Abstract
Purpose
Graft failure (GF) after hematopoietic cell transplant (HCT) occurs in 5 – 30% of patients. GF can be accompanied by neutropenia (NGF) or can result with adequate neutrophils, but loss of donor chimerism (non-neutropenic graft failure, NNGF). In this report we describe the outcomes of 95 patients treated with a second HCT for GF at the University of Minnesota; 62 with NGF and 33 with NNGF.
Findings
The cumulative incidence of neutrophil recovery at 42 days after second HCT was 45% for NGF and 88% for NNGF. A second GF occurred in 34 NGF (55%) and in 9 NNGF (27%) patients. The incidence of grade III–IV acute graft versus host disease (GVHD) was 8% (95% confidence interval (CI), 1 – 16%) and 12% (95% CI, 1 – 23%) for NGF and NNGF, respectively. From the 2nd HCT, 1-year overall survival (OS) was 44% (95% CI, 34 – 54%), [NNGF: 76% (95% CI, 57 – 87%) and NGF: 27% (95% CI, 17 – 39%)]. The most common cause of death after second HCT was infection (52%).
Conclusions
In summary, the outcomes of second HCT after NGF and NNGF are different with much worse outcomes for NGF necessitating new approaches for this complication.
Keywords: stem cell transplantation, neutropenia, outcomes research
Introduction
Hematopoietic cell transplant (HCT) is uniquely curative for high-risk malignant and non-malignant disease. The successful engraftment of hematopoietic stem cells is a primary goal of any transplant. While transplantation has become safer with improved outcomes, graft failure (GF) still occurs with an incidence of 5 – 30%[1–4]. Many factors contribute to GF including conditioning regimen intensity (myeloablative versus reduced intensity), type of disease (malignant versus non-malignant), the presence of HLA antibodies, the number of infused hematopoietic cells, the degree of HLA-(mis)matching, and co-existent infections [5, 6].
GF has been defined several ways, with the most accepted definition of primary GF as being a lack of achieving a donor-derived absolute neutrophil count (ANC) greater than 500 cells per microliter by 6 weeks (42 days). If the ANC remains below 500 cells per microliter, this would be considered neutropenic GF (NGF). Alternatively, patients may recover with an adequate neutrophil count derived from autologous hematopoiesis. This most often occurs in patients with non-malignant conditions such as thalassemia, sickle-cell, or metabolic storage diseases and is termed non-neutropenic GF (NNGF). Patients may also achieve transient donor engraftment, but then at a later time, donor derived engraftment is lost, leading to persistent neutropenia (NGF). If loss of neutrophils or donor chimerism occurs after 42 days, this is often called secondary graft failure. Untreated NGF is usually fatal, as patients cannot survive long-term without functioning hematopoietic and immune recovery. Outcomes after autologous recovery or loss of chimerism are varied and depend on the primary indication for transplant. In non-malignant diseases, life-threatening conditions may not arise as acutely compared to patients who have a malignant disease as the primary indication for transplant.
A second transplant has been used as salvage therapy for GF employing both myeloablative (MA) and reduced intensity conditioning (RIC) regimens and various hematopoietic stem cell sources. The reported outcomes after second HCT are wide-ranging, with overall survival from 11 – 70%[7–17]. To date, no study has examined outcomes of second HCT based upon NGF versus NNGF. Here, we describe a large single institution experience with second HCT for GF, evaluating outcomes for NGF and NNGF separately.
Methods
Patient Selection
For this retrospective cohort study, patients were identified from the University of Minnesota Blood and Marrow Transplant Database. We evaluated 2045 consecutive allogeneic transplants at the University of Minnesota from January 2000 – July 2013. Cases included patients who underwent an allogeneic HCT for hematologic malignancy, bone marrow failure, and non-malignant diseases (including Hurler syndrome, sickle cell disease, and thalassemia). Cases were excluded if the second transplant was performed for relapsed malignancy. There were 201 patients identified as having NGF or NNGF after their first transplant. Of those 201 GF patients, we identified 95 patients that received a second HCT. Of these, 62 patients had NGF and 33 had NNGF after first transplant. All clinical and laboratory data was obtained from the database and supplemented with review of the medical record. This study was approved by the University of Minnesota’s Institutional Review Board. Informed consent was obtained from all subjects.
