Abstract
The treatment of human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer has been revolutionized by trastuzumab. However, longer survival of these patients now predisposes them to forming HER2 positive brain metastases, as the therapeutic antibodies cannot cross the blood brain barrier. The current oncologic repertoire does not offer a rational, non-toxic targeted therapy for brain metastases. In this study, we utilized an established human neural stem cell line, HB1.F3 NSCs, and generated a stable pool of cells secreting a high amount of functional full-length anti-HER2 antibody, equivalent to trastuzumab. Anti-HER2Ab secreted by the NSCs (HER2Ab-NSCs) specifically binds to HER2 overexpressing human breast cancer cells and inhibits PI3K-Akt signaling. This translates to HER2Ab-NSC inhibition of breast cancer cell growth in vitro. Pre-clinical in vivo experiments using HER2Ab overexpressing NSCs in a breast cancer brain metastases (BCBM) mouse model demonstrate that intracranial injection of HER2Ab-NSCs significantly improves survival. In effect, these NSCs provide tumor localized production of HER2Ab, minimizing any potential off-target side effects. Our results establish HER2Ab-NSCs as a novel, non-toxic and rational therapeutic approach for the successful treatment of HER2 overexpressing BCBM, which now warrants further preclinical and clinical investigation.
Keywords: Neural stem cells, Breast cancer brain metastasis, Human epidermal growth factor receptor 2, HER2 overexpressing breast cancer, Trastuzumab, Blood brain barrier, Monoclonal antibody therapy
Graphical Abstract
Introduction
Brain metastases are a fatal complication of breast cancer with a median survival time of 4-12 months [1]. Surgical resection in addition to whole brain / stereotactic radiation therapy are the only available options which provide limited survival benefits. Moreover, some lesions, such as diffuse micro-metastases and those located in eloquent cortex or critical structures, are not amenable to surgical resection. At present, there is a dearth of targeted treatment modalities for the treatment of breast cancer brain metastases (BCBM), warranting the development of novel therapies in this domain.
Human epidermal growth factor receptor 2 (HER2) is a tyrosine kinase receptor that is overexpressed in about 30% of breast cancer patients and is associated with advanced disease and poor prognosis [2]. This overexpression of HER2 in breast cancer patients increases the propensity for CNS metastases, which ranges from 30.7% - 53% in various cohorts [3, 4]. Therefore, HER2 protein is a suitable target for the treatment of BCBM. Trastuzumab (trade name Herceptin®) is a humanized monoclonal antibody which is effective in the treatment of systemic metastatic disease [5]. However, it is futile in treatment of BCBM because of poor drug penetration through the blood-brain barrier (BBB)[6]. Furthermore, trastuzumab treatment is strongly associated with increased incidence of brain metastases, an observation that has been documented in a number of reports [4, 6-8]. This is attributable to the systemic control of the disease through trastuzumab, which prolongs survival, allowing the outgrowth of cancer cells at a sanctuary site i.e. brain. A recent clinical trial of Herceptin® (HERA) documents that, of all the patients who died, 53% had CNS involvement [9].
The BBB is a major obstacle in the treatment of brain malignancies. To overcome this limitation, our group has developed neural stem cells (HB1F3), which are tumor tropic and can cross the BBB when injected systemically [10, 11]. These NSCs have been employed as carriers of therapeutic molecules and oncolytic viruses [12-15] to achieve significant survival benefits for brain malignancies. Moreover, NSCs (HB1.F3.CD) are FDA approved for phase-I clinical studies in patients with glioblastoma (completed safety study NCT01172964; phase I dose escalation study in progress NCT02015819). Our group has previously demonstrated the ability of modified NSCs to deliver functional, anti-HER2-antibody to non-CNS, orthotopic breast cancer cells in vivo [16]. However the potential therapeutic implication of NSCs secreting anti-HER2Ab in a brain metastatic breast cancer model has not been evaluated.
In this report, NSCs secreting stable and high amount of anti-HER2 antibody (HER2Ab-NSCs) were generated. Using these genetically modified NSCs, we performed intracranial xenograft studies using HER2 overexpressing, human brain metastatic cells. Our results demonstrate significant improvement in the survival of mice injected with HER2Ab-NSCs. Hence our work provides compelling evidence for the use of HER2Ab-NSCs to treat HER2 overexpressing BCBM.
Materials and Methods
Cell culture
The HB1.F3 human NSC line was derived from primary cultures of fetal telencephalon by immortalization with an amphotropic, replication incompetent retrovirus encoding the v-myc gene as previously described [17, 18]. NSC, BT474 (ATCC, Manassas, VA), BT474M1BrM3 [19] (will be referred to as BT474Br), Lenti-X 293T cells (Clonetech, Mountain View, CA), MCF7 cells (Dr. Suzanne Conzen, University of Chicago) were maintained in DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) in a humidified (5%) CO2 incubator. MDA-MB-361 (Dr. Seungpyo Hong, University of Illinois at Chicago) cells were maintained in L-15 media supplemented with 20% FBS at 0% CO2. SKBR3 and ZR-75-30 (Dr. Olufunmilayo I. Olopade, University of Chicago) cells were maintained in RPMI medium supplemented with 10% FBS. All cells were supplemented with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA).
