects, and fear of distracting providers predicted analgesic adherence for African Americans but not for Whites.
Introduction
The Institute of Medicine (IOM) report, Relieving Pain in America, finds that one of the most robust findings on differential pain outcomes pertain to African Americans.16 Previous IOM reports41, accumulated reviews, 1, 8, 11, 12, 26 and a meta-analysis,23 compelingly demonstrates that African American patients are less likely to receive analgesia for pain in cancer and non-cancer settings. There is also strong evidence from studies conducted independently in different geographical regions in the United States (U.S.) that pharmacies in predominantly African American and minority zip codes do not carry opioids needed to treat moderate to severe pain.13,30
While provider and system level factors have been documented in the literature, suprisingly little is known about adherence to analgesia for cancer pain among African Americans. This issue is important since analgesics remain the predominant and consistently reimbursable clinical paradigm for managing cancer pain. While the National Comprehensive Cancer Network guidelines for adult cancer pain31 include a number of complementary and alternative modalities, they are not consistently reimbursed or lack rigorous data on clinical effectiveness for cancer pain.4,20 Thus differential analgesic adherence may be conceptualized as an important explanatory variable in cancer pain outcomes.28
The majority of the studies on analgesic adherence for cancer pain have been conducted predominantly or exclusively with White samples.27, 28, 32, 43, 47, 53, 55 The very limited existing studies with African Americans are cross-sectional (e.g., computed adherence for the past 24 hours)38 and are based on self-reported measures of adherence.2, 22, 38, 50 Studies in non-cancer settings, comparing self-reported measures of adherence with objective measures such as electronic monitoring, have found that subjective adherence measures are not sufficiently accurate and overestimate rates of adherence by 10%–30%.3, 7, 10, 14, 19, 54 Thus, we compared analgesic adherence for cancer pain between African Americans and Whites longitudinally using Medication Event Monitoring System [MEMSTM]. The specific aims were to:
Compare adherence to prescribed around-the-clock (ATC) analgesic between African Americans and Whites with cancer-related pain over a 3-month period.
Identify unique predictors of ATC analgesic adherence for cancer pain for African Americans and Whites.
Methods
Design and Study Population
The study was a 3-month observational design with repeated measures at two time-points, i.e., baseline (T1) and 3-months (T2). Patients were recruited from two outpatient medical oncology clinics of an academic medical center in Philadelphia between December 2009- August 2011. Inclusion was based on self-identified African Americans or Whites, at least 18 years of age, diagnosed with solid tumors or multiple myeloma, with cancer-related pain, and at least one prescription of oral ATC analgesic. Patients were excluded if they were prescribed ATC analgesics using a transdermal system (e.g. fentanyl patch) due to limitations of MEMS vials. The study was approved by the Institutional Review Board of the University of Pennsylvania and all patients provided informed consent.
Study Measures
Index Analgesic
The information regarding prescribed ATC analgesics (index medication) was gathered based on patient self-report during the baseline T1 interview and triangulated with electronic medical records review. Index analgesics were coded according to the World Health Organization’s (WHO) analgesic ladder. 51, 52 This includes Step 1 (non-opioid analgesics e.g., ibuprofen, acetaminophen, naproxen); Step 2 (weak opioids e.g., codeine); and Step 3 (strong opioids (e.g., morphine, oxycodone, methadone). The Step 3 analgesics were further coded according to immediate release and extended or sustained release (long acting) opioids due to evidence of both differential prescription and use of long acting opioids by race.50 We computed Pain Management Index (PMI) for each patient based on WHO guidelines for treating cancer pain.51, 52 The PMI measure is based on the most potent analgesic prescribed to a patient relative to the level of their reported pain. PMI is calculated by subtracting patient’s pain levels (“pain worst” score from the Brief Pain Inventory coded as mild, moderate, or severe) from the most potent analgesia prescribed. A negative PMI implies inadequate analgesic prescription relative to the reported pain level.
MEMS Analgesic Adherence
Analgesic adherence was captured using [MEMSTM AARDEX Group Ltd]. MEMS is a medication bottle cap with a microprocessor that records the occurrence and time of bottle opening in real time. The primary measure of ATC analgesic adherence in our study was “dose adherence” (percentage of the total number of prescribed doses that were actually taken). For example, if a patient took 60 out of 80 prescribed doses over the study period, ‘dose adherence’ measure would be 75%.
Patients were instructed on the correct use of MEMS bottle during the baseline T1 interview. A follow-up phone call was made to each participant within 7 days of the T1 to allow participants to ask any questions they may have about proper usage of the MEMS bottle. Patients were instructed to use the bottle for the duration of the study period, and only use the bottle to take the index medication including any refills for the index medication. They were asked to notify the study staff of any changes in the medication dose or frequency as well as document this information in a medication log, where they also maintained a record of any instances of bottle opening other than when taking the index medications.
The PowerView software was used to record and compute MEMS adherence. If a frequency or medication change occurred during the study period, a new medication entry (phase) was created as a denominator, with the previous phase ending at PowerView’s default time, 2:59am the day of the change and the next phase beginning at 3:00am. If a dosage change occurred, the average of the two (or more) dosages was reported, and no new phase was created. If a patient reported (in writing on the event log or verbally with reasonable certainty during the T2 interview) having taken doses that the bottle did not record, the events were added to the MEMS data. For example, added events might occur if a patient took out two pills at one time and took the second later in the day, or if the patient took out 6 pills for a 3-day trip. Likewise, if a patient reported extra openings for reasons other than taking the medication, the extra openings were excluded from the MEMS adherence calculation. Excluded events included accidental openings, openings only to count pills or refill the bottle.
Also, hospitalization periods were adjusted in the analysis as a non-monitored period beginning on the calendar day of admission at 3:00am and ending on the calendar day after discharge at 2:59am. Hospitalization information (including facility name, dates, and primary and secondary diagnoses) was obtained from self-report between the T1 and T2 dates, self-report at the T2 interview, and review of patient charts. Hospitalization duration was calculated by subtracting the admission date from the discharge date.
