Abstract
Aim
To determine whether the low C-peptide levels (<50 pmol/l) produced by the pancreas for decades after onset of Type 1 diabetes have clinical significance.
Methods
We evaluated fasting C-peptide levels, duration of disease and age of onset in a large cross-sectional series (n=1272) of people with Type 1 diabetes. We then expanded the scope of the study to include the relationship between C-peptide and HbA1c control (n=1273), as well as diabetic complications (n=324) and presence of hypoglycaemia (n=323). The full range of C-peptide levels was also compared with 1,5-Anhydroglucitol, a glucose responsive marker.
Results
C-peptide levels declined for decades after diagnosis, and the rate of decline was significantly related to age of onset (P<0.0001), after adjusting for disease duration. C-peptide levels > 10 pmol/l were associated with protection from complications (e.g. nephropathy, neuropathy, foot ulcers and retinopathy; P=0.03). Low C-peptide levels were associated with poor metabolic control measured by HbA1c (P<0.0001). Severe hypoglycaemia was associated with the lowest C-peptide levels compared with mild (P=0.049) or moderate (P=0.04) hypoglycaemia. All levels of measurable C-peptide were responsive to acute fluctuations in blood glucose levels as assessed by 1,5-Anhydroglucitol (P<0.0001).
Conclusions
Low C-peptide levels have clinical significance and appear helpful in characterizing groups at-risk for faster C-peptide decline, complications, poorer metabolic control and severe hypoglycaemia. Low C-peptide levels may be a biomarker for characterizing at-risk patients with Type 1 diabetes.
Introduction
Improved biomarkers that predict patient vulnerability to diabetic complications might enable targeted therapeutic strategies to be implemented. Over the last three decades, large and/or prospective studies have provided evidence of an association between elevated HbA1c levels and diabetic microvascular complications [1–3]. Complications include diabetic retinopathy, nephropathy, including albuminuria, neuropathy including pain and foot ulcers/amputations [4]. A progressive fall in endogenous insulin production, monitored by the loss of C-peptide, makes it difficult to maintain tight glucose control in the majority of patients, and this poor glycaemic control affects HbA1c measurements and hypoglycaemia [5,6].
Recent evidence shows that pancreatic β cells are frequently still viable and functional decades after onset of Type 1 diabetes based on new, ultrasensitive C-peptide assays [7–9]. C-peptide is secreted at a 1:1 molar ratio to insulin, thus representing a direct measure of endogenous insulin. The prevailing dogma has been that for the majority of people with Type 1 diabetes, β cells are destroyed after a short, 1- to 2-year ‘honeymoon period’ after diagnosis [10]. The identification of this honeymoon period provided the rationale for only testing new immunotherapies in patients with new-onset diabetes. People with Type 1 diabetes with established disease were defined, using the current definition, as a disease with absolute insulin deficiency [11]. The regular C-peptide assays used by most healthcare systems have lower limits of detection of ~50 pmol/l. By contrast, ultrasensitive assays now enable C-peptide to be monitored at levels as low as 1.5–2.5 pmol/l.
With the advent of methods of measuring low C-peptide levels, important questions arise about the usefulness of this biomarker in the management of diabetes. First, is there clinical value in measuring low levels of C-peptide and does it identify heterogeneity in patients with Type 1 diabetes? Second, does the measurement of low levels of C-peptide tassist in predicting protection from, or risk of, complications? Do low levels of C-peptide protect from severe hypoglycaemia? Finally, do low levels of C-peptide maintain or provide HbA1c regulation? The data reported in the present study begin to provide answers to such questions.
Patients and methods
Patients and blood sampling
At the Immunobiology Research Clinics of the Massachusetts General Hospital, we surveyed 1273 patients with Type 1 diabetes accrued over an 8-year period (2005–2013) for fasting C-peptide levels in a cross-sectional manner. The study was gradually expanded to examine the relationship between C-peptide and HbA1c, accruing patients over a 5-year period (2008–2013), and for C-peptide and presence or absence of diabetes-related complications and hypoglycaemia, the study accrued 324 patients over a 1.5-year period (2011–2013). These studies had full institutional approval and informed consent was obtained from all patients (Protocol #2001P001379).
Design
All patients included in the study were research-only volunteers, predominantly from the USA and Canada (98% total), and were of non-Hispanic white ethnicity (99%). All patients met the American Diabetes Association classification of Type 1 diabetes, which includes continuous use of insulin from the time of diagnosis, history of ketones and history of autoantibodies. Patients were aged 8–90 years. The patients with Type 1 diabetes were receiving frequent medical care and had well-controlled diabetes with a mean HbA1c concentration of 56 mmol/mol (7.3%). More details on our research clinic and patient demographics can be found in the Supplementary Methods (File S1).
