Abstract
Low voltage EEG (LVEEG) is a heritable phenotype that differs depending on ancestral heritage, yet its impact on brain networks and cognition remain relatively unexplored. In this study we assessed energy and task related phase locking of event-related oscillation (EROs), behavioral responses, measures of IQ and personality, and expected responses to alcohol in a large sample of individuals with LVEEG compared to those with higher voltage variants. Participants (n=762) were recruited from a Native American community and completed a diagnostic interview, the Quick Test, the Subjective High Assessment Scale Expectation Version (SHAS-E) and the Maudsley Personality Inventory. Clinical and spectral analyzed EEGs were collected for determination of the presence of a LVEEG variant. EROs were generated using a facial expression recognition task. Participants with LVEEG (n=451) were significantly more likely to be older, married and have higher degrees of Native American heritage but did not differ in gender, income or education. Individuals with LVEEG were also found to have decreased energy in their alpha EROs, increased phase locking between stimulus trials, and increased phase-locking between cortical brain areas. No significant differences in the cognitive tests, personality variables or alcohol dependence or anxiety diagnoses were found, however, individuals with LVEEG did report a larger number of drinks ever consumed in a 24-hr period and a less intense expected response to alcohol. These data suggest that alpha power in the resting EEG is highly associated with energy and cortical connectivity measures generated by event-related stimuli, as well as potentially increased risk for alcohol use.
Keywords: EEG phase synchrony, event-related oscillations, event-related potentials, low voltage EEG
1.1 Introduction
EEG alpha oscillations are the single most salient feature of the waking electroencephalogram (EEG); they are most prominently seen in the resting state EEG in the occipital areas when the eyes are closed. Resting state alpha oscillations have historically been viewed as an “idling” rhythm associated with relaxation or non-focused attention that are reduced in amplitude during cognitive or sensorimotor stimulation or replaced by slower waves during drowsiness (for a recent review see Basar, 2012; Bazanova and Vernon, 2013; Niedermeyer and Lopes de Silva, 1999). Changes in the amplitude and phase of alpha oscillations in the EEG can also be measured during task conditions and the synchronization or enhancement of ongoing EEG alpha oscillations by a time locked cognitive and/or sensory process is termed an event-related oscillation (ERO) (Basar et al., 2000, 2001a; Begleiter and Porjesz, 2006; Roach and Mathalon, 2008). Event-related oscillations in the alpha frequency range have been attributed to a number of cognitive processes (Basar, 2012; Basar et al., 1997, 2001b; Klimesch et al., 1994, 1997, 2007) and individual differences in alpha oscillations have also been associated with neuropsychiatric disorders (see Basar and Guntekin, 2013).
Over the last decade a prevailing theory has emerged that has viewed alpha oscillations as an important top-down mechanism of neuronal inhibition that exerts control over the timing of neuronal discharges in nearby populations (see Klimesch, 2012; Klimesch et al., 2007) and thus may play a prominent role in the control of cortical excitability. In the past few years several studies have recorded both EEG alpha power and functional MRI, as well as EEG alpha power modulation of fMRI resting-state connectivity (see Scheeringa et al., 2012). It has been demonstrated that alpha power correlates with decreased activity in the dorsal attention system of superior frontal and intraparietal regions (Laufs et al., 2003; Mantini et al., 2007), and increased activity in the cognitive control/alerting network, comprised of the dorsal anterior cingulate cortex, frontal operculum/anterior insula and thalamus and that in this capacity it is important in maintaining sustained cognitive control and alertness (Dosenbach et al., 2007; Sadaghiani et al., 2010). Using a combination of EEG and event-related optical signal, it has also been demonstrated that suppression of posterior alpha is preceded by increased activity in the regions of the dorsal attention network and decrease activity in regions of the cingulo-opercular network (Mathewson et al., 2014).
Alpha oscillations have also been suggested to provide a basic mechanism subserving a number of cognitive processes including attentional, executive and contextual functions (see Palva and Palva, 2011). Most prominent have been studies that have evaluated EROs in the alpha frequency range to investigate working memory (see Guntekin and Basar, 2014). Basar and Stampfer (1985) first described the association between alpha activity and working memory. A series of studies have provided strong support for the idea that alpha power increases reflect: enhanced control of access to working memory (see Klimesch, 2012; Manza et al., 2014; Roux and Uhlhaas, 2014), the gating of task-irrelevant sensory information (Jensen and Mazaheri, 2010), and the clearing and updating of incoming information (Sadaghiani et al., 2010). Alpha oscillations have also been utilized to investigate responses to affective stimuli using the presentation of faces and facial expressions (see Balconi and Pozzoli, 2007, 2008; Guntekin and Basar, 2014, for review). In one study, it was demonstrated that alpha EROs were significantly higher following presentation of angry faces than following happy faces at posterior locations (Guntekin and Basar, 2007). Alpha responses to different facial expressions have even been demonstrated in a patient with cortical blindness (Del Zotto et al., 2013), suggesting that alpha oscillations may reflect unconscious process of facial expressions. While it is tempting to speculate on a specific role for alpha EROs in cognitive processing of sensory and emotional information, as well as working memory, it has also been suggested that it may not be possible to launch a specific hypothesis for alpha oscillations as they may serve a more general function in sensory, motor and memory functions than what has been suggested by some authors (Basar and Guntekin, 2012).
One of the important factors that is often not taken into consideration when theorizing about the functional significance of power in alpha oscillations is the fact that the amplitude of the alpha rhythm is a trait variable that varies greatly between individuals, and in some individuals it is virtually absent. This phenotype, called low voltage EEG (LVEEG), was first described by Adrian and Mathews (1934) based on visual inspection of the EEG. Early genetic studies also identified LVEEG as being highly heritable, suggested it might have an autosomal dominant mode of inheritance, and provided data in a few families for linkage to a marker on chromosome 20q (see Anokhin et al., 1992; Steinlein et al., 1992; Vogel, 1962, 1970, 2000). If alpha oscillations represent a powerful inhibitory mechanism subserving a host of important brain network operations then how is this accomplished in individuals who have little or no measurable alpha rhythm? It has been suggested that in individuals with LVEEG that alpha oscillations are not absent but rather there is insufficient synchronization of local cortical alpha rhythms for the signal to be recorded on the scalp (Anokhin et al., 1992). It has also been suggested that LVEEG is a result of weakened thalamo-cortical links leading to insufficient pacemaking activity of cortical neurons (Bazanova and Vernon, 2013; Vogel et al., 1979a, 1979b). To date no studies have recorded EROs in the alpha frequency range or evaluated phase-locking or synchronization of EROs between cortical regions specifically in individuals identified with this EEG variant, as compared to those with higher amplitude variants, in order to determine if they have a relative deficiency of neuronal synchronization.
Although the psychological and cognitive characteristics of individuals with LVEEG have not been adequately explored some studies have found significant associations with another genetically regulated trait, the risk for alcoholism. For instance, in early studies, a cross-sectional association between having a relatively lower voltage EEG and alcohol dependence was found in primarily EuroAmerican populations (Arentsen and Sindrup, 1963; Coger et al., 1978; Jones and Holmes, 1976; Varga and Nagy, 1960). More recently it has been demonstrated that LVEEG records may be four times more common in some types of alcoholics, particularly those with anxiety, as compared to non-alcoholics (Enoch et al., 1995, 1999). The presence of a low voltage EEG has also been demonstrated to be associated with differing subjective responses to acute alcohol administration (Ehlers et al., 1999; Propping, 1980).
