Abstract
Research on opioid analgesics such as morphine suggests that expression of abuse-related effects increases with repeated exposure. Repeated exposure to opioids often occurs clinically in the context of pain management, and a major concern for clinicians is the risk of iatrogenic addiction and dependence in patients receiving opioids for treatment of pain. This study compared abuse-related morphine effects in male rats in an intracranial self-stimulation (ICSS) procedure after repeated treatment either with morphine alone or with morphine in combination with a repeated noxious stimulus (intraperitoneal administration of dilute acid). The study also permitted comparison of morphine potency and effectiveness to block acid-induced depression of ICSS (antinociception) and to produce enhanced facilitation of ICSS (abuse-related effect). There were three main findings. First, initial morphine exposure to drug naïve rats did not produce abuse-related ICSS facilitation. Second, repeated daily treatment with 3.2 mg/kg/day morphine for six days increased expression of ICSS facilitation. This occurred whether morphine was administered in the absence or presence of the noxious stimulus. Finally, a lower dose of 1.0 mg/kg/day morphine was sufficient to produce antinociception during repeated acid treatment, but this lower dose did not reliably increase abuse-related morphine effects. Taken together, these results suggest that prior morphine exposure can increase abuse liability of subsequent morphine treatments even when that morphine exposure occurs in the context of a pain state. However, it may be possible to relieve pain with relatively low morphine doses that do not produce increases in abuse-related morphine effects.
Keywords: Morphine, opioid, intracranial self-stimulation, drug abuse, pain
INTRODUCTION
Abuse potential limits the therapeutic deployment of opioid analgesics such as morphine (Dart et al., 2015; Gutstein & Akil, 2005; Passik & Kirsh, 2011). Preclinical procedures used to examine abuse-related effects of opioids include intracranial self-stimulation (ICSS), an operant procedure in which operant responding is maintained by electrical pulses delivered to components of the brain reward system. Drug-induced increases in ICSS rates are often interpreted as abuse-related effects, whereas drug-induced decreases in ICSS rates may be indicative of abuse-limiting effects associated with anhedonia or motor impairment (Carlezon & Chartoff, 2007; Negus & Miller, 2014). Morphine and other mu opioid agonists produce both ICSS rate-increasing and rate-decreasing effects, and the relative expression of these effects is determined by variables including history of opioid exposure. Specifically, in morphine-naïve animals, morphine predominately produces abuse-limiting rate-decreasing effects, whereas repeated morphine treatment results in tolerance to rate-decreasing effects and increased expression of abuse-related rate-increasing effects (Adams, Lorens, & Mitchell, 1972; Altarifi, Miller, & Negus, 2012; Altarifi & Negus, 2011; Carlezon & Wise, 1993; Lorens & Mitchell, 1973). Other preclinical assays, including self-administration procedures (Carrera, Schulteis, & Koob, 1999; Negus & Rice, 2009; O’Connor, Chapman, Butler, & Mead, 2011; Thompson & Schuster, 1964; Yanagita, 1978) have yielded similar results. Moreover, examination of opioid abuse-related effects in humans has revealed enhanced expression after repeated opioid exposure (Comer, Sullivan, Vosburg, Kowalczyk, & Houser, 2010; Cooper et al., 2012; Lasagna, 1955).
Repeated exposure to opioids often occurs clinically in the context of pain management, and as a result, a major concern for clinicians is the risk of iatrogenic addiction and dependence in patients receiving opioids for treatment of pain. An emerging preclinical literature has focused largely on changes in expression of abuse-related opioid effects during ongoing pain, and a consistent pattern has yet to emerge. For example, models of sustained inflammatory or neuropathic pain increased potency of morphine to facilitate ICSS (Leitl et al., 2014) or to produce place preferences in place conditioning procedures (Cahill et al., 2013; Sufka, 1994), and these effects were attributed to higher potency of morphine to produce rewarding alleviation of pain than to produce rewarding effects in the absence of pain. However, a spinal nerve ligation model of neuropathic pain decreased both potency of opioids to maintain self-administration and effectiveness of opioids to facilitate ICSS in rats (Martin, Kim, Buechler, Porreca, & Eisenach, 2007; Ewan & Martin 2011a, 2011b), and sciatic nerve ligation also reduced morphine place preferences in both rats and mice (Ozaki et al., 2002, 2003). Finally, oral fentanyl self-administration was increased in rats by an arthritis model of chronic inflammation but not by a sciatic nerve ligation model of neuropathy (Kupers & Gybels, 1995; Colpaert et al., 2001), and chronic inflammation failed to alter place conditioning by morphine in rats (Shippenberg, Stein, Huber, Millan, & Herz, 1988).
In contrast to these studies of opioid effects during putative pain states, no studies have yet evaluated whether opioid treatment during a pain state alters subsequent sensitivity to abuse-related opioid effects after termination of pain. This is a pertinent clinical issue given that many patients receive transient opioid treatment for transient pain (e.g. postsurgical pain), and little is known regarding the degree to which such regimens of opioid exposure might increase subsequent vulnerability to opioid abuse. Accordingly, the major goal of this study was to compare abuse-related morphine effects in an ICSS procedure after repeated treatment either with morphine alone or with morphine in combination with a repeated noxious stimulus (intraperitoneal administration of dilute acid (IP acid)). Two different doses of repeated morphine were examined to evaluate potency of morphine to enhance subsequent morphine-induced facilitation of ICSS. IP acid was used as the repeated noxious stimulus for two reasons (Stevenson, Bilksy, & Negus, 2006; Pereira Do Carmo, Stevenson, Carlezon, & Negus 2009; Altarifi, Rice, & Negus., 2015). First, IP acid effects are transient (1-2 hr) and permit more precise temporal control of noxious stimulation onset and offset than can be achieved with other more sustained inflammatory or neuropathic insults. Second, we have shown previously that IP acid produces a pain-related decrease in ICSS, and morphine produces a dose-dependent antinociceptive blockade of this IP acid effect. Accordingly, morphine potency and effectiveness to block acid-induced depression of ICSS could be compared to morphine potency and effectiveness to produce enhanced facilitation of ICSS.
METHODS
Subjects and ICSS Electrode Implantation
Male Sprague-Dawley rats (Harlan, Fredrick, MD, USA) weighing 310-350 g and approximately 11-12 weeks old at the time of surgery were used for these studies. Rats were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animal subjects in research (National Research Council, 2011), and all animal use protocols were approved by the Virginia Commonwealth University Institutional Care and Use Committee.