Graft Failure
Graft failure was defined as NGF if either of two scenarios occurred. If there was persistent neutropenia (ANC< 500/μL) for ≥ 42 days after the first transplant regardless of any chimerism status, or if there was neutrophil engraftment (defined as achieving an ANC of ≥ 500 neutrophils/μl before day 42) followed by later development of persistent neutropenia with or without loss of chimerism. NNGF was defined as engraftment, but with chimerism ≤ 10% as measured in CD15 positive cells (after June 2009) or mononuclear fractioned cells (prior to June 2009) anytime after transplant without later recovery, necessitating a second HCT.
Characteristics and Outcomes
The administration of any conditioning regimen or immunosuppressive therapy, as well as the cell source used for second transplant, was determined by contemporaneous protocols as modified by the attending physician and donor availability. The presence of a cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus-6 (HHV6) viral infection between first and second transplants was determined by positive viral load on PCR-based testing or by CMV antigenemia direct immunofluorescence assay (prior to 2004). Viral reactivation based on PCR positivity was based on values of > 200 copies/mL for CMV, >1000 copies/mL for EBV, and any detectable level of HHV6 (initiated after 2006).
Statistical Analysis
Patient and transplant characteristics were summarized using descriptive statistics. Statistical comparisons between graft failure types were completed using the Wilcoxon rank-sum test for continuous and Chi-square test for categorical factors. All patients were followed longitudinally until death or last follow up. Cumulative incidence rates and their 95% confidence intervals were estimated for engraftment, grade II–IV and grade III–IV acute GVHD treating death as the competing risk. Event times for neutrophil engraftment were measured from the date of transplantation to the date of neutrophil recovery censoring at the time of death. Kaplan-Meier analysis was used to estimate overall survival (OS). All statistical analyses used Statistical Analysis System statistical software version 9.3 (SAS Institute Inc., Cary, NC).
Results
The characteristics of the patients and conditions of second HCT are shown in Table 1. The NGF and NNGF groups are quite different with the median age of NGF patients was significantly older at 22 years versus 3 years in the NNGF group (p < 0.01). Only 26 of 62 NGF (44%) patients had a non-malignant diagnosis, all of the NNGF had non-malignant conditions, including metabolic disease, hemoglobinopathies, and immunodeficiencies. The median Karnofsky performance scores at the time of second transplant were similar in both groups at 90 and 100 for the NGF and NNGF groups, respectively (p = 0.18). The median time to second HCT was 60 days for the entire cohort. Patients with NGF were transplanted earlier than NNGF patients (median 47.5 days versus 167 days, p < 0.01). For patients receiving a second HCT, the use of a different donor was more likely in the NGF versus NNGF patients (63% vs 28% respectively, p = 0.03), though this is probably accounted for by more UCB use in the NGF group (54% vs 31% in the NNGF group) which would nearly always result in a different donor from the 1st HCT. If one evaluates marrow or PBSCT exclusively, 23 of 29 NGF patients received the same donor, while 5 of 22 NNGF patients received the same donor. Because the overall survival is so poor for NGF patients (and numbers were small), donor source played little role in modifying the outcome.
Table 1.