Self-reported Analgesic Barriers
Barriers Questionnaire-II49 was used at baseline to assess patients’ beliefs about management of cancer pain. BQ-II is a 27 item instrument that elicits pain management concerns in 8 domains: 1) fear of addiction, 2) fear of tolerance, 3) fear of side effects, 4) fatalism about cancer pain, 5) desire to be a good patient, 6) fear of distracting health provider from treating cancer, 7) fear that the analgesics impair the immune system and 8) concern that analgesics may mask ability to monitor illness symptoms. For each item, the responses range from 0 (do not agree) to 5 (agree very much). The recommended scoring is based on mean scores on the total scale (27-items) and subscales. The internal consistency of the scale is excellent at 0.8949.
Analgesic Side-effects
Analgesic side-effects were captured at baseline using Medication Side-effects Checklist (MSEC)48 that elicits information on presence, type and severity of eight common analgesic side-effects during the past week (0–10; no severity-extreme severity). The reported internal consistency reliability (Cronbach’s alpha) is greater than 0.80.
Pain Severity and Pain Impact
Pain severity and pain impact was measured at baseline using the Brief Pain Inventory (BPI).9 The tool assesses pain at its worst, least, average over the past week and pain currently experienced (pain now) on a 0–10 scale (no pain-pain as bad as you can imagine). The psychometrics of the BPI is well-established with cancer patients, including minority patients with cancer. Its Cronbach’s alpha ranges 0.77 to 0.91.
Intentional vs. Unintentional Non-adherence
Morisky Medication Adherence Scale (MMAS),29 a structured, 4-item self-report measure was used at baseline to distinguish between both intentional (active) and unintentional (passive) dimensions of non-adherence. Statements corresponding to unintentional non-adherence include “I sometimes forget to take my pain medicine” and “I am sometimes careless about taking my pain medicine.” Statements that correspond to intentional non-adherence include “When I feel better I sometimes stop taking my pain medicine” and “If I feel worse when I take the pain medicine, sometimes I stop taking it.” The participants were asked to indicate the extent to which they agree with each statement on the MMAS 4-point scale. This score for each of the four items are aggregated to give a score ranging from 0 to 4, where higher scores indicate higher levels of reported adherence.29 MAMS has established concurrent and predictive validity and its Cronbach’s alpha in different studies have ranged 0.61 to 0.86.
Demographic and Illness Variables
Self-reported demographic data were gathered on age, gender, self-identified race and ethnicity, marital status, education, income, and type of health insurance. Illness-related variables collected from patients’ medical records included type of cancer; stage of cancer, time since cancer diagnosis, past history of drug or substance abuse, comorbidities, and history of depression.
Statistical Analysis
All data were analyzed using SAS version 9.3.40 A prediction model was constructed using a backward elimination method considering as potential predictors all variables that were significant at the bivariate level (p-value < 0.2). The backward elimination method involved starting with all candidate variables in the model, then deleting the variable (if any) that improves the model the most by being deleted, and repeating this process until no further improvement is possible (i.e. all remaining variables in the model are significant at the alpha = 0.05 level).
Separate models were run for African Americans and Whites to understand unique predictors of analgesic adherence. The rational for running separate models by race rather than an overall model of adherence was to identify potential intervention targets, which may be unique to each subgroup.
To assess potential bias due to confounding, we generated a series of bivariate analyses with adherence as the outcome and several key variables obtained at the initial visit as potential predictors. All variables that were found to be statistically significant at the 0.2 level were then considered as covariates in the final analysis. Once the multivariable model was derived, each of the original variables were re-entered into the model, one variable at a time, by testing the most significant to least significant variable to allow a previously insignificant variable to become significant in the final model and retaining any variable that yielded a P-value < 0.05.
Furthermore, to assess for potential bias due to lost to follow-up at month 3, we created a binary (yes/no) indicator variable for retention. We then ran a series of bivariate analyses considering several key variables obtained at the initial visit as potential predictors of retention status. We found no statistically significant predictors of dropout, which supports the statistical missing at random data assumption, suggesting no significant bias due to retention.
Sensitivity Analysis for the Observer Effect
A critique of MEMS monitoring is that due to the awareness of being observed, the MEMS monitoring may lead some individuals to modify aspects of their medication taking behavior.44, 45 To account for this potential source of bias, we created two separate variables to determine the internal consistency between the “dose adherence” outcomes containing data from all the days monitored to the outcome containing data with the first 30 days of observation removed. The Spearman correlation between adherence scores for all days monitored and the adherence scores with the first 30 days excluded was 0.97 (p < 0.001) for African Americans and 0.95 (p < 0.001) for Whites.
Because all Spearman correlations were significantly large, there was strong internal consistency between total adherence scores and the total adherence scores with the first 30 days of observations removed. Similar trends in parameter estimates were observed when the outcome with first 30 days removed was used with little difference in the available data between the all monitored data and 30 days of observations removed. Based on this, the outcome containing the adherence scores for all days monitored was chosen for the final analysis.