One reason that the LVEEG phenotype has been understudied is the fact that LVEEG is not highly prevalent in most populations (~4% in EuroAmerican populations, see Anokhin et al., 1992; Niedermeyer, 1987) so that large scale EEG screening would be required to obtain a relatively small number of cases. We have previously reported that LVEEG is more prevalent in an American Indian community sample than what has been documented in other select Asian or EuroAmerican populations (see Ehlers and Phillips, 2007; Ehlers et al., 1999). The prevalence of alcohol use disorders also differs among population groups. Although use of alcohol varies among tribes, as a whole, Native Americans suffer high rates of alcohol and drug dependence and higher alcohol-related death rates than any other U.S. ethnic group, and alcohol dependence rates up to five times that of the general U.S. population (Beals et al., 2005a, 2005b; Ehlers et al., 2004a, 2006; Kunitz, 2006; May, 1982; May and Smith, 1988; Robin et al., 1998; Shalala et al., 1999). The present report is part of a larger family study exploring risk factors for substance dependence in a community sample of American Indians (Ehlers et al., 2001a, 2001b, 2001c, 2001d, 2004b, 2008c; Gilder et al., 2004, 2006, 2007, 2009). The lifetime prevalence of substance dependence in this Indian population is high and genetic and environmental risk factors for substance dependence have been identified (Ehlers and Wilhelmsen, 2005, 2007; Ehlers et al., 2004b, 2006b, 2007a, 2007b, 2007c, 2008a, 2008b, 2009b, 2010a, 2010b, 2011b, 2012; Gizer et al., 2011; Wall et al., 2003; Wilhelmsen and Ehlers, 2005).
We have also demonstrated that LVEEG is highly prevalent in this population and that alpha EEG phenotypes are substantially heritable and are linked to specific areas of the genome also demonstrated to harbor genes associated with substance dependence (see Ehlers et al., 2010a, 2010b). We have additionally demonstrated that event related alpha oscillations elicited by a facial recognition task were significantly associated with externalizing diagnoses in this population (Criado et al., 2012). Using data from this unique population, several fundamental questions regarding the LVEEG phenotype were evaluated in the present study: (1) We assessed energy in event-related oscillations, in a range of frequencies, that were generated to a facial recognition task in those individuals with LVEEG compared to those with higher voltage variants; (2) We determined whether task-related phase locking of EROs were significantly different within scalp locations and between the frontal and posterior cortical networks in a range of frequencies in individuals with LVEEG; (3) We evaluated demographic characteristics, behavioral responses to the facial recognition task, IQ, and personality variables in those individuals with LVEEG and higher voltage variants; and (4) We tested for associations between anxiety disorders, alcohol dependence, the largest number of drinks ever consumed in a 24-hr period, and expected level of response to alcohol in those individuals with LVEEG and higher voltage variants.
2.0 Methods
2.1 Experimental participants
Participants were recruited from eight geographically contiguous Indian reservations, as previously described (Ehlers et al., 2004a; Gilder et al., 2004). To be included in the study, participants had to be an Indian indigenous to the catchment area, between the age of 18 and 70 years, and be mobile enough to be transported from his or her home to The Scripps Research Institute (TSRI). Participants were excluded from electrophysiological analyses if they had a history of head trauma or were currently using medications that could bias the EEG recording. The protocol for the study was approved by the Institutional Review Board (IRB) of TSRI, and the Indian Health Council, a tribal review group overseeing health issues for the reservations where recruitment was undertaken. The studies have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Potential participants first met individually with research staff to have the study explained and give written informed consent. During a screening period, participants had blood pressure and pulse taken, and completed a questionnaire that was used to gather information on demographics and personal medical history (Schuckit, 1985). Participants were asked to refrain from alcohol and drug usage for 24 hours prior to the testing. No individuals with detectable breath alcohol levels were included in the study dataset. Each participant also completed an interview with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994, Hesselbrock et al., 1999) which was used to determine Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) alcohol dependence as well as the presence of any DSM-IV anxiety disorder (panic disorder with or without agoraphobia, agoraphobia without panic, social phobia, obsessive-compulsive disorder), and the largest number of drinks ever consumed in a 24-hr period. Personality variables were assessed using the Maudsley personality Inventory, IQ was estimated using the Quick Test. Self-reported response to alcohol was assessed using the Subjective High Assessment Scale Expectations version (SHAS-E). The SHAS-E consists of 14 items rated on Likert scales ranging from 0 (normal) to 36 (extreme effect). The participants were asked to rate how they would expect to feel 30 minutes after drinking 3 standard drinks for the following items: buzzed, clumsy, dizzy, drunk, effects of alcohol, energy, good, high, nauseated, sleepy, talkative, uncomfortable, terrible overall and great overall. A total score that was a sum of all of the responses for the first 12 SHAS-E items was also calculated. The intersession reliability of the SHAS, from which the SHAS-E was constructed, is approximately 0.80 with a Cronbach’s alpha of 0.96 overall (Schuckit et al., 2000). The items cluster together with an overall item-to-total correlation of 0.80 or higher and a Cronbach’s alpha of 0.96. Values on the SHAS-E have been demonstrated to significantly (p<0.0001) correlate with responses on the SHAS at 30 and 60 minutes following alcohol ingestion (r2=0.49, 30 min; r2=0.51, 60 min) (Schuckit and Smith, personal communication).
2.2 EEG collection and analyses
For resting EEG recordings, used to identify LVEEG, six channels of bipolar EEG (F3-C3, C3-P3, P3-01 and F4-C4, C4-P4, P4-02, international 10–20 system) were obtained using an electrode cap (impedance < 5K ohms), as described. A forehead ground electrode was used. An electrode placed left lateral infra-orbitally and referenced to the left earlobe was used to monitor both horizontal and vertical eye movement. The combined gain of the EEG amplifiers and the analog-to-digital multiplexer amplifier was 50 K. Resting EEG was recorded in a temperature and noise controlled room while a participant was comfortably sitting on a chair. Participants were instructed to relax and keep their eyes closed, but to remain awake throughout the EEG recording. Ten to 15 minutes of EEG was collected on paper (high-low pass filters 1–70 Hz) and also digitized for subsequent analyses. EEG records were continuously monitored during all recordings for signs of drowsiness or artifact. Ten minutes of artifact-free, drowsiness-free EEG, as defined by Daly and Pedley (1990), was computer analyzed for each channel using spectral analyses as previously described (Ehlers and Havstad, 1982; Ehlers et al., 1989, 2009). Muscle and movement artifact are identified by a computer driven algorithm and verified by the user and epochs containing artifact are removed prior to processing. Participants were classified as LVEEG both by clinical evaluation of the data by an EEG technologist and by spectral analyses (voltage in the 9–12 Hz range in occipital leads is less than 30 microvolts squared per octave) as described previously (Ehlers et al., 1999).
2.3. ERP collection and analyses
For ERP recordings seven channels of ERP data (FZ, CZ, PZ, F3, F4, F7, and F8, referenced to linked ear lobes with a forehead ground, international 10–20 system) were obtained using gold-plated electrodes with impedance held below 5 kΩ. An electrode placed left lateral infraorbitally and reference to the left earlobe was used to monitor both horizontal and vertical eye movements. ERP recording signals were amplified (high pass 0.5 Hz, 35 Hz low pass) and were transferred on-line to a PC for digitation. The present study used a facial discrimination task (Erwin et al., 1992; Gur et al., 1992; Heimberg et al., 1992) that was adapted for use in an ERP paradigm (Orozco and Ehlers, 1998) to generate ERO data. The stimuli were realistic digital photographs of happy, neutral and sad faces presented on a computer screen for 1000 ms with an inter-trial interval of 1000–1500 ms. The pre-stimulus interval was 150 ms. Participants were instructed to discriminate, by pressing a counter, between happy and sad faces (36 trials each) and not to respond to neutral faces (144 trials). There were 36 unique faces (12 each of happy, neutral, and sad) presented in random order for a total of 216 trials. The number of male and female faces presented was also equally distributed among neutral, sad and happy stimuli.