Rats were anesthetized with isoflurane gas (2.5-3% in oxygen; Webster Veterinary, Phoenix, AZ) for the implantation of stainless steel electrodes. The cathode (0.25 mm, insulated) of each bipolar electrode (Plastics One, Roanoke, VA) was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from bregma, and 8.8 mm below the skull). The anode (0.124 mm, uninsulated) was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured to the skull with orthodontic resin. Animals were allowed to recover for at least 7 days prior to commencing ICSS training.
Experimental Procedure
Apparatus
Experiments were conducted in sound attenuating chambers that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow and green positioned 7.6 cm directly above the lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables routed through a commutator (Model SL2C, Plastics One, Roanoke, VA). A computer and software (Med Associates, St. Albans, VT) controlled the stimulator, programming parameters and data collection.
Training
Rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using procedures similar to those described previously (Altarifi et al., 2012). During the initial phase of training, the frequency of stimulation was held constant at 2.2 Log Hz, and the stimulation intensity was adjusted to the lowest value that would sustain at least 30 stimulations per minute during 60-min training sessions. Once this criterion was met, frequency manipulations were introduced during sessions that consisted of sequential 10 min components. During each component, a descending series of 10 current frequencies (2.2-1.75 Log Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial began with a 5-s time out followed by a 5-s “priming” phase during which animals received five non-contingent stimulations with a 0.5-s interval between each stimulation. This non-contingent stimulation was followed by a 50-s “response” period during which responding produced electrical stimulation under the FR 1 schedule. During this phase of training, the current intensity was adjusted until rats reliably responded during the first four to five frequency trials of all components for at least three consecutive days. This intensity was held constant for the duration of the study. Once training was completed, three “Pre-drug Baseline” sessions were conducted over three consecutive days to establish baseline ICSS performance. Experimental sessions each day consisted of three ICSS components. The first component was considered an acclimation component and data were discarded. Data from the second and third components were averaged for the three sessions to yield Pre-Drug Baseline Data shown in Table 1 and the figures. Rats were then divided into six groups (N=6−7 each), and testing was initiated using the experimental design described below.
Table 1.
Pre-experiment MCR and Total Stimulations.
| Group | MCR ± SE | Total Stimulations ± SE |
|---|---|---|
| Repeated Saline + Water | 58.9 ± 2.0 | 257.6 ± 27.7 |
| Repeated 1.0 mg/kg + Water | 58.9 ± 4.2 | 283.0 ± 32.7 |
| Repeated 3.2 mg/kg + Water | 58.3 ± 3.0 | 283.0 ± 12.0 |
| Repeated Saline + IP acid | 61.4 ± 2.6 | 279.9 ± 29.2 |
| Repeated 1.0 mg/kg + IP acid | 55.6 ± 5.0 | 285.6 ± 37.3 |
| Repeated 3.2 mg/kg + IP acid | 56.5 ± 4.0 | 267.1 ± 28.4 |
Testing
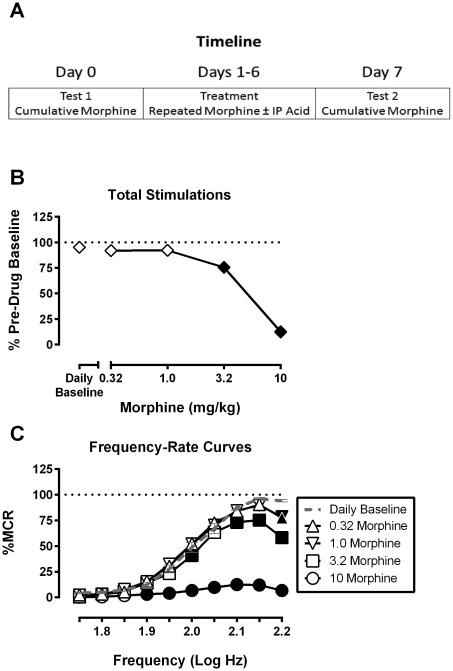
Experiments were conducted using an eight-day treatment protocol (Figure 1A). On Days 0 and 7, rats received cumulative doses of morphine (0.32-10 mg/kg), and on intervening days 1-6, rats received daily injections of (1) morphine (1.0 or 3.2 mg/kg/day) or its vehicle paired with (2) 1.8% lactic acid or its vehicle. Treatment groups are outlined in Table 1. Major goals of the study were to evaluate (a) impact of the Day 1-6 treatment on effects of cumulative morphine and (b) stability of treatment effects on Days 1 to 6.
Figure 1.
Study timeline and initial effects of cumulative morphine under morphine-naïve conditions for all rats used in this study. Figure 1A, schematic of the experimental timeline. Figure 1B shows effects of morphine on ICSS expressed as Percent Pre-drug Baseline total stimulations delivered across all frequencies of brain stimulation. Abscissa: morphine dose in mg/kg. Ordinate: Percent Pre-drug Baseline total stimulations. Filled symbols represent ICSS rates that were statistically different from Daily Baseline as determined by one-way ANOVA followed by a Dunnett’s post hoc test, P<0.05. Figure 1C shows morphine effects on full ICSS frequency–rate curves. Abscissa: Frequency of electrical brain stimulation in Log Hz. Ordinate: Percent maximum control reinforcement rate (%MCR). Filled points represent frequencies at which reinforcement rates were statistically different from Daily Baseline as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, P < 0.05. Statistical results as follows: (B) significant effect of morphine dose [F(4, 160) = 184.7, P < 0.05]. (C) significant main effects of morphine dose [F(4, 160) = 165.3, P < 0.05] and frequency [F(9, 360) = 344.7, P < 0.05], and a significant interaction [F(36, 1440) = 49.58, P < 0.05]. Data show mean ± SEM for 41 rats.
Each test session on Days 0-7 began with three “Daily Baseline” components. The first component of each session was considered an acclimation component, and data were discarded. Data from the second and third components were averaged to yield Daily Baseline data shown in the figures. During cumulative-dosing sessions on Day 0 and Day 7, Daily Baseline components were followed by cumulative morphine administration (0.32-10 mg/kg). Specifically, a series of increasing morphine doses was administered at 50 min intervals, and each sequential dose increased the total cumulative morphine dose by 0.5 log units. ICSS was evaluated during two consecutive 10-min test components starting 30 min after each dose. During treatment sessions on Days 1-6, the Daily Baseline components were followed by treatment according to group assignments shown in Table 1. Thus, rats were treated first with saline, 1.0 mg/kg or 3.2 mg/kg morphine and then 30 min later with 1.8% lactic acid or water before being returned immediately to the operant chambers for two 10-min ICSS test components.