Results
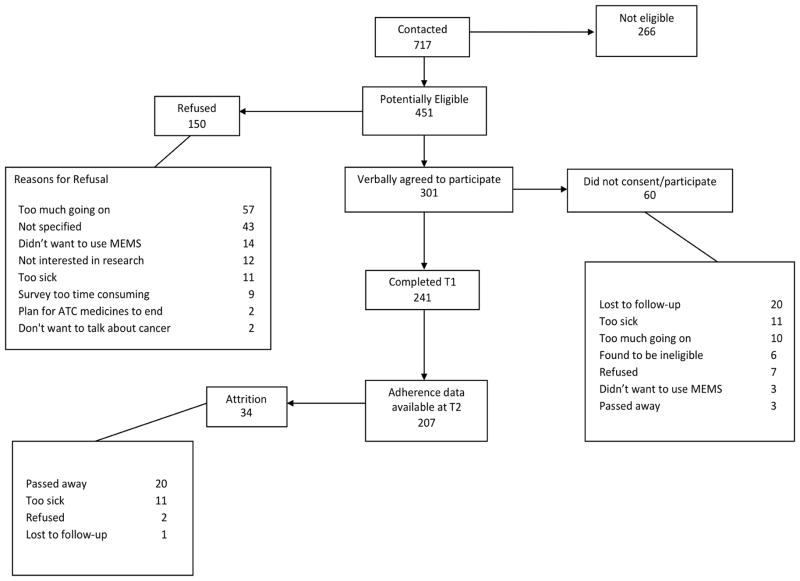
Figure 1 presents participant and recruitment flowchart. Adherence data using MEMS were available for 207 patients (non-Hispanic Whites =121; non-Hispanic African Americans = 86). There was no differential attrition from T1 to T2 based on key variables such as race (P=0.496) or participants’ general health status (P=0.612). The mean age of the group was 54 years (SD=11). There were significant differences between African Americans and Whites based on education, income, type of health insurance, and presence of metastasis (Table 1). However, there were no significant differences between the groups based on age, gender, type of cancer, time since cancer diagnosis, comorbidity burden, and past history of substance or alcohol abuse (Table 1).
Figure 1.
Participant and recruitment flow chart.
Table 1.
Demographic and Illness Characteristics (N=207)
| Variable | Total (N=207) | Whites (N=121) | African Americans (N=86) | p-values† |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age | 54(11) | 54(12) | 53(10) | .392 |
| Time since cancer diagnosis (months) | 37(35) | 36(35) | 38(36) | .784 |
| Charlson Comorbidity Index | 4(3) | 4(2) | 4(3) | .260 |
| Frequency (%) | ||||
| Gender | ||||
| Male | 90(43) | 59(49) | 31(36) | .069 |
| Female | 117(57) | 62(51) | 55(64) | |
| Marital Status | <.001 | |||
| Married | 110(53) | 84(69) | 26(30) | |
| Separated/Divorced/Widowed | 56(27) | 19(16) | 37(43) | |
| Never Married | 41(20) | 18(15) | 23(27) | |
| Education | .016 | |||
| Elementary | 3(1) | 1(1) | 2(2) | |
| High School | 70(34) | 35(29) | 35(41) | |
| College/Trade School | 101(49) | 58(48) | 43(50) | |
| More Than College | 33(16) | 27(22) | 6(7) | |
| Income (US$) | <.001 | |||
| < 30, 000 | 73(35) | 24(20) | 49(57) | |
| 30–50,000 | 36(17) | 15(12) | 21(24) | |
| 50–70,000 | 37(18) | 26(21) | 11(13) | |
| 70–90,000 | 24(12) | 21(17) | 3(3) | |
| >90,000 | 37(18) | 35(29) | 2(2) | |
| Health Insurance | <.001 | |||
| Private | 107(52) | 81(68) | 26(30) | |
| Medicaid | 27(13) | 5(4) | 22(26) | |
| Medicare | 41(20) | 21(18) | 20(23) | |
| Multiple | 25(12) | 12(10) | 13(15) | |
| Other | 6(3) | 1(1) | 5(6) | |
| Cancer Type | .907 | |||
| Lung | 32(15) | 21(17) | 11(13) | |
| Breast | 38(18) | 21(17) | 17(20) | |
| Gastrointestinal | 31(15) | 19(16) | 12(14) | |
| Genitourinary/Reproductive | 25(12) | 15(12) | 10(12) | |
| Multiple Myeloma | 34(16) | 17(14) | 17(20) | |
| Other Solid tumors | 47(23) | 28(23) | 19(22) | |
| Presence of Metastasis | .008 | |||
| Yes | 148 (72) | 95 (78) | 53 (62) | |
| No | 59 (28) | 26 (22) | 33 (38) | |
| History of Substance Abuse | .131 | |||
| Yes | 35(17) | 16(13) | 19(22) | |
| No | 172(83) | 105(87) | 67(78) | |
| History Alcohol Abuse | .636 | |||
| Yes | 20(10) | 13 (11) | 7(8) | |
| No | 187(90) | 108 (89) | 79(92) | |
| History of Depression | .236 | |||
| Yes | 87(42) | 55(45) | 32(37) | |
| No | 120(58) | 66(55) | 54(63) |
p-values are based on t-tests for continuous variables and chi-squared for categorical variables.
WHO =World Health Organization
Pain and Analgesic Prescription
When compared to Whites, African Americans reported significantly greater cancer pain including higher BPI’s “pain worst” scores (P<.001), higher “pain least” scores indicating lower pain relief (P<.001) and negative PMI indicating inadequate analgesic prescription given the pain levels (P<.001) (Table 2). There were no differences in African Americans and Whites in analgesic prescription according to the WHO analgesic step. However, within WHO Step 3 analgesics, African Americans were less likely to be prescribed long acting opioids for pain relief (P<.001). There was a significant difference between groups on Morisky non-adherence items. More specially, a larger percentage of African Americans reported being forgetful (41% vs. 27%, p= .043) and intentionally stopping to take pain medicine when feeling better (58% vs. 40%; p= .009).
Table 2.
