Abstract
Epidemiological studies report that arsenic exposure via drinking water adversely impacts cognitive development in children and, in adults, can lead to greater psychiatric disease susceptibility, among other conditions. While it is known that arsenic toxicity alters the epigenome, very few studies have investigated its effects on chromatin architecture in the brain. We have previously demonstrated that exposure to a low level of arsenic (50 ppb) during all three trimesters of fetal/neonatal development induces deficits in adult hippocampal neurogenesis in the dentate gyrus (DG), depressive-like symptoms, and alterations in gene expression in the adult mouse brain. As epigenetic processes control these outcomes, here we assess the impact of our developmental arsenic exposure (DAE) paradigm on global histone posttranslational modifications and expression of associated chromatin-modifying proteins in the dentate gyrus and frontal cortex (FC) of adult male and female mice. DAE influenced histone 3 K4 trimethylation with increased levels in the male DG and FC and decreased levels in the female DG (no change in female FC). The histone methyltransferase MLL exhibited a similar sex- and region- specific expression profile as H3K4me3 levels, while histone demethylase KDM5B expression trended in the opposite direction. DAE increased histone 3 K9 acetylation levels in the male DG along with histone acetyltransferase (HAT) expression of GCN5 and decreased H3K9ac levels in the male FC along with decreased HAT expression of GCN5 and PCAF. DAE decreased expression of histone deacetylase enzymes HDAC1 and HDAC2, which were concurrent with increased H3K9ac levels but only in the female DG. Levels of H3 and H3K9me3 were not influenced by DAE in either brain region of either sex. These findings suggest that exposure to a low, environmentally relevant level of arsenic during development induces alterations in the adult brain via histone modifications and chromatin modifiers a sex- and region-specific manner.
INTRODUCTION
Arsenic exposure is a worldwide health concern as several millions of people are exposed to this environmental toxicant via natural and anthropogenic sources each year (Naujokas et al., 2013). Efforts to minimize exposure have resulted in allowance of 10 μg/L (parts-per-billion, ppb) arsenic in water as stipulated by the Environmental Protection Agency (EPA) and World Health Organization (WHO); however, in several countries (including in the U.S. prior to 2006), 50 ppb arsenic remains the standard allotment (WHO, 2008). Additionally, there are places where access to drinking water containing arsenic within the WHO limits is simply not possible, and populations within these regions are exposed to excessive arsenic (in the parts-per-million range) resulting in damage to almost every organ system, including the brain (Jiang et al., 2013), (Bustaffa et al., 2014). Epidemiological studies have demonstrated that even low levels of arsenic exposure can negatively impact the body, including increasing the propensity toward developing psychiatric disorders and cognitive dysfunction (Zierold et al., 2004, Brinkel et al., 2009). Importantly, in utero and developmental arsenic exposure results in learning and memory deficits in children and may underlie long-lasting susceptibility to disease later in life (reviewed in (Tyler and Allan, 2014). However, relatively little is known about the long-term influence of low levels of arsenic exposure, particularly in the brain.
Research over the past decade has provided evidence that arsenic alters the epigenetic landscape in various cell types. The epigenome consists of DNA methylation and histone modifications that collectively constitute chromatin structure and ultimately chromatin function, conferring regulation of gene expression (Kouzarides, 2007). Of particular interest are studies on histone posttranslational modifications (HPTM), as histone modifications can be dynamic in response to the extrinsic environment and are paramount for proper neurogenesis and differentiation of neural stem cells in the brain (Hsieh and Eisch, 2010, Day and Sweatt, 2011). Indeed, epigenetic dysregulation of HPTMs has been postulated as a molecular mechanism underpinning psychiatric disorders such as depression (Mateus-Pinheiro et al., 2011, Sun et al., 2013). However, the impact of arsenic on the epigenetic status of the brain has not been thoroughly investigated, particularly in the context of developmental exposure. To date, there have been three studies on the effects of developmental exposure to arsenic in the brain, suggesting an impact of arsenic on histone acetylation and DNA methylation with concurrent deficits in proteins that may underlie learning and memory deficits (Zarazua et al., 2010, Martinez et al., 2011, Cronican et al., 2013). Conversely, the literature on the effect of arsenic on the epigenome is quite extensive in the context of cancer (Ray et al., 2014). In vitro studies have demonstrated arsenic exposure influences histone methylation, acetylation, and phosphorylation along with the protein expression of chromatin modifying enzymes that impart these modifications in human carcinoma cell lines (Zhou et al., 2008, Ren et al., 2011, Chervona et al., 2012a). Further, in vitro arsenic exposure in human PBMCs influences canonical H3 protein expression potentially underlying arsenic-induced sensitivity to DNA damage (Brocato et al., 2014). While very few epidemiological studies have assessed the effect of arsenic exposure (via drinking water) on the epigenome, those that have report a particularly strong correlation between arsenic exposure and altered histone methylation and acetylation, with a differential influence of arsenic dependent on sex (Chervona et al., 2012b). To our knowledge, there have been no reports on the long-term epigenetic consequences of developmental arsenic exposure in the brain, particularly of HPTMs and their associated chromatin modifying enzymes.
We are interested in the mechanisms that mediate the long-lasting toxicity of developmental arsenic exposure into adulthood when the presence of arsenic is quite low. Using a perinatal exposure paradigm to 50 ppb sodium arsenate through all three trimesters of fetal/neonatal development, we have previously demonstrated that arsenic reduces adult hippocampal neurogenesis, particularly differentiation of neural progenitor cells, leading to greater susceptibility to developing stress-induced depressive-like symptoms in adult male mice (Tyler and Allan, 2013, Tyler et al., 2014). Adult neurogenesis is comprised of several processes that include the continual mitotic activity of dentate gyrus neural progenitor cells and their subsequent maturation and integration into the hippocampal circuitry (Ming and Song, 2005). These processes are finely orchestrated by several epigenetic factors, including histone modifications (Ma et al., 2010). We have provided evidence that developmental arsenic exposure (DAE) alters the expression of many neurogenesis-related genes in the adult dentate gyrus (Tyler and Allan, 2013); however, the mechanism by which arsenic induces this damage is currently not known. Based on the extensive literature investigating the effects of arsenic on the epigenome in relation to cancer, we hypothesize that DAE leads to altered epigenetic processes, specifically histone modifications, in the adult mouse brain.
Here, we report an influence of DAE on levels of trimethylation of histone 3 lysine 4 (H3K4me3) and acetylation of histone 3 lysine 9 (H3K9ac) and on associated chromatin-modifying proteins including MLL, KDM5B, GCN5, PCAF, HDAC1, and HDAC2 in a sex- and region-dependent manner. No changes in levels of total H3 or H3K9me3 levels in either tissue of either sex were observed. This is the first report to demonstrate that low-level arsenic exposure during development influences the epigenetic landscape of the brain in adulthood long after arsenic exposure has ceased.
MATERIALS AND METHODS
Chemical Hazards
Arsenic is classified as a human co-carcinogen; all arsenicals were handled with caution in accordance with MSDS standards.