Subcloning of anti-HER2Ab cDNA construct in Lentiviral (pLVX-zsGreen1) vector and generation of NSCs secreting anti-HER2Ab using lentivirus
The cDNA of anti-HER2Ab was amplified from pBOB-anti-HER2Ab plasmid [16] using flanking primers containing EcoR1 and BamH1 restriction enzymes for directional cloning. Following amplification, the PLVX-IRES-ZsGreen1 plasmid (Clonetech, Mountain View, CA) and the PCR product was digested with EcoR1 and BamH1 restriction enzymes (NEB, Ipswich, MA). After overnight ligation of products using T4DNA ligase, E. Coli BJ5183 electrocompetent cells (Agilent Technologies, Santa Clara, CA) were transformed using the ligated product. Plasmid purification after amplification from the colonies revealed a 2.2 kb fragment when subjected to digestion with EcoR1 and BamH1, indicating release of anti-HER2Ab cDNA. Lentivirus plasmid PLVX-GFP/PLVX-GFP-Anti-HER2Ab and packaging plasmids (containing gag, pol, VSV-G gene) were co-transfected at 2:1:1 ratio in Lenti-X293T cells cultured in 100mm culture dish using FugeneHD transfection reagent (Promega, Madison, WI) to generate lentiviral particles. Cell supernatants were collected at 24 and 48 hr time points, concentrated using LentiX concentrator (Clonetech, Mountain View, CA) and stored at −80°C. For transduction of HB1.F3 cells, NSCs were plated in 35mm dish at a density of 5×105 and next day transduced with viral concentrate in the presence of 8μg/ml polybrene (Sigma, St.Louis). The NSCs with lentivirus were incubated overnight and the following day media was changed. The NSCs were then expanded and subjected to live cell sorting based on GFP expression. NSCs pools were isolated and expanded in culture.
Enzyme linked immunosorbant assay (ELISA)
ELISA plates (Corning, Pittsburg, PA) were coated overnight with 2μg/mL of recombinant human HER2 Fc chimera (R&D Systems, Minneapolis, MN). The next day, different amounts of trastuzumab (1ng-5μg/mL) and culture supernatant were applied and incubated for 2hr. Non-specific binding was eliminated by vigorous washes with TBS-Tween (Boston Bioproducts, MA). The plates were then incubated with secondary antibody, anti-human IgG (Fab specific)-alkaline phosphatase conjugate (Sigma, St Louis, MO) for 2hr. After washing with TBS-Tween, the plates were incubated with pNPP substrate (Sigma, St. Louis, MO) for 5-15 minutes and the optical density was measured at 405nM.
Western blotting and immunofluorescence microscopy
Protein estimation and western blotting was carried out using standard protocols. Antigen-antibody reaction was detected using ECL prime kit according to manufacturer's instructions (GE Healthcare, Buckinghamshire, UK). The primary antibodies used were anti-human HRPO (Sigma, St. Louis, MO), pAKT, AKT, pERK1/2, Keratin and HER2 (Cell signaling technololgies, MA). Secondary antibodies used were anti-mouse and anti-rabbit HRPO conjugate (Santacruz biotechnologies, Santacruz, CA), goat anti-Human Alexa Fluor ® −647 (Sigma, St.Louis, MO), and anti-rabbit PE (Molecular Probes, Eugene, OR). For immunofluorescence microscopy, breast cancer cells were grown in chamber slides (Thermo scientific, Rochester, NY) for 48 hrs, washed with PBS and fixed with ice cold methanol at −20°C for 10 min. Nonspecific binding sites were blocked using 5% BSA for 30 min and then incubated with anti-HER2 antibody secreted by NSCs for 1hr at RT. After washing with PBS, cells were incubated with secondary antibody for 1hr at RT. After intermittent washes, the slides were mounted using anti-quenching mounting agent (Molecular Probes, Eugene, OR). Confocal images were obtained using a Marianas Confocal system.
Determination of growth inhibition by secreted antibody in HER2 positive BT474 cells
BT474 cells were plated at a density of 1×104 per well in a 96 well plate and the next day, 48hr supernatant from control HB1.F3 NSCs and anti-HER2Ab secreting HB1.F3 NSCs (HER2Ab-NSCs) were added to the BT474 cells. As a positive control trastuzumab was added at different concentrations to assess the quantity of effective anti-HER2Ab produced by the HER2Ab-NSCs. MTT cell viability assay (Roche, Indianapolis, IN) was performed after incubation with respective reagents, according to manufacturer's protocol.
Animal experiments
All animal studies were approved by the Institutional Committee on Animal Use at the University of Chicago. Six to eight-week-old Hsd:athymic Nude female mice were obtained from Harlan laboratories and maintained in a specific pathogen free facility at The University of Chicago. All surgical procedures were completed in accordance with NIH guidelines on the care and use of laboratory animals for research purposes. Mice were anaesthetized with an intraperitoneal injection (ip.) of 0.1 ml of a stock solution containing ketamine HCl (25 mg/ml), xylazine (2.5 mg/ml) and 14.25 % ethyl alcohol (diluted 1:3 in 0.9 % NaCl). For the stereotactic intracranial injection, the surgical site was prepared with 70% ethyl alcohol. A midline incision was made and a 1mm diameter parietal burr hole, centered 2mm posterior to the coronal suture and 2mm lateral to the sagittal suture was drilled. Mice were placed in a stereotactic frame and 2×105 BT474Br cells resuspended in 5μl of sterile PBS were injected intracranially with a 26-gauge needle at a depth of 3mm. The needle was removed and the skin was sutured with a 4-0 absorbable, synthetic braided suture. Following tumor implantation and verification, NSCs (Vector control/ HER2Ab-NSCs cells) were injected in the same burr hole, after 7 and 14 days. Mice were monitored for a period of 3 months and sacrificed when meeting established IACUC criteria for euthanasia.