Blood serum was collected and frozen at −80°C. In cases where recent HbA1c values were not available from the clinic charts, blood was sent out for HbA1c testing. Only serum tubes that had never been thawed were included in the C-peptide analysis. Samples were typically thawed and analysed within 3–4 months.
Evaluation of reduced awareness of hypoglycaemia in patients across all stages of disease was conducted using the survey by Clarke et al. [12]. A total of 324 patients with Type 1 diabetes (mean age 40.3 years) completed the survey. The survey included eight questions (Fig. S4). If four or more responses on the survey fell into the ‘R’ category, then the patient was classified as having reduced awareness. When the responses fell into ≤ 2 ‘R’ reduced awareness categories, the patient was classified as having awareness. Moderate hypoglycaemia was defined as lethargy, confusion or requiring assistance for treatment. Severe hypoglycaemia was defined as unconsciousness, seizure or requiring glucagon or intravenous glucose.
C-peptide assay methods
Serum samples were assayed for C-peptide using the Mercodia regular (Cat. No 10-1136-01) or ultrasensitive C-peptide ELISA kits (Cat. No 10-1141-01), both from Mercodia AB (Uppsala, Sweden). Both assays were calibrated against the International Reference Reagent for C-peptide (IRR C-peptide 84/510; a WHO standard) and listed with the US Food and Drug Administration as Class I IVD devices. The lower limit of sensitivity of the Mercodia ultrasensitive assay for these studies was calibrated to 2.5 pmol/l. Additional details are provided in the Supplementary Methods (File S1).
Statistics
Data on disease duration, fall in C-peptide level, and HbA1c measurements were not normally distributed. We therefore used the non-parametric Mann–Whitney U-test to determine the statistical significance of differences in measurements. Rates of complications between two C-peptide strata were compared, adjusting for disease duration using a logistic regression model that related a binary outcome to multiple categorical or continuous explanatory variables. We applied a multivariable logistic regression model using complication event as the binary dependent variable and C-peptide stratum and duration of disease as independent variables. The duration-adjusted comparisons were represented by the P value and odds ratio of the C-peptide stratum term in the multivariable model. Percent of patients with undetectable C-peptide and hypoglycaemia degree were compared using a chi-squared test. Scatter plots and bar plots were used to illustrate visually distributions of variables. We used SAS version 9.3 (SAS Institute, Cary, NC, USA) for statistical analyses. Statistical significance was established using a P value threshold of 0.05.
Results
C-peptide decline in patients with Type 1 diabetes is frequently decades-long
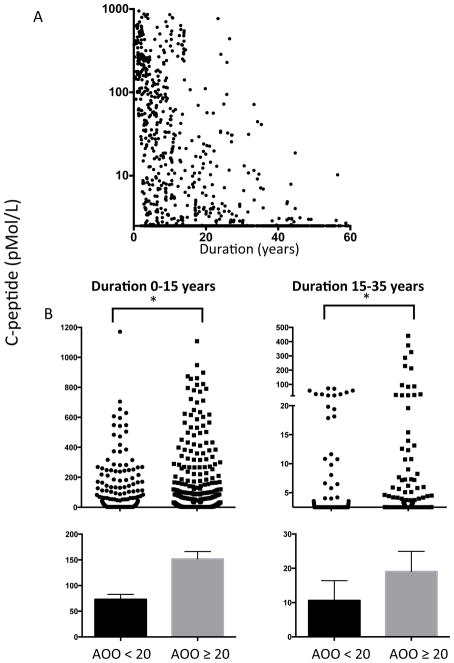
Figure 1a shows the decline in fasting C-peptide levels in 1272 patients with diabetes in relation to disease duration. The patients’ clinical characteristics are shown in Fig. S1. The data show the persistence of C-peptide as it gradually declines over decades after diagnosis. Note that the dataset is cross-sectional and not longitudinal. Nevertheless, the association between C-peptide and disease duration is clear. As previous studies have observed a relationship between C-peptide decline and age of onset, we first examined our data for this association [13]. The differences in the C-peptide rates of decline were examined for patients with a disease duration of <15 years (Fig. 1b, left) and those with a disease duration of 15–30 years (Fig. 1b, right). Regardless of disease duration, age of onset was a risk factor for a more rapid decline in C-peptide levels (duration <15 years, P=0.0001; duration 15–30 years, P=0.0001). The association with disease duration is shown in Fig. 1a and is further analysed in Fig. S2 and is considered in the context of previous data [14].