Developmental arsenic exposure paradigm
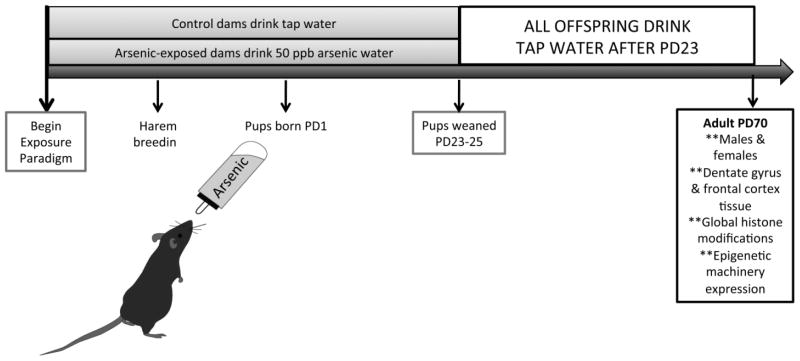
The Institutional Animal Care and Use Committee at the University of New Mexico (UNM) approved the animal protocols, including the arsenic exposure paradigm, used in this study. C57BL/6 mice obtained from Jackson Labs were maintained on a reverse light/dark cycle (lights off at 0800) with ad libitum access to food and water in the Animal Resource Facility at UNM. Arsenic exposure was performed as previously described (Tyler et al., 2014), as depicted in Figure 1. Briefly, singly-housed female mice aged 55 days were acclimated to drinking 50 parts-per-billion arsenic water (sodium arsenate, Sigma Aldrich) for 10 days prior to mating. Arsenic water was prepared weekly using standard tap and MilliQ water. Control mice were administered tap water from UNM, which contains approximately 2–5 ppb arsenic. Mating occurred for five days; dams continued to drink arsenic-laced water throughout pregnancy until offspring were weaned at postnatal day (PD) 23. Offspring were group housed separately by sex, four per cage, with ad libitum access to food and tap water. At PD70, animals were euthanized via rapid decapitation, and the frontal cortex and dentate gyrus from both male and female animals were microdissected and snap frozen and stored at −80°C until further analysis. Female animals were exposed to male bedding prior to euthanizing. Synchronicity of cycle was confirmed via vaginal cytology, and only brain tissue from females in the proestrus phase was used, as some biochemical and behavioral measures are impacted by the phase of the estrous cycle (Warren and Juraska, 1997). Multiple rounds of breeding were performed for sufficient numbers of litters for arsenic or control groups. For each experiment, at least 6 different litters from different dams were used; e.g. n = 6 represents the number of different litters used with one animal per litter to avoid litter effects.
Figure 1. Developmental Arsenic Exposure (DAE) Paradigm.
Exposure to 50 parts-per-billion arsenic via drinking water occurs throughout the perinatal period, which includes all three trimesters of development in rodents. The DAE paradigm is initiated 10 days prior to mating, and arsenic consumption is monitored. Control dams drink tap water from UNM, which contains approximately 2–5 ppb arsenic. Dams drink arsenic-laced water until pups are weaned at approximately postnatal day (PD) 23. Both control and arsenic-exposed offspring are group housed with ad libitum access to food and tap water; brain tissue is retrieved via microdissection at PD70 for analysis.
Histone extraction
For preparation of extracted histones, microdissected tissue derived from one animal, either the dentate gyrus or the frontal cortex, was homogenized in a Biomasher II disposable microhomogenizer (Kimble Chase) using PBS buffer containing 0.5% Triton-X 100 (v/v), 2 mM phenylmethylsulfonyl fluoride (PMSF), 0.02% (w/v) sodium azide (NaN3), 5 mM sodium butyrate (NaB), and 1 μg/μl protease inhibitor cocktail (Sigma, #P8340). Tissue homogenates were centrifuged at 6500 × g for 10 minutes at 4°C; nuclei were washed and centrifuged as before. The pellet was resuspended in 1 N HCl and acid extraction was allowed to occur overnight at 4°C; 1 N NaOH was added the following day to neutralize the acid. Lysates were centrifuged as before and the supernatant saved for protein quantification. Bradford assays were performed to determine the concentration of histone protein. Aliquots were stored at −80°C until further use.
Histone modification assessment
Approximately 8–12 μg histone protein, determined by antibody optimization, was separated using NUPAGE 10% bis-tris gels (Invitrogen, NP0316) and transferred to a PVDF membrane (Millipore Corporation, IPFL00010). Membranes were incubated overnight at 4°C using the following primary antibodies diluted in PBS-T: H3 (1:1000 for DG and FC; Cell Signaling, 3638), H3K4 trimethyl (1:1000 for DG and FC; Abcam, ab8580), H3K9 trimethyl (1:2000 for DG; 1:1000 for FC; Epigentek, A-4036), and H3K9 acetyl (1:500 for DG and FC; Epigentek, A-4022). Membranes were incubated for 45 min in their respective secondary antibodies (1:15,000) from LiCOR: rabbit IRDye 680RD and mouse IRDye 800CW. Quantification of protein expression was performed using Image Studio, and values are expressed as each individual histone mark normalized to H3 for individual immunoblots, after it was determined that H3 was not altered by DAE. Evaluation of each histone modification was performed on separate gels and blots to avoid confounds. The n = 6–10 litters depending on histone mark and will be expressed for each individual blot in the results.
Evaluation of chromatin modifying proteins
Immunoblotting for chromatin-modifying proteins was conducted essentially as previously described using our established protocols (Goggin et al., 2012, Tyler et al., 2014). Dentate gyrus or frontal cortex tissue lysates from one animal were prepared, and the nuclear fraction was isolated for protein expression analysis. Total protein concentration was determined by Bradford assay. Antibody and protein optimization was performed for each target protein in both types of tissues; Coomassie staining was demonstrated to be linear for all protein concentrations used. Membranes were incubated overnight at 4°C using the following primary antibodies diluted in PBS-T: MLL (1:1000 in DG, 1:500 in FC, Santa Cruz, sc-20153); KDM5B (1:500 in DG and FC, Abcam, ab-181089); GCN5 (1:000 in DG and FC, Santa Cruz, sc-20698); PCAF (1:000 in DG and FC, Santa Cruz, sc-13124); HDAC1 (1:500 in DG and FC, Active Motif 39531); and HDAC2 (1:500 in DG and FC, Santa Cruz, sc-81599). Membranes were washed, then incubated for 45 min in their respective secondary antibodies (1:15,000) from LiCOR: rabbit IRDye 680RD and mouse IRDye 800CW. Quantification of protein expression was performed using Image Studio version 3.1; all blots were normalized by Coomassie staining using IRDye® Blue Protein Stain (LI-COR; #3343C056) (Perrone-Bizzozero et al., 1996). Values are presented as protein expression normalized to Coomassie staining and renormalized to controls.
Rationale for selection of chromatin modifying proteins
At a conservative estimate, over 60 different residues in histone proteins have the potential for modification; and for some of these, there are several different enzymes each with the ability to impart the covalent modification (Kouzarides, 2007, Bannister and Kouzarides, 2011). This amounts to at least hundreds of different histone modifying proteins that can be responsible for the placement and removal of these groups. For each histone modification we found altered by arsenic exposure, we assessed the protein expression of a few modifiers that may be responsible for conferring or removing the modification. Selection was based on previous literature either 1) demonstrating the role of the modifier in depression or adult neurogenesis or 2) arsenic exposure directly impacting the modifier.
Histone methyltransferase proteins (HMT) have greater specificity than other chromatin modifiers as their catalytic domains, such as the SET domain for H3K4, recognize specific amino acids, and may allow for multiple methylated substrates to proceed from mono- to tri-methylated products (Bannister and Kouzarides, 2011). Of the several HMTs that impart three methyl groups on H3K4, mixed lineage leukemia factor (MLL1) is of particular interest as it is essential to proper neurogenesis (Lim et al., 2009), and we have demonstrated that our DAE paradigm inhibits adult hippocampal neurogenesis in the dentate gyrus (Tyler and Allan, 2013, Tyler et al., 2014). Additionally, the impact of arsenic on MLL expression has not been measured to date. Similarly, histone demethylase enzymes (HDM), including Jumonji-containing proteins, have been shown to be altered by toxin exposure (Chen et al., 2010); KDM5B (JARID1B), one of several histone demethylase enzymes responsible for removing methyl groups from H3K4, protects against aberrant H3K4me3 during development (Albert et al., 2013) and is involved in neuronal differentiation (Schmitz et al., 2011). Thus, we chose to assess both MLL and KDM5B expression in accordance with H3K4me3.