Statistical Analysis
All statistical analyses were performed using Graphpad Prism 4 (GraphPad Software Inc., San Diego CA). Sample size for each group was ≥ 3 and numerical data was reported as Mean ±SEM. The RNA levels of stem cell/differentiation markers were assessed using one-sample t-tests. Comparisons between more than two groups were conducted using one-way ANOVA with Dunnett's post-hoc test. For animal survival experiments, Kaplan-Meier survival curves were generated and log rank test was applied to compare survival distributions. All reported p values were two-sided and were considered to be statistically significant at *p<0.05, **p<0.01, ***p<0.001.
Results
Generation and characterization of HB1.F3 NSCs secreting functional anti-HER2 antibody
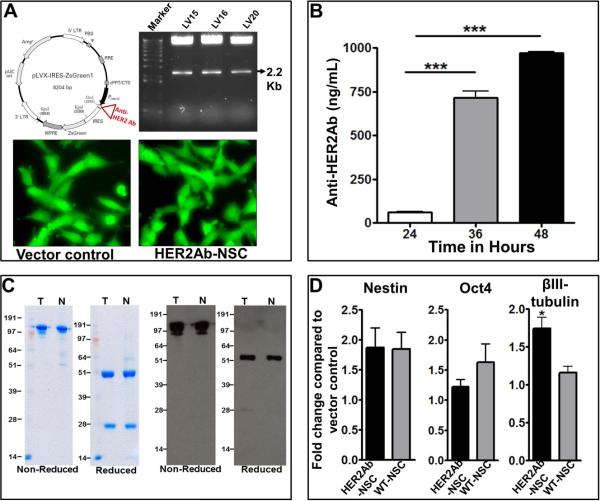
To generate a lentiviral vector encoding anti-HER2Ab, the cDNA sequence [16] of anti-HER2Ab was sub-cloned into PLVX-IRES-GREEN-1 vector using flanking primers (Figure 1A). After generation of lentivirus, HB1.F3 NSCs were transduced with either empty vector control containing GFP or anti-HER2Ab (with GFP) lentiviruses. The transduced NSCs were sorted based on GFP fluorescence and the cells were plated in a culture dish (will be referred to as HER2Ab-NSCs). To ascertain the amount of antibody secreted by the NSCs, vector control and HER2Ab-NSCs were plated at a density of 1×106 cells in a 35mm dish in DMEM containing 10% FBS. At various time points, the supernatant was collected and ELISA was conducted using ERBB2 Fc chimera as a coating antigen. Quantitative analysis revealed that HER2Ab-NSCs secreted 60, 675, and 962 ng/mL after 24, 36 and 48hr after plating (Figure 1B). To further evaluate the stable assembly of heavy and light chains of anti-HER2Ab, western blotting was conducted using purified anti-HER2Ab. Figure 1C shows a 150KDa band equivalent to trastuzumab, demonstrating antibody release by NSCs under non-reducing conditions, whereas a 50KDa band corresponds to the heavy chain of the antibody under reducing conditions (Figure 1C). The results show the release and assembly of high amounts of anti-HER2 antibody produced by HER2Ab-NSCs. Moreover anti-HER2Ab induces similar gene expression as trastuzumab (Supplementary Figure 1) and is equivalent to commercially available trastuzumab based on size exclusion chromatography and mass-spectrometry of heavy and light chains of antibody (Supplementary Figure 2, 3, 4). To further assess the stem cell characteristics of HER2Ab-NSCs, qRT-PCR was conducted. Wild type, vector control and HER2Ab-NSCs exhibited no significant difference in the levels of stem cell markers Nestin and Oct4. There was a minor difference in the level of differentiation marker βIII-tubulin (Figure 4D). We reason that, given the poor transduction efficiency of NSCs with HER2-IgG lentiviral construct (3-5%), there are more cell divisions in sorted NSC after transduction. Hence, there could be differentiation of HER2Ab-NSC. However the maintenance of stem cell markers indicates that, even if there is any differentiation, it is minimal.
Figure 1.
Generation of NSCs secreting anti-HER2Ab. (A) Anti-HER2Ab cDNA clone was obtained as previously described [16]. The cDNA was cloned in PLVX-IRES-ZsGreen-1 vector using flanking primers. Left panel indicates the vector map and right panel represents release of anti-HER2Ab cDNA followed by digestion of PLVX vector with EcoR1 and BamH1 restriction endonucleases. The lower panel shows PLVX vector control NSCs and NSCs endoding anti-HER2Ab, sorted based on GFP fluorescence. (B) Temporal secretion of anti-HER2Ab by HER2Ab-NSCs in cell supernatants using ELISA. Note a high production of anti-HER2Ab (~1μg) in 48 hrs. The experiments were repeated three times in triplicates and before in vivo injections of NSCs . (C) Determination of stable assembly of anti-HER2Ab secreted by NSC. First two panel shows SDS-PAGE seperation of trastuzumab (T) and anti-HER2Ab released by NSCs (N) under non-reducing and reducing condition respectively. Next two panels demonstrates western blotting of T and N using anti-Human-HRPO. (D) Quantitative RT-PCR of Nestin, Oct4 and βIII tubulin demonstrating preservation of stemness of HER2Ab-NSCs. The experiments were repeated three times in triplicates. * indicates p<0.05 and *** indicates p<0.001.