2.4. ERO, PLI, and PDLI analyses
ERO energy (peak magnitude of the S transform output, squared, in a time frequency region of interest), PLI (phase lock index) and PDLI (phase difference lock index) analyses were accomplished from the same datasets that were used to generate ERP data (Criado and Ehlers, 2007; Ehlers et al., 2007a). The methods for these analyses have been described in detail elsewhere (Ehlers et al., 2012, 2014).
The ERO trials were digitized at a rate of 256 Hz. Trials containing excessive artifact were eliminated prior to averaging (<5% of the trials). An artifact rejection program was utilized to eliminate individual trials in which the EEG exceeded ± 400 μV. Data from single trials generated by the stimuli were entered into the time frequency analyses algorithm. The S-transform (ST), a generalization of the Gabor transform (1946), was used (see Stockwell et al.,1996).
The S-transform mathematically resembles the continuous wavelet transform but it uses Gaussian windows which do not meet a requirement of wavelet analysis, and it includes a “phase correction” that is not part of wavelet analysis. The actual use of the S-transform was simplified by performing first a forward Fourier transform of the time series. Then, for each frequency of the Fourier transform, summing the results of multiplication by a set of Fourier transforms of Gaussian windows of varying width. Finally, for each of these sums, taking the inverse Fourier transform. The equation for calculation of the S-transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The S-transform results in a time-frequency representation of the data. The exact code we used is a C language, S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). This code is specifically for use with real time series, so it sets the input imaginary values, required by the S-transform, to zero, and it always uses the Hilbert transform so that each of the complex output time series is an analytic signal.
To reduce anomalies in the S-transform output at the beginning and the end of the output time series, we used a Hanning window over the initial and final 100 msec of the input time series. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. To quantify S-transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. The time-frequency points saved from each S-transformation are from 100 ms before to 900 ms after the onset of the stimulus, and from 1 Hz through 50 Hz at intervals of 0.5 Hz. Energy is the square of the magnitude of the S-transform output in a time frequency region of interest. The S-transform output for a time/frequency ROI, for a specific EEG lead, is proportional to the input voltage of the lead over the time/frequency interval. The S-transform magnitude squared for a time/frequency interval is therefore proportional to volts squared. These analyses are similar to what has been previously described (Ehlers et al., 2014).
An S transformation at time t and frequency f has real and imaginary parts
where i is the square root of minus 1. The cosine and sine of the phase angle at this time-frequency point are:
where the vertical bar pair indicates magnitude, here and below. The cosine and sine of phase angle are calculated from the S transformation without having to calculate the phase angle.
PLI is a measure of synchrony of phase angle over trials, as a function of frequency and of time relative to the start of the stimulus for each trial. The range of PLI is from zero to 1.0, with high values at a time and frequency indicating little variation, among trials, of phase angle at that time and frequency. PLI is defined as:
where the angle bracket pair indicates mean value over eligible trials, here and in the equation below. This definition is based on cosine and sine of phase angles that are calculated from S transformations without calculating phase angles. This definition is mathematically equivalent to the definition in Schack and Klimesch (2002).
PDLI is a measure of constancy over trials of the difference in phase angle between two channels, as a function of frequency and of time relative to the start of the stimulus for each trial. The range of PDLI is from zero to 1.0, with high values at a time and frequency indicating little variation, among trials, of phase angle difference between channels of the pair, at that time and frequency. PDLI is defined for frequency f at time t as:
where φA and φB are phase angles of channels A and B, respectively. This definition of PDLI is
equivalent to a definition of PLV, phase lock value, in Brunner and colleagues (2005). By means of some standard trigonometric identities the equation above is equivalent to the following, which, as for PLI, does not require that the phase angles be calculated:
Six rectangular regions of interest (ROIs) were defined within the time-frequency analysis plane by specifying, for each ROI, a band of frequencies and a time interval relative to the stimulus onset time. The ROI frequencies were: delta (1–4 Hz), theta (4–7 Hz), alpha (7–13 Hz), and beta (13–30 Hz) frequencies. The frequencies selected represented the major EEG rhythms within the filter frequencies available from EEG collection. The ROI time intervals were: delta (200–500 ms), theta (400–800 ms), alpha (0–300, and 300–800 ms) and beta (0–300 ms). ROI time intervals were selected based on ERO energy in specific ERP component locations (N1, P3) identified in previous ERP/ERO studies (Criado and Ehlers, 2007; Criado et al., 2012; Ehlers et al., 2007a). Using mean values over trials, the maximum values were calculated for each ROI, for each electrode location or, for PDLI, for a pair of electrode locations (FZ-PZ) for energy (E), PLI amplitude, and PDLI amplitude.
2.5 Statistical analyses
The statistical analyses were focused on the 4 specific aims: In the first aim we compared ERO energy between those individuals with LVEEG and those with higher voltage variants. In the second aim we determined whether task-related phase locking of EROs were significantly different in individuals with LVEEG as compared to those with higher voltage variants. For aims one and two, Multivariate Analysis of Variance (MANOVA) with repeated measures was used. To determine if the values for energy, differed between those with low voltage EEG as compared to those with higher voltage variants, energy values for the two groups (High vs low voltage) for each of the three electrode locations (FZ, CZ, PZ) for the 3 stimuli (happy sad, neutral) in the 5 RO1s were calculated in a 2 (group) X 3 (electrode location) X 3 (stimulus type) X 5 (ROIs) repeated measures design. To determine if the values for phase locking (PLI, and PDLI) differed between those individuals with LVEEG compared to higher voltage variants the same analyses were carried out using PLI and PDLI values. Post-hocs analyses, which co-varied for age, were conducted on significant main effects found for group.
To control for multiple comparisons, p values were set at ≤ 0.01 in these analyses. The third and fourth aims were more exploratory in that they investigated potential associations between behavioral and demographic variables and the LVEEG phenotype. In the third aim we compared demographic variables (gender, age, education, Native American blood degree, marital status, economic status): the number of correct responses in the visual recognition task, the scores on the extroversion and neuroticism scales on the Maudsley, and scores on the Quick test, between those individuals with LVEEG and those with higher voltage recordings. In the fourth aim we tested whether there was an association between having: any anxiety disorder, alcohol dependence, number of drinks consumed in a 24-hr period, SHAS responses to alcohol, and the presence of a low voltage EEG phenotype. To test aims 3 and 4 Chi Square or Fishers exact test were used for dichotomous variables and ANOVA was used for continuous variables. The significant demographic and behavioral variables identified in aims 3 and 4 were then entered into backward stepwise (Wald) logistic regression for the LVEEG phenotype in order to determine which of these variables remained significant in a larger statistical model. P values for these analyses were set at p<0.05.
3.0 Results
3.1 LVEEG and participant demographics
Seven hundred and sixty-two participants’ records were available for these analyses. Four hundred and fifty-one of the participants met criteria for classification as having a low voltage EEG variant as defined previously (see Ehlers and Phillips, 2007; Ehlers et al., 1999). As seen in Table 1, there were no significant gender differences between those individuals with LVEEG and those with higher voltage variants. However, those individuals with LVEEG were older (ANOVA: p<0.001) more likely to be married (Fishers exact test: p<0.001), and have higher degrees of Native American heritage (>50%, Fishers exact test p<0.0001), but were not more likely to differ in income or number of years of education.
Table 1.