H3K9ac levels and associated histone acetyltransferase (HAT) and deacetylase (HDAC) protein expression was assessed. Of the GNAT, MYST, and CBP/p300 families (Bannister and Kouzarides, 2011) of HAT proteins, exposure to high concentrations of arsenic has been shown to alter expression of members of the GNAT family, specifically GCN5 (Nelson et al., 2009). In addition to GNC5, PCAF (p300/CREB associated factor in the CPB/p300 family) is also responsible for H3K9 acetylation. Members of the CBP/p300 family of histone acetyltransferases are paramount for proper learning and memory and have been implicated in the response to stress and depressive-like behaviors (Maurice et al., 2008). Additionally, members of the histone deacetylase families, including HDAC1 and HDAC2, have been implicated in depressive-like symptoms, with HDACi inhibitors not only alleviating these symptoms but also increasing learning and memory in several mouse models. Thus, in addition to assessment of H3K9ac, we measured expression of GCN5, PCAF, HDAC1, and HDAC2 in the brains of arsenic-exposed animals.
Statistical Analysis
H3 protein expression was determined to be similar among exposure groups in both tissue types and in both sexes and was normalized to Coomassie staining. Histone protein expression, measured via western blotting, was normalized to H3 protein expression for each sample, and each histone mark was run on a separate gel. Chromatin modifying protein expression was normalized to the average of total protein expression evaluated by Coomassie staining. All data are presented as mean ± SEM normalized to control values, and a p value of <.05 was set for statistical significance. All data were analyzed by Student’s t-test using GraphPad software (GraphPad Software, v. 6.0; San Diego, CA). The number of litters is reported for each experiment, with only one animal per litter used to avoid litter confounds. All studies contain at least three different breeding rounds per assay, with at least n = 6 per assay.
RESULTS
Validation of H3 for normalization of histone proteins
To validate histone 3 as a proper control, we assessed the impact of developmental arsenic exposure on H3 protein expression in dentate gyrus and frontal cortex tissue derived from adult male and female mice (PD70). We observed no effect of DAE on H3 protein expression (Supplementary Figure 1A: Male DG, t(11) = 0.46, p > .05; Female DG, t(11) = 0.30, p > .05, and Figure 1B: Male FC, t(10) = 0.26, p > .05; Female FC, t(12) = 0.75, p > .05). Previous studies have reported cleavage of histone 3 in both humans and several other species in multiple tissue types, particularly during mitotic activity (Sauve et al., 1999, Duncan et al., 2008, Howe and Gamble, 2015); thus, we assessed the impact of DAE on H3 cleavage for the 17 kDa and 15 kDa fragments (Supplemental Figure 2C, D). We observed cleavage of H3 in both control and arsenic exposed animals in both brain regions and both sexes; however, DAE had no impact on the amount of cleavage of H3 in the dentate gyrus (C) or frontal cortex (D) compared with controls. Thus, the 17 kDa H3 band expression was used to normalize expression for all subsequent histone modifications. (n = 6–7 litters per assay)
Developmental arsenic exposure does not influence methylation of H3K9me3
To determine the long-term epigenetic consequences of developmental arsenic exposure (Figure 1), we assessed histone methylation at postnatal day (PD) 70 in the dentate gyrus (DG) and the frontal cortex (FC) of both male and female mice. DAE did not alter levels of histone 3 lysine 9 trimethylation (H3K9me3), a repressive posttranslational modification, in either brain region of either sex (Figure 2A: Male DG, t(14) = 0.06, p > .05; Female DG, t(16) = 0.23, p > .05; Figure 3B: Male FC, t(14) = 1.6, p = .13; Female FC, t(12) = 0.54, p > .05). While there was a modest increase of H3K9me3 expression in the adult male frontal cortex, this was not significant. (n = 7–9 litters per assay; histone blots were normalized to H3; protein expression was normalized to Coomassie staining)
Figure 2. Developmental arsenic exposure does not impact histone 3 lysine 9 trimethylation (H3K9me3) levels in adult mouse brain.
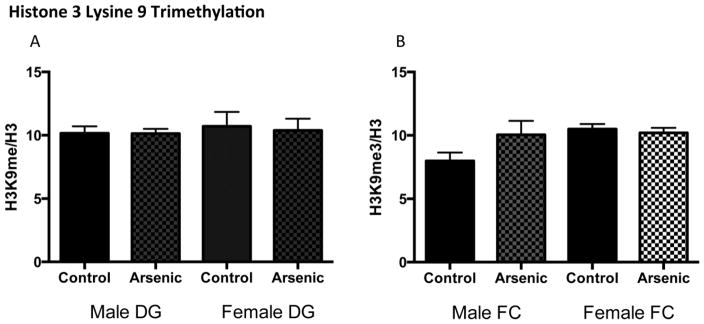
Developmental arsenic exposure does not significantly alter levels of trimethylation of H3K9 in (A) the dentate gyrus or (B) the frontal cortex of males or females. DG = dentate gyrus; FC = frontal cortex
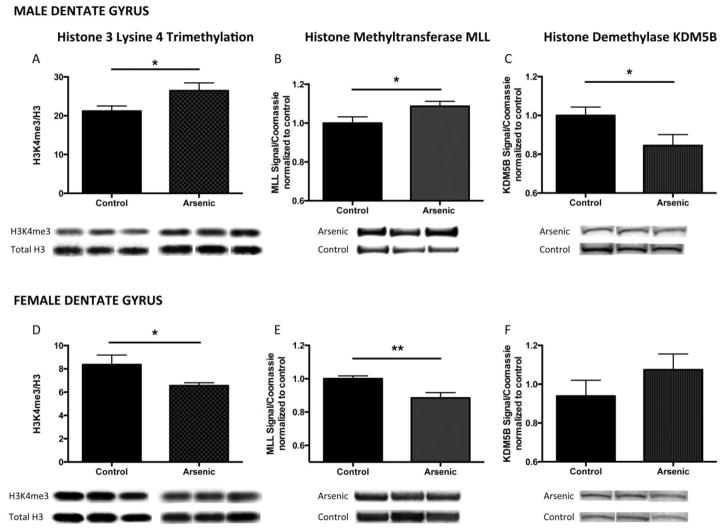
Figure 3. Developmental arsenic exposure alters trimethylation of histone 3 lysine 4 (H3K4me3) and its associated chromatin modifying proteins in a sex-specific manner in the adult dentate gyrus.
Developmental arsenic exposure significantly increases levels of H3K4me3 in (A) the adult male dentate gyrus with concurrent increased protein expression of (B) MLL, the histone methyltransferase responsible for trimethylation of H3K4, and decreased protein expression of (C) KDM5B, the demethylase that removes methyl groups from H3K4. Conversely, DAE significantly reduces levels of H3K4me3 in (D) the adult female dentate gyrus with concurrent decreased protein expression of (E) MLL, and no change, though an increased trend in (F) KDM5B. *p<.05; **p<.01
Trimethylation of H3K4 and associated chromatin-modifying enzymes in the dentate gyrus are impacted by developmental arsenic exposure based on sex
Developmental arsenic exposure differentially influenced the levels of histone 3 lysine 4 trimethylation (H3K4me3), an activating HPTM, in a sex-specific manner in adult mice (Figures 3 and 4). We observed increased expression levels of H3K4me3 in the dentate gyrus of adult male mice (Figure 3A: Male DG, t(14) = 2.24, p < .05) and decreased expression in the dentate gyrus of adult female mice (Figure 3D: Female DG, t(15) = 2.18, p < .05). To determine a potential mechanism by which this histone modification is impacted by arsenic exposure, the protein expression profiles of chromatin modifiers were assessed.