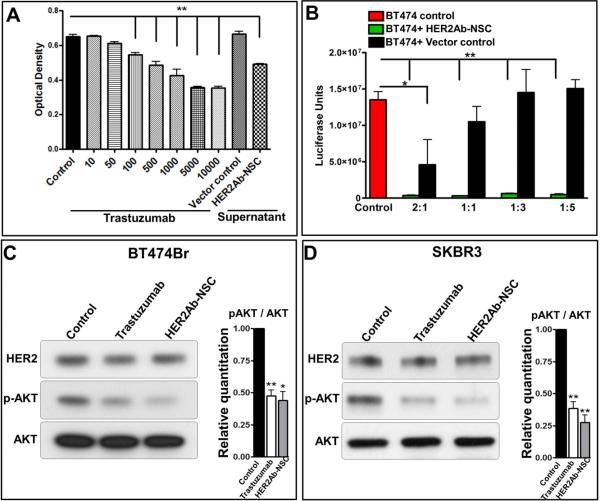
Figure 4.
Inhibition of growth and HER2 signaling by HER2Ab-NSCs cells. (A) Inhibition in growth of BT474 cells using supernatant of HER2Ab-NSCs. (B) Co-Culture assay using BT474 cells with either vector control or HER2Ab-NSCs cells. (C-D) Inhibition of PI3K-AKT signaling using purified anti-HER2Ab released by NSC. Right panel in C and D demonstrates significant decrease in relative densitometric units. Trastuzumab was used as positive control in A, C and D. The experiments were repeated three times. * indicates p<0.05 and ** indicates p<0.01.
Anti-HER2Ab secreted by NSCs specifically binds to HER2 overexpressing breast cancer cells
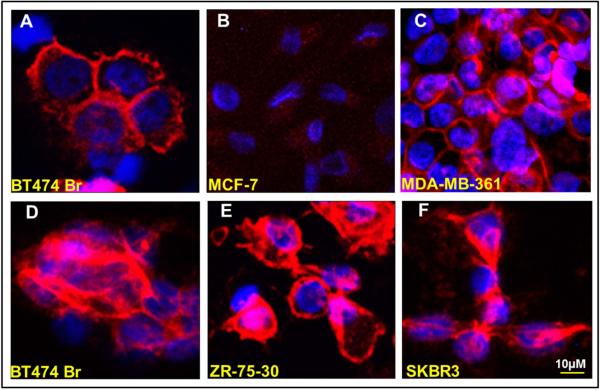
To evaluate if the antibody secreted by the HER2Ab-NSCs binds to HER2 overexpressing breast cancer cells, immunofluorescence microscopy was conducted. Five different breast cancer cells were employed for this experiment (HER2 overexpressing BT474Br, MDA-MB-361, ZR-75-30, SKBR3 and HER2 negative/low, MCF-7 cells) including BT474Br cells which are brain metastatic cells derived from BT474 cells [19]. Following the plating of breast cancer cell lines on glass slides, the cells were fixed and incubated with secreted HER2Ab-NSCs supernatant. Anti-HER2 antibody binding was visualized using secondary antibody conjugated with Alexa Fluor®-647(anti-human IgG). As shown in Figure 2, HER2 negative MCF-7 cells demonstrate absence of cell membrane staining using HER2Ab-NSCs supernatant, whereas the anti-HER2Ab secreted by the NSCs showed positive membrane staining, demonstrating binding to HER2 overexpressing breast cancer cells (BT474Br, MDA-MB-361, ZR-75-30, SKBR3). Trastuzumab was used as a positive control for BT474Br cells. To further assess the ability of HER2Ab-NSCs cells to deliver the antibody to breast cancer cells in vitro, immunofluorescence was conducted on co-culture experiments using HER2Ab-NSCs and BT474Br cells. BT474Br cells were stained with keratin to differentiate the cancer cells from the NSCs. As depicted in the Figure 3, HER2Ab-NSCs cells deliver the anti-HER2Ab (Red) to the BT474Br cells (Figure 4 A-E), whereas vector control NSCs exhibited absence of anti-HER2Ab staining (Figure 4 F-I). These results demonstrate that the antibody secreted by the NSCs binds specifically to HER2 overexpressing breast cancer cells.
Figure 2.
Binding of anti-HER2Ab to HER2 overexpressing breast cancer cells. (A) BT474Br were stained with trastuzumab as a positive control (B-F) MCF 7, MDA-MB-361, BT474Br, ZR-75-30 and SKBR3 cells stained with anti-HER2Ab secreted by NSC. The experiments were repeated two times.
Figure 3.