FIGURE 1.
Decades-long persistence of C-peptide in patients with Type 1 diabetes (n=1272) and more rapid fall in C-peptide levels with younger age of onset. (a) Graph showing gradual, decades-long decline in C-peptides detectable using an ultrasensitive assay. (b) Graph showing that the decline in C-peptide levels in patients with long-term diabetes is related to the age of onset of the disease. Data are stratified by diabetes duration <15 years (left) or 15–30 years (right). For a Type 1 diabetes duration of < 15 years, an age of onset of <20 years old was associated with a more rapid decline in C-peptide level (age of onset <20 years, n=292; age of onset >20 years, n=294). With a Type 1 diabetes duration of 15–30 years, a more rapid decline in C-peptide level was again associated with an age of onset of < 20 years (age of onset <20 years, n=196; age of onset >20 years, n=165). P values were calculated using a Mann–Whitney U-test (Wilcoxon rank-sum test) and the data are represented as mean ± SEM. ***P<0.0001.
As there was a major difference in C-peptide decline based on the adult vs. adolescent onset of diabetes, we also stratified the data across many age-of-onset intervals. Fig. S2 shows that children with a younger age of onset (either with disease onset at age <5 years or with onset at age < 10 years), have very rapid C-peptide declines in parallel with diabetes duration. By contrast, C-peptide decline was more moderate within the disease onset age groups 20–30 or 30–40 years. Statistical trends in C-peptide decline based on small age of onset intervals are shown in Fig. S2b. The data show the more rapid decline in C-peptide levels in the youngest children (disease onset at < 5 years of age or < 10 years of age), compared with adolescents and young adults. The data also show that in patients with disease onset at >40 years of age, C-peptide levels again decline at a faster rate, as previously reported [7].
Relationship between complications and HbA1c values with C-peptide levels
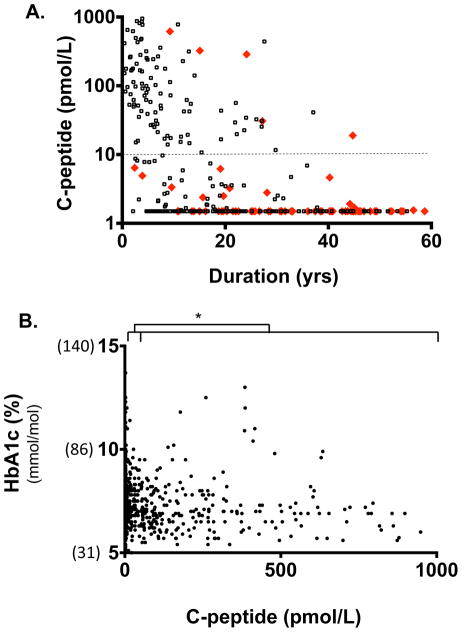
An examination of the clinical histories of 324 patients with Type 1 diabetes showed that 76 had at least one Type 1 diabetes-related complication (Fig. 2a, red diamonds) compared with 248 patients with Type 1 diabetes without complications (Fig. 2a, black circles). Importantly, when the data were plotted against duration, and the known association of diabetes duration and complications was statistically removed, an independent association was found between low levels of C-peptide and complications. More specifically, a C-peptide level > 10 pmol/l, regardless of diabetes duration, was protective from complications, and a C-peptide value of < 10 pmol/l presented a risk of complications (P=0.03). This is seen in Fig. 2a, where the majority of the red diamonds are below the dashed line, indicating the 10 pmol/l C-peptide level. For the values from 2.5 to 10 pmol/l, 40 patients were without complications (black) and 13 of the patients were with complications (red). Of the patients with undetectable C-peptide levels, 110 had no complications (black) and 58 had complications (red). Patients with C-peptide levels <10 pmol/l were 3.1 times more likely to develop a diabetic complication (odds ratio 3.1, confidence interval 1.1–8.6). The clinical characteristics of patients studied for complications are shown in Fig. S3a.
FIGURE 2.