Data Analysis
Data were analyzed using an approach described previously (Negus & Miller, 2014). First, to evaluate performance over the entire range of frequency magnitudes, the total number of stimulations earned per component in each rat was calculated as the average of the total stimulations delivered across all 10 frequency trials of each component. Daily Baseline and Test data during each experimental day in each rat were expressed as a percentage of the total stimulations per component earned during the “Pre-drug Baseline” components in that rat before initiation of morphine and/or acid exposure. Thus, % Pre-drug Baseline Total Stimulations was calculated as: (Mean Total Stimulations during Daily Baseline or Test Components ÷ Mean Total Stimulations during Pre-drug Baseline Components) × 100. These data were analyzed by repeated-measures one-way or two-way ANOVA. For cumulative dosing test sessions (Days 0 and 7), the factors were morphine dose and test day. For treatment sessions (Days 1-6), the factors were treatment and treatment day. A significant ANOVA was followed by a Dunnett’s or Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
The analysis described above was complemented by analysis of full frequency-rate curves for selected conditions. For this analysis, raw ICSS rates at each frequency in each rat were first converted to Percent Maximum Control Rate (%MCR) for that rat, with the maximum control rate (MCR) defined as the mean of the maximal rates observed in any frequency trial during each of the Pre-drug Baseline components. Thus, %MCR for each trial was calculated as: (ICSS Rate During a Frequency Trial ÷ Maximum Control Rate) × 100. Normalized data for Daily Baseline and Test components were then averaged across rats for statistical analysis using repeated-measures two-way ANOVA, with brain stimulation as one factor and either morphine dose or treatment day as the second factor. A significant ANOVA was followed by a Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
Drugs
Morphine sulfate was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA), dissolved in saline, and delivered subcutaneously in a volume of 1 ml/kg body weight. Lactic acid was purchased from Sigma Chemical Co. (St. Louis, MO), diluted in bacteriostatic water to a concentration of 1.8%, and delivered IP in a volume of 1 ml/kg body weight.
RESULTS
Baseline ICSS performance and effects of morphine in drug-naïve rats
Table 1 shows “Pre-drug Baseline” values from each group for (a) maximum control rate (MCR) at the most reinforcing frequency, and (b) total stimulations per component earned across all frequencies. These baseline measures did not significantly differ across groups, and subsequent data were expressed as a percent of these Pre-drug Baseline data. Figure 1 shows the Day 0 cumulative morphine dose-effect curve determined in all 41 rats included in this study. Data for total stimulations per component are shown in Figure 1B, and full frequency-rate curves are shown in Figure 1C. Under these conditions morphine exclusively decreased ICSS rates, and ICSS was not facilitated at any frequency by any cumulative morphine dose.
Effects of cumulative morphine on ICSS before and after repeated saline or morphine
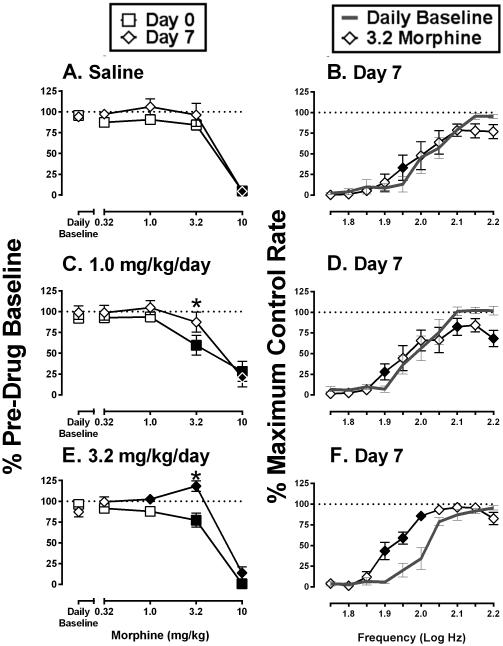
Figure 2 shows effects of cumulative morphine (0.32-10 mg/kg) before and after six days of treatment with saline, 1.0 mg/kg/day morphine, or 3.2 mg/kg/day morphine in combination with lactic acid vehicle. Data for total stimulations per component on Day 0 and Day 7 are compared in the left panels (Figure 2A,C,E), and right panels highlight effects of cumulative 3.2 mg/kg morphine on full frequency-rate curves on Day 7 (Figures 2B,D,F). Repeated saline treatment did not alter cumulative morphine effects on total stimulations per component (Figure 2A). On Day 7, cumulative 3.2 mg/kg morphine had little effect on the ICSS frequency-rate curve, producing significant but small facilitation of ICSS at one brain-stimulation frequency (1.95 log Hz) and a non-significant trend toward depression of ICSS at higher frequencies (2.15 and 2.20 log Hz) (Figure 2B). Repeated 1.0 mg/kg/day morphine produced a small but significant change in morphine effects on Day 0 vs. Day 7. On Day 0, before 1.0 mg/kg/day morphine treatment, doses of 3.2 and 10 mg/kg morphine significantly depressed total stimulations per component; however, on Day 7, 3.2 mg/kg morphine no longer depressed ICSS (Figure 2C). Cumulative treatment with 3.2 mg/kg morphine on Day 7 produced mixed effects on full frequency-rate curves, with significant facilitation of ICSS at one low frequency (1.90 log Hz) and significant depression of ICSS at two high frequencies (2.10 and 2.20 log Hz) (Figure 2D). Repeated 3.2 mg/kg/day morphine produced a greater change in morphine effects on Day 0 vs. Day 7. On Day 0, cumulative 3.2 and 10 mg/kg morphine significantly depressed total stimulations per component; however, on Day 7, 3.2 mg/kg morphine significantly increased this measure of ICSS (Figure 2E). Moreover, Figure 2F shows that cumulative 3.2 mg/kg morphine on Day 7 produced a leftward shift in the ICSS frequency curve and facilitated ICSS at three intermediate frequencies (1.90-2.0 log Hz) without evidence for ICSS depression at higher frequencies. Thus, relative to treatment with saline or 1.0 mg/kg/day morphine, treatment with 3.2 mg/kg/day morphine on Days 1-6 reduced expression of rate-decreasing effects and increased expression of rate-increasing effects by morphine on Day 7.