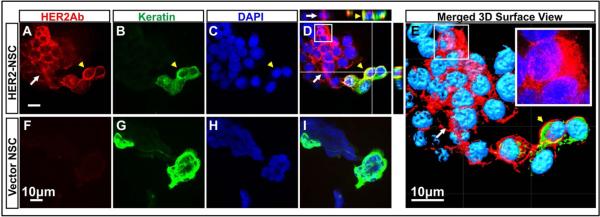
Co-culture of HER2Ab-NSCs with BT474Br cells. BT474Br cells were stained with epithelia specific keratins (Pseudo colour Green) to differentiate NSC (Kertatin negative cells). HER2Ab was visualized using anti-Human Alexa Fluor®-647antibody (Far Red). Top panel demonstrates the production of antibody (Red) by HER2Ab-NSCs and cell membrane staining of breast cancer cells with anti-HER2Ab (Red) (A-E). Yellow arrow head indicates breast cancer cells stained with keratin, whereas white arrows indicate HER2Ab-NSCs. (D) Composite image of NSCs with breast cancer cells, (E) 3D surface rendition of the Z stack composite image, inset shows enlarged views of white boxes of D and E indicating cytoplasmic staining in HER2Ab-NSCs. Vector control NSCs showed absence of anti-HER2Ab signal (F-I). The experiments were repeated two times. Scale bar = 10μM
Anti-HER2Ab secreted by NSCs inhibit the growth of breast cancer cells
To analyze the growth inhibitory property of HER2Ab-NSCs, an MTT assay was conducted. BT474 cells were plated at a density of 1×104 per well in a 96 well plate and next day, supernatant from control vector control and HER2Ab-NSCs cells was added to the BT474 cells (respective NSC supernatants were obtained after 48 hrs). As a positive control, trastuzumab was added at different concentrations to assess the effective anti-HER2Ab produced by the HER2Ab-NSCs. An MTT cell proliferation assay was performed which demonstrated that supernatants from HER2Ab-NSCs showed growth inhibition equivalent to 500ng/mL trastuzumab. These results demonstrate that anti-HER2Ab in the supernatant inhibits the growth of breast cancer cells overexpressing HER2 (Figure 4A). To further assess the ability of HER2Ab-NSCs to inhibit HER2 positive BT474 cells, we performed co-culture experiments with luciferase positive BT474 cells. As depicted in the Figure 4B, BT474 cells were incubated with different ratios of vector control/ HER2Ab-NSCs and the luciferase activity was measured. Control NSCs at higher ratio (2 NSCs: 1 BT474) demonstrated inhibition of growth of BT474 cells as compared to the no NSC control, possibly because NSC themselves have the ability to inhibit the growth of cancer cells (a phenomenon also observed in glioma co-culture experiments). However, at a 1:5 ratio of NSCs: BT474 cells no inhibition of BT474 cells was observed as depicted by relative luciferase units. At a 1:5 ratio of NSCs: BT474 cells, HER2Ab-NSCs showed dramatic reduction in luciferase units indicating a potent therapeutic effect by inhibition of breast cancer cell growth.
Anti-HER2Ab secreted by NSCs inhibits cell signaling of HER2 overexpressing human breast cancer cells
The dimerization of membrane bound HER2 protein results in activation of PI3 kinase which subsequently results in activation of Akt and ERK1/2. To evaluate the inhibition of HER2 signaling by HER2Ab-NSCs, western blotting was performed. The anti-HER2Ab was purified using protein A/G columns and the antibody was used to assess the inhibition in HER2 signaling. BT474Br HER2 overexpressing breast cancer cells that metastasize to the brain were employed in the experiment. Using an equivalent amount of trastuzumab as a positive control, we performed western blot analysis to assess the activation of Akt (S473) which is a classical reader of PI3K activity. Trastuzumab (20μg/mL) and HER2Ab-NSCs (20μg/mL) demonstrated decreased phosphorylation of Akt (S473) in both BT474Br and SKBR3 cells, indicating decrease in PI3K-Akt signaling (Figure 4C and D). These results demonstrate that anti-HER2Ab expressed by the NSCs can inhibit Akt signaling in HER2 overexpressing cells.
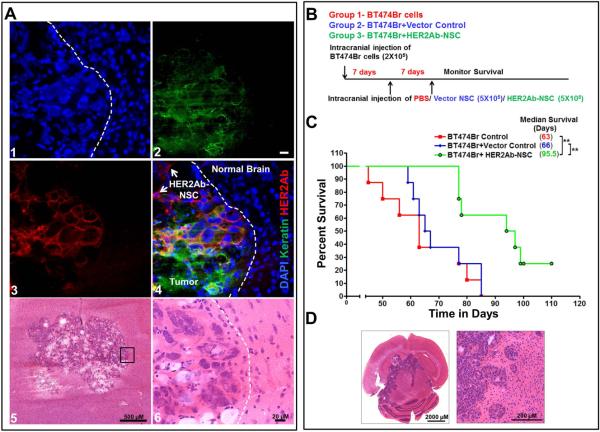
Intracranial delivery of therapeutic NSCs secreting anti-HER2Ab improves survival in a HER2 overexpressing brain metastasis model
The tumor tropic property of NSCs and their migratory ability is critical for targeted delivery of anticancer agents [20-24]. These properties give the NSCs selectivity, specificity [25] and ability to track the invasive tumor cells in vivo [26]. To evaluate if the HER2Ab-NSCs can deliver the anti-HER2Ab to tumor cells in the brain, BT474Br cells were implanted intracranially in mice and after 20 days of tumor growth, HER2Ab-NSCs were injected at the same site. Our preliminary results demonstrated that, intracranial implantation of BT474Br cells alone, exhibits a median survival of 64 days. This implied that the BT474Br tumors grow slowly in mice. Hence to visualize the tumors and the delivery of antibody, NSCs were injected after 20 days of tumor growth. The mice were sacrificed 3 days post-NSCs and frozen sections were stained to visualize the release of anti-HER2Ab. BT474Br cells were stained with keratin antibody (Pseudo color-green) and the anti-HER2Ab is stained with antihuman-IgG (Alexa Fluor®-647, Red). As depicted in the Figure 5A the HER2Ab-NSCs deliver and localize the anti-HER2Ab to surrounding BT474Br cells. To assess if the therapeutic NSCs provide survival benefit in a preclinical mouse model, we performed intracranial xenograft studies using brain metastatic, BT474Br cells that naturally overexpress HER2 protein [19]. For these experiments three groups of 8 mice each were subjected to survival analysis for a period