Characteristics of patients with graft failure receiving a second HCT.
| All GF (n = 95) | NGF (n = 62) | NNGF (n = 33) | ||
|---|---|---|---|---|
| Age, median in years | 10 | 22 | 3 | <.01 |
| (range) | (0.9 – 69) | (0.9 – 69) | (1 – 15) | |
| Sex | .67 | |||
| Male | 55 (60%) | 36 (62%) | 19 (58%) | |
| Karnofsky | .18 | |||
| median | 90 | 90 | 100 | |
| (range) | (10–100) | (10–100) | (30–100) | |
| missing | 11 | 7 | 4 | |
| Diagnosis | <.01 | |||
| Malignant | 36 (40%) | 36 (58%) | ||
| AML | 15 (18%) | 15 (24%) | 0 | |
| Other Leukemia | 9 (9%) | 9 (15%) | 0 | |
| MDS | 4 (4%) | 4 (7%) | 0 | |
| NHL | 2 (2%) | 2 (3%) | 0 | |
| Other Malignancy | 6 (6%) | 6 (10%) | 0 | |
| Non-Malignant | 59 (62%) | 26 (42%) | 33 (100%) | |
| Metabolic Disorder | 32 (34%) | 11 (18%) | 21 (64%) | |
| Aplastic Anemia | 18 (19%) | 15 (24%) | 3 (9%) | |
| Hemoglobinopathy | 6 (6%) | 0 | 6 (18%) | |
| Immunodeficiency | 3 (3%) | 0 | 3 (9%) | |
| 2nd HCT Graft Source | .06 | |||
| Double UCB | 19 (20%) | 16 (26%) | 3 (9%) | |
| Single UCB | 25 (26%) | 17 (27%) | 8 (24%) | |
| PBSCT | 18 (19%) | 13 (21%) | 5 (15%) | |
| Marrow | 33 (35%) | 16 (26%) | 17 (52%) | |
| 2nd HCT Graft Relationship | .13 | |||
| Sibling Marrow | 9 (10%) | 6 (10%) | 3 (9%) | |
| Haploidentical Marrow | 7 (7%) | 7 (11%) | 0 | |
| URD | 79 (83%) | 49 (79%) | 30 (91%) | |
| 2nd HCT Donor | .03 | |||
| Same | 28 (30%) | 23 (37%) | 5 (15%) | |
| Different | 67 (71%) | 39 (63%) | 28 (85%) | |
| Time to 2nd HCT | <.01 | |||
| Median, days | 60 | 47.5 | 167 | |
| (range) | 19 – 1751 | 19 – 1012 | 38 – 1751 | |
| Conditioning Regimen | <.01 | |||
| No chemotherapy | 48 (51%) | 37 (60%) | 1 (3%) | |
| Cyclophosphamide (Cy) | 8 (8%) | 2 (3%) | 6 (18%) | |
| Fludarabine | 6 (5%) | 5 (8%) | 1 (3%) | |
| Busulfan/Cy (MA) | 5 (5%) | 0 | 5 (15%) | |
| Busulfan/fludarabine | 17 (18%) | 2 (3%) | 15 (45%) | |
| Fludarabine/melphalan | 2 (2%) | 0 | 2 (6%) | |
| Fludarabine/Cy | 4 (4%) | 2 (3%) | 2 (6%) | |
| Clofarabine | 7 (7%) | 6 (15%) | 1 (3%) | |
| Clofarabine/melphalan | 1 (1%) | 0 | 1 (3%) | |
| Radiation at 2nd HCT | <.01 | |||
| TBI | 29 (31%) | 5 (8%) | 24 (73%) | |
| TLI | 2 (2%) | 1 (2%) | 1 (3%) | |
| None | 63 (66%) | 55 (89%) | 8 (24%) | |
| Missing | 1 (1%) | 1 (2%) | 0 | |
| Serotherapy | <.01 | |||
| ATG | 65 (68%) | 50 (81%) | 15 (46%) | |
| Rituximab | 12 (13%) | 12 (19%) | 0 | |
| Alemtuzumab | 3 (3%) | 0 | 3 (9%) | |
| Second Graft Failure | 43 (45%) | 34 (55%) | 9 (27%) | <.01 |
| NGF | 26 (27%) | 22 (36%) | 4 (12%) | |
| NNGF | 17 (18%) | 12 (19%) | 5 (15%) | |
| Underwent 3rd HCT | 24 (25%) | 15 (24%) | 9 (27%) | .74 |
GF, graft failure; NGF, neutropenic graft failure; NNGF, non-neutropenic graft failure; AML; acute myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin’s lymphoma; UCB, umbilical cord blood; URD, unrelated donor; ATG, anti-thymocyte globulin; MA, myeloablative regimen; TBI, total body irradiation; TLI, total lymphoid irradiation
Conditioning regimens varied significantly prior to second HCT. There were 48 patients (51%) who received only anti-thymocyte globulin (ATG), but no chemotherapy. The remaining patients received various chemotherapy regimens; the majority were RIC regimens. Five patients in the NNGF group received a second myeloablative transplant with busulfan and cyclophosphamide; all had non-malignant and non-bone marrow failure diagnoses. Six NGF patients received total body irradiation (TBI) or total lymphoid irradiation (TLI) versus 25 in the NNGF group. More than half of the entire cohort received some kind of serotherapy (either as single agent or in combinations) using rituximab, ATG, or alemtuzumab.