Demographic and Behavioral Characteristics
| Low Voltage EEG (n=451) | Higher Voltage EEG (n=311) | |||
|---|---|---|---|---|
| Native American Heritage | <50% | 247 (32%) | 209 (28%) | p<0.001 |
| ≥50% | 204 (27%) | 102 (13%) | ||
|
| ||||
| Gender | Male | 200 (26%) | 122 (16%) | ns |
| Female | 251 (33%) | 189 (25%) | ||
|
| ||||
| Married | Yes | 88 (12%) | 33 (4%) | p<0.001 |
| No | 363 (48%) | 278 (36%) | ||
|
| ||||
| Income | <$20k/Yr | 209 (29%) | 114 (16%) | ns |
| ≥$20k/Yr | 219 (31%) | 167 (24%) | ||
|
| ||||
| Alcohol Dep (DSM4) | Yes | 205 (27%) | 134 (18%) | ns |
| No | 246 (32%) | 177 (23%) | ||
|
| ||||
| Any Anxiety (DSM4) | Yes | 61 (8%) | 50 (7%) | ns |
| No | 390 (51%) | 261 (34%) | ||
|
| ||||
| Age (years, x̄ ±SD) | 34.04 ±13.75 | 27.76 ±12.19 | p<0.001 | |
|
| ||||
| Years of Education (x̄ ±SD) | 11.56 ±1.66 | 11.59 ±1.38 | ns | |
|
| ||||
| Maudsley Impulsivity (x̄ ±SE) | 4.61 ±0.08 | 4.53 ±0.10 | ns | |
|
| ||||
| Maudsley Extraversion (x̄ ±SE) | 12.64 ±0.16 | 12.46 ±0.19 | ns | |
|
| ||||
| Maudsley Neuroticism (x̄ ±SE) | 9.52 ±0.25 | 9.26 ±0.30 | ns | |
|
| ||||
| Max drinks in 24 hours (x̄ ±SE) | 27.73 ±1.16 | 23.70 ±1.41 | p=0.029 | |
|
| ||||
| SHAS Total (x̄ ±SE) | 122.40 ±4.76 | 140.95 ±5.73 | p=0.014 | |
|
| ||||
| Quick Test (QT) (x̄ ±SE) | 106.99 ±0.42 | 107.81 ±0.51 | ns | |
|
| ||||
| Correct Response Happy (%, x̄ ±SE) | 89.27 ±0.52 | 89.69 ±0.63 | ns | |
|
| ||||
| Correct Response Neutral (%,x̄ ±SE) | 92.65 ±0.49 | 93.86 ±0.59 | ns | |
|
| ||||
| Correct Response Sad (%,x̄ ±SE) | 84.11 ±0.74 | 85.59 ±0.90 | ns | |
3.2 LVEEG and ERO energy
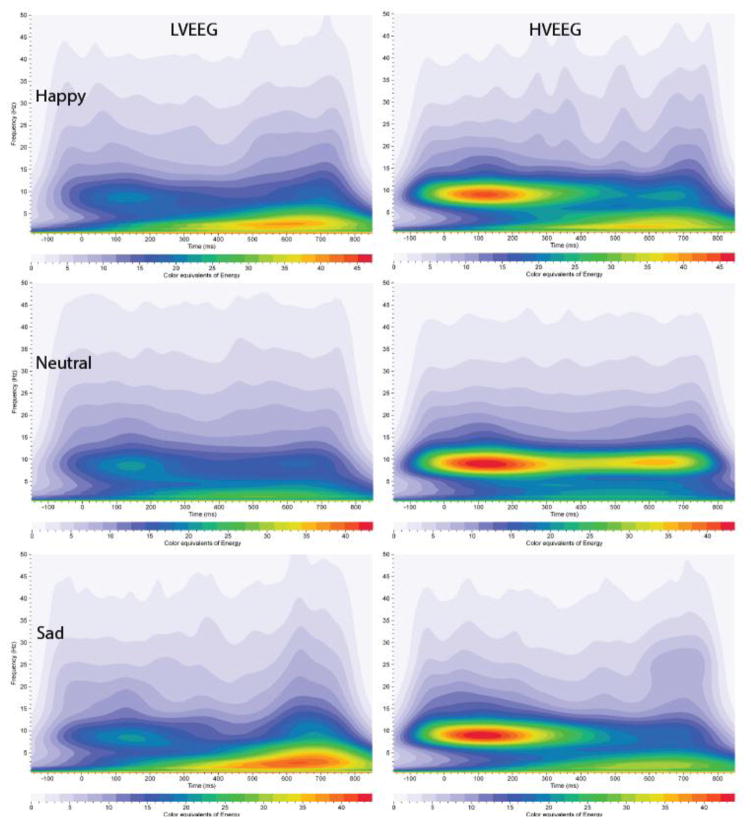
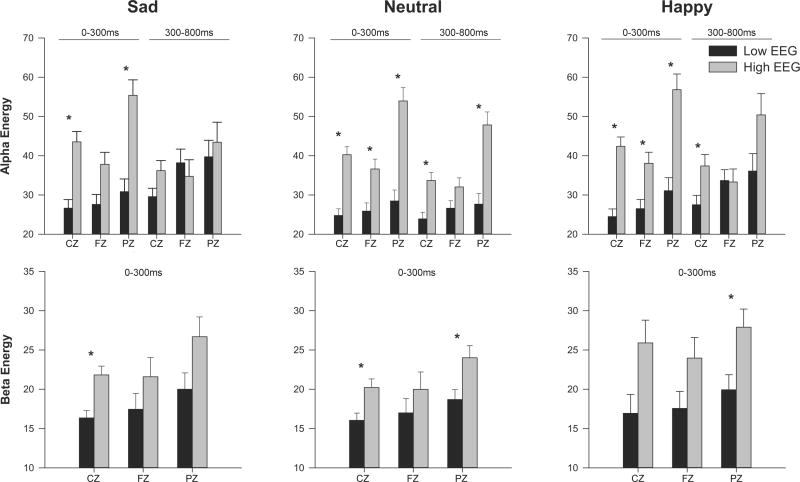
The first major research question concerned whether those participants with the LVEEG phenotype also had reduced energy in their event-related oscillations, generated to a facial recognition task, as compared to those participants with higher voltage variants. Multivariate ANOVA revealed a significant main effect of energy for the LVEEG phenotype in the alpha frequencies for both ROIs (0–300 msec: F=34.0, p<0.0001; 300–800 msec: F=7.7, p<0.006), and in the beta frequencies (F=6.9, p<0.009). As seen in Figure 1 and 2, post-hoc one way ANOVA, that co-varied for age, revealed that those participants with LVEEG were found to have significantly lower energy in their alpha oscillations in the 0–300 msec range in all three leads analyzed (FZ, CZ, PZ) for all stimuli (happy, sad, neutral) (F value ranges: 9.7 - 33.0, df=1,761, p<0.002 - 0.0001) except for the sad stimuli in FZ. Significantly lower energy alpha oscillations were also found in participants in the 300–800 msec range to neutral and happy stimuli in CZ and to neutral stimuli in PZ (F value ranges:6.7– 21.5, df=1,761, p<0.01- 0.0001) Those participants with LVEEG also had significantly lower beta oscillations in the 0–300 msec range to sad and neutral stimuli in CZ, and to happy and neutral stimuli in PZ (F value ranges 6.9–13.7, df=1,761, p<0.009–0.0001).
Figure 1. Grand averages of Energy for Event–Related Oscillations (EROs).
Time-frequency representation of energy shown for electrode location PZ following the visual recognition task for low and higher voltage EEG variants (HVEEG). Responses to the three task conditions (rows) are shown for the LVEEG (left column) and higher EEG voltage (right column) variants. In each graph, frequency (Hz) is presented on the Y-axis, time regions of interest on the X-axis (msec). Energy is presented as color/shade equivalents as indicated on the bar at the bottom of each graph.
Figure 2. ERO Energy in Alpha and Beta Frequency Bands.
Adjusted mean values (ANOVA co-varied for age) for energy of event-related oscillations (EROs) following the visual recognition task for individuals with LVEGG (black bars) and those with higher EEG voltage (grey bars). Channels shown on the x-axis (CZ, FZ, and PZ) for each time window indicated. * indicates p<0.01. Error Bars = S.E.M.