of 3 months. Animal groups included 1) BT474Br cells alone + PBS, 2) BT474Br cells + Vector control NSCs and 3) BT474Br cells + HER2Ab-NSCs. 2×105 BT474Br cells were injected into the right frontal lobe of nu/nu mice. PBS, 5×105 Vector Control NSC, or NSC-HER2IgG cells were injected twice, 1 and 2 weeks subsequently at the same site of tumor cell implantation (Figure 5B). Mice were monitored for 3 months and once the mice demonstrated signs of morbidity consistent with IACUC/AAALAC Euthanasia Criteria, they were euthanized and brains harvested. The BT474Br + PBS control group exhibited a median survival of 63 days whereas the vector control group showed a median survival of 66 days. Mice treated with HER2Ab-NSCs exhibited a median survival of 95.5 days (p<0.01). The difference in median survival was found to be statistically significant by Kaplan-Meyer curve analysis (Figure 5C). These results demonstrate that HER2Ab-NSCs therapy significantly improves survival in the BCBM model. Taken together, our results provide a foundation toward the clinical utility of HER2Ab-NSCs as a potent inhibitor of BCBM progression.
Figure 5.
Survival analysis using intracranial injection of HER2Ab-NSCs. (A) Confocal microscopic images demonstrating the delivery of antiHER2Ab secreted by NSC to BT474Br cells in vivo. Tumor cells were stained with epithelia specific mouse monoclonal antibody to keratin (pseudo colour Green). antiHER2Ab released by NSCs was stained with anti-Human IgG Alexa Fluor®-647 (Red). Nucleus was counter stained DAPI. Bar = 20 μM (A) Schematic diagram of experimental design. 2×105 were injected intracranially and survival was monitored for 120 days. (B) Kaplan Meier survival curve of PBS, vector control and HER2Ab-NSCs injected. Mice were monitored weekly and sacrificed when moribund. Log rank test was applied to compare mice survival. (n=8). ** indicates p<0.01. (C) Representative image demonstrating preexisting brain metastasis.
Discussion
The treatment of breast cancer brain metastases (BCBM) is an enormous challenge considering physical limitations to current drug delivery methods as well as the poor prognosis of patients with the disease. At present, there is a paucity of targeted therapeutic modalities for this oncologic dissemination in the brain.
HER2 overexpression drives oncogenic signaling in breast cancer cells [27]. The dimerization of HER2 leads to hyper-activation of PI3K-Akt signaling and subsequent activation of various downstream mediators [28]. This aberrant activation of PI3K-Akt signaling increases invasion which is responsible for metastasis of breast cancer. The inhibition of HER2 using trastuzumab (humanized anti-HER2 antibody) in a number of epithelial malignancies (breast [5]/gastric [29]) has met with significant clinical success (although about 7% of patients exhibit cardiac dysfunction as a side effect, due to the expression of HER2 in cardiomyocytes [5, 30]). However, these clinical benefits of trastuzumab are rendered inefficacious for HER2 overexpressing brain metastases as it fails to penetrate the BBB. Moreover, HER2 overexpression in breast cancer patients increases the incidence of brain metastases [4, 6-8] and its levels are further increased in brain metastases as compared to matched primary breast cancers [31]. Hence, HER2 is an ideal target for the treatment of BCBM.
Neural stem cells, on the other hand, can be modified to produce therapeutic proteins or viruses for the treatment of brain malignancies [18, 26, 32]. Hence the modification of NSCs to produce therapeutic protein/s presents a viable option for the treatment brain metastases. In the last decade, a number of reports have demonstrated that NSCs can be modified to deliver therapeutic proteins like PEX (Proliferation and angiogenesis inhibitor) [33], TRAIL [34] (Pro- apoptotic protein), IL12 [35] (Pro-inflammatory) which improve the survival of mice in preclinical models. NSCs are also employed as a “Trojan horse” for the delivery of oncolytic adenovirus to brain malignancies [36]. Moreover, it has been demonstrated that NSCs can facilitate suicide gene mediated enzyme/prodrug cytotoxicity, specifically to glioma with no apparent systemic toxicity. This encouraging work led to first-in-human NSC enzyme/prodrug clinical trials in recurrent high grade glioma patients [37]. These reports thus demonstrate that NSCs can be used as vehicles to deliver a wide range of therapeutic agents for the treatment of brain associated malignancies.
We have previously reported NSCs as delivery vehicles for anti-HER2Ab and its functional binding to the HER2 overexpressing breast cancer cells [16]. We demonstrated that using adenovirus mediated production of anti-HER2Ab in the NSCs, delivered the therapeutic antibody to mouse orthotopic xenografts of MCF7/HER2 cells in vivo. However, to generate a stable cell line of NSCs with high production of anti-HER2Ab was of immense interest. Towards this end we have cloned the anti-HER2Ab cDNA into different vectors (pSEB-C3F with EF1a/HLTV hybrid promoter for gene of interest or PLVX-IRES-ZS-Green1 with CMV promoter) and found that transduction of NSCs using a CMV promoter driven anti-HER2Ab in the lentiviral vector increased the antibody production by 20 fold as compared to previous report [16].