Hematopoietic Recovery
A lower cumulative incidence of neutrophil recovery was observed for patients with NGF versus NNGF at 45% (95% CI, 34 – 58%) and 88% (95% CI, 74 – 96%) although median time to recovery was similar in both groups [15 days (range 4 – 29 days) versus 15 days (range 7 – 30 days), respectively]. There were 43 (45%) patients from the entire cohort who suffered a second graft failure; 34 were from the NGF group and 9 were from the NNGF group. Two patients (both from the NGF group) died early at 6 and 11 days post second HCT.
Graft failure after the second HCT in the NGF group was a second NGF in 22 patients, while 12 had NNGF which was surprising being that they did not have count recovery after their first HCT. Of these 37 second GF patients, there were 15 patients from this group that were clinically well enough to undergo a third HCT. In the NNGF group, four patients developed a neutropenic graft failure while 5 developed a second non-neutropenic graft failure; all (9) second graft failure patients from the NNGF group underwent a third HCT. For the entire group of 24 third transplant patients, only six patients survived greater than 1 year after the 3rd HCT.
Overall Survival
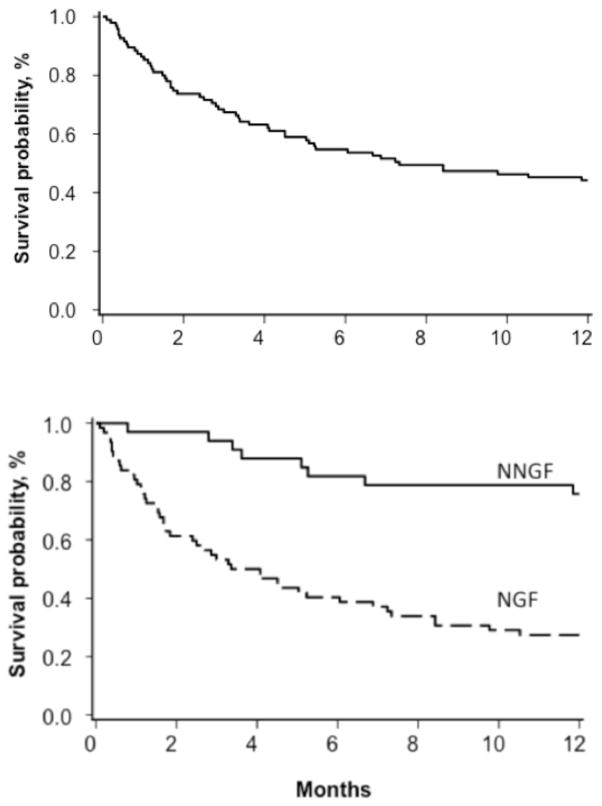
The 1-year overall survival (OS) was 44% (95% CI, 34–54%) for the entire cohort. However, 1-year OS was only 27% (95% CI, 17–39%) for NGF, yet 76% (95% CI, 57–87%) for NNGF patients. The rates of grade III–IV acute graft versus host disease were 12% (95% CI, 1 – 23%) and 8% (95% CI, 1 – 16%) for NGF and NNGF, respectively.