3.3 LVEEG and ERO phase locking
The second research question concerned whether those participants with LVEEG phenotype had less phase-locking of EROs across trials or between electrode locations as compared to those participants with higher voltage variants. Multivariate ANOVA revealed a significant main effect of PLI for the LV EEG phenotype in the theta (F=7.75, p<0.006) and alpha frequencies (0–300 msec: F=21.67, p<0.0001; 300–800 msec: F=18.1, p<0.0001). ANOVA, which co-varied for age, revealed that those participants with LVEEG did not significantly differ from those individuals with higher voltage variants in the phase locking of their EROs across theta frequencies. However, as seen in Figure 3, participants with LVEEG had significantly higher values of alpha phase locking across trials in the 0–300 msec range, in all leads, to all stimuli except to happy stimuli in PZ (F value ranges: 6.8–13.4, df=1,761, p<0.009-p<0.0001).
Figure 3. Phase Lock Index in Alpha Frequency Band.
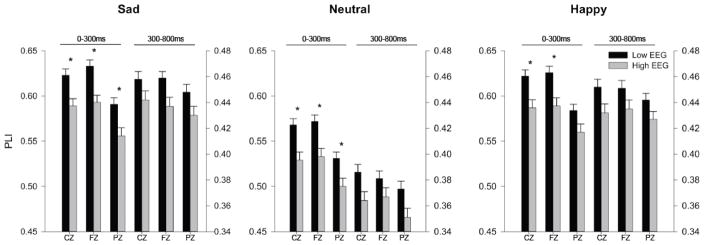
Adjusted mean values (ANOVA co-varied for age) for phase lock index (PLI) of event-related oscillations (EROs) following the visual recognition task for individuals with LVEGG (black bars) and those with higher EEG voltage (grey bars). Channels shown on the x-axis (CZ, FZ, and PZ) for each time window indicated. Early time window scale on the left y-axis, late time window scale on the right y-axis. * indicates p<0.01. Error Bars = S.E.M.
An evaluation of phase locking values between the electrode locations FZ-PZ were also analyzed using multivariate ANOVA and significant main effects were seen for the delta (F=26.62, p<0.0001), theta (F=28.6, p<0.0001) alpha (0–300 msec:F=35.7, p<0.0001; 300–800 msec: F=38.0, p<0.0001) and beta (F=21.4, p<0.0001) frequencies. A post-hoc one way ANOVA that co-varied for age revealed that a significant increase in phase locking between electrode locations was seen in those individuals with LVEEG, as compared to those with higher voltage variants, in delta, theta, alpha and beta ROIs to all three stimuli (F value ranges 7.1–20.4, df=1,761, p<0.009-p<0.0001), as seen in Figure 4.
Figure 4. Phase difference lock index for all frequency bands.
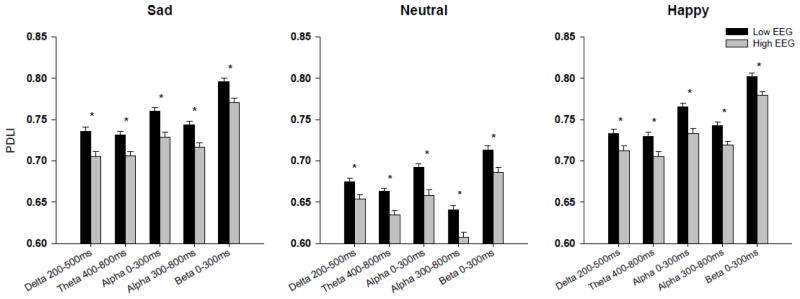
Adjusted mean values (ANOVA co-varied for age) for phase difference lock index (PDLI) of event-related oscillations (EROs) following the visual recognition task for individuals with LVEGG (black bars) and those with higher EEG voltage (grey bars). PDLI shown between channels FZ and PZ for frequency band and time windows indicated on the x-axis. * indicates p<0.01. Error Bars = S.E.M.
3.4 LVEEG and participant behavioral characteristics
We also tested whether those participants with LVEEG differed in their motor responses to the facial recognition task that was used to generate the EROs. There were no significant differences in the percentage of stimuli (happy [F=0.25, p<0.62], sad [F=1.56, p<0.21 or neutral [F=2.4, p<0.12) correctly identified, by pressing the appropriate button, between participants with LVEEG and those with higher voltage variants. We also compared scores on a picture naming task that is correlated with verbal IQ (The Quick Test) and found no significant differences between participants with LVEEG and those with higher voltage variants (F=1.54, p<0.22). Additionally, we evaluated scores on the Maudsley Personality Inventory for the Extroversion (F=0.5, p<0.48) and Neuroticism (F=0.43, p<0.52) scales and found no significant differences in scores on those scales between participants with LVEEG and those with higher voltage variants. These data are presented in Table 1. We also found no significant differences in the number of individuals with DSM-IV anxiety disorders (Fisher exact test p<0.36) or alcohol dependence (Fisher exact test p<0.56) between those participants with LVEEG and those with higher voltage variants (Fisher’s exact test p<0.14). However those participants with LVEEG did report consuming a larger number of drinks over a 24-hr period (F=4.8, p<0.029) and also had lower scores on the SHAS-E total (F=6.04, p<0.014) than those participants with higher voltage variants, as seen in Table 1. A logistic regression was performed by entering all the significant demographic variables (age, marital status, Native American blood degree) and the two significant alcohol use related variables (Max drinks in 24 hours, SHAS total) into a backward (Wald) stepwise analysis. The final model revealed that age (Wald=30.7, B=.96, p<0.0001) blood degree (Wald=5.7, B=.67, p<0.017) and SHAS total (Wald=6.4, B=1.0, p<0.01) remained significant.
4.0 Discussion
A body of knowledge is beginning to emerge that suggests that the phase locking of frequency specific, neuro-oscillatory activity within and between neural assemblies may underlie the processes whereby the brain organizes and communicates information (Basar et al., 1999a, 1999b, 1999c; Roach and Mathalon, 2008; Sauseng and Klimesch, 2008). The phase locking of event-related oscillations (EROs) therefore represents a methodology whereby neuronal synchrony can be quantified. Studies that have estimated the phase locking of alpha oscillations concurrent with fMRI have found that phase locking in the upper alpha band is selectively associated with activity in a well defined intrinsic functional connectivity network comprised of frontal and parietal lobe regions that are involved in cognitive control (Sadaghiani et al., 2012). These authors further suggest that this network is separate and distinct from the networks associated with global alpha power (Sadaghiani et al., 2012). In the present study, we investigated whether individuals identified as having a LVEEG variant, a global LV alpha power phenotype, differed in their even-related oscillations, in a range of frequencies, following presentation of a facial expression recognition task. We found a significant association between lower amplitude global alpha power and energy in event-related oscillations in the alpha and beta (but not other frequency) ranges. This suggests that alpha power in the resting EEG is highly correlated with energy that is generated to event-related stimuli but does not necessarily suggest that they represent the same network.
It has been suggested, that in individuals with LVEEG, that alpha oscillations are not absent but rather there is insufficient synchronization of local cortical alpha rhythms for the signal to be recorded on the scalp (Anokhin et al., 1992). It has also been suggested that LVEEG is a result of weakened thalamo-cortical links leading to insufficient pacemaking activity of cortical neurons (Bazanova and Vernon, 2013; Vogel et al., 1979a, 1979b). In the present study, we evaluated the ability of individuals with LVEEG to “phase-lock” their alpha oscillations in response to a cognitive task. We found that the phase locking index of alpha oscillations, a measure of synchrony of oscillations across stimulus presentation trials, was actually higher in individuals with LVEEG as compared to those individuals with higher amplitude global EEG phenotypes. We also evaluated whether the connectivity between frontal and posterior networks was significantly different between those individuals with LVEEG and those with higher voltage records. In this case we found that phase locking of EROs in all frequency bands were increased in individuals with LVEEG. These findings are partially supported by fMRI-based connectivity studies by Scheeringa et al. (2012) compared measures of fMRI-based connectivity and EEG measures of alpha power. They demonstrated that when alpha power in the EEG increases, BOLD connectivity between the primary visual cortex and occipital brain regions decreases, the negative correlation between the visual cortex and the anterior/medial thalamus decreases, and the ventral-medial prefrontal cortex is reduced in strength. Thus these findings are consistent with ours and further suggest that alpha activity may serve to reduce connectivity between brain sites both as a state phenomena and a trait variable.