Low levels of C-peptide < 10 pmol/l were predictive of diabetes-related complications (a), and levels of 2.5 pmol/l identified patients with Type 1 diabetes with poor HbA1c control (b). (a) Development of diabetes-related complications of retinopathy, foot ulcer amputations, neuropathy or kidney disease (nephropathy or micro-albuminuria) was associated with a C-peptide level of < 10 pmol/l. This significant association (P=0.03, n = 324) was independent of disease duration. Red triangles, patients with Type 1 diabetes with complications; black circles, patients with Type 1 diabetes without any diabetes-related complications. (b) Lower risk of elevated HbA1c was associated with a C-peptide range of >50–100 pmol/l, while higher risk was associated with a C-peptide range of >2.5–50 pmol/l (P=0.0001). A total of 1273 patients were studied.
We studied the impact of C-peptide levels in 1273 patients with Type 1 diabetes on metabolic control by examining HbA1c values (Fig. 2b). Low C-peptide levels (2.5–50 pmol/l) were associated with higher HbA1c values [median 7.2, interquartile range (IQR) 6.8–7.9] compared with substantially more C-peptide at levels of 50–100 pmol/l (median 6.9, IQR 6.3–7.8; P<0.0001). For C-peptide levels ≤2.5 pmol/l the stratum median was equal to 7.2, IQR=6.8–7.9. For C-peptide levels 2.5–50 pmol/l the stratum median (IQR) was 7.2 (6.9–8.9). By contrast, for C-peptide levels >50 pmol/l the stratum median (IQR) was 7.0 (6.3–7.6). The P values for HbA1c using a Wilcoxon rank-sum test (also known as the Mann–Whitney U-test) on new stratifications of C-peptide were also calculated. The P value for C-peptide ≤2.5 vs. >50 pmol/l was 0.004, and for 2.5–50 vs. >50 pmol/l it was 0.002. These data show that low levels of C-peptide are associated with poorer metabolic control, but levels > 50 pmol/l appear to be associated with improved HbA1c control.
There is a well established goal of normalizing HbA1c concentration to < 58 mmol/mol (7.5%) to prevent complications. These data reinforce the value of even lower levels of C-peptide in assisting metabolic control. The clinical characteristics of the patients included in the present analysis are shown in Fig. S3b. This newly demonstrated value of preserved C-peptide < 50 pmol/l to prevent complications and to possibly control blood sugars, as measured by HbA1c, is supportive of the recently identified linear relationship between HbA1c and stimulated C-peptides above the 200 pmol/l range [15]. Our data show that a similar linear relationship exists even for lower C-peptide values.
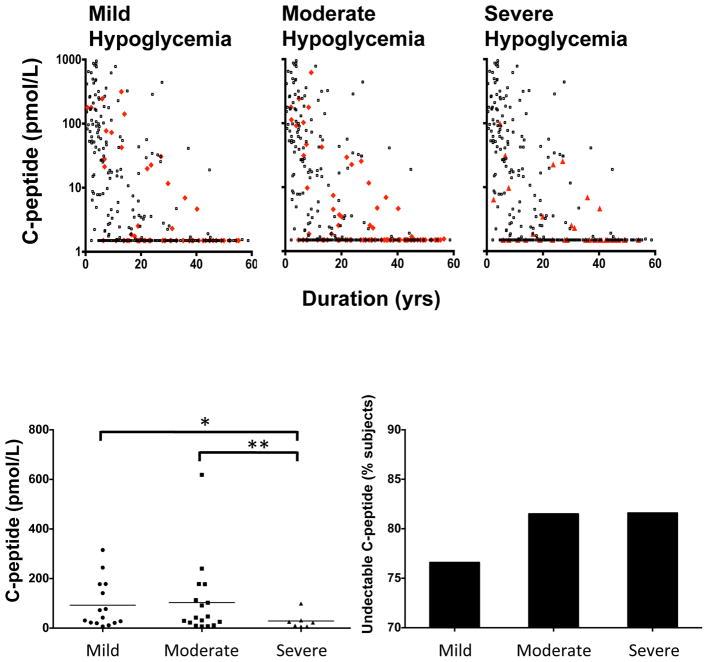
We evaluated the frequency, severity and consequences of hypoglycaemia unawareness in relation to low C-peptide levels. We used the survey method of Clarke et al. [12] (Fig. S4) to begin to determine if there were any trends in the severity of hypoglycaemia in relation to low C-peptide levels. Of the 324 patients completing the survey, 64 reported moderate hypoglycaemia and 38 reported severe hypoglycaemia (Fig. 3a). We attempted to find an absolute cut-off value for C-peptide vs. severe hypoglycaemia but could not identify one with this sample size. This implies that this clinical symptom may be more related in a linear fashion to the symptoms of severe hypoglycaemia. The clinical characteristics of these patients are presented in Fig. S5.