The most frequent cause of death (n = 27) after 2nd HCT was overwhelming infection (Table 2). Other primary causes of death were underlying malignancy, pulmonary failure, non-malignant disease progression and GVHD (Table 2).
Table 2.
Cause of Death (COD) 1-year after Second HCT
| Evaluable patients | n = 52 |
|---|---|
| Bacterial Infection | 12 |
| Fungal Infection | 10 |
| Viral Infection | 5 |
| Underlying Malignancy | 3 |
| Pulmonary Failure | 3 |
| Underlying Non-Malignant Disease | 3 |
| Acute GVHD | 2 |
| Other | 14 |
Viral Reactivation
Because prolonged aplasia may be associated with viral infection, we also evaluated the incidence of viral reactivation occurring associated with graft failure (Table 3). There was no difference in the incidence of CMV reactivation between NGF and NNGF groups (7 % and 18%, respectively, p = 0.08) or EBV reactivation (19% and 6%, respectively, p = 0.30). However, there was more frequent HHV6 reactivation, with 44% of the NGF group versus 9% of the NNGF group (p < 0.01) having reactivation. This is of interest as HHV6 has been linked to graft failure in some [18, 19], but not all reports [20]. Of the 27 NGF patients with HHV6 reactivation, 15 patients developed a second graft failure, two patients died prior to 42 days post 2nd HCT, and only three patients were alive at 1-year post 2nd HCT. Of the three NNGF patients with HHV6 reactivation, all three engrafted and two survived beyond 1-year post 2nd HCT.
Table 3.
Viral reactivation between first and second HCT.
| All GF (n = 95) | NGF (n = 62) | NNGF (n = 33) | ||
|---|---|---|---|---|
| HHV6 Reactivation | <.01 | |||
| Yes | 30 (32%) | 27 (44%) | 3 (9%) | |
| No | 29 (31%) | 18 (29%) | 11 (33%) | |
| Not Tested | 36 (38%) | 17 (27%) | 19 (58%) | |
| EBV Reactivation | .30 | |||
| Yes | 7 (12%) | 5 (19%) | 2 (6%) | |
| No | 35 (57%) | 14 (52%) | 21 (62%) | |
| Not Tested | 19 (31%) | 8 (30%) | 11 (32%) | |
| CMV Reactivation | .08 | |||
| Yes | 10 (11%) | 4 (7%) | 6 (18%) | |
| No | 85 (90%) | 58 (94%) | 27 (82%) |
Discussion
Second HCT is frequently used to treat primary GF occurring after allogeneic HCT. In this analysis, we distinguished outcomes in two different cohorts of patients: NGF and NNGF, which have not previously been reported. The NNGF group was composed of younger, non-malignant diagnosed patients with greater time span between first and second HCT. We observed a promising 76% 1-year survival in NNGF, but disappointing 27% survival in those with NGF. This poor survival in NGF patients is despite nearly half (43%) achieving neutrophil recovery, although 12 of these patients had autologous recovery (NNGF), indicating that neutrophil recovery alone was insufficient to give a survival benefit and many of these patients dies of infectious causes.
As mentioned, our cohorts contain patients of different ages. Those with NGF were more likely to be of older age and have malignant disease (median age = 22 years), while those with NNGF were more likely to have undergone transplant for a metabolic disease and therefore much younger (median age = 3 years). Age could play a role in outcome after second transplant, but has never been studied independently. Schriber et al reported on 122 patients with NGF that were between ages 2 – 61 years (median 30 years), though that cohort contained only 3 non-malignant, non-aplastic patients. No effect of age on overall survival was found[11]. In a study of second transplant for relapsed malignancy (age range 1 – 59, median 30 years), Platzbecker found a significant increase in transplant-related mortality (TRM) overall survival for patients >50 years versus those < 50 years of age[21]. In a another study evaluating second transplant for relapsed malignancy (range 1.5 – 46 years, median 35 years), Michallet found that children < 16 years of age at second transplant had significantly better OS and improved TRM versus older children in a multivariate analysis[22]. The median age of second transplant for our NGF patients who survived was 7 years while for those who did not survive it was 25 years (p = 0.01). The median age of second transplant for our NNGF patients who survived was 6 years while for those who did not survive it was 2 years (p = 0.05). This observation suggests that age may be an outcome factor in NGF (with a malignant diagnosis), but is less robust in the NNGF setting (with a non-malignant diagnosis).