Figure 2.
Effects of cumulative morphine on ICSS before and after repeated saline or repeated morphine without the acid noxious stimulus. Figure 2A and 2B: repeated vehicle + vehicle treatment group, Figure 2C and 2D: repeated 1.0 mg/kg/day morphine + vehicle treatment group, Figure 2E and 2F: repeated 3.2 mg/kg/day morphine + vehicle treatment group. Left column of panels (A,C,E) shows effects of morphine on ICSS expressed as Percent Pre-drug Baseline total stimulations delivered across all frequencies of brain stimulation. Abscissae: morphine dose in mg/kg. Ordinates: Percent Pre-drug Baseline total stimulations. Filled symbols represent ICSS rates that were statistically different from Daily Baseline, and asterisks represent ICSS rates that were statistically different across test days (Day 0 vs Day 7) as determined by two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Right column of panels (B,D,F) shows full ICSS frequency–rate curves on Day 7 for the Daily Baseline and after cumulative 3.2 mg/kg morphine. Abscissae: Frequency of electrical brain stimulation in Log Hz. Ordinate: Percent maximum control reinforcement rate (%MCR). Filled points represent frequencies at which reinforcement rates were statistically different from the Daily Baseline as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, P < 0.05. Statistical results are as follows: (A) significant effect of morphine dose [F(4, 20) = 54.4, P < 0.05], no effect of test day [F(1, 5) = 6.6, P = 0.05], and no interaction [F(4, 20) = 1.0, P > 0.05]. (B) significant effects of morphine [F(4, 20) = 20.6, P < 0.05] and frequency [F(9, 45)=37.9, P < 0.05], and a significant interaction [F(36, 180) = 7.5, P < 0.05]. (C) significant effect of morphine dose [F(4, 24) = 42.8, P < 0.05], no effect of test day [F(1, 6) = 1.37, P > 0.05], but a significant interaction [F(4, 24) = 2.9, P < 0.05]. (D) significant effects of morphine [F(4, 24) = 50.0, P < 0.05] and frequency [F(9, 54) = 35.2, P < 0.05], and a significant interaction [F(36, 216) = 8.3, P < 0.05]. (E), significant effects of morphine dose [F(4, 24) = 68.2, P < 0.05] and test day [F(1, 6) = 30.42, P < 0.05], and a significant interaction [F(4, 24) = 8.5, P < 0.05]. (F) significant effects of morphine [F(4, 24) = 46.4, P < 0.05] and frequency [F(9, 54) = 149.9, P < 0.05], and a significant interaction [F(36, 216) = 8.5, P < 0.05]. Data show mean ± SEM for 6-7 rats.
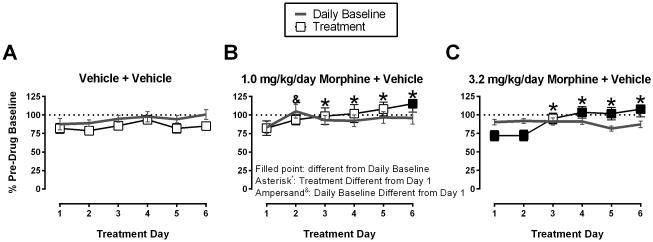
Effects of Day 1-6 treatment with saline or morphine
Figure 3 shows ICSS on Days 1-6 before (Daily Baseline) and after (Treatment) each daily treatment with saline, 1.0 mg/kg/day morphine or 3.2 mg/kg/day morphine. Daily baselines were stable across days in all groups with the exception that the Daily Baseline on Treatment Day 2 differed from Day 1 in the 1.0 mg/kg/day morphine + vehicle group. Daily saline treatment produced modest decreases in total stimulations relative to Daily Baseline, and there was no change in saline effects across days. Treatment with 1.0 mg/kg/day morphine (Figure 3B) significantly increased total stimulations compared to the Daily Baseline on Day 6 of treatment, and total stimulations were elevated on Days 3-6 compared to Day 1. Treatment with 3.2 mg/kg/day morphine (Figure 3C) decreased total stimulations compared to the Daily Baseline on Days 1 and 2, but increased total stimulations earned on Days 4-6. In addition, total stimulations were elevated on Days 3-6 compared to Day 1.
Figure 3.
Effects of Day 1-6 treatment with saline or morphine + acid vehicle. Figure 3A: repeated vehicle + vehicle group, Figure 3B: repeated 1.0 mg/kg/day morphine + vehicle group, Figure 3C: repeated 3.2 mg/kg/day morphine + vehicle group. Abscissae: treatment day. Ordinates: Percent Pre-drug Baseline total stimulations per component. Filled symbols represent ICSS rates that were statistically different from the Daily Baseline, and asterisks represent ICSS rates that were statistically different from Day 1 as determined by two-way ANOVA followed by a Holm-Sidak post hoc test, P < 0.05. Statistical results are as follows: (A) significant effect of treatment [F(1, 5) = 11.3, P < 0.05], no significant effect of treatment day [F(5, 25) = 1.9, P > 0.05], no significant interaction [F(5, 25) = 0.8, P > 0.05]. (B) significant effects of treatment [F(1, 6) = 1.3, P > 0.05] and treatment day [F(5, 30) = 4.6, P < 0.05], and a significant interaction [F(5, 30) = 3.6, P < 0.05]. (C) no significant main effect of treatment [F(1, 6) = 0.3, P > 0.05], significant main effect of treatment day [F(5, 30) = 4.9, P < 0.05], and a significant interaction [F(5, 30) = 14.3, P < 0.05]. Data show mean ± SEM for 6-7 rats.