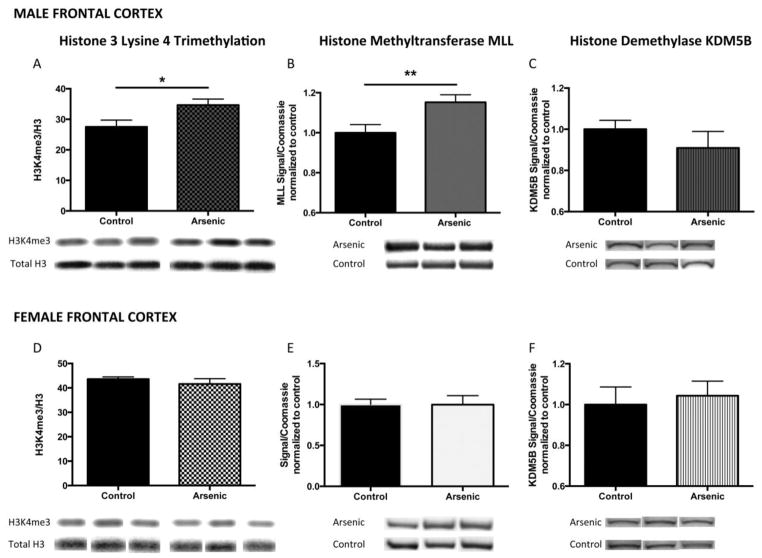
Figure 4. Developmental arsenic exposure alters trimethylation of histone 3 lysine 4 (H3K4me3) and its associated chromatin modifying proteins in the frontal cortex in a similar pattern as the dentate gyrus.
As observed in the dentate gyrus, developmental arsenic exposure significantly increases levels of H3K4me3 in (A) the adult male frontal cortex with concurrent increased protein expression of (B) MLL, the histone methyltransferase responsible for trimethylation of H3K4, and decreased protein expression of (C) KDM5B, the demethylase that removes methyl groups from H3K4. Unlike the observations in the dentate gyrus, DAE does not impact (D) H3K4me3 levels or protein expression of (E) MLL or (F) KDM5B in the adult female frontal cortex. *p<.05; **p<.01
Similar to the pattern of H3K4me3 levels in males and females, protein expression of MLL was increased in the male dentate gyrus (Figure 3B: Male DG, t(19) = 2.12, p < .05) and decreased in the female dentate gyrus (Figure 3E: Female DG, t(15) = 3.1, p < .01). Developmental arsenic exposure resulted in reduced protein expression of KDM5B in the male dentate gyrus (Figure 3C: Male DG, t(11) = 2.13, p < .05) but had no effect in the female dentate gyrus (Figure 3F: Female DG, t(15) = 1.17, p = .26). (n = 6–10 litters per assay)
Trimethylation of H3K4 in the frontal cortex of adult males and females
As observed in the male dentate gyrus, levels of H3K4me3 were increased in the male frontal cortex (Figure 4A: Male FC, t(14) = 2.41, p < .05) as was protein expression of MLL (Figure 4B: Male FC, t(22) = 2.73, p < .01), yet no change was observed in KDM5B protein expression (Figure 4C: Male FC, t(12) = 0.10, p = .34). Conversely, no changes in H3K4me3 levels nor its associated chromatin modifying proteins were observed in the female frontal cortex: H3K4me3 (Figure 4D: Female FC, t(12) = 0.84, p > .05), MLL (Figure 4E: Female FC, t(12) = 0.01, p > .05), and KDM5B (Figure 4F: Female FC, t(16) = 0.37, p > .05). (n = at least 9 litters per assay) A summary of the impact of DAE on H3K4me3 levels and protein expression of MLL and KDM5B in both brain regions of both sexes is shown in Figure 9. (n = 7–13 litters per assay)
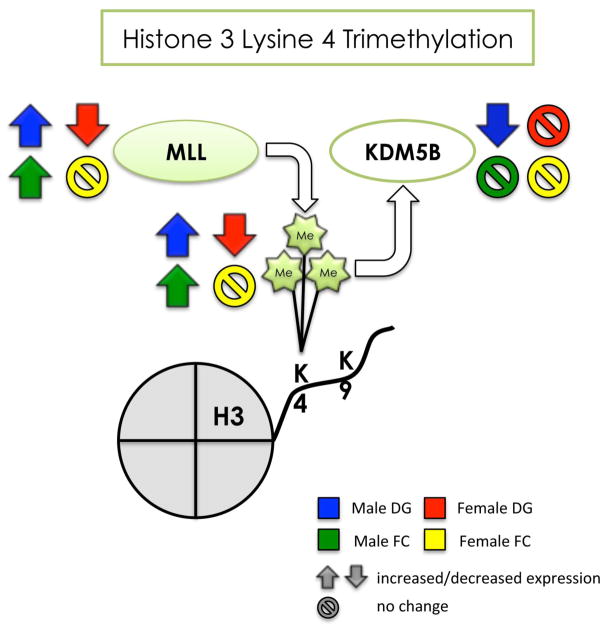
Figure 9. Summary of the effects of developmental arsenic exposure on trimethylation of H3K4 and its associated chromatin modifying proteins in the adult brain.
This figure displays the significant effects of DAE on levels of H3K4me3 (next to the Me groups) and protein expression of MLL (histone methyltransferase) and KDM5B (histone demethylase) in the adult brain. DAE impacts H3K4me3 levels and MLL protein expression in a similar manner in both tissue types of the male brain (blue and green arrows), suggesting increased transcriptional activation in the male brain. The effect of DAE in KDM5B is only apparent in the male dentate gyrus (blue), and inversely correlates with protein expression levels of MLL. DAE results in the opposite effect in the female brain, with decreased H3K4me3 and MLL protein expression (red arrows), but only in the dentate gyrus; the female frontal cortex is relatively unaffected by DAE (yellow). DAE impacts H3K4me3 primarily in a sex- specific manner with regional differences apparent in the female brain.
Up arrow = significantly increased expression
Down arrow = significantly decreased expression
Circle w/ line = no significant change in levels or expression
blue = male dentate gyrus; green = male frontal cortex red = female dentate gyrus; yellow = female frontal cortex
H3K9 acetylation in the dentate gyrus of adult males and females
Acetylation of histone 3 lysine 9 has been shown to be positively associated with transcriptionally active gene loci. As we observed increased levels of H3K4me3 in the adult male brain, we wanted to further assess the hypothesis that arsenic induces transcriptional activation in the adult male brain. Developmental arsenic exposure differentially impacted lysine 9 acetylation (H3K9ac) based on brain region and sex. H3K9ac levels in the male dentate gyrus were increased after DAE (Figure 5A: Male DG, t(13) = 2.22, p < .05), as was the protein expression of histone acetyltransferase, GCN5 (Figure 5B: Male DG, t(18) = 2.46, p < .05) with no impact on PCAF protein expression (Figure 5B: Male DG, t(23) = 0.41, p > .05). Additionally, HDAC1 and HDAC2 protein expression were not impacted by developmental arsenic exposure in the adult male dentate gyrus (Figure 5C: Male DG HDAC1, t(14) = 0.16, p > .05; HDAC2, t(15) = 0.16, p > .05). Conversely, in the female dentate gyrus, levels of H3K9ac were significantly decreased (Figure 6A: Female DG, t(20) = 6.88, p < .0001). Interestingly, we observed no change in GCN5 or PCAF protein expression in the female DG (Figure 6B: GCN5 Female DG, t(11) = 0.14, p > .05; PCAF t(12) = 1.45, p = .17); however, increased protein expression of both histone deacetylase enzymes was measured in the female dentate gyrus (Figure 6C: HDAC1 Female DG, t(11) = 2.78, p < .05; HDAC2 Female DG, t(11) = 2.27, p < .05). (n = 7–11 litters per assay)
Figure 5. Developmental arsenic exposure increases acetylation of histone 3 lysine 9 (H3K9ac) and protein expression of the histone acetyltransferase, GCN5, in the adult male dentate gyrus.