The NNGF outcome is similar to the limited data reported in patients with thalassemia who had graft failure (11 of 16 had NNGF). After 2nd HCT, 94% of 16 patients had sustained engraftment and a 5-year overall survival of 79% [23]. HCT for non-malignant disease is associated with up to 3-fold higher risks of GF (primarily NNGF), as described by Olsson et al in 54 GF out of 967 HCTs [24]. Many patients with non-malignant diseases are chemotherapy naïve and thus more immunocompetent at initial HCT possibly leading to immune-based GF. Some also have hypercellular hematopoiesis or have been heavily transfused, resulting in allo-sensitization which further enhances risks of GF [23, 25].
The outcomes of 2nd HCT as salvage therapy for NGF that we observed are similar, though slightly better than CIBMTR data reported by Schriber et al, who described 122 patients with primary GF and underwent a second HCT [11]. They reported day 28 engraftment of 66%, 100-day mortality of 75%, and 1-year overall survival of only 11%. The primary cause of death was second graft failure and leading secondary causes were infection, organ failure, and GVHD. Although not stated in that study, those patients most likely had NGF without autologous recovery, since the vast majority had malignant diseases or aplastic anemia and only 3 had other non-malignant diagnoses. A registry study from the Japanese Society for Hematopoietic Cell Transplantation evaluated 220 cases of graft failure (91% primary, and 9% secondary GF)[26]. Second HCT was performed using umbilical cord blood (UCB), mobilized peripheral blood stem cells (PBSC), or bone marrow (BM) grafts with day-30 neutrophil engraftment rates of 39%, 71% and 75%, respectively. The probability of 1-year overall survival after second HCT was 28% for UCB, 58% for mobilized PBSC, and 38% for BM grafts. Multivariate analysis showed that engraftment and survival were significantly better using PBSC for the salvage HCT.
We observed that patients with NGF undergoing 2nd HCT had compromised engraftment rates versus those patients with NNGF. Patients with NGF have prolonged bone marrow failure and are at high risk for infections, a major cause of their high morbidity and mortality[11]. We found that patients with NGF were likely to experience a second graft failure (36% in our cohort). This is very similar to earlier reports on severe aplastic anemia or malignant disease, in which up to 30 – 40% of patients have repeat graft failure after 2nd HCT[11, 12]. Patients with NNGF had better outcomes after 2nd HCT, most likely due to fewer days of neutropenia and reduced infection risk, as they frequently had a non-malignant diagnosis.
Earlier case series and reports have employed various chemotherapy regimens and donor sources, including haploidentical HCT, as salvage therapy after graft failure. Survival rates ranging from 36 – 80% are described[7, 9, 15, 27–30]. In our analysis, neither a donor type nor a specific conditioning regimen led to superior outcomes after the 2nd HCT, though this was confounded by the variability in diagnoses, conditioning regimens and graft sources used.
Finally, our data show that the majority of patients with NNGF after their first HCT can be salvaged with a second HCT, but the preferred graft source or conditioning regimen is still not determined as we saw no clear advantage to any particular regimen (though there were insufficient patient numbers to perform a multivariate analysis). Overall, most GF patients are spared the toxicity of a second fully ablative preparative regimen as a common practice and treated with a reduced intensity regimen, but strategies to determine an ideal conditioning regimen are needed recognizing that infection remains a major cause of death after second HCT with or without neutrophil recovery.