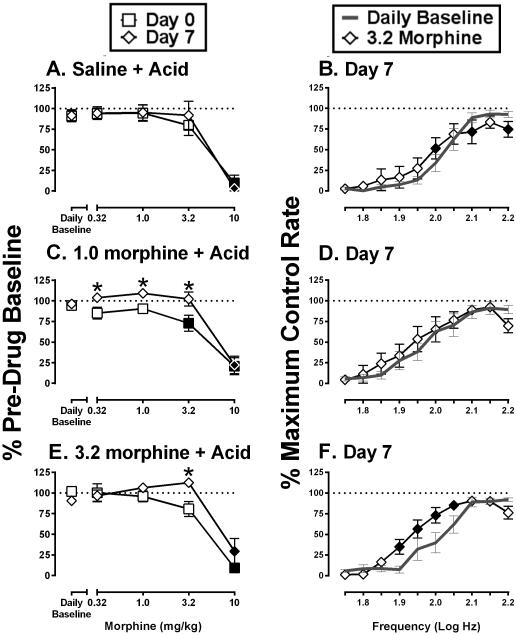
Effects of cumulative morphine on ICSS before and after repeated saline or morphine + IP acid
Figure 4 shows effects of cumulative morphine (0.32-10 mg/kg) before and after six days of treatment with saline, 1.0 mg/kg/day morphine, or 3.2 mg/kg/day morphine in combination with IP acid. Repeated saline+acid did not alter cumulative morphine effects on total stimulations per component (Figure 4A), and on Day 7, cumulative 3.2 mg/kg morphine produced a significant but small facilitation of ICSS at one brain-stimulation frequency (2.0 log Hz) and significant depression of ICSS at two higher frequencies (2.10 and 2.20 log Hz) (Figure 4B). Repeated 1.0 mg/kg/day morphine+acid attenuated the rate-decreasing effects of morphine. In particular, cumulative doses of both 3.2 and 10 mg/kg morphine significantly depressed total stimulations per component on Day 0, but on Day 7 after six days of 1.0 mg/kg/day morphine+acid, 3.2 mg/kg morphine no longer depressed ICSS (Figure 4C). Cumulative treatment with 3.2 mg/kg morphine on Day 7 did not alter ICSS at any frequency of the frequency-rate curve on Day 7 (Figure 4D). Repeated 3.2 mg/kg/day morphine+acid also modified morphine effects on Day 0 vs. Day 7. Total stimulations per component were significantly higher after cumulative 3.2 mg/kg morphine on Day 7 than on Day 0 (Figure 4E). Moreover, Figure 4F shows that cumulative 3.2 mg/kg morphine on Day 7 produced a leftward shift in the ICSS frequency curve and facilitated ICSS at four intermediate frequencies (1.90-2.05 log Hz) without producing significant depression of ICSS at higher frequencies.
Figure 4.
Effects of cumulative morphine on ICSS before and after repeated saline or morphine + IP acid. Figure 4A and 4B: repeated vehicle + acid group, Figure 4C and 4D: repeated 1.0 mg/kg/day morphine + acid group, Figure 4E and 4F: repeated 3.2 mg/kg/day morphine + acid group. Left column of panels (A,C,E) shows effects of morphine on ICSS expressed as Percent Pre-drug Baseline total stimulations delivered across all frequencies of brain stimulation. Abscissae: morphine dose in mg/kg. Ordinates: Percent Pre-drug Baseline total stimulations. Filled symbols represent ICSS rates that were statistically different from Daily Baseline, and asterisks represent ICSS rates that were statistically different across test days (Day 0 vs Day 7) as determined by two-way ANOVA followed by a Holm-Sidak post hoc test, P<0.05. Right column of panels (B,D,F) shows full ICSS frequency–rate curves on Day 7 for the Daily Baseline and after cumulative 3.2 mg/kg morphine. Abscissae: Frequency of electrical brain stimulation in Log Hz. Ordinate: Percent maximum control reinforcement rate (%MCR). Filled points represent frequencies at which reinforcement rates were statistically different from the Daily Baseline as determined by a two-way ANOVA followed by a Holm–Sidak post hoc test, P < 0.05. Statistical results are as follows: (A) significant effect of morphine dose [F(4, 24) = 41.9, P < 0.05], no effect of test day [F(1, 6) = 0.1, P > 0.05], and no interaction [F(4, 24) = .5, P>0.05]. (B) significant effect of morphine [F(4, 24) = 27.7, P<0.05] and frequency [F(9, 54) = 51.9, P < 0.05], and a significant interaction [F(36, 216) = 12.6, P < 0.05]. (C) significant effect of morphine dose [F(4, 24) = 48.5, P < 0.05] and test day [F(1, 6) = 9.9, P < 0.05], and a significant interaction [F(4, 24) = 4.9, P < 0.05]. (D) significant effect of morphine [F(4, 24) = 27.3, P < 0.05] and frequency [F(9, 54) = 36.9, P < 0.05], and a significant interaction [F(36, 216) = 5.1, P < 0.05]. (E) significant effect of morphine dose [F(4, 24) = 38.4, P < 0.05], no significant main effect of test day [F(1, 6) = 3.2, P > 0.05], but a significant interaction [F(4, 24) = 4.0, P < 0.05]. (F), significant main effect of morphine [F(4, 24) = 13.9, P < 0.05] and frequency [F(9, 54) = 58.7, P < 0.05] and a significant interaction [F(9, 54) = 7.5, P < 0.05]. Data show mean ± SEM for 7 rats.
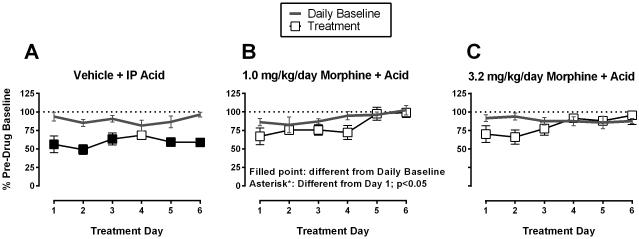
Effects of Day 1-6 treatment with saline or morphine + acid
Figure 5 shows ICSS on Days 1-6 before (Daily Baseline) and after (Treatment) each daily treatment with saline, 1.0 mg/kg/day morphine or 3.2 mg/kg/day morphine + IP acid. Treatment with saline+acid (Figure 5A) decreased total stimulations per component on five of the six treatment days, and IP acid effects on total stimulations did not differ across treatment days. Treatment with 1.0 mg/kg/day morphine+acid (Figure 5B) did not reduce ICSS relative to the Daily Baseline, and total stimulations per component were elevated on Days 5 and 6 compared to Day 1. Treatment with 3.2 mg/kg/day morphine+acid (Figure 5C) also did not reduce ICSS relative to the Daily Baseline, and effects did not significantly differ across treatment days. Overall, IP acid produced a repeatable and pain-related depression of ICSS in the saline+acid group, and both morphine doses produced a repeatable blockade of acid-induced ICSS depression that tended to be more robust at the last days of treatment than the first days of treatment.
Figure 5.