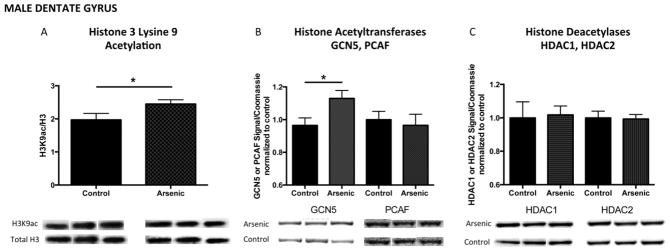
H3K4me3 is associated with histone acetylation for transcriptionally active genes. As observed with H3K4me3, developmental arsenic exposure significantly increases levels of H3K9ac in (A) the male dentate gyrus. DAE increases protein expression of histone acetyltransferase (B, left) GCN5 but not histone acetyltransferase (C, right) PCAF, both capable of acetylation of H3K9. Protein expression of histone deacetylases (C) HDAC1 and HDAC2 is not impacted by DAE in the male dentate gyrus. *p<.05; **p<.01
Figure 6. Developmental arsenic exposure differentially alters acetylation of histone 3 lysine 9 (H3K9ac) and associated chromatin modifying enzymes in the female dentate gyrus.
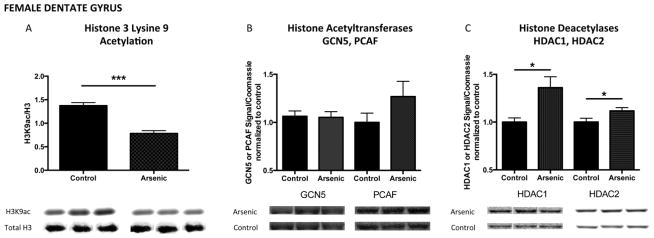
Similar to the differential impact of DAE observed for H3K4me3 among males and females, developmental arsenic exposure significantly decreases levels of acetylation of histone 3 lysine 9 (H3K9ac) in (A) the female dentate gyrus. Unlike the impact of DAE in the male dentate gyrus, no changes in protein expression of (B) GCN5 or PCAF are observed in the female dentate gyrus. However, DAE increases protein expression of histone deacetylases (C, left) HDAC1 and (C, right) HDAC2. *p<.05; **p<.01
H3K9 acetylation in the frontal cortex of adult males and females
As we previously observed increased H3K9ac in the male dentate gyrus (and both the DG and FC had increased H3K4me3), we expected to measure increased H3K9ac in the male frontal cortex. However, developmental arsenic exposure resulted in decreased H3K9ac levels in the male frontal cortex (Figure 7A: Male FC, t(14) = 2.47, p < .05), with associated decreased protein expression of GCN5 (Figure 7B: Male FC, t(20) = 2.28, p < .05) and decreased protein expression of PCAF (Figure 7B: Male FC, t(24) = 3.6, p < .01). Additionally, we observed a trend of decreased, but not significant changes in protein expression of HDAC1 (Figure 7C: Male FC, t(17) = 1.42, p = .17) or HDAC2 (Figure 7C: Male FC, t(16) = 1.49, p = .16) in the male frontal cortex after arsenic exposure. As observed with H3K4me3, MLL, and KDM5B protein expression, the female frontal cortex was exempt from developmental arsenic exposure toxicity. We observed no changes in H3K9ac levels (Figure 8A: Female FC, t(11) = 0.98, p > .05) nor protein expression of associated chromatin modifying enzymes GCN5 (Figure 8B: Female FC, t(12) = 0.34, p > .05) and PCAF (Figure 8B: Female FC, t(12) = 0.88, p > .05). However, we did observe a trend of increased, but not significant, histone deacetylase protein expression for HDAC1 (Figure 8C: Female FC, t(12) = 1.43, p = .18) but no change in HDAC2 protein expression (Figure 8C: Female FC, t(12) = 0.61, p = .55). This is the opposite trend observed in the male frontal cortex for histone deacetylase protein expression. (n = 7–13 litters per assay)
Figure 7. Developmental arsenic exposure differentially alters acetylation of histone 3 lysine 9 (H3K9ac) and its associated chromatin modifying proteins in the frontal cortex compared to the dentate gyrus.
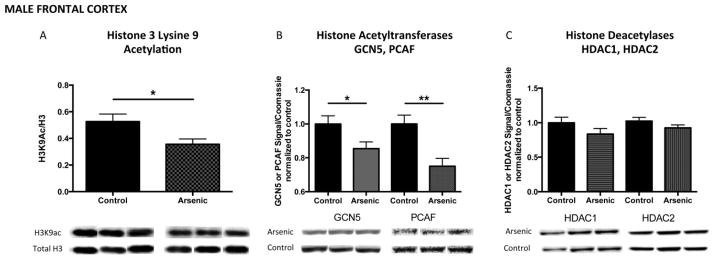
Unlike the pattern of H3K4me3 the male dentate gyrus and frontal cortex, DAE decreases levels of acetylation of H3K9 (H3K9ac) in (A) the adult male frontal cortex with concurrent decreased protein expression of (B, left) GCN5 and (B, right) PCAF, both histone acetyltransferases capable of H3K9 acetylation. Similar to observations in the male dentate gyrus, DAE does not impact (C) HDAC1 or HDAC2 protein expression in the male frontal cortex. *p<.05; **p<.01
Figure 8. Developmental arsenic exposure does not impact acetylation of histone 3 lysine 9 (H3K9ac) and associated chromatin modifying enzymes in the female frontal cortex.
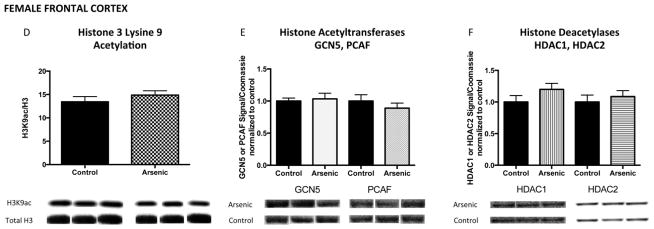
Similar to the lack of effect of DAE on H3K4me3 in the female frontal cortex, DAE does not impact acetylation of H3K9 (H3K9ac) in (A) the female frontal cortex. Additionally, protein expression levels of (B) GCN5 and PCAF and (C) HDAC1 and HDAC2 are not significantly altered by DAE in the female frontal cortex. *p<.05; **p<.01
DISCUSSION
While the impact of arsenic exposure on the epigenome has been extensively studied in the context of cancer research, the influence of this toxicant in the brain, particularly as it relates to epigenetics, is not well understood. Additionally, as the developing brain can be vulnerable to several insults, including arsenic, the genetic programs that are initiated, executed, and subsequently terminated via epigenetic processes may be sensitive as well (Calkins and Devaskar, 2011). Thus, determining the long-term impact of fetal exposures on the status of factors associated with epigenetic control of genetic programs could provide insight into how fetal programming leads to disease susceptibility in adulthood. This study provides the first step in demonstrating that exposure to 50 ppb arsenic, throughout all three trimesters of fetal/neonatal development, influences histone modifications and the protein expression of chromatin-modifying proteins in the adult mouse brain long after subjection to arsenic has diminished. Notably, the impact of this exposure paradigm is related to the type of histone modification (e.g. methylation or acetylation), the location of the modification (e.g. the dentate gyrus or frontal cortex), and the sex in which the modification is present (e.g. male or female arsenic-exposed offspring). Such specification of arsenic toxicity may shed light on its potential mechanisms of action in altering the adult brain.
We have previously shown that our arsenic exposure model alters expression of several genes associated with neurogenesis in the adult dentate gyrus (Tyler and Allan, 2013). The genetic programs involved in the processes of adult neurogenesis are highly orchestrated by epigenetic factors (Covic et al., 2010). In order to evaluate the regulation of neurogenesis, we sought to first demonstrate that arsenic impacts epigenetic processes in this brain region, possibly leading to aberrant gene expression. We chose to assess H3K4me3 and H3K9me3 levels, as both of these modifications are influenced by arsenic (Zhou et al., 2008) and each is present on either actively transcribed or repressed genes, respectively (Kouzarides, 2007). Surprisingly, we found no differences in H3K9me3 levels, as anticipated based on cell culture studies; potential reasons for this include the dose and timing of arsenic exposure or the cell type, as in vitro studies use direct application of high concentrations of arsenite on cancer cells (Chervona et al., 2012a, Hong and Bain, 2012). We did observe an increase in H3K4me3 levels in the dentate gyrus of adult males, and a decrease in levels in adult females in the same region; this is directly opposite of the expression patterns for this HPTM measured in PBMCs isolated from humans chronically exposed arsenic-contaminated drinking water (Chervona et al., 2012b). However, it should be noted that epigenetic modifications in the brain cannot be predicted based on assessment of the epigenetic landscape in other cell types; in keeping with other reports, results provided here demonstrate that tissue specificity is paramount of determining epigenetic mechanisms of toxicity (Begum et al., 2012). Additionally, while arsenic exposure seems to impact molecular biology across species in a similar manner in cancer studies (Wang et al., 2002), polymorphisms in factors that are responsible for metabolism of methyl groups can directly impact the epigenetic landscape (da Costa et al., 2006). Thus, it is possible that the genetic background of C57Bl/6 mice could confer specificity on arsenic metabolism, which directly impacts methyl donor production, and thus epigenetic processes.