FIGURE 3.
Various degrees of hypoglycaemia (mild vs. moderate vs. severe) were compared with simultaneously evaluated C-peptide levels (n=324). Using the survey method of Clarke et al. [12] to evaluate mild, moderate and severe hypoglycaemia unawareness, the patients with Type 1 diabetes were divided into three categories and their mean C-peptide levels quantified (a). Patients with symptoms of hypoglycaemia are indicated as red dots; patients without symptoms of hypoglycaemia are represented as black dots. An absolute value for residual C-peptide level related to hypoglycaemic symptoms could not be identified in this dataset. (b) Determination of detectable C-peptide levels in patients with symptoms of mild, moderate and severe hypoglycaemia yielded a statistically significant trend when detectable C-peptide levels were compared. Mean ± SEM C-peptide levels for mild hypoglycaemia: 92.6 ± 24.8 pmol/l; moderate hypoglycaemia: 103.2 ± 36.4 pmol/l; and severe hypoglycaemia: 28.8 ± 12.3 pmol/l (*P=0.049; **P=0.04).
All patients with diabetes with detectable C-peptide at any level were grouped based on the hypoglycaemic survey results into mild, moderate and severe hypoglycaemia (Fig. 3b, c). The groups were then compared using the Mann–Whitney U-test. Patients with mild and moderate hypoglycaemia had higher levels of C-peptide than those with severe hypoglycaemia and the differences were significant (mild vs severe P=0.043; moderate vs severe P=0.043): mild: mean ± SEM 92.6 ± 24.8 pmol/l, median (IQR) 42.4 (21.3–177.5) pmol/l; moderate: mean ± SEM 103.2 ± 36.4 pmol/l, median (IQR) 42.4, (17.1–145.3) pmol/l; and severe: mean ± SEM 28.8 ± 12.3 pmol/l, median (IQR) 22.6, (6.9–31.2) pmol/l.
In the mild hypoglycaemia group 76.9% of patients had undetectable C-peptide levels, whereas in the moderate and severe groups 81.5 and 81.6% had undetectable C-peptide levels, respectively.
Relationship of C-peptide with the 1,5-Anhydroglucitol metabolic marker of glucose control
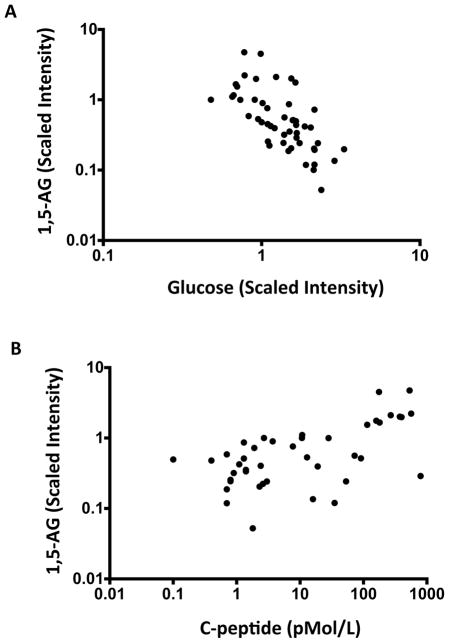
The clinical standard for evaluating glycaemic control is HbA1c levels, which represents a chronic marker of glucose control over a 3-month period. A more dynamic or acute marker of glucose control is 1,5-Andryoglucitol (1,5-AG). This rapidly changing marker is found in high concentration in the blood of people with normoglycaemia and low in those with hyperglycaemia. 1,5-AG represents glycaemia over the preceding 1–2 weeks. In a subset of 50 patients with Type 1 diabetes, we measured 1,5-AG, serum glucose and C-peptide levels. Figure 4a shows the known association of 1,5-AG with serum glucose levels: as 1,5-AG rises, blood sugars fall (Pearson r=-0·464; P=0.007). Figure 4b shows 1,5-AG levels compared with the full range of diabetic C-peptide levels, including low levels of C-peptide in the ultrasensitive assay range of 2.5–50 pmol/l. Like high levels of C-peptide, ultra-low levels of C-peptide were observed to respond in a moderate way to hyperglycaemia in a linear and statistically significant manner (Pearson r=0.566; P<0.0001). Low ranges of C-peptide showed a dynamic pancreas response measured though 1,5-AG, even when there were small pancreatic reserves of insulin secretion in response to blood sugars [7,8].
FIGURE 4.