A number of studies have provided evidence to support the idea that LVEEG phenotype is genetically regulated and differs in prevalence between different ethnic populations (see Anokhin, 2014; Anokhin et al., 1992 for reviews). In a study that evaluated German military pilots and Japanese train drivers, which included close to 8,000 individuals, only ~4% of the individuals had a LVEEG record (Vogel and Fujiya, 1969). This was a similar incidence to what was reported in a large patient sample obtained in the United States of 7,000 individuals (Niedermeyer, 1987). However, we have demonstrated that certain U.S. ethnic populations have much higher rates of LVEEG records. In the present community sample of Native Americans 59% of the sample had a LVEEG record. Additionally, having a low voltage record was significantly associated with having a higher percentage of Native American ancestry. In a previous study of young African American adults, conducted in our laboratory, 32% of the sample was found to have a LVEEG variant (Ehlers and Phillips, 2003). Low rates of LVEEG records were found in Hispanic and EuroAmerican samples (<9%) also investigated in our laboratory (Ehlers et al., 2004c, 2004d). Taken together, these studies suggest that significant population stratification exists in the presence of LVEEG variants.
In early studies by Vogel et al., (1979a, 1979b), modest but significant differences were seen between a number of different EEG variants in cognitive performance and personality, in a population in Europe. It has also been speculated, based on theoretical considerations, that individuals with “non-dominant” alpha activity may have a different cognitive processing mode (Klimesch, 2012). In the present study we compared performance in the facial recognition task used to generate EROs between individuals with LVEEG and those with higher frequency variants, and found no significant differences in the number of stimuli correctly identified. We also found no significant differences in performance on the Quick Test, a picture-word recognition task that has been demonstrated to be correlated with verbal IQ. Additionally, we found no differences on the neuroticism or extroversion scales on the Maudsley personality inventory between those individuals with LVEEG and those with higher amplitude variants. These studies suggest that individual differences in alpha phenotype may impart subtle effects on brain and behavior that are not related to global measures of personality or IQ in this population group. However, studies using more subtle measures of cognition and personality might result in significant associations.
Although few studies have investigated the psychological and cognitive characteristics of individuals with LVEEG there have been some studies have found significant associations with some psychiatric diagnoses. For instance, early studies have found an association between having LV EEG and alcohol dependence in primarily EuroAmerican populations (Arentsen and Sindrup, 1963; Coger et al., 1978; Jones and Holmes, 1976; Varga and Nagy, 1960), and more recently this was verified in a Hispanic population (Ehlers and Phillips, 2007). In another study a subgroup of alcoholics with anxiety disorders were found to be more likely to have a low voltage alpha EEG, as compared to non-alcoholics (Enoch et al., 1995, 1999). In the present study, we did not confirm that in this population sample that the LVEEG phenotype was associated with anxiety disorders or alcohol dependence. We did find that participants with LVEEG reported drinking a larger number of drinks over a 24-hr period and also reported less expected subjective feelings of alcohol intoxication when they drink. Several previous studies have also reported that those individuals with low voltage EEG can have a lower subjective response to acute alcohol administration (Ehlers et al., 1999; Propping, 1980). Low level of response to alcohol is perhaps one of the best predictors of risk for development of alcohol-related problems over the life span (Schuckit and Smith, 1996). How a LVEEG might lead to a low level of response to alcohol is not currently known. However, we have shown previously that acute alcohol administration results in significant reductions in phase locking of EROs both within and between brain sites, in both rats and humans, and that those reductions in phase locking in the alpha frequencies in the parietal cortex were found to be correlated with blood ethanol concentrations (Ehlers et al., 2012). We interpreted these findings as being consistent with the hypothesis that ethanol’s intoxicating actions in the brain include reducing synchrony within and between neuronal networks, perhaps by increasing the level of noise in key neuromolecular interactions. Therefore the current findings demonstrating that individuals with LVEEG have higher levels of synchrony both within and between brain sites at baseline may mean that it would take more alcohol to reduce that phase synchrony and thus those individuals would report a low level of response to alcohol to a standard dose of the drug.
In conclusion, these data represent the first association analysis of measures of brain connectivity in individuals with LVEEG phenotype. Individuals with LVEEG were found to have decreased energy in their alpha EROs, increased phase locking between stimulus trials, and increased phase-locking between cortical brain areas in response to a facial recognition task as compared to individuals with higher voltage EEGs. No differences in cognitive tests or personality variables were found between those individuals with LVEEG and those with higher voltage variants. However, LVEEG was associated with higher levels of drinking and low level of response to alcohol. The results of this study should, however, be interpreted in the context of several limitations. First, the findings may not generalize to other Native Americans other ethnic groups. Second, comparisons of association findings to non-Native American populations may be limited by differences in a host of potential genetic and environmental variables, including prevalence of heavy drinking and alcohol dependence that could influence EEG voltage levels. This study reports associations between LVEEG and connectivity and such associations may or may not be causative. A bipolar montage was used to determine the spectral power level that determined the “cut off” for the designation of a low voltage record. Bipolar montages can reduce the amplitude of signals common to the two reference electrodes. However, our studies have demonstrated a high correlation between visual inspection and characterization of LVEEG by a registered electroencephalographer and the spectral determination of the designation. Never-the-less, different labs may characterize LVEEG in different ways and thus our results may not be entirely comparable to other large studies. Despite these limitations, this report represents an important step in an ongoing investigation to understand the genetic and environmental determinants of EEG variants.
Highlights.
EROs were used to evaluate 762 participants with and without low voltage EEGs.
Individuals with LVEEG were found to have decreased energy in their alpha EROs
LVEEG was associated with increased phase locking between cortical brain areas.
LVEEG was associated with a less intense expected response to alcohol.
Acknowledgments
Role of Funding
Funding for this study was provided by grants from the National Institutes of Health (NIH);from the National Institute on Alcoholism and Alcohol Abuse (NIAAA) 5R37 AA010201, (CLE). NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors wish to acknowledge the technical support of David Gilder, Philip Lau, Shirley Sanchez and Gina Stouffer.