To determine the potential mechanism by which arsenic alters histone modifications, we assessed the expression several chromatin-modifying proteins in the same brain regions in which we found altered histone modifications. We analyzed the protein expression of MLL, a histone methyltransferase responsible for trimethylation of H3K4 and KDM5B, a histone demethylase responsible for removing the trimethyl groups from H3K4 (Bannister and Kouzarides, 2011). Both of these modifiers have been shown to play a role in adult neurogenesis, for which we have previously reported arsenic impacts. We found increased protein expression of MLL in arsenic-exposed adult males and decreased expression in females (both in the dentate gyrus). As expected, KDM5B protein expression was inversely related to H3K4me3 levels: reduced in the male dentate gyrus and slightly, though not significantly, increased in the female dentate gyrus. MLL has been implicated in subventricular zone neurogenesis and mediates the conversion of silent bivalent domains to active domains containing H3K4me3 (Lim et al., 2009); thus, increased protein expression of MLL and H3K4me3 could be a compensatory mechanism to counteract the deleterious effects of arsenic, which are perpetuated into adulthood. Conversely, depletion of KDM5B has been associated with improper differentiation of stem cells, particularly those associated with neuronal lineage (Schmitz et al., 2011). These data (summarized in Figure 9) demonstrate that arsenic influences histone methylation likely due altered expression of the associated histone writer or eraser; this pattern is repeated throughout these studies in other brain regions.
H3K4 trimethylation has been associated with histone acetylation, creating crosstalk and a particular “histone code” for regulation of gene expression (Kouzarides, 2007, Jobe et al., 2012). As observed with H3K4me3, DAE increased levels of H3K9 acetylation in the male dentate gyrus while decreasing levels in the female dentate gyrus (summarized in Figure 10). This was unexpected, as human studies have demonstrated that arsenic exposure is inversely correlated with H3K9ac levels in blood with no effect based on sex (Chervona et al., 2012b); however, another study demonstrated that arsenic exposure was directly correlated to H3K9ac levels in leukocytes (Cantone et al., 2011). Additionally, increased acetylation occurs in response to oxidative stress induced by arsenic exposure in vitro; as such, the expression profile of H3K9ac in the dentate gyrus may be a residual response to arsenic toxicity during the embryonic period, though this has yet to be assessed (Li et al., 2001, Sun et al., 2009). GNC5 and PCAF are histone acetyltransferase enzymes found in multi-subunit mammalian complexes, SAGA (STAGA, TFTC) and ATAC, and either complex can use either protein as the acetyltransferase for particular acetyl modifications in vitro (Wang et al., 2008). Conditional deletion of GCN5/PCAF together significantly reduces H3K9ac (Jin et al., 2011); thus, we assessed the protein expression of both GCN5 and PCAF to determine their correlation to H3K9ac levels. Increased H3K9ac in the male dentate gyrus was concurrent with increased GCN5, similar to previously observed effects of arsenic on GCN5 (Nelson et al., 2009) with no effect of arsenic on PCAF. Additionally, no significant differences of either protein were found in the female dentate gyrus. As with histone demethylase proteins, histone deacetylase proteins can impact the levels of histone modifications. Class I histone deacetylase 1 and 2 (HDAC1, HDAC2) are paramount for proper neuronal development (Montgomery et al., 2009) and are both able to remove the acetyl group from H3K9ac. Interestingly, we found no differences in HDAC1 or HDAC2 protein expression in the male dentate gyrus, suggesting that aberrant H3K9ac levels after DAE are likely due to increased GCN5 protein expression in males. However, in the female dentate gyrus, both HDAC1 and HDAC2 protein expression were increased, suggesting that arsenic-induced decreases in H3K9ac in the females may be mediated through HDACs. This is the first study to assess the impact of arsenic on HDAC1 and HDAC2; a previous study demonstrated inhibition of HDAC activity after 5 μM arsenic exposure in vitro (Qu et al., 2012), suggesting that the effects we observe here could be compensatory for developmental arsenic exposure. The effects of arsenic on H3K9ac in the male and female dentate gyrus are summarized in Figure 10; as with H3K4me3, arsenic impacts H3K9ac and its associated chromatin modifying enzymes based on sex, with increased levels of the modification in the males and decreased levels in the females. Additionally, DAE is preferentially impacting HAT protein expression in males and HDAC protein expression in females. This pattern of opposite epigenetic profiles between the sexes has been observed in other arsenic toxicity studies (Chervona et al., 2012b, Bailey and Fry, 2014), though this is the first to observe sex differences in histone modifications in the brain resulting from developmental arsenic exposure.
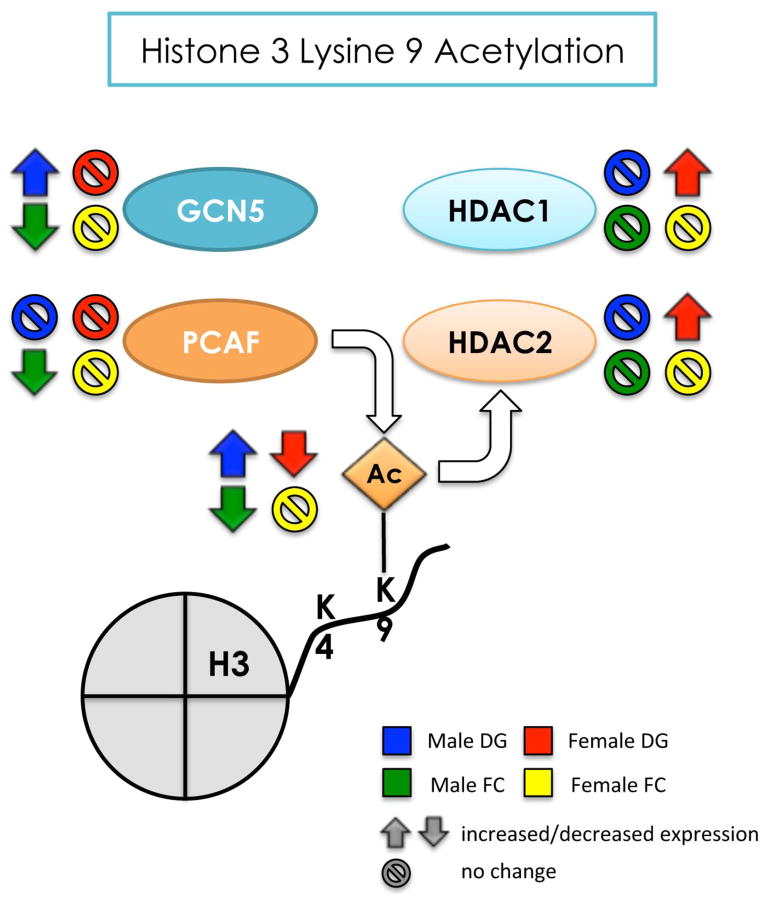
Figure 10. Summary of the effects of developmental arsenic exposure on acetylation of H3K9 and its associated chromatin modifying proteins in the adult brain.