Analgesic Prescription and Pain Management Variables (N=207)
| Variable | Total (N=207) | Whites (N=121) | African Americans (N=86) | p-values† |
|---|---|---|---|---|
|
| ||||
| Frequency (%) | ||||
|
| ||||
| Index Analgesic | .111 | |||
|
| ||||
| WHO Step 1 | 19 (9.2) | 7 (5.8) | 12 (14.0) | |
|
| ||||
| WHO Step 2 | 22 (10.6) | 12 (9.9) | 10 (11.6) | |
|
| ||||
| WHO Step 3 | 166 (80.2) | 102 (84.3) | 64 (74.4) | |
|
| ||||
| Negative Pain Management Index | <.001 | |||
|
| ||||
| Yes | 18 (8.7) | 5 (4.13) | 13 (15.1) | |
|
| ||||
| No | 189 (91.3) | 116 (95.9) | 73 (84.9) | |
|
| ||||
| Prescription of long acting opioids | <.001 | |||
|
| ||||
| Yes | 117 (56.5) | 82 (67.8) | 35 (40.7) | |
| No | 90 (43.5) | 39 (32.2) | 51 (59.3) | |
|
| ||||
| MMAS unintentional; forgetfulness | ||||
| Yes | 68 (32.9) | 33 (27.3) | 35 (40.7) | 0.043 |
| No | 139 (67.1) | 88 (72.7) | 51 (59.3) | |
|
| ||||
| MMAS unintentional; carelessness | ||||
| Yes | 35 (16.9) | 21 (17.4) | 14 (16.3) | 0.839 |
| No | 172 (83.1) | 100 (82.6) | 72 (83.7) | |
|
| ||||
| MMAS intentional; stop when feel better | ||||
| Yes | 98 (47.3) | 48 (39.7) | 50 (58.1) | 0.009 |
| No | 109 (52.7) | 73 (60.3) | 36 (41.9) | |
|
| ||||
| MMAS intentional; stop when feel worse | ||||
| Yes | 34 (16.4) | 19 (15.7) | 15 (17.4) | 0. 739 |
| No | 173 (83.6) | 102 (84.3) | 71 (82.6) | |
|
| ||||
| Mean (SD) | ||||
|
| ||||
| Pain worst (BPI, 0–10) | 6.4(3) | 5.9(3) | 7.0(2) | <.001 |
|
| ||||
| Pain least (BPI, 0–10) | 3.3(2) | 2.8 (2) | 4.0(2) | <.001 |
|
| ||||
| Pain average (BPI, 0–10) | 4.7(2) | 4.1(2) | 5.3(2) | <.001 |
|
| ||||
| Pain interference (BPI, 0–70) | 35.2(16) | 33.6 (15) | 37.6(16) | .086 |
|
| ||||
| Severity of side-effects (MSEC, 0–80) | 25.2 (15) | 23.8 (13) | 27.1(17) | .130 |
|
| ||||
| Barriers Questionnaire (BQ-II, 0–135) | 66.8 (20) | 64.5(19) | 70.0 (21) | .052 |
|
| ||||
| Number of index medication changes during the study period | .05 (0.24) | .06 (0.23) | .05 (0.26) | .744 |
|
| ||||
| Number of medication frequency changes during the study period | .14 (0.40) | 0.18 (0.46) | .09 (0.29) | .094 |
|
| ||||
| Total number of analgesics prescribed (excluding co-analgesics) | 2.1 (0. 80) | 2.1 (0.79) | 2.0 (082) | .711 |
|
| ||||
| Total number co-analgesics prescribed | 0.24 (0.50) | 0.24 (0.51) | 0.23 (0.47) | .920 |
|
| ||||
| % Overall adherence | 65.1 (34.5) | 73.7 (31.5) | 52.8 (34.9) | <.001 |
|
| ||||
| Number of MEMS days monitored | 87.6(16.7) | 86.8 (15.5) | 88.4(17.9) | .486 |
|
| ||||
| % Adherence by WHO step | ||||
|
| ||||
| WHO Step 1 | 50.6 (33.5) | 59.5 (37.5) | 45.4 (31.5) | .391 |
|
| ||||
| WHO Step 2 | 45.2 (31.8) | 54.9 (28.6) | 33.6 (33.0) | .121 |
|
| ||||
| WHO Step 3 | 69.3 (33.7) | 76.9 (30.7) | 57.3 (35.1) | .000 |
|
| ||||
| % Adherence by long acting opioids only | 73.6 (31.0) | 78.1 (29.2) | 62.9 (32.9) | .015 |
p-values are based on t-tests for continuous variables and chi-squared for categorical variables.
BPI= Brief Pain Inventory; Morisky Medication Adherence Scale; MSEC= Medication Side-effects Checklist; BQ= Barriers Questionnaire; WHO =World Health Organization
MEMS Analgesic Adherence
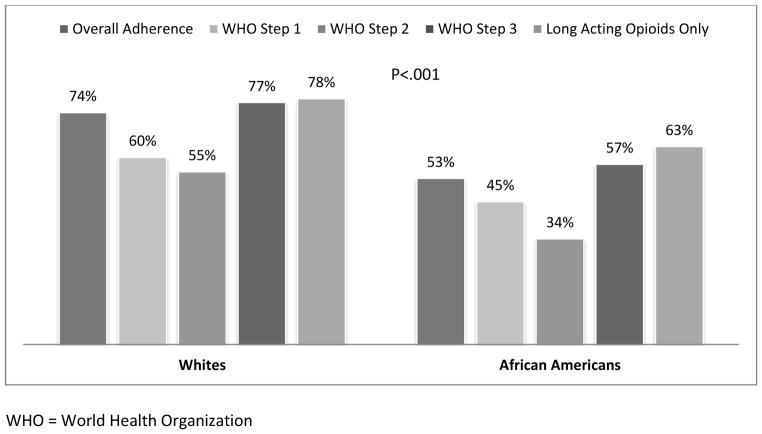
Patients’ adherence was monitored for an average of 88 days (SD=17) using MEMS. There was no difference between African Americans and Whites in the number and frequency of medication changes during the index period (Table 2). However, there were considerable differences between African Americans and Whites in the overall analgesic adherence (53% vs. 74%, P< .001) as well as adherence according to the WHO analgesic step (Table 2). On sub-analysis, analgesic adherence rates for African Americans ranged from 34% (for weak opioids) to 63% for long acting opioids. For Whites, adherence ranged from 55% (for weak opioids) to 78% for long acting opioids (see Figure 2).
Figure 2.
MEMS Dose Adherence by Race and Type of Analgesic
WHO = World Health Organization
Unique Predictors of MEMS Analgesic Adherence
Tables 3 and 4 present the findings of the unique predictors of overall adherence (dose adherence) for African Americans and Whites, respectively.
Table 3.