Here, we have combined the anti-tumor effects of the anti-HER2 antibody and the tumor tropic properties of NSC by genetically modifying NSCs to secrete high levels of functional anti-HER2Ab (Figure 1A-C), equivalent to the effects of trastuzumab. These v-myc immortalized NSCs retain their stemness properties as shown by expression of nestin and Oct4 (Figure 1D). Additionally, the purified anti-HER2Ab induces similar gene expression (genes related to adhesion, angiogenesis, apoptosis, cell cycle progression, cell signaling, chemokine and growth factors, extracellular matrix proteins and metastasis regulatory genes) as in the case of commercially available trastuzumab (Supplementary Figure 1). We also demonstrated that the antibody secreted by the NSCs can bind specifically to various HER2 overexpressing breast cancer cells (BT474Br, MDA-MB-361, SKBR3, and ZR-75-30) (Figure 2). We further demonstrated that breast cancer cell growth can be inhibited using supernatant from HER2Ab-NSCs cells (Figure 4A). Moreover, the antibody secreted by NSC is functionally active and able to inhibit classical HER2 downstream signaling of HER2 overexpressing cells (Figure 4C and D).
In the literature there exist various breast cancer cell models to understand the biology of HER2 protein. However, very few cell models which overexpress HER2 and metastasize to the brain (MDA-MB-231-Br, MDA, MB-453 and BT474Br cell models) [19, 38, 39]. Among these models, MDA-MB-231-Br cells were derived after transfection of MDA-MB-231 cells with HER2 to isolate the brain metastatic cells. These cells, however, are not sensitive to trastuzumab treatment, and MDA-MB-453 cells were also found to be resistant to trastuzumab treatment [39]. On the other hand BT474Br cells have been derived by injecting the parental BT474 cells systemically to subsequently isolate the brain metastatic cells from intracranial tumors [19]. To our knowledge this is the only cell model which naturally over-expresses HER2 protein and predominantly metastasizes to the brain, along with being responsive to antiHER2 therapies. Therefore although a single model is a limitation of our study, it is the most suitable model to evaluate the effectiveness of anti-HER2 therapies for brain metastasis. Hence, to assess the in vivo efficacy of HER2Ab-NSCs we employed BT474Br cells, which naturally overexpress HER2 protein (BT474Br). These cells maintain HER2 expression and are sensitive to trastuzumab treatment (Supplementary figure 5). To evaluate the in vivo efficacy of HER2Ab-NSCs, brain metastatic BT474Br cells were injected intracranially to establish brain metastases. This was followed by injection of PBS, vector control or HER2Ab-NSCs and survival analysis was conducted. Our results demonstrate that mice treated with HER2Ab-NSCs had a significant improvement in survival over controls, establishing these cells as potent inhibitor of BCBM progression.
Our preclinical data has vast clinical implications considering the consistent survival benefits in the HER2 overexpressing breast cancer model. (1) HER2Ab-NSCs may offer improvement in prognosis of HER2 positive BCBM, either through intracranial injection at the time of biopsy or resection, or local delivery via setereotactic injection. (2) Given prior biodistribution and efficacy data demonstrating the tumor-tropic properties of intravenously administered NSCs in a metastatic breast cancer model [40] HER2Ab-NSCs may also be employed for the treatment of systemic HER2 overexpressing breast cancer metastases (for example lung and bone metastasis of breast cancer), (3) By selectively localizing the anti-HER2Ab to tumor sites, this NSC delivery stategy should also minimize the cardiotoxicity observed with intravenously administered trastuzamab. Additionally, NSCs can be modified to express other therapeutic proteins and antibodies, offering a novel clinical entity for the clinical treatment of breast cancer, and potentially other metastatic cancers.
Our results present compelling evidence for the feasibility and effectiveness of combining these two clinically relevant therapeutic agents, anti-HER2Ab and NSCs, for the treatment of HER2 positive BCBM.
Conclusions
In conclusion, we have presented evidence that NSCs secreting a trastuzumab equivalent antibody effectively binds to HER2 overexpressing breast cancer cells and inhibits their growth. Intracranial injection of these clinically relevant NSCs provides a significant survival benefit in a dose dependent manner, in a preclinical model of HER2 positive BCBM. Given the overexpression of HER2 in multiple epithelial malignancies, these genetically modified NSCs may have numerous clinical benefits.
Supplementary Material
Acknowledgements
We would like to thank Dr. Suzanne Conzen (University of Chicago) and Dr. Seungpyo Hong (University of Illinois at Chicago) for providing MCF-7 and MDA-MB-361 cells respectively. We would also like to thank Dr. Olufunmilayo I. Olopade (University of Chicago) for providing SKBR3 and ZR-75-30 cells. This study was supported in part by NIH/NINDS U01NS069997 to MSL.