Significant correlations between all ranges of C-peptide with glucose, C-peptide and 1,5-Anhydroglucitol (1,5AG) in patients with Type 1 diabetes (n=50). (a) Graph showing the well-known inverse correlation between glucose levels and 1,5-AG levels, which was also observed in the present study population (Pearson r=-0.464; P=0.007). (b) Graph showing that C-peptide levels at all ranges from 1.5 to 1000 pmol/l were linearly related to 1,5-AG levels.
Discussion
Our data show a gradual, decades-long decline of pancreatic function measured by a sensitive C-peptide assay in 1272 patients with Type 1 diabetes. The study confirms the results of smaller studies: C-peptide commonly exhibits a decline over decades after disease onset [7,8]. The present study also showed that an early age of onset of Type 1 diabetes was a risk factor for undetectable C-peptide levels and a later age of onset was commonly associated with a slower decline in C-peptide levels. This observation is supported by previous studies in patients with new-onset diabetes using less sensitive C-peptide assays. In 1978, Madsbad et al. [16] reported residual β-cell function in insulin-dependent people with diabetes in relation to age of onset shortly after diagnosis. The Diabetes Control and Complications Trial (DCCT) confirmed an association of early age of onset with faster decline in C-peptide levels immediately after disease onset [13]. Wang et al. [7] used C-peptide assays with lower limits of detection combined with a decades-long evaluation of the disease course and observed that age of onset was also related to decades-long preservation of C-peptide. This year two additional studies similarly reinforced this concept in new-onset disease [17,18]. A very clear protective factor for a slow C-peptide decay, therefore, was older age of onset and a very clear risk factor for a rapid decline in C-peptide was a younger age of onset. The only exception was with age of onset of diabetes > 40 years; C-peptide levels again started to decay faster, and this subgroup of patients is also notable for the fact that the majority had long-standing hypothyroidism before diabetes onset [7].
Our cross-sectional data suggest that low levels of residual fasting C-peptide may have clinical significance in preventing complications and impact on HbA1c. In an analysis of 324 patients, preservation of C-peptide levels > 10 pmol/l was associated with protection from the onset of diabetes-specific complications. These data suggest that preserved C-peptide levels lead to better glycaemic control as measured with HbA1c. Early clinical data suggest that an immunotherapy given to people with diabetes with an average of 15 years’ duration led to a small but increased C-peptide release [19]. Previous DCCT data showed that C-peptide levels of > 200 pmol/l near the time of diagnosis could be considered a meaningful threshold, and a new pilot study shows stimulated C-peptide levels of >30 pmol/l are beneficial in preventing complications [20,21]. The present study shows low levels and decades-long insulin secretion may also be beneficial.
In Type 1 diabetes the standard of care with insulin and glucose-monitoring devices has continued to improve. We saw a flat association between broad ranges of fasting C-peptide and HbA1c control. There was one exception. At the very lowest and new ranges of C-peptide detectable with this sensitive assay (2.5–50 pmol/l), as compared with higher levels (51–200 pmol/l) of C-peptide, we saw worsening of HbA1c control. These data indirectly support the concept that low levels of C-peptide secretion might be beneficial for patients with very advanced diabetes. The data also show that only at very low levels of C-peptide was there an impact on HbA1c control that was not affected by the improved standards of care. A single newly identified value of C-peptide < 50 pmol/l also does not rule out a linear relationship with these gradual declines. Using established survey methods of assessing the degree of hypoglycaemia unawareness, this dataset also shows that severe hypoglycaemia was associated with the lowest unstimulated levels of C-peptide. This finding was statistically significant. Islet transplant studies have also shown that low levels of endogenous stimulated C-peptide (> 30 pmol/l) may assist in maintaining fasting blood glucose values, lower HbA1c and prevent severe hypoglycaemia [22].
The present study uses metabolic characteristics to look at low levels of C-peptide (<50 pmol/l) in relation to 1,5-AG. The 1,5-AG blood metabolite identifies patients with more frequent and extreme glycaemic excursions and low 1,5 AG levels stratifies patients at higher risk of complications [8,23–28]. We show a strong linear and non-threshold relationship between 1,5 AG and low levels of C-peptide < 50 pmol/l (P<0.0001). Low levels of C-peptide therefore not only correlate with high risk of complications but also relate to the known association of 1,5 AG with acute glycaemic excursions over a 2-week period. If it were possible to preserve low levels of C-peptide, both marked excursions in blood glucose and complications could potentially be avoided. This suggests again that low levels of C-peptide are responding (albeit inadequately) to serum glucose fluctuations as previous studies have shown with fasting or stimulated C-peptide measurements [7,8].