Unique Predictors of Analgesic Adherence for African Americans
| Variable | Beta Coefficients* | Standard Error | P-Value |
|---|---|---|---|
|
| |||
| Household Income | |||
| <$10,000 | −41.828 | 9.207 | <0.001 |
| $10,000–$50,000 | −25.894 | 8.188 | 0.002 |
| >$50,000 (reference) | ------ | ------ | ------ |
|
| |||
| Feel the need to receive further information about pain medication | |||
| Yes | 25.629 | 9.381 | 0.008 |
| No (reference) | ------ | ------ | ------ |
|
| |||
| Intentional non-adherence (When I feel better I sometimes stop taking my pain medicine) | |||
| Yes | −22.174 | 6.131 | <0.001 |
| No (reference) | ------ | ------ | ------ |
|
| |||
| Total number of analgesics prescribed (excluding co-analgesics) | 10.720 | 3.836 | 0.007 |
|
| |||
| Number of analgesic side-effects | 9.812 | 2.675 | <0.001 |
|
| |||
| Fear that if doctors have to deal with pain they won’t concentrate on curing the disease (0 = do not agree at all, 5 = agree very much) | −7.440 | 2.256 | 0.002 |
|
| |||
| Fear that doctors might find it annoying to be told about pain (0 = do not agree at all, 5 = agree very much) | 5.911 | 2.394 | 0.016 |
|
| |||
| Severity of analgesic side-effects | −1.389 | 0.406 | <0.001 |
|
| |||
| Model: (F(9,76) = 6.65, p < 0.001, R2 = 0.441) | |||
MEMS =Medication Event Monitoring System
The beta coefficients from the final prediction model represent slope coefficients for the continuous predictors, and the difference from the reference category for the categorical predictors. A large value implies a large effect size.
Table 4.
Unique Predictors of Analgesic Adherence for Whites
| Variable | Beta coefficients | Standard Error | P-Value |
|---|---|---|---|
|
| |||
| Intentional non-adherence (when I feel better I sometimes stop taking my pain medicine) | |||
| Yes | −23.672 | 5.315 | <0.001 |
| No (reference) | ------ | ------ | ------ |
|
| |||
| Intentional non-adherence (if I feel worse when I take the pain medicine, sometimes I stop taking it) | |||
| Yes | −18.557 | 7.054 | 0.010 |
| No (reference) | ------ | ------ | ------ |
|
| |||
| Pain “least” in last week (0 = no pain, 10 = pain as bad as you can imagine) | −2.876 | 1.394 | 0.041 |
|
| |||
| Length it has been since the diagnosis of cancer (months) | 0.160 | 0.071 | 0.026 |
|
| |||
| Model: (F(4,116) = 12.34, p < 0.001, R2 = 0.299) | |||
MEMS =Medication Event Monitoring System
The beta coefficients from the final prediction model represent slope coefficients for the continuous predictors, and the difference from the reference category for the categorical predictors. A large value implies a large effect size.
African Americans
Income level was the strongest predictor of analgesic adherence for cancer pain among African Americans (Table 3). Compared to those who reported a household income of more than $50,000 a year, those between $10,000 and $50,000 a year had a 25.89 lower percentage of adherence (P=0.002) and those with less than $10,000 a year had a 41.83 lower percentage of dose adherence (P< 0.001). Also, clinical variables were significant in explaining non-adherence in African Americans. For instance, for each unit increase in the severity of analgesic side effects, the percentage of dose adherence decreased by 1.39 (P<0.001). Similarly, for each unit increase in the concern of distracting doctor from curing the disease, the percentage of dose adherence decreased by 7.44 (P= 0.002). The Morisky subscale of intentional non-adherence was also a strong predictor of dose adherence for African Americans. Those who reported intentional non-adherence (i.e., stopping to use analgesics when feeling better), had a −22.17 lower percentage of dose adherence (P< 0.001). On the other hand, number of analgesic side-effects reported and number of analgesics prescribed were associated positively with dose adherence. This model was statistically significant and explained 44% of the variance for dose adherence for African Americans.
Whites
Intentional non-adherence subscale (i.e., stopping to use prescribed analgesics when feeling better or worse) was the strongest predictor of dose adherence for Whites (Table 4). Those who reported stopping to use analgesics when feeling better had a 23.67 lower percentage of dose adherence (P< 0.001). Similarly, those who reported stopping to use analgesics when feeling worse, had a 18.56 lower percentage of dose adherence (P= 0.010). Clinical variables such as length of pain due to cancer and pain levels also predicted dose adherence for Whites. For every unit increase in time since cancer diagnosis (in months), dose adherence increased by 0.16 percent (P=0.026). Whereas for every unit increase in “pain least” (higher scores indicate lower pain relief), the percentage of dose adherence decreased by 2.88 (P= 0.041). This model was statistically significant and explained 30% of the variance for dose adherence for Whites.
Discussion
“Drugs don’t work in patients who don’t take them” (C. Everett Koop). By the same token, not taking medication is a behavioral representation of what may be right or wrong for the patient in a medication treatment setting. We found that analgesic adherence was low for both Whites and African Americans but it was considerably lower for African Americans.
Most existing interventions to improve cancer pain outcomes are conceived within a psychoeducational paradigm, which focuses on knowledge transfer to address attitudes and barriers to opioid use.17, 39, 42 A systematic review of the effectiveness of such interventions for cancer pain management found that while the interventions improved knowledge about cancer pain management in the majority of the studies (73%), most did not improve reported adherence to analgesics.35 These findings were confirmed in another meta-analysis that found no benefit of educational interventions on analgesic adherence or pain-related interference.5 This indicates that the knowledge path to improving analgesic adherence or cancer pain outcomes may be inadequate.
Consitently, we found that most common analgesic-related fears (including addiction concerns) did not explain objective analgesic adherence for cancer pain for African Americans or Whites. Rather, most of the identified predictors of objective adherence may be thought of as circumstantial or experiential likely based on patients’ previous clinical experience of cancer pain management or clinician-patient interaction. Further, African Americans had more of such barriers (e.g., need for more information about pain medications, and fear of distracting or annoying clinicians, and concern for side-effects) than Whites.