Figure 1. Incidence of neutrophil recovery after second HCT for graft failure.
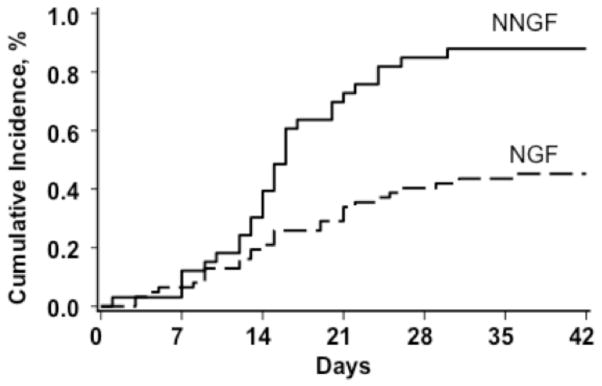
The incidence of neutrophil recovery for NNGF patients was 88% (95% CI, 74 – 96%) versus NGF patients at 45% (95% CI, 34 – 58%).
Figure 2. Overall survival at 1-year following second HCT.
For the entire cohort (top), the overall survival at 1-year was 44% (95% CI, 34–54%). The NNGF overall survival was 76% (95% CI, 57–87%) versus NGF at 27% (95% CI, 17–39%) shown in the bottom panel.
Acknowledgments
This work was supported in part by grants from the Children’s Cancer Research Fund and National Cancer Institute P01 CA65493.
Footnotes
Conflicts of Interest
No Author has any conflict of Interest
References
- 1.Passweg JR, Zhang MJ, Rocha V, et al. Donor characteristics affecting graft failure, graft-versus-host disease, and survival after unrelated donor transplantation with reduced-intensity conditioning for hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1869–1873. doi: 10.1016/j.bbmt.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satwani P, Jin Z, Duffy D, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19:552–561. doi: 10.1016/j.bbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Davies SM, Kollman C, Anasetti C, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood. 2000;96:4096–4102. [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page KM, Zhang L, Mendizabal A, et al. The Cord Blood Apgar: a novel scoring system to optimize selection of banked cord blood grafts for transplantation (CME) Transfusion. 2012;52:272–283. doi: 10.1111/j.1537-2995.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 7.Park JA, Koh KN, Choi ES, et al. Successful rescue of early graft failure in pediatric patients using T-cell-depleted haploidentical hematopoietic SCT. Bone Marrow Transplant. 2014;49:270–275. doi: 10.1038/bmt.2013.163. [DOI] [PubMed] [Google Scholar]
- 8.Waki F, Masuoka K, Fukuda T, et al. Feasibility of reduced-intensity cord blood transplantation as salvage therapy for graft failure: results of a nationwide survey of adult patients. Biol Blood Marrow Transplant. 2011;17:841–851. doi: 10.1016/j.bbmt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Kanda J, Horwitz ME, Long GD, et al. Outcomes of a 1-day nonmyeloablative salvage regimen for patients with primary graft failure after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumi M, Shimizu I, Sato K, et al. Graft failure in cord blood transplantation successfully treated with short-term reduced-intensity conditioning regimen and second allogeneic transplantation. International journal of hematology. 2010;92:744–750. doi: 10.1007/s12185-010-0714-6. [DOI] [PubMed] [Google Scholar]
- 11.Schriber J, Agovi MA, Ho V, et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transplant. 2010;16:1099–1106. doi: 10.1016/j.bbmt.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horan JT, Carreras J, Tarima S, et al. Risk factors affecting outcome of second HLA-matched sibling donor transplantations for graft failure in severe acquired aplastic anemia. Biol Blood Marrow Transplant. 2009;15:626–631. doi: 10.1016/j.bbmt.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arfons LM, Tomblyn M, Rocha V, et al. Second hematopoietic stem cell transplantation in myeloid malignancies. Curr Opin Hematol. 2009;16:112–123. doi: 10.1097/MOH.0b013e3283257a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan KW, Grimley MS, Taylor C, et al. Early identification and management of graft failure after unrelated cord blood transplantation. Bone Marrow Transplant. 2008;42:35–41. doi: 10.1038/bmt.2008.40. [DOI] [PubMed] [Google Scholar]