The present study has some limitations. Our data and conclusions are based on a cross-sectional study of patients with diabetes using fasting C-peptide. In general, studies looking at associations with fasting C-peptide require larger sample sizes compared with those assessing stimulated C-peptide, although in a recent report by Lachin et al. [15] similar data trends with preservation of C-peptide to 200 pmol/l for both fasting or stimulated C-peptide were observed. The lack of statistical significance of low C-peptide with mild hypoglycaemia that we observed could be further examined with a future study of stimulated C-peptide or non-survey data collections. Although expensive to perform, a longitudinal study of individuals who have experienced decline in C-peptide levels over decades before complications or worsening HbA1c levels would be of value.
The measurement of low levels of C-peptide appears helpful in defining the natural history of Type 1 diabetes as a decades-long decline in insulin secretion. The low levels of C-peptide appear biologically important and are associated with HbA1c values and protection from diabetic complications. It is of historical interest to point out that it had been known since 1902 that occasionally the examination of the pancreas at autopsy of a patient with long-standing diabetes revealed islets of Langerhans structures. Unfortunately, without accompanying functional data, those islet structures were not known to secrete and thus insulin production was thought to cease for the majority of patients [9]. Our data now additionally show that, not only does C-peptide production commonly persist, but that prolonged C-peptide production is more frequent in people with Type 1 diabetes with onset in adulthood rather than in childhood. During the course of the review of this paper, two studies have also observed this age association [18,29]. Future studies of low levels of C-peptide production could be important in Type 2 as well as Type 1 diabetes [30]. These findings start to establish the clinical relevance of long-term C-peptide secretion in established Type 1 diabetes.
Supplementary Material
Figure S1. Clinical characteristics of the patients with Type 1 diabetes included in Fig. 1 to study C-peptide decline.
Figure S2. Age of onset of Type 1 diabetes influences the rate of decline in C-peptide. (a) To show in more detail what happens to C-peptide as a function of the age of onset, patients with Type 1 diabetes (n=1272) were subdivided into different groups based on their age of onset. C-peptide was then plotted against disease duration. (b) Mann–Whitney U-test comparison of the groups shown in (a). Mann–Whitney U-test P values are shown. Number of subjects in each group: age of onset <5 years, n=130; age of onset <10 years, n=319; age of onset 10–20 years, n=387; age of onset 20–30 years, n=260; age of onset 30–40 years, n=150; age of onset >40 years, n=170.
Figure S3. Clinical characteristics for the patients with Type 1 diabetes shown in Fig. 2 to study the rates of complications or HbA1c control.
Figure S4. Hypoglycaemia survey [12] used to generate the data in Fig. 3.
Figure S5. Clinical characteristics for the patients with Type 1 patients included in Fig. 3 to study the incidence and severity of hypoglycaemia.
Supplemental methods.
What’s new?
We report the measurement of small amounts of residual insulin secretion, via an ultrasensitive C-peptide assay, identifies and stratifies people with Type 1 diabetes who are at risk of or have protection from complications and hypoglycaemia.
Measurement of low levels of C-peptide may clinically identify at-risk patients.
The data suggest a new therapeutic strategy of maintaining low C-peptide levels, even in advanced disease, to avoid or prevent complications, improve HbA1c and lessen severe hypoglycaemia.
Acknowledgments
We thank Dr Miriam Davis, from the Immunobiology Laboratory of the Massachusetts General Hospital, for her critique of this paper and Ms Lynne Murphy for manuscript preparation.
Funding sources
This paper presents work supported by The Iacocca Foundation and NIH BADRC grant support (P30 DK057521) and we thank them for their generosity.
Footnotes
Competing interests
None declared.