Effects of Day 1-6 treatment with saline or morphine + acid. Figure 5A: repeated vehicle + IP acid group, Figure 5B: repeated 1.0 mg/kg/day morphine + IP acid group, Figure 5C: repeated 3.2 mg/kg/day morphine + IP acid group. Abscissae: treatment day. Ordinates: Percent Pre-drug Baseline total stimulations per component. Filled symbols represent ICSS rates that were statistically different from the Daily Baseline, and asterisks represent ICSS rates that were statistically different from Day 1 as determined by two-way ANOVA followed by a Holm-Sidak post hoc test, P < 0.05. Statistical results are as follows: (A) significant effect of treatment [F(1, 6) = 34.3, P < 0.05], no effect of treatment day [F(5, 30) = 0.7, P > 0.05], and a significant interaction [F(5, 30) = 2.7, P < 0.05]. (B) no effect of treatment [F(1, 6) = 6.1, P = 0.05], but a significant effect of treatment day [F(5, 30) = 6.5, P < 0.05], and no significant interaction [F(5, 30) = 1.1, P > 0.05]. (C) no significant main effect of treatment [F(1, 6) = 1.7, P > 0.05] or treatment day [F(5, 30) = 1.1, P > 0.05] and no significant interaction [F(5, 30) = 2.5, P = 0.05]. Data show mean ± SEM for 7 rats.
DISCUSSION
This study used an intracranial self-stimulation procedure to evaluate abuse-related effects of morphine determined before and after a regimen of repeated morphine in the absence or presence of a noxious stimulus. There were three main findings. First, as reported previously, initial morphine exposure to drug naïve rats produced primarily rate-decreasing effects and did not produce abuse-related ICSS facilitation. Second, repeated daily treatment with 3.2 mg/kg/day morphine for six days reduced expression of ICSS rate-decreasing effects by subsequent morphine treatment and increased expression of ICSS facilitation. This occurred whether morphine was administered in the absence or presence of the noxious stimulus, indicating that noxious stimulation did not prevent effectiveness of morphine exposure to increase subsequent expression of abuse-related morphine effects. Finally, a lower dose of 1.0 mg/kg/day morphine was sufficient to produce antinociception during repeated IP acid treatment, but this lower dose did not reliably increase abuse-related morphine effects. Taken together, these results suggest that prior morphine exposure can increase abuse liability of subsequent morphine treatments even when that morphine exposure occurs in the context of a pain state. However, it may be possible to relieve pain with relatively low morphine doses that do not produce increases in abuse-related morphine effects.
Drug-induced increases in low ICSS rates maintained by low frequencies of brain stimulation are often interpreted as evidence of abuse potential, whereas drug-induced decreases in higher ICSS rates maintained by higher brain-stimulation frequencies are interpreted as evidence of abuse-limiting effects that may be related to anhedonia or motor impairment (Carlezon & Chartoff, 2007; Negus & Miller, 2014). In this study, morphine exclusively decreased ICSS rates in morphine-naïve rats, and repeated morphine treatment produced a dose-dependent tolerance to these initial rate-decreasing effects and increased expression of rate-increasing effects. These findings are consistent with previous work with both morphine and other mu agonist analgesics in ICSS procedures (Altarifi et al., 2012; Altarifi, Rice, & Negus, 2013; Altarifi & Negus, 2011; Carlezon Jr & Wise, 1993; Lorens & Mitchell, 1973), and these results suggest that one consequence of repeated opioid exposure is a transition from expression of primarily abuse-limiting effects to expression of primarily abuse-related effects. Similar increases in abuse-related morphine effects after regimens of opioid exposure have been reported from other preclinical assays such as place conditioning (Shippenberg, Heidbreder, & Lefevour, 1996) and drug self-administration (Carrera et al., 1999; Negus & Rice, 2009; Thompson & Schuster, 1964; Yanagita, 1978), and in humans using measures of subjective effects and drug self-administration (Comer et al., 2010; Cooper et al., 2012; Lasagna, 1955). A major goal of the present study was to evaluate the degree to which a noxious stimulus might alter effectiveness of morphine exposure to enhance subsequent expression of abuse-related morphine effects.
Morphine and other mu agonists are effective analgesics, and pain management is one context in which individuals experience repeated exposure to these drugs. A major concern in opioid pain management is whether opioid exposure to treat pain might increase risk for opioid abuse and development of iatrogenic addiction (Dart et al., 2015; Passik & Kirsh, 2011); however, it remains unclear whether repeated opioid exposure in the context of pain management exposes individuals to increased risk (Minozzi, Amato, & Davoli, 2013). Such questions have fueled preclinical efforts to examine the role of pain as a determinant of opioid effects related to abuse-potential, and the present study examined morphine effects both during and after exposure to a noxious stimulus. Our results suggest that abuse-related morphine effects were reduced during a pain state. Specifically, when 1.0 or 3.2 mg/kg/day morphine was administered in the absence of IP acid, there was at least one treatment day during which morphine significantly increased ICSS rates above that day’s Daily Baseline rates. In contrast, when 1.0 or 3.2 mg/kg/day morphine was administered in combination with daily acid injections, morphine never increased ICSS rates above the Daily Baseline rates. This finding is consistent with other studies reporting pain-related decreases in the acute abuse-related opioid effects in assays of opioid self-administration (Martin et al., 2007), opioid-induced place conditioning (Narita et al., 2004; Ozaki et al., 2003; Ozaki et al., 2002), and opioid-induced increases in nucleus accumbens dopamine release (Narita et al., 2004). It should be noted, however, that these results contrast with other reports suggesting either no decrease, or an increase, in abuse-related opioid effects in assays of self-administration (Colpaert et al., 2001; Kupers & Gybels, 1995) and place conditioning (Cahill et al., 2013; Sufka, 1994).