- 15.Byrne BJ, Horwitz M, Long GD, et al. Outcomes of a second non-myeloablative allogeneic stem cell transplantation following graft rejection. Bone Marrow Transplant. 2008;41:39–43. doi: 10.1038/sj.bmt.1705882. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes J, Rocha V, Robin M, et al. Second transplant with two unrelated cord blood units for early graft failure after haematopoietic stem cell transplantation. Br J Haematol. 2007;137:248–251. doi: 10.1111/j.1365-2141.2007.06562.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002;29:545–552. doi: 10.1038/sj.bmt.1703389. [DOI] [PubMed] [Google Scholar]
- 18.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone marrow transplantation. 2010;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 19.Dulery R, Salleron J, Dewilde A, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1080–1089. doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 20.Betts BC, Young JA, Ustun C, et al. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1562–1568. doi: 10.1016/j.bbmt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platzbecker U, Binder M, Schmid C, et al. Second donation of hematopoietic stem cells from unrelated donors for patients with relapse or graft failure after allogeneic transplantation. Haematologica. 2008;93:1276–1278. doi: 10.3324/haematol.12798. [DOI] [PubMed] [Google Scholar]
- 22.Michallet M, Tanguy ML, Socie G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de moelle (SFGM) Br J Haematol. 2000;108:400–407. doi: 10.1046/j.1365-2141.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 23.Gaziev J, Sodani P, Lucarelli G, et al. Second hematopoietic SCT in patients with thalassemia recurrence following rejection of the first graft. Bone Marrow Transplant. 2008;42:397–404. doi: 10.1038/bmt.2008.175. [DOI] [PubMed] [Google Scholar]
- 24.Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:537–543. doi: 10.1038/bmt.2012.239. [DOI] [PubMed] [Google Scholar]
- 25.Storb R, Prentice RL, Thomas ED, et al. Factors associated with graft rejection after HLA-identical marrow transplantation for aplastic anaemia. Br J Haematol. 1983;55:573–585. doi: 10.1111/j.1365-2141.1983.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuji S, Nakamura F, Hatanaka K, et al. Peripheral blood as a preferable source of stem cells for salvage transplantation in patients with graft failure after cord blood transplantation: a retrospective analysis of the registry data of the Japanese Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2012;18:1407–1414. doi: 10.1016/j.bbmt.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Moscardo F, Romero S, Sanz J, et al. T Cell-Depleted Related HLA-Mismatched Peripheral Blood Stem Cell Transplantation as Salvage Therapy for Graft Failure after Single Unit Unrelated Donor Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Tan YM, Fu HR, Luo Y, et al. Haploidentical allogeneic haematopoietic stem cell transplantation as salvage therapy for engraftment failure after unrelated and autologous stem cell transplantation: a case report and review of the literature. The Journal of international medical research. 2011;39:950–959. doi: 10.1177/147323001103900330. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino T, Sakura T, Miyawaki K, et al. Successful engraftment of a second transplant from unrelated cord blood identifying acceptable HLA Ag mismatches as treatment for primary graft failure possibly mediated by anti-HLA Abs after ‘mega-dose’ haploidentical PBSC transplantation. Bone Marrow Transplant. 2010;45:1665–1667. doi: 10.1038/bmt.2010.30. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed N, Leung KS, Rosenblatt H, et al. Successful treatment of stem cell graft failure in pediatric patients using a submyeloablative regimen of campath-1H and fludarabine. Biol Blood Marrow Transplant. 2008;14:1298–1304. doi: 10.1016/j.bbmt.2008.09.003. [DOI] [PubMed] [Google Scholar]