This figure displays the significant effects of DAE on levels of H3K9ac (next to the Ac group) and protein expression of GCN5 and PCAF (histone acetyltranferases) and HDAC1 and HDAC2 (histone deacetylases) in the adult brain. DAE has a differential impact on H3K9ac levels and histone acetyltransferase protein expression in the male brain: DAE increases levels of H3K9ac in the male dentate gyrus likely due to increases in GCN5 (blue) and decreased H3K9ac in the frontal cortex, likely due to both decreased GCN5 and PCAF (green). As observed with H3K4me3, DAE results in the opposite patterns of expression in the female dentate gyrus for H3K9ac and associated chromatin modifying proteins compared to the male dentate gyrus. DAE decreases H3K9ac in the female DG like due to increased protein expression of HDAC1 and HDAC2 (red arrows) with no impact on GCN5 or PCAF. Interestingly, DAE preferentially alters histone deacetylase protein expression in females and histone acetyltransferase protein expression in males, with levels of H3K9ac affected by DAE dependent on both region and sex.
Up arrow = significantly increased expression
Down arrow = significantly decreased expression
Circle w/ line = no significant change in levels or expression
blue = male dentate gyrus; green = male frontal cortex
red = female dentate gyrus; yellow = female frontal cortex
Arsenic can accumulate in particular brain regions, including the hippocampus (in which the dentate gyrus is located) and the frontal cortex (Sanchez-Pena et al., 2010). To demonstrate region specificity of arsenic toxicity for the dentate gyrus, we assessed histone modifications in the frontal cortex of both males and females. Additionally, the frontal cortex has been implicated in the pathophysiology of depression, and we have previously shown our model of arsenic exposure induces depressive-like behaviors in adulthood (Martinez et al., 2008, Tyler et al., 2014). Thus, in the male frontal cortex, we observed increased levels of both H3K4me3 and associated MLL, similar to the pattern observed in the male dentate gyrus, though KDM5B protein expression was only slightly decreased. MLL has been found in GABAergic and cortical neurons, and remodeling mechanisms in the frontal cortex that involve both H3K4me3 and MLL have been implicated in the etiology of schizophrenia (Huang et al., 2007). However, the role of MLL and H3K4me3 in the frontal cortex, especially as it pertains to depression and metal toxicity like arsenic, is unknown. Opposite expression profiles of H3K9ac were observed in the male frontal cortex compared to the male dentate gyrus: arsenic exposure resulted in decreased H3K9ac levels in the male frontal cortex, along with significantly decreased protein expression of both GCN5 and PCAF, with no effect on HDAC1 or HDAC2. Aberrant epigenetic processes have been implicated in the etiology of psychiatric disorders, such as depression, but have been specific to measurements in the hippocampus and the amygdala (Sun et al., 2013). While the frontal cortex and hippocampus are part of a cooperative neural network, they subserve different processes associated with memory, plasticity, and depression; as each region is comprised of different types of neurons, it’s likely that each has a unique epigenetic profile to drive the specific roles they play in cognition. Thus, it is possible that concurrent decreased acetylation in the frontal cortex and increased levels in the dentate gyrus of the hippocampus may be one molecular mechanism by which arsenic increases susceptibility to depression.
Sex-dependent changes in molecular components, including histone modifications, in response to arsenic exposure, have previously been demonstrated. Here, we report that developmental arsenic exposure influenced acetylation in a sex-specific manner similar to that of methylation, with no effect of arsenic on H3K4me3 or H3K9ac levels in the female frontal cortex. Protein expression of all sets of modifiers, including MLL and KDM5B, GCN5 and PCAF, and HDAC1 and HDAC2 mirrored that of their respective histone modifications, with no change in the female frontal cortex. It is possible that this brain region is somehow protected in females from the deleterious effects of arsenic, as observed in other studies concerning developmental toxicity (Faulk et al., 2013, Kundakovic et al., 2013, Tyler and Allan, 2014). In humans exposed to low to moderate levels of arsenic (50–500 ppb), urinary arsenic levels are correlated with high levels of H3K4me3 and H3K27me3 in peripheral blood mononuclear cells from females, but the reverse pattern is present in males (Chervona et al., 2012b). In utero arsenic exposure alters global DNA methylation profiles in cord blood with a greater influence in males than females (Broberg et al., 2014). Indeed, many of the changes that we observed using our developmental arsenic exposure paradigm have been demonstrated in males (Martinez-Finley et al., 2011, Goggin et al., 2012). Finally, several sex-dependent alterations in cognition and intelligence measures have been observed among males and females, suggesting that arsenic may disrupt endocrine function, possibly mediating differences observed in these studies (Vahter, 2009, Hughes et al., 2011, Kundakovic et al., 2013).
In conclusion, we have provided evidence suggesting that perinatal arsenic exposure impacts histone modifications in the brain during adulthood. We have shown that levels of H3K4me3 and H3K9ac are altered in a region- and sex-specific manner and that the chromatin modifying proteins responsible for these modifications also have altered expression profiles that parallel histone modification levels. Additionally, we observed sex-specific influences of arsenic that are antithetical to prior observations in human studies, likely due to cell type (brain versus blood) in which histone modifications were measured and the extent of arsenic exposure. Indeed, we did not observe changes in many histone modifications, including H3K9me3, as expected, suggesting that the timing and dose of exposure used in cell culture studies as compared to our in vivo studies plays a role in the damage arsenic elicits. In the dentate gyrus, arsenic may be activating a transcriptional program by increasing the levels of H3K4me3 and H3K9ac histone modifications, along with the protein expression of their chromatin modifiers. Importantly, results presented here demonstrate region specificity and suggest a new avenue of research for arsenic toxicity: epigenetic regulation of frontal cortex functionality. Overall, these findings are the first step in analyzing the effect of developmental arsenic exposure on epigenetic programming of gene expression in the brain; further studies will evaluate the impact of histone modifications on locus-specific gene expression and the potential aberrant fetal programming of these epigenetic modifications that appear to be perpetuated into adulthood.
Supplementary Material
To establish validity as a control for immunoblotting, H3 levels were assessed in PD70 control and arsenic-exposed male and female offspring in both tissue types. DAE does not alter the protein expression levels of total histone protein 3 (at 17 kDa) in the (A) dentate gyrus or the (B) frontal cortex of either sex and can be used to normalize other histone modifications localized to histone 3. Additionally, H3 cleavage can occur in embryonic stem cells in mouse and other species; thus, we assessed if arsenic impacted levels of the 17 kDa and 15 kDa fragments of H3. Arsenic exposure has no impact on cleavage of H3 in the dentate gyrus (C) nor frontal cortex (D). Representative immunoblots showing cleavage products of H3 in both brain regions are below graphs. DG = dentate gyrus, FC = frontal cortex
Separate antibody validation studies were performed for each antibody used in the immunoblotting analysis. As most histone antibodies provide information for modifications present at the 17 kDa band for H3, all the histone modifications were assessed on separate gels (i.e. one gel for H3K9me3, one gel for H3K9ac, etc). A representative image depicts the ladder (left, in red with molecular weights) and H3K9me3 (yellow) and H3 (green) in the male frontal cortex to demonstrate typical cross-reactivity of antibody binding; Co = control samples; As = developmental arsenic exposure samples.
Research Highlights.
Brain tissue from adult mice with developmental arsenic exposure (DAE) was used
DAE impacted histone methylation and associated methyltransferases based on sex
DAE differentially altered histone acetylation based on brain region
DAE altered HATs in males and HDACs in females
Epigenetic modifier expression correlated with the associated histone modification
Acknowledgments
FUNDING
This work was supported by grants from the National Institute of Environmental Health Sciences [RO1ES019583 to AMA], the National Institute of Mental Health [F31 101984 to CRT], and the Pilot Project Award from the UNM HSC Environmental Health Signature Program [AMA & CRT].