One factor that may contribute to these apparent discrepancies is the interpretative challenge in distinguishing between aspects of drug reward that are independent of pain state from those that depend on alleviation of an aversive pain state. Although interpretations may differ regarding pain effects on opioid reward, there is more general agreement that morphine and other opioids are effective to reduce aversive aspects of pain. In the present study, for example, morphine blocked pain-related depression of ICSS produced by daily acid injections. This is consistent with previous reports that pain-related depression of ICSS and some other behaviors can be blocked by morphine and other mu opioid analgesics (Altarifi et al., 2015; Matson et al., 2007; Miller, Picker, Umberger, Schmidt, & Dykstra, 2012; Rutten, Robens, Read, & Christoph, 2014), as well as by non-steroidal anti-inflammatory drugs (Kwilasz & Negus, 2012; Matson et al., 2007; Rutten et al., 2014). Morphine also blocks pain-related place aversion (Pedersen & Blackburn-Munro, 2006; van der Kam, Vry, Schiene, & Tzschentke, 2008) and pain-related punishment of operant responding (Neubert et al., 2005). The present study expands on these earlier studies in two ways. First, this study found sustained effectiveness of the acid noxious stimulus to depress ICSS during repeated daily treatments for six days. This suggests a lack of tolerance to acid effects and supports use of repeated acid administration to model sustained pain. Second, there was also a lack of tolerance to morphine antinociception expressed as a blockade of acid-induced ICSS depression. Previous studies have provided evidence that tolerance to morphine’s antinociceptive effects develops at different rates in assays of pain-stimulated and pain-depressed behavior (Altarifi & Negus, 2015). Moreover, these findings are consistent with experimental (Cooper et al., 2012) and clinical human data (Cowan, Allan, Libretto, & Griffiths, 2001; Watson, 2012) suggesting opioids often maintain analgesic efficacy after repeated treatment. Though no evidence of antinociceptive tolerance was observed in this study, there was qualitative evidence of enhanced antinociceptive efficacy over the course of the treatment regimen. Effects observed in Figures 5B and C may reflect the development of tolerance to morphine’s rate-decreasing effects, such as sedation, over the course of the treatment regimen. Sedation and respiratory depression limit the therapeutic potential of opioids, and tolerance to these effects can be viewed as a desirable consequence of repeated opioid exposure (Benyamin et al., 2008; Labianca et al., 2012).
The interaction between rewarding effects of analgesics and aversive effects of pain states is an important consideration for pain treatment; however, it is also important to consider whether opioid exposure during a pain state might increase vulnerability to abuse-related opioid effects after the pain state has resolved. Results of the present study which used 1.8% lactic acid as a noxious stimulus suggest that the answer to this question depends more on the morphine dose than on presence or absence of this noxious stimulus. The low dose of 1.0 mg/kg morphine was sufficient to block acid-induced depression of ICSS during repeated morphine+acid treatment, and this regimen of opioid exposure produced little change in subsequent expression of morphine effects after termination of acid treatment. This finding suggests that morphine can be administered repeatedly at doses sufficient to produce antinociception without producing large changes in subsequent vulnerability to abuse-related morphine effects. Though definitive data are lacking, this conclusion may also be consistent with relatively low rates of iatrogenic opioid addiction in patients who receive transient opioid treatment for transient pain (Compton & Volkow, 2006). However, this low morphine dose also produced little change in subsequent vulnerability to abuse-related morphine effects when it was administered repeatedly in the absence of the acid noxious stimulus. Moreover, in contrast to results with the low dose of 1.0 mg/kg/day morphine, repeated treatment with the higher dose of 3.2 mg/kg/day morphine did increase subsequent expression of abuse-related morphine effects regardless of whether this morphine dose was administered in the absence or presence of repeated acid. Taken together, these results suggest two conclusions. First, under these conditions, morphine is more potent to produce antinociceptive effects than to produce increases in subsequent sensitivity to abuse-related morphine effects. Second, the IP acid noxious stimulus is not sufficient to block the increase in abuse-related morphine effects produced by repeated exposure to higher morphine doses. Additional studies using pain manipulations of different modality, duration, and magnitude are needed to further bridge the gaps in our knowledge of how pain states modulate opioid reward and abuse potential.
Acknowledgments
This research was supported by NIH grants R01-NS070715, T32-DA007027, F32-DA033920, and Jordan University of Science and Technology. The funding sources had no other role than financial support
The authors wish to thank Christina Johnson for outstanding technical support.
Footnotes
Disclosures
All authors contributed in a significant way to the manuscript, and read and approved the final manuscript.
All authors report no conflicts of interest.
REFERENCES
- Adams WJ, Lorens SA, Mitchell CL. Morphine Enhances Lateral Hypothalamic Self-Stimulation in the Rat. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1972;140(3):770–771. doi: 10.3181/00379727-140-36549. doi:10.3181/00379727-140-36549. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, Miller LL, Negus SS. Role of μ-opioid receptor reserve and μ-agonist efficacy as determinants of the effects of μ-agonists on intracranial self-stimulation in rats. Behavioural Pharmacology. 2012;23(7):678–692. doi: 10.1097/FBP.0b013e328358593c. doi:10.1097/FBP.0b013e328358593c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behavioural Pharmacology. 2011;22(7):663–673. doi: 10.1097/FBP.0b013e32834aff54. doi:10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. European Journal of Pharmacology. 2015;748:76–82. doi: 10.1016/j.ejphar.2014.12.011. doi:10.1016/j.ejphar.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Abuse-related effects of μ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behavioural Pharmacology. 2013;24(5-6):459–470. doi: 10.1097/FBP.0b013e328364c0bd. doi:10.1097/FBP.0b013e328364c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Effects of μ-Opioid Receptor Agonists in Assays of Acute Pain-Stimulated and Pain-Depressed Behavior in Male Rats: Role of μ-Agonist Efficacy and Noxious Stimulus Intensity. The Journal of Pharmacology and Experimental Therapeutics. 2015;352(2):208–217. doi: 10.1124/jpet.114.219873. doi:10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–120. [PubMed] [Google Scholar]