Abbreviations
- NSC
Neural stem cells
- HER2
Human epidermal growth factor receptor 2
- Ab
Antibody
- BBB
Blood brain barrier
Footnotes
Authors Contributions :
Deepak Kanojia : Performed experiments, data analysis and interpretation
Irina V. Balyasnikova: Design of experiments, data analysis and interpretation
Ramin A. Morshed : Performed experiments, editing of manuscript
Richard T. Frank : Performed experiments, editing of manuscript
Dou Yu: Performed experiments
Lingjiao Zhang : Statistical Analysis
Drew Spencer : Performed experiments, editing of manuscript
Julius W. Kim : Provision of study material, editing of manuscript
Yu Han : Performed experiments
Dihua Yu : Provision of study material
Karen S. Aboody : Conception, design and financial support, editing of manuscript
Maciej S. Lesniak : Conception, design, financial support and final approval of manuscript
Disclosure of Potential Conflicts of Interest: KSA is a share-holder and officer of TheraBiologics, a clinical stage biopharmaceutical company focused on the development of stem cell-mediated cancer therapy.
References
- 1.Maher EA, Mietz J, Arteaga CL, et al. Brain metastasis: opportunities in basic and translational research. Cancer research. 2009;69:6015–6020. doi: 10.1158/0008-5472.CAN-08-4347. [DOI] [PubMed] [Google Scholar]
- 2.Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26:6577–6592. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5278–5286. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 4.Lin NU, Amiri-Kordestani L, Palmieri D, et al. CNS metastases in breast cancer: old challenge, new frontiers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6404–6418. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Melisko ME, Glantz M, Rugo HS. New challenges and opportunities in the management of brain metastases in patients with ErbB2-positive metastatic breast cancer. Nature clinical practice Oncology. 2009;6:25–33. doi: 10.1038/ncponc1243. [DOI] [PubMed] [Google Scholar]
- 7.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast cancer research and treatment. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs IB, Loebbecke M, Buhler H, et al. HER2 in brain metastases: issues of concordance, survival, and treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:4130–4133. doi: 10.1200/JCO.2002.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2- positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). The lancet oncology. 2013;14:244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 10.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Lee JE, Kim SU, et al. Stereological analysis on migration of human neural stem cells in the brain of rats bearing glioma. Neurosurgery. 2010;66:333–342. doi: 10.1227/01.NEU.0000363720.07070.A8. discussion 342. [DOI] [PubMed] [Google Scholar]
- 12.Thaci B, Ahmed AU, Ulasov, et al. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer gene therapy. 2012;19:431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed AU, Thaci B, Alexiades NG, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balyasnikova IV, Ferguson SD, Han Y, et al. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer letters. 2011;310:148–159. doi: 10.1016/j.canlet.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene therapy. 2009;16:262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank RT, Edmiston M, Kendall SE, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PloS one. 2009;4:e8314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology : official journal of the Japanese Society of Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 18.Danks MK, Yoon KJ, Bush RA, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer research. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Huang WC, Zhang L, et al. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer research. 2013;73:5764–5774. doi: 10.1158/0008-5472.CAN-12-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi BR, Hwang KA, Kang NH, et al. Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta via their tumor tropism to selectively target human hepatocarcinoma cells. Cancer gene therapy. 2012;19:644–651. doi: 10.1038/cgt.2012.45. [DOI] [PubMed] [Google Scholar]
- 21.Kim KY, Yi BR, Lee HR, et al. Stem cells with fused gene expression of cytosine deaminase and interferon-beta migrate to human gastric cancer cells and result in synergistic growth inhibition for potential therapeutic use. International journal of oncology. 2012;40:1097–1104. doi: 10.3892/ijo.2011.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KY, Kim SU, Leung PC, et al. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer science. 2010;101:955–962. doi: 10.1111/j.1349-7006.2009.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SK, Kim SU, Park IH, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Ahn Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed AU, Alexiades NG, Lesniak MS. The use of neural stem cells in cancer gene therapy: predicting the path to the clinic. Current opinion in molecular therapeutics. 2010;12:546–552. [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed AU, Thaci B, Tobias AL, et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. Journal of the National Cancer Institute. 2013;105:968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the Treatment of Breast Cancer. Annual review of medicine. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 28.Ursini-Siegel J, Schade B, Cardiff RD, et al. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nature reviews Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 29.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 30.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature medicine. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 31.Duchnowska R, Sperinde J, Chenna A, et al. Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro-oncology. 2015 doi: 10.1093/neuonc/nov012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims TL, Jr., Hamner JB, Bush RA, et al. Neural progenitor cell-mediated delivery of osteoprotegerin limits disease progression in a preclinical model of neuroblastoma bone metastasis. Journal of pediatric surgery. 2009;44:204–210. doi: 10.1016/j.jpedsurg.2008.10.041. discussion 210-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 34.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer research. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 35.Ehtesham M, Kabos P, Kabosova A, et al. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer research. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 36.Tobias AL, Thaci B, Auffinger B, et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem cells translational medicine. 2013;2:655–666. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Science translational medicine. 2013;5:184ra159. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer research. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 39.Nanni P, Nicoletti G, Palladini A, et al. Multiorgan Metastasis of Human HER-2(+) Breast Cancer in Rag2(−/−);Il2rg(−/−) Mice and Treatment with PI3K Inhibitor. PloS one. 2012;7:e39626. doi: 10.1371/journal.pone.0039626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao D, Najbauer J, Annala AJ, et al. Human neural stem cell tropism to metastatic breast cancer. Stem Cells. 2012;30:314–325. doi: 10.1002/stem.784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.