We thank Dr. Daniel Purcell, Dr. Mary Ann Osley, and Samantha Goggin for assistance, helpful discussions, and critical reading of the manuscript. We thank Dr. Russell Morton for assistance with illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert M, Schmitz SU, Kooistra SM, Malatesta M, Morales Torres C, Rekling JC, Johansen JV, Abarrategui I, Helin K. The histone demethylase Jarid1b ensures faithful mouse development by protecting developmental genes from aberrant H3K4me3. PLoS genetics. 2013;9:e1003461. doi: 10.1371/journal.pgen.1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KA, Fry RC. Arsenic-Associated Changes to the Epigenome: What Are the Functional Consequences? Current environmental health reports. 2014;1:22–34. doi: 10.1007/s40572-013-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell research. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum G, Stevens A, Smith EB, Connor K, Challis JR, Bloomfield F, White A. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1694–1703. doi: 10.1096/fj.11-198762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkel J, Khan MH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. International journal of environmental research and public health. 2009;6:1609–1619. doi: 10.3390/ijerph6051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg K, Ahmed S, Engstrom K, Hossain MB, Jurkovic Mlakar S, Bottai M, Grander M, Raqib R, Vahter M. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. Journal of developmental origins of health and disease. 2014;5:288–298. doi: 10.1017/S2040174414000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocato J, Fang L, Chervona Y, Chen D, Kiok K, Sun H, Tseng HC, Xu D, Shamy M, Jin C, Costa M. Arsenic induces polyadenylation of canonical histone mRNA by down-regulating stem-loop-binding protein gene expression. The Journal of biological chemistry. 2014;289:31751–31764. doi: 10.1074/jbc.M114.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustaffa E, Stoccoro A, Bianchi F, Migliore L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Archives of toxicology. 2014;88:1043–1067. doi: 10.1007/s00204-014-1233-7. [DOI] [PubMed] [Google Scholar]
- Calkins K, Devaskar SU. Fetal origins of adult disease. Current problems in pediatric and adolescent health care. 2011;41:158–176. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantone L, Nordio F, Hou L, Apostoli P, Bonzini M, Tarantini L, Angelici L, Bollati V, Zanobetti A, Schwartz J, Bertazzi PA, Baccarelli A. Inhalable metal-rich air particles and histone H3K4 dimethylation and H3K9 acetylation in a cross-sectional study of steel workers. Environmental health perspectives. 2011;119:964–969. doi: 10.1289/ehp.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kluz T, Zhang R, Costa M. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis. 2010;31:2136–2144. doi: 10.1093/carcin/bgq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervona Y, Arita A, Costa M. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics : integrated biometal science. 2012a;4:619–627. doi: 10.1039/c2mt20033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervona Y, Hall MN, Arita A, Wu F, Sun H, Tseng HC, Ali E, Uddin MN, Liu X, Zoroddu MA, Gamble MV, Costa M. Association Between Arsenic Exposure and Global Post-translational Histone Modifications Among Adults in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2012b doi: 10.1158/1055-9965.EPI-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic M, Karaca E, Lie DC. Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity (Edinb) 2010;105:122–134. doi: 10.1038/hdy.2010.27. [DOI] [PubMed] [Google Scholar]
- Cronican AA, Fitz NF, Carter A, Saleem M, Shiva S, Barchowsky A, Koldamova R, Schug J, Lefterov I. Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. PloS one. 2013;8:e53478. doi: 10.1371/journal.pone.0053478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk C, Barks A, Liu K, Goodrich JM, Dolinoy DC. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics. 2013;5:487–500. doi: 10.2217/epi.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–1345. doi: 10.1016/j.neuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong GM, Bain LJ. Sodium arsenite represses the expression of myogenin in C2C12 mouse myoblast cells through histone modifications and altered expression of Ezh2, Glp, and Igf-1. Toxicology and applied pharmacology. 2012;260:250–259. doi: 10.1016/j.taap.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG, Gamble MV. Enzymatic cleavage of histone H3: a new consideration when measuring histone modifications in human samples. Clinical epigenetics. 2015;7:7. doi: 10.1186/s13148-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiology of disease. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicological sciences : an official journal of the Society of Toxicology. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JQ, Ashekuzzaman SM, Jiang A, Sharifuzzaman SM, Chowdhury SR. Arsenic contaminated groundwater and its treatment options in Bangladesh. International journal of environmental research and public health. 2013;10:18–46. doi: 10.3390/ijerph10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, Wang C, Brindle PK, Dent SY, Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. The EMBO journal. 2011;30:249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe EM, McQuate AL, Zhao X. Crosstalk among Epigenetic Pathways Regulates Neurogenesis. Frontiers in neuroscience. 2012;6:59. doi: 10.3389/fnins.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Molecular and cellular biology. 2001;21:8213–8224. doi: 10.1128/MCB.21.23.8213-8224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nature neuroscience. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–655. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L, Jimenez V, Garcia-Sepulveda C, Ceballos F, Delgado JM, Nino-Moreno P, Doniz L, Saavedra-Alanis V, Castillo CG, Santoyo ME, Gonzalez-Amaro R, Jimenez-Capdeville ME. Impact of early developmental arsenic exposure on promotor CpG-island methylation of genes involved in neuronal plasticity. Neurochem Int. 2011;58:574–581. doi: 10.1016/j.neuint.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Goggin SL, Labrecque MT, Allan AM. Reduced expression of MAPK/ERK genes in perinatal arsenic-exposed offspring induced by glucocorticoid receptor deficits. Neurotoxicology and teratology. 2011;33:530–537. doi: 10.1016/j.ntt.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus-Pinheiro A, Pinto L, Sousa N. Epigenetic (de)regulation of adult hippocampal neurogenesis: implications for depression. Clinical epigenetics. 2011;3:5. doi: 10.1186/1868-7083-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Celerier A, Jacquet C, Copois V, Mechti N, Ozato K, Gongora C. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annual review of neuroscience. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental health perspectives. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM, Ahlborn GJ, Allen JW, Ren H, Corton JC, Waalkes MP, Kitchin KT, Diwan BA, Knapp G, Delker DA. Transcriptional changes associated with reduced spontaneous liver tumor incidence in mice chronically exposed to high dose arsenic. Toxicology. 2009;266:6–15. doi: 10.1016/j.tox.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14182–14187. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Du J, Zhang C, Fu W, Xi H, Zou J, Hou J. Arsenic trioxide exerts antimyeloma effects by inhibiting activity in the cytoplasmic substrates of histone deacetylase 6. PloS one. 2012;7:e32215. doi: 10.1371/journal.pone.0032215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environmental health perspectives. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pena LC, Petrosyan P, Morales M, Gonzalez NB, Gutierrez-Ospina G, Del Razo LM, Gonsebatt ME. Arsenic species, AS3MT amount, and AS3MT gene expression in different brain regions of mouse exposed to arsenite. Environmental research. 2010;110:428–434. doi: 10.1016/j.envres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Sauve DM, Anderson HJ, Ray JM, James WM, Roberge M. Phosphorylation-induced rearrangement of the histone H3 NH2-terminal domain during mitotic chromosome condensation. The Journal of cell biology. 1999;145:225–235. doi: 10.1083/jcb.145.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz SU, Albert M, Malatesta M, Morey L, Johansen JV, Bak M, Tommerup N, Abarrategui I, Helin K. Jarid1b targets genes regulating development and is involved in neural differentiation. The EMBO journal. 2011;30:4586–4600. doi: 10.1038/emboj.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Molecular and cellular biology. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. Adult hippocampal neurogenesis and mRNA expression are altered by perinatal arsenic exposure in mice and restored by brief exposure to enrichment. PloS one. 2013;8:e73720. doi: 10.1371/journal.pone.0073720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Current environmental health reports. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Solomon BR, Ulibarri AL, Allan AM. Fluoxetine treatment ameliorates depression induced by perinatal arsenic exposure via a neurogenic mechanism. Neurotoxicology. 2014;44:98–109. doi: 10.1016/j.neuro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Effects of arsenic on maternal and fetal health. Annual review of nutrition. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- Wang JP, Qi L, Moore