Similar findings were supported in a previous study of adherence to analgesia for cancer pain (employing subjective measures of adherence and African American patients only). The authors found that addiction concerns were not correlated with adherence for WHO step 2 or step 3 analgesics; rather pain intensity, side-effects, and fear of distracting clinicians were associated with analgesic adherence in African Americans with cancer pain.38
Similarly, in our study, an increase in the severity of side-effects was associated with lower adherence to analgesia for African Americans but not for Whites. Moreover, more adherent African Americans reported greater number of analgesic side-effects at baseline suggesting disparites in analgesic adverse effects management in African Americans. The higher burden of side-effects in African Americans may also be related to the choice of analgesics in African Americans. In a recent study, authors found that controlling for the type of health insurance, African Americans with cancer pain had 71% lower odds of receiving a prescription of oxycodone than White patients (P <.001) and they were more likely to be prescribed morphine even in the presence of renal insufficiency.25 Authors further demonstrated that the type of analgesics prescribed partially mediated the reported adverse analgesic effects.25
Among Whites, lower pain relief (higher pain least scores) predicted lower adherence to analgesia whereas length since cancer diagnosis, possibly indicating disease severity, predicted greater analgesic adherence. Consistently, in a previous analysis to understand trade-offs African Americans and Whites employ in making cancer pain decisions, we found that African Americans were more likely to make analgesic use decisions based on side-effects whereas Whites were more likely to make analgesic use decisions based on amount of relief expected from using pain medications.24
Another important finding of this study is the strong negtive linear relationship in the levels of income and adherence to analgesia for cancer pain among African Americans. Studies in non-pain settings have found that higher out of pocket cost and household income less than $20,000 are associated with medication non-adhrence behaviors including decreasing the dose or frequency of medications, failing to refill or extending time between the refills.15, 36, 37, 46 In the setting where patients refill their pain medications, they may save pain medications until they cannot stand pain or hoard pain medications for when pain is severe; a behavior termed medication triaging.21 While studies of medication triaging in the context of pain are limited, there is some evidence that patients may be non-adherent to pain medications in order to be able to afford medications for other chronic conditions such as diabetes.18 Thus, low income patients may compromise on taking pain medications to be able to afford medications considered as “more important” or “lifesaving” or even resort to less expensive but also less potent over-the counter alternative therapies.18, 21
The fact that African Americans with lower incomes were less adherent, brings to the forefront the importance of discussing cost and ability to pay when writing an analgesic prescription. In the current clinical scenario, management of multiple conditions and symtoms occur in isolation and by multiple health care providers resulting in accumulated cost and complexity for the patients. In a national study, majority (two-thirds) of patients with chronic illnesses reporting underusing medications due to cost-related concerns never discussed these concerns with their clinicians.37 Of those reporting cost-related non-adhrence said clinicians never asked them about their ability to pay for medications or did not believe that clinicians could help.37 Clinicians may take a more proactive role in assessing cost-related issues potentially contributing to analgesic non-adherence and provide assistance such as reviewing overall medication regimens, simpligying regimens, changing medications to less expensive alternatives when clinically appropriate, or providing information about programs that may assist with prescription medication cost.
Finally, consistent with Rhee et al., study,38 overuse of analgesia among African Americans is not supported in our study. Unlike adherence for some other chronic conditions where there is more agreement on adherence cut-off rates, there is no agreement about which cut-off is valid for analgesic use for cancer pain.34 Previous studies have employed 70%32 to 100%33, 34 and in a non-U.S. study However, regardless of the cut-off used, the analgesic adherence rates of 34%–63% in African Americans are considerably lower. Similar lower analgesic adherence rates for cancer pain in African Americans were also identified in another study (46%)38 even using subjective measures that typically overestimate adherence. These findings should be a call for concern for the goal of achieving equity in clinical cancer pain outcomes.
Strengths and Limitations
This is the first study to our knowledge that has compared adherence to analgesia for cancer pain and its unique predictors between African Americans and Whites while employing objective measures of adherence over time. However, some findings of our study are limited. First, we limited objective monitoring of analgesics to one ATC analgesic. Since we used MEMS vial for electronic monitoring, it was not feasible to monitor ATC prescription in a patch form. However, we have no reason to believe that analgesics in a patch form would be prescribed disproportionately to African Americans- an assumption that is needed to nullify our findings of differential prescription of long acting opioids by race.
Furthermore, while MEMS allows for long-term assessment of adherence and detailed information about patterns of prescription use, it does not guarantee ingestion of medication. Vial opening other than for medication taking, medication changes within the study period, and medication holidays (e.g., secondary to hospitalization) may result in inaccuracies in adherence measurement. We minimized this potential source of bias by accounting for cap openings other than for medication taking (e.g., for refills), a change in frequency and dose of analgesics during the study period, or medication holidays due to hospitalizations (see study measures). Despite these limitations, studies in non-cancer pain setting comparing MEMS with a variety of subjective measures have concluded that MEMS is one of the more accurate adherence measurement approaches.6
Our study is limited in that there were unmeasured cancer (cancer treatment, medications other than analgesics and co-analgesics, caner treatment related side-effects, cancer-related functional impairments), and psychiatric variables (such as cancer-related anxiety, cancer treatment-related posttraumatic symptoms) that may confound the findings. Furthermore, we included history of depression from patients’ medical records and did not use self-report measure of depression. Also, to create predictive models, we used self-reported data from baseline. Since our main goal was to assess patients’ actual adherence behaviors, we believed that multiple contacts by the study staff would create an observational effect resulting in alteration of patient’s actual behavior. It is conceivable that some of the predictors of interest changed over the 3-month course of the study. Finally, although we computed PMI for adequacy of analgesic prescription given patient’s levels of pain, we did not compare doses of analgesics between African Americans and Whites. Despite these limitations, our findings add to a very scarce body of literature to understand differences in analgesic adherence and preliminary understanding of sources of those differences as a way to explain the widely observed clinical disparities in cancer pain outcomes.