List of Abbreviations
- ANOVA
analysis of variance
- BOLD
Blood Oxygenation Level-Dependent Signal
- CNS
central nervous system
- COGA
collaborative study on the genetics of alcoholism
- DNA
deoxyribonucleic acid
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth edition
- EEG
electroencephalogram
- EROs
event-related oscillations
- ERPs
event-related potentials
- FHAM
family history assessment module
- fMRI
Functional magnetic resonance imaging
- Hz
cycles/second
- HVEEG
high voltage EEG
- IRB
Institutional review board
- LVEEG
low voltage EEG
- NAH
Native American heritage
- PLI
phase lock index
- PDLI
phase difference lock index
- ROI
region of interest
- SHAS-E
subjective high assessment scale expectations
- SSAGA
semi-structured assessment for the genetics of alcoholism
Footnotes
Conflicts of Interests
The author(s) declare that they have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian ED, Matthews BHC. The Berger Rhythm: Potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Anokhin AP. Genetic psychophysiology: Advances, problems, and future directions. Int J Psychophysiol. 2014;93:173–197. doi: 10.1016/j.ijpsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin A, Steinlein O, Fischer C, Mao Y, Vogt P, Schalt E, Vogel F. A genetic study of the human low-voltage electroencephalogram. Hum Genet. 1992;90:99–112. doi: 10.1007/BF00210751. [DOI] [PubMed] [Google Scholar]
- Arentsen K, Sindrup E. Electroencephalographic investigation of alcoholics. Acta Psychiatr Scand. 1963;39:371–383. doi: 10.1111/j.1600-0447.1963.tb07471.x. [DOI] [PubMed] [Google Scholar]
- Balconi M, Pozzoli U. Event-related oscillations (EROs) and event-related potentials (ERPs) comparison in facial expression recognition. J Neuropsychol. 2007;1:283–294. doi: 10.1348/174866407x184789. [DOI] [PubMed] [Google Scholar]
- Balconi M, Pozzoli U. Event-related oscillations (ERO) and event-related potentials (ERP) in emotional face recognition. Int J Neurosci. 2008;118:1412–1424. doi: 10.1080/00207450601047119. [DOI] [PubMed] [Google Scholar]
- Basar E. A review of alpha activity in integrative brain function: fundamental physiology, sensory coding, cognition and pathology. Int J Psychophysiol. 2012;86:1–24. doi: 10.1016/j.ijpsycho.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999a;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng Med Biol Mag. 1999b;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001b;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Demiralp T, Schurmann M, Basar-Eroglu C, Ademoglu A. Oscillatory brain dynamics, wavelet analysis, and cognition. Brain Lang. 1999c;66:146–183. doi: 10.1006/brln.1998.2029. [DOI] [PubMed] [Google Scholar]
- Basar E, Guntekin B. A short review of alpha activity in cognitive processes and in cognitive impairment. Int J Psychophysiol. 2012;86:25–38. doi: 10.1016/j.ijpsycho.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Basar E, Guntekin B. Review of delta, theta, alpha, beta and gamma response oscillations in neuropsychiatric disorders. In: Basar E, Basar B-EC, Ozerdem A, Rossini PM, Yener GG, editors. Application of Brain Oscillations in Neuropsychiatric Diseases; Selected Papers from “Brain Oscillations in Cognitive Impairment and Neurotransmitters” Conference; Istanbul, Turkey. 29 April–1 May 2011; Elsevier BV; 2013. pp. 303–341. Supplements to Clinical Neurophysiology. [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Demiralp T, Basar-Eroglu C, Ademoglu A. Event-related oscillations are 'real brain responses'--wavelet analysis and new strategies. Int J Psychophysiol. 2001a;39:91–127. doi: 10.1016/s0167-8760(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Basar E, Stampfer HG. Important associations among EEG-dynamics, event-related potentials, short-term memory and learning. Int J Neurosci. 1985;26:161–180. doi: 10.3109/00207458508985615. [DOI] [PubMed] [Google Scholar]
- Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci Biobehav Rev. 2013;44C:94–110. doi: 10.1016/j.neubiorev.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Beals J, Manson SM, Whitesell NR, Spicer P, Novins DK, Mitchell CM. Prevalence of DSM-IV disorders and attendant help-seeking in 2 American Indian reservation populations. Arch Gen Psychiatry. 2005a;62:99–108. doi: 10.1001/archpsyc.62.1.99. [DOI] [PubMed] [Google Scholar]
- Beals J, Novins DK, Whitesell NR, Spicer P, Mitchell CM, Manson SM. Prevalence of mental disorders and utilization of mental health services in two American Indian reservation populations: mental health disparities in a national context. Am J Psychiatry. 2005b;162:1723–1732. doi: 10.1176/appi.ajp.162.9.1723. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Brunner C, Graimann B, Huggins JE, Levine SP, Pfurtscheller G. Phase relationships between different subdural electrode recordings in man. Neurosci Lett. 2005;375:69–74. doi: 10.1016/j.neulet.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Coger RW, Dymond AM, Serafetinides EA, Lowenstam I, Pearson D. EEG signs of brain impairment in alcoholism. Biol Psychiatry. 1978;13:729–739. [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Electrophysiological responses to affective stimuli in Southwest California Indians: relationship to alcohol dependence. J Stud Alcohol Drugs. 2007;68:813–823. doi: 10.15288/jsad.2007.68.813. [DOI] [PubMed] [Google Scholar]
- Criado JR, Gizer IR, Slutske WS, Phillips E, Ehlers CL. Event-related oscillations to affective stimuli: heritability, linkage and relationship to externalizing disorders. J Psychiatr Res. 2012;46:256–263. doi: 10.1016/j.jpsychires.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly DD, Pedley TA. Current practice of clinical electroencephalography. 2. Raven Press; New York: 1990. [Google Scholar]
- Del Zotto M, Deiber MP, Legrand LB, De GB, Pegna AJ. Emotional expressions modulate low alpha and beta oscillations in a cortically blind patient. Int J Psychophysiol. 2013;90:358–362. doi: 10.1016/j.ijpsycho.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Cloutier D, Phillips E. Electroencephalographic responses to alcohol challenge in Native American Mission Indians. Biol Psychiatry. 1999;45:776–787. doi: 10.1016/s0006-3223(98)00113-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Gizer IR, Wilhelmsen KC. Heritability and a genome-wide linkage analysis of a Type II/B cluster construct for cannabis dependence in an American Indian community. Addict Biol. 2009;14:338–348. doi: 10.1111/j.1369-1600.2009.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Phillips E. P3 components of the event-related potential and marijuana dependence in Southwest California Indians. Addict Biol. 2008c;13:130–142. doi: 10.1111/j.1369-1600.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Slutske WS, Lind PA, Wilhelmsen KC. Externalizing disorders in American Indians: Comorbidity and a genome wide linkage analysis. Am J Med Genet B Neuropsychiatr Genet. 2008b;147B:690–698. doi: 10.1002/ajmg.b.30666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. Am J Med Genet B Neuropsychiatr Genet. 2004b;129B:110–115. doi: 10.1002/ajmg.b.30057. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Gilder DA, Wilhelmsen KC. Linkage analyses of stimulant dependence, craving, and heavy use in American Indians. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:772–780. doi: 10.1002/ajmg.b.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Phillips E, Wilhelmsen KC. EEG alpha phenotypes: linkage analyses and relation to alcohol dependence in an American Indian community study. BMC Med Genet. 2010a;11:43. doi: 10.1186/1471-2350-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacology Bull. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the mu opioid receptor gene (OPRM1) and self-reported responses to alcohol in American Indians. BMC Med Genet. 2008a;9:35. doi: 10.1186/1471-2350-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. EEG low-voltage alpha and alpha power in African American young adults: relation to family history of alcoholism. Alcohol Clin Exp Res. 2003;27:765–772. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. Association of EEG alpha variants and alpha power with alcohol dependence in Mexican American young adults. Alcohol. 2007;41:13–20. doi: 10.1016/j.alcohol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol Teratol. 2007a;29:153–163. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Gizer IR, Gilder DA, Wilhelmsen KC. EEG spectral phenotypes: Heritability and association with marijuana and alcohol dependence in an American Indian community study. Drug Alcohol Depend. 2010b;106:101–110. doi: 10.1016/j.drugalcdep.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Schuckit MA. EEG alpha variants and alpha power in Hispanic American and white non-Hispanic American young adults with a family history of alcohol dependence. Alcohol. 2004d;33:99–106. doi: 10.1016/j.alcohol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wall TL, Wilhelmsen K, Schuckit MA. EEG alpha and level of response to alcohol in Hispanic- and non-Hispanic-American young adults with a family history of alcoholism. J Stud Alcohol. 2004c;65:301–308. doi: 10.15288/jsa.2004.65.301. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the cannabinoid receptor gene (CNR1) and impulsivity in Southwest California Indians. Twin Res Hum Genet. 2007b;10:805–811. doi: 10.1375/twin.10.6.805. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Betancourt M, Gilder DA. The clinical course of alcoholism in 243 Mission Indians. Am J Psychiatry. 2004a;161:1204–1210. doi: 10.1176/appi.ajp.161.7.1204. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Corey L, Lau P, Gilder DA, Wilhelmsen K. Heritability of illicit drug use and transition to dependence in Southwest California Indians. Psychiatr Genet. 2007c;17:171–176. doi: 10.1097/01.ypg.0000242201.56342.1a. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Auditory P3 findings in mission Indian youth. J Stud Alcohol. 2001a;62:562–570. doi: 10.15288/jsa.2001.62.562. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. EEG asymmetry: relationship to mood and risk for alcoholism in Mission Indian youth. Biol Psychiatry. 2001b;50:129–136. doi: 10.1016/s0006-3223(01)01132-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Effects of age and parental history of alcoholism on EEG findings in mission Indian children and adolescents. Alcohol Clin Exp Res. 2001c;25:672–679. [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001d;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic scan for alcohol craving in Mission Indians. Psychiatr Genet. 2005;15:71–75. doi: 10.1097/00041444-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for substance dependence and body mass index in Southwest California Indians. Genes Brain Behav. 2007;6:184–191. doi: 10.1111/j.1601-183X.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Desikan A, Phillips E, Havstad J. Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Dev Neurosci. 2014;36:175–195. doi: 10.1159/000358484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Havstad J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. doi: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Rohrbaugh JW, Davis EZ, Harris CR, Ellingson RJ, Andreason P, Moore V, Varner JL, Brown GL, Eckardt MJ. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am J Med Genet. 1995;60:400–408. doi: 10.1002/ajmg.1320600510. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin Exp Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of Communication. J Inst Elec Eng. 1946;93:429–457. [Google Scholar]
- Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from cannabis dependence in Southwest California Indians. J Addict Dis. 2007;26:23–30. doi: 10.1300/J069v26n04_04. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Dixon M, Corey L, Phillips E, Ehlers CL. Co-morbidity of select anxiety, affective, and psychotic disorders with cannabis dependence in Southwest California Indians. J Addict Dis. 2006;25:67–79. doi: 10.1300/J069v25n04_07. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Ehlers CL. Item response theory analysis of lifetime cannabis-use disorder symptom severity in an American Indian community sample. J Stud Alcohol Drugs. 2009;70:839–849. doi: 10.15288/jsad.2009.70.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder DA, Wall TL, Ehlers CL. Comorbidity of select anxiety and affective disorders with alcohol dependence in Southwest California Indians. Alcohol Clin Exp Res. 2004;28:1805–1813. doi: 10.1097/01.alc.0000148116.27875.b0. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Edenberg HJ, Gilder DA, Wilhelmsen KC, Ehlers CL. Association of Alcohol Dehydrogenase Genes with Alcohol-Related Phenotypes in a Native American Community Sample. Alcohol Clin Exp Res. 2011;35:2008–2018. doi: 10.1111/j.1530-0277.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntekin B, Basar E. Gender differences influence brain's beta oscillatory responses in recognition of facial expressions. Neurosci Lett. 2007;424:94–99. doi: 10.1016/j.neulet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Guntekin B, Basar E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia. 2014;58C:33–51. doi: 10.1016/j.neuropsychologia.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Gur RE, Erwin RJ, Shtasel DL, Gur RC. Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psychiatry Res. 1992;42:253–265. doi: 10.1016/0165-1781(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FW, Holmes DS. Alcoholism, alpha production, and biofeedback. J Consult Clin Psychol. 1976;44:224–228. doi: 10.1037//0022-006x.44.2.224. [DOI] [PubMed] [Google Scholar]
- Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Kunitz SJ. Life-course observations of alcohol use among Navajo Indians: natural history or careers? Med Anthropol Q. 2006;20:279–296. doi: 10.1525/maq.2006.20.3.279. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del GC, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Hau CL, Leung HC. Alpha Power Gates Relevant Information during Working Memory Updating. J Neurosci. 2014;34:5998–6002. doi: 10.1523/JNEUROSCI.4641-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Beck DM, Ro T, Maclin EL, Low KA, Fabiani M, Gratton G. Dynamics of alpha control: preparatory suppression of posterior alpha oscillations by frontal modulators revealed with combined EEG and event-related optical signal. J Cogn Neurosci. 2014;26:2400–2415. doi: 10.1162/jocn_a_00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA. Substance abuse and American Indians: prevalence and susceptibility. Int J Addict. 1982;17:1185–1209. doi: 10.3109/10826088209056349. [DOI] [PubMed] [Google Scholar]
- May PA, Smith MB. Some Navajo Indian opinions about alcohol abuse and prohibition: a survey and recommendations for policy. J Stud Alcohol. 1988;49:324–334. doi: 10.15288/jsa.1988.49.324. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, da Silva FL, editors. Electroencephalography, basic principles, clinical applications, and related fields. Urban & Schwarzenberg; Baltimore: 1987. pp. 97–117. [Google Scholar]
- Niedermeyer E, Lopes da Silva FH. Electroencephalography: basic principles, clinical applications, and related fields. 4. Lippincott Williams & Wilkins; Baltimore, MD: 1999. [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44:281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propping P. Genetic aspects of alcohol action on the electroencephalogram (EEG) Adv Exp Med Biol. 1980;126:589–602. doi: 10.1007/978-1-4684-3632-7_45. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcohol Clin Exp Res. 1998;22:518–523. [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn Sci. 2014;18:16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, D'Esposito M, Kleinschmidt A. alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 2012;32:14305–14310. doi: 10.1523/JNEUROSCI.1358-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Kleinschmidt A, Jensen O, Bastiaansen MC. EEG alpha power modulation of fMRI resting-state connectivity. Brain Connect. 2012;2:254–264. doi: 10.1089/brain.2012.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Genetics and the risk for alcoholism. JAMA. 1985;254:2614–2617. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol Alcohol. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Shalala DE, Trujillo MH, Hartz PE, Paisano EL. Trends in Indian health 1998–99. Washington, D.C: United States Department Health and Human Services; Indian Health Service; Division of Program Statistics; 1999. [Google Scholar]
- Steinlein O, Anokhin A, Yping M, Schalt E, Vogel F. Localization of a gene for the human low-voltage EEG on 20q and genetic heterogeneity. Genomics. 1992;12:69–73. doi: 10.1016/0888-7543(92)90408-k. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44:998–1001. [Google Scholar]
- Varga B, Nagy T. Analysis of alpha rhytims in the electroencephalgram of alcoholics. Electroencephalogr Clin Neurophysiol. 1960;12:933. [Google Scholar]
- Vogel F. Erganzende Untersuchungen zur Genetik des menschlichen Niederspannungs-EEG. Deutsch Z Nervenheilk. 1962;185:105–111. [Google Scholar]
- Vogel F. The genetic basis of the normal human electroencephalogram (EEG) Humangenetik. 1970;10:91–114. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics and the electroencephalogram. Springer; New York: 2000. [Google Scholar]
- Vogel F, Fujiya Y. The incidence of some inherited EEG variants in normal Japanese and German males. Humangenetik. 1969;7:38–42. doi: 10.1007/BF00278691. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examinations in healthy males with various inherited EEG variants. II Results. Hum Genet. 1979a;47:47–80. doi: 10.1007/BF00295570. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J, Propping P, Lehnert KF. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examinations in healthy males with various inherited EEG variants. I Rationale of the study Material Methods Heritability of test parameters. Hum Genet. 1979b;47:1–45. doi: 10.1007/BF00295569. [DOI] [PubMed] [Google Scholar]
- Wall TL, Carr LG, Ehlers CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am J Psychiatry. 2003;160:41–46. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Ehlers C. Heritability of substance dependence in a Native American population. Psychiatr Genet. 2005;15:101–107. doi: 10.1097/00041444-200506000-00006. [DOI] [PubMed] [Google Scholar]