- Cahill CM, Xue L, Grenier P, Magnussen C, Lecour S, Olmstead MC. Changes in morphine reward in a model of neuropathic pain. Behavioural Pharmacology. 2013;24(3):207–213. doi: 10.1097/FBP.0b013e3283618ac8. doi:10.1097/FBP.0b013e3283618ac8. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. doi:10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacology. 1993;111(4):402–408. doi: 10.1007/BF02253528. doi: 10.1007/BF02253528. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Research. 1993;620(2):339–342. doi: 10.1016/0006-8993(93)90177-o. doi:10.1016/0006-8993(93)90177-O. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology. 1999;144(2):111–120. doi: 10.1007/s002130050983. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91(1–2):33–45. doi: 10.1016/s0304-3959(00)00413-9. doi:10.1016/S0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug and Alcohol Dependence. 2010;109(1-3):130–138. doi: 10.1016/j.drugalcdep.2009.12.018. doi:10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug and Alcohol Dependence. 2006;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. doi:10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Comer SD. Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behavioural Pharmacology. 2012;23(3):271–279. doi: 10.1097/FBP.0b013e3283536d6f. doi:10.1097/FBP.0b013e3283536d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DT, Allan LG, Libretto SE, Griffiths P. Opioid Drugs: A Comparative Survey of Therapeutic and “Street” Use. Pain Medicine. 2001;2(3):193–203. doi: 10.1046/j.1526-4637.2001.01026.x. doi:10.1046/j.1526-4637.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. The New England Journal of Medicine. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. doi:10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology. 2011a;114:624–632. doi: 10.1097/ALN.0b013e31820a4edb. doi: 10.1097/ALN.0b013e31820a4edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids. Anesthesiology. 2011b;115:1271–1280. doi: 10.1097/ALN.0b013e3182330448. doi: 10.1097/ALN.0b013e3182330448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein HB, Akil H. Opioid Analgesics. In: Parker KL, Brunton LL, Lazo JS, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11 McGraw-Hill Professional; New York: 2005. pp. 547–590. [Google Scholar]
- Kupers R, Gybels J. The consumption of fentanyl is increased in rats with nociceptive but not with neuropathic pain. Pain. 1995;60(2):137–141. doi: 10.1016/0304-3959(94)00106-O. doi:10.1016/0304-3959(94)00106-O. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. The Journal of Pharmacology and Experimental Therapeutics. 2012;343(2):389–400. doi: 10.1124/jpet.112.197780. doi:10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clinical Drug Investigation. 2012;32(Suppl 1):53–63. doi: 10.2165/11630080-000000000-00000. doi:10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lasagna L,FJ. Drug-induced mood changes in man: 1. observations on healthy subjects, chronically ill patients, and “postaddicts. Journal of the American Medical Association. 1955;157(12):1006–1020. doi: 10.1001/jama.1955.02950290026009. doi:10.1001/jama.1955.02950290026009. [DOI] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Molecular Pain. 2014;10:62. doi: 10.1186/1744-8069-10-62. doi:10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorens SA, Mitchell CL. Influence of morphine on lateral hypothalamic self-stimulation in the rat. Psychopharmacologia. 1973;32:271–277. doi: 10.1007/BF00422149. doi:10.1007/BF00422149. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106(2):312–322. doi: 10.1097/00000542-200702000-00020. Retrieved from http://anesthesiology.pubs.asahq.org. [DOI] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-Induced Reduction of Spontaneous Activity by Adjuvant: A Novel Model to Study the Effect of Analgesics in Rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320(1):194–201. doi: 10.1124/jpet.106.109736. doi:10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- Miller LL, Picker MJ, Umberger MD, Schmidt KT, Dykstra LA. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. The Journal of Pharmacology and Experimental Therapeutics. 2012;342(1):177–187. doi: 10.1124/jpet.112.191478. doi:10.1124/jpet.112.191478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction. 2013;108(4):688–698. doi: 10.1111/j.1360-0443.2012.04005.x. doi:10.1111/j.1360-0443.2012.04005.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. Direct Evidence for the Involvement of the Mesolimbic [kappa]-Opioid System in the Morphine-Induced Rewarding Effect Under an Inflammatory Pain-Like State. Neuropsychopharmacology. 2004;30(1):111–118. doi: 10.1038/sj.npp.1300527. doi:10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- National Research Council Guide for the Care and Use of Laboratory Animals: Eighth Edition. 2011 Retrieved from http://www.nap.edu/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth.
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological Reviews. 2014;66(3):869–917. doi: 10.1124/pr.112.007419. doi:10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34(4):899–911. doi: 10.1038/npp.2008.127. doi:10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116(3):386–395. doi: 10.1016/j.pain.2005.05.011. doi:10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neuroscience and Biobehavioral Reviews. 2011;35(3):912–938. doi: 10.1016/j.neubiorev.2010.10.012. doi:10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Miyoshi K, Suzuki T. Suppression of the morphine-induced rewarding effect and G-protein activation in the lower midbrain following nerve injury in the mouse: involvement of G-protein-coupled receptor kinase 2. Neuroscience. 2003;116(1):89–97. doi: 10.1016/s0306-4522(02)00699-1. doi:10.1016/S0306-4522(02)00699-1. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. Journal of Neurochemistry. 2002;82(5):1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. doi:10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL. Addictions in pain clinics and pain treatment. Annals of the New York Academy of Sciences. 2011;1216:138–143. doi: 10.1111/j.1749-6632.2010.05897.x. doi:10.1111/j.1749-6632.2010.05897.x. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Blackburn-Munro G. Pharmacological characterisation of place escape/avoidance behaviour in the rat chronic constriction injury model of neuropathic pain. Psychopharmacology. 2006;185(2):208–217. doi: 10.1007/s00213-005-0281-3. doi:10.1007/s00213-005-0281-3. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS, Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144(1-2):170–177. doi: 10.1016/j.pain.2009.04.010. doi:10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Robens A, Read S. j., Christoph T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. European Journal of Pain. 2014;18(2):213–222. doi: 10.1002/j.1532-2149.2013.00359.x. doi:10.1002/j.1532-2149.2013.00359.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. European Journal of Pharmacology. 1996;299(1-3):33–39. doi: 10.1016/0014-2999(95)00852-7. doi:10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A. Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain. 1988;35(2):179–186. doi: 10.1016/0304-3959(88)90225-4. doi:10.1016/0304-3959(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS, Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. The Journal of Pain. 2006;7(6):408–416. doi: 10.1016/j.jpain.2006.01.447. doi:10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58(3):355–366. doi: 10.1016/0304-3959(94)90130-9. doi:10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR. Morphine self-administration, food-reinforced, and avoidance behaviors in rhesus monkeys. Psychopharmacologia. 1964;5:87–94. doi: 10.1007/BF00413045. doi: 10.1007/BF00413045. [DOI] [PubMed] [Google Scholar]
- Van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136(3):373–379. doi: 10.1016/j.pain.2007.07.027. doi:10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Watson CPN. Opioids in chronic noncancer pain: more faces from the crowd. Pain Research & Management: The Journal of the Canadian Pain Society. 2012;17(4):263–275. doi: 10.1155/2012/495781. Retrieved from www.ncbi.nlm.nih.gov/pmc/articles/PMC3411376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T. Drug dependence studies in laboratory animals. NIDA Research Monograph. 1978;19:179–190. Retrieved from archives.drugabuse.gov/pdf/monographs/19.pdf. [PubMed] [Google Scholar]