Conclusions
Our salient findings indicate that 1) there are significant disparities between African Americans and Whites in the treatment of cancer pain and adherence to analgesia captured using MEMS over a 3-months period; 2) analgesic-related beliefs commonly implicated in analgesic and opioid-related non-adherence (e.g., addiction concerns) do not explain objective analgesic taking in both groups; 3) rather, clinical pain management variables explain objective analgesic adherence in this sample of African Americans and Whites; 4) the unique predictors of analgesic adherence vary by race, specially socioeconomic variables, fear of distracting providers, and analgesic side-effects predict analgesic adherence for African Americans but not for Whites; 5) these additional variables may explain differential analgesic adherence and consequent disparities in cancer pain outcomes in African Americans. The greater burden of unmet cancer pain management needs in African Americans deserves correspondingly greater attention and perhaps greater intensity of interventions with this group, however, majority of the existing interventions have been both conceptualized and investigated predominantly with White patients.
Perspective.
Despite evidence of disparities in cancer pain outcomes among African Americans, surprisingly little research exists on African Americans’ adherence to analgesia for cancer pain. This prospective study employs objective measures to compare adherence to prescribed pain medications between African American and White patients with cancer pain.
Footnotes
Presented in part at the 32nd Annual Scientific Meeting of the American Pain Society, New Orleans, May 8–11, 2013.
Conflicts of Interest:
No authors declared any financial or non-financial conflicts of interest.
Disclosures:
Supported by NIH Challenge Grant to Dr. Salimah Meghani (NIH/NINR RC1-NR011591).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain. 2009;10:1187–204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KO, Mendoza TR, Payne R, Valero V, Palos GR, Nazario A, Richman SP, Hurley J, Gning I, Lynch GR, Kalish D, Cleeland CS. Pain education for underserved minority cancer patients: A randomized controlled trial. J Clin Oncol. 2004;22:4918–25. doi: 10.1200/JCO.2004.06.115. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Buono D, Eckholdt H, Howard AA, Schoenbaum EE. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. J Clin Oncol. 2006;24:5457–64. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- 5.Bennett MI, Bagnall AM, Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143:192–9. doi: 10.1016/j.pain.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF, Naya I. Compliance with an oral asthma medication: A pilot study using an electronic monitoring device. Respir Med. 2000;94:852–8. doi: 10.1053/rmed.2000.0813. [DOI] [PubMed] [Google Scholar]
- 8.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J of Pall Med. 2006;9:1454–73. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 10.Daniels T, Goodacre L, Sutton C, Pollard K, Conway S, Peckham D. Accurate assessment of adherence: Self-report and clinician report vs electronic monitoring of nebulizers. Chest. 2011;140:425–32. doi: 10.1378/chest.09-3074. [DOI] [PubMed] [Google Scholar]
- 11.Ezenwa MO, Ameringer S, Ward SE, Serlin RC. Racial and ethnic disparities in pain management in the united states. J Nurs Scholarsh. 2006;38:225–33. doi: 10.1111/j.1547-5069.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 12.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 13.Green CR, Ndao-Brumblay SK, West B, Washington T. Differences in prescription opioid analgesic availability: Comparing minority and white pharmacies across Michigan. J Pain. 2005;6:689–99. doi: 10.1016/j.jpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs. 2003;2:219–28. doi: 10.1016/S1474-5151(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 15.Heisler M, Wagner TH, Piette JD. Patient strategies to cope with high prescription medication costs: Who is cutting back on necessities, increasing debt, or underusing medications? J Behav Med. 2005;28:43–51. doi: 10.1007/s10865-005-2562-z. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Relieving Pain in America: A blueprint for transforming prevention, care, education, and research. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- 17.Kravitz RL, Tancredi DJ, Grennan T, Kalauokalani D, Street RL, Jr, Slee CK, Wun T, Oliver JW, Lorig K, Franks P. Cancer health empowerment for living without pain (Ca-HELP): Effects of a tailored education and coaching intervention on pain and impairment. Pain. 2011;152:1572–82. doi: 10.1016/j.pain.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Kurlander JE, Kerr EA, Krein S, Heisler M, Piette JD. Cost-related nonadherence to medications among patients with diabetes and chronic pain: Factors beyond finances. Diabetes Care. 2009;32:2143–8. doi: 10.2337/dc09-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaFleur J, Oderda GM. Methods to measure patient compliance with medication regimens. J Pain Palliat Care Pharmacother. 2004;18:81–7. [PubMed] [Google Scholar]
- 20.Lu W, Rosenthal DS. Acupuncture for cancer pain and related symptoms. Curr Pain Headache Rep. 2013;17:321. doi: 10.1007/s11916-013-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meghani SH. Corporatization of pain medicine: Implications for widening pain care disparities. Pain Med. 2011;12:634–44. doi: 10.1111/j.1526-4637.2011.01074.x. [DOI] [PubMed] [Google Scholar]
- 22.Meghani SH, Bruner DW. A pilot study to identify correlates of intentional versus unintentional nonadherence to analgesic treatment for cancer pain. Pain Manag Nurs. 2013;14:e22–30. doi: 10.1016/j.pmn.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13:150–74. doi: 10.1111/j.1526-4637.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 24.Meghani SH, Chittams J, Hanlon AL, Curry J. Measuring preferences for analgesic treatment for cancer pain: How do African-Americans and whites perform on choice-based conjoint (CBC) analysis experiments? BMC Med Inform Decis Mak. 2013;13:11. doi: 10.1186/1472-6947-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meghani SH, Kang Y, Chittams J, McMenamin E, Mao JJ, Fudin J. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: A mediation analysis study. J Clin Oncol. 2014;32:2773–9. doi: 10.1200/JCO.2013.54.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: Directions for health policy, education, practice, and research. Pain Med. 2012;13:5–28. doi: 10.1111/j.1526-4637.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 27.Miaskowski C, Dodd M, West C, Schumacher K, Paul SM, Tripathy D, Koo P. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22:1713–20. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 28.Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. J Clin Oncol. 2001;19:4275–9. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- 29.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. “We don't carry that”--failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med. 2000;342:1023–6. doi: 10.1056/NEJM200004063421406. [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. [Accessed October 6, 2014];Clinical practice guidelines in oncology: Adult cancer pain. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive.