References
- 1.Genuth SM. The case for blood glucose control. Advances Intern Med. 1995;40:573–623. [PubMed] [Google Scholar]
- 2.DCCT.Research.Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group [see comments] N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 4.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:625–640. doi: 10.1016/j.ecl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen JS, Johannesen J, Pociot F, Kristensen K, Thomsen J, Hertel NT, et al. Residual beta-Cell function 3–6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013;36:3454–3459. doi: 10.2337/dc13-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care. 2012;35:465–470. doi: 10.2337/dc11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faustman DL. Why were we wrong for so long? The pancreas of type 1 diabetic patients commonly functions for decades. Diabetologia. 2014;57:1–3. doi: 10.1007/s00125-013-3104-9. [DOI] [PubMed] [Google Scholar]
- 10.Eisenbarth GS. Type I diabetes mellitus: A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Executive summary: Standards of medical care in diabetes–2014. Diabetes Care. 2014;37 (Suppl 1):S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 12.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- 13.DCCT. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) J Clin Endocrinol Metab. 1987;65:30–36. doi: 10.1210/jcem-65-1-30. [DOI] [PubMed] [Google Scholar]
- 14.Giordano C, Galluzzo A, Panto F, Caruso C, Bompiani G. Prevalence of residual B-cell function related to age at onet and genetic profile in newly diagnosed type 1 diabetics. Acta Diabetologica. 1987;24:317–323. doi: 10.1007/BF02742964. [DOI] [PubMed] [Google Scholar]
- 15.Lachin JM, McGee P, Palmer JP, Group DER. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63:739–748. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsbad S, Faber OK, Binder C, McNair P, Christiansen C, Transbol I. Prevalence of residual beta-cell function in insulin-dependent in relation to age at onset and duration of diabetes. Diabetes. 1978;27 (Suppl 1):262–264. doi: 10.2337/diab.27.1.s262. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson J, Carlsson A, Deli A, Forsander G, Ivarsson SA, Kockum I, et al. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013;100:203–209. doi: 10.1016/j.diabres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Barker A, Lauria A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, et al. Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014;16:262–267. doi: 10.1111/dom.12216. [DOI] [PubMed] [Google Scholar]
- 19.Faustman DL, Wang L, Okubo Y, Burger D, Ban L, Man G, et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PloS One. 2012;7:e41756. doi: 10.1371/journal.pone.0041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 21.McGee P, Steffes M, Nowicki M, Bayless M, Gubitosi-Klug R, Cleary P, et al. Insulin secretion measured by stimulated C-peptide in long-established Type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabet Med. 2014;31:1264–1268. doi: 10.1111/dme.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vantyghem MC, Raverdy V, Balavoine AS, Defrance F, Caiazzo R, Arnalsteen L, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (beta-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (beta-score greater than 3) J Clin Endocrinol Metab. 2012;97:E2078–2083. doi: 10.1210/jc.2012-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanouchi T, Kawasaki T, Yoshimura T, Inoue T, Koshibu E, Ogata N, et al. Relationship between serum 1,5-anhydroglucitol and urinary excretion of N-acetylglucosaminidase and albumin determined at onset of NIDDM with 3-year follow-up. Diabetes Care. 1998;21:619–624. doi: 10.2337/diacare.21.4.619. [DOI] [PubMed] [Google Scholar]
- 24.Kim WJ, Park CY, Park SE, Rhee EJ, Lee WY, Oh KW, et al. Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet Med. 2012;29:1184–1190. doi: 10.1111/j.1464-5491.2012.03613.x. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T. Serum 1,5-anhydro-D-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis. 2011;216:477–483. doi: 10.1016/j.atherosclerosis.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Juraschek SP, Steffes MW, Miller ER, 3rd, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265–2270. doi: 10.2337/dc12-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 28.Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, et al. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31:1534–1535. doi: 10.2337/dc08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, et al. Prevalence of Detectable C-peptide According to Age at Diagnosis and Duration of Type 1 Diabetes. Diabetes Care. 2015;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 30.Lowe WL, Jr, Bain JR. “Prediction is very hard, especially about the future”: new biomarkers for type 2 diabetes? Diabetes. 2013;62:1384–1385. doi: 10.2337/db13-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clinical characteristics of the patients with Type 1 diabetes included in Fig. 1 to study C-peptide decline.
Figure S2. Age of onset of Type 1 diabetes influences the rate of decline in C-peptide. (a) To show in more detail what happens to C-peptide as a function of the age of onset, patients with Type 1 diabetes (n=1272) were subdivided into different groups based on their age of onset. C-peptide was then plotted against disease duration. (b) Mann–Whitney U-test comparison of the groups shown in (a). Mann–Whitney U-test P values are shown. Number of subjects in each group: age of onset <5 years, n=130; age of onset <10 years, n=319; age of onset 10–20 years, n=387; age of onset 20–30 years, n=260; age of onset 30–40 years, n=150; age of onset >40 years, n=170.
Figure S3. Clinical characteristics for the patients with Type 1 diabetes shown in Fig. 2 to study the rates of complications or HbA1c control.
Figure S4. Hypoglycaemia survey [12] used to generate the data in Fig. 3.
Figure S5. Clinical characteristics for the patients with Type 1 patients included in Fig. 3 to study the incidence and severity of hypoglycaemia.
Supplemental methods.