- 32.Nguyen LM, Rhondali W, De la Cruz M, Hui D, Palmer L, Kang DH, Parsons HA, Bruera E. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage. 2013;45:506–16. doi: 10.1016/j.jpainsymman.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldenmenger WH. To be in pain or not: Research to improve cancer-related pain management. Rotterdam, The Netherlands: Erasmus University Rotterdam; 2011. [Google Scholar]
- 34.Oldenmenger WH, Echteld MA, de Wit R, Sillevis Smitt PA, Stronks DL, Stoter G, van der Rijt CC. Analgesic adherence measurement in cancer patients: Comparison between electronic monitoring and diary. J Pain Symptom Manage. 2007;34:639–47. doi: 10.1016/j.jpainsymman.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Oldenmenger WH, Sillevis Smitt PA, van Dooren S, Stoter G, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: A critical appraisal. Eur J Cancer. 2009;45:1370–80. doi: 10.1016/j.ejca.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: The treatments people forgo, how often, and who is at risk. Am J Public Health. 2004;94:1782–7. doi: 10.2105/ajph.94.10.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse: Do patients with chronic illnesses tell their doctors? Arch Intern Med. 2004;164:1749–55. doi: 10.1001/archinte.164.16.1749. [DOI] [PubMed] [Google Scholar]
- 38.Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: Factors related to adherence to analgesics. J Immigr Minor Health. 2012;14:1045–51. doi: 10.1007/s10903-012-9582-x. [DOI] [PubMed] [Google Scholar]
- 39.Rustoen T, Valeberg BT, Kolstad E, Wist E, Paul S, Miaskowski C. The Pro-Self(c) pain control program improves patients' knowledge of cancer pain management. J Pain Symptom Manage. 2012;44:321–30. doi: 10.1016/j.jpainsymman.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 40.SAS Institute Inc. SASÆ Component Language 9.3. SAS Institute Inc; Cary: NC: 2011. [Google Scholar]
- 41.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: 2003. [PubMed] [Google Scholar]
- 42.Street RL, Jr, Tancredi DJ, Slee C, Kalauokalani DK, Dean DE, Franks P, Kravitz RL. A pathway linking patient participation in cancer consultations to pain control. Psychooncology. 2014 doi: 10.1002/pon.3518. [DOI] [PubMed] [Google Scholar]
- 43.Syrjala KL, Abrams JR, Polissar NL, Hansberry J, Robison J, DuPen S, Stillman M, Fredrickson M, Rivkin S, Feldman E, Gralow J, Rieke JW, Raish RJ, Lee DJ, Cleeland CS, DuPen A. Patient training in cancer pain management using integrated print and video materials: A multisite randomized controlled trial. Pain. 2008;135:175–86. doi: 10.1016/j.pain.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Moum T, Rustoen T. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24:627–36. doi: 10.1097/AJP.0b013e31816fe020. [DOI] [PubMed] [Google Scholar]
- 45.Wagner GJ, Ghosh-Dastidar B. Electronic monitoring: Adherence assessment or intervention? HIV Clin Trials. 2002;3:45–51. doi: 10.1310/XGXU-FUDK-A9QT-MPTF. [DOI] [PubMed] [Google Scholar]
- 46.Wagner TH, Heisler M, Piette JD. Prescription drug co-payments and cost-related medication underuse. Health Econ Policy Law. 2008;3:51–67. doi: 10.1017/S1744133107004380. [DOI] [PubMed] [Google Scholar]
- 47.Ward S, Donovan HS, Owen B, Grosen E, Serlin R. An individualized intervention to overcome patient-related barriers to pain management in women with gynecologic cancers. Res Nurs Health. 2000;23:393–405. doi: 10.1002/1098-240x(200010)23:5<393::aid-nur6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 48.Ward SE, Carlson-Dakes K, Hughes SH, Kwekkeboom KL, Donovan HS. The impact on quality of life of patient-related barriers to pain management. Res Nurs Health. 1998;21:405–13. doi: 10.1002/(sici)1098-240x(199810)21:5<405::aid-nur4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Ward SE, Goldberg N, Miller-McCauley V, Mueller C, Nolan A, Pawlik-Plank D, Robbins A, Stormoen D, Weissman DE. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–24. doi: 10.1016/0304-3959(93)90165-L. [DOI] [PubMed] [Google Scholar]
- 50.Wieder R, Delarosa N, Bryan M, Hill AM, Amadio WJ. Prescription coverage in indigent patients affects the use of long-acting opioids in the management of cancer pain. Pain Med. 2014;15:42–51. doi: 10.1111/pme.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. Cancer pain relief. Geneva: 1986. [Google Scholar]
- 52.World Health Organization. Cancer pain relief and palliative care. Geneva, Switzerland: 1996. [Google Scholar]
- 53.Yoong J, Traeger LN, Gallagher ER, Pirl WF, Greer JA, Temel JS. A pilot study to investigate adherence to long-acting opioids among patients with advanced lung cancer. J Palliat Med. 2013;16:391–6. doi: 10.1089/jpm.2012.0400. [DOI] [PubMed] [Google Scholar]
- 54.Zeller A, Ramseier E, Teagtmeyer A, Battegay E. Patients' self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31:2037–43. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]
- 55.Zhukovsky DS, Gorowski E, Hausdorff J, Napolitano B, Lesser M. Unmet analgesic needs in cancer patients. J Pain Symptom Manage. 1995;10:113–9. doi: 10.1016/0885-3924(94)00072-s. [DOI] [PubMed] [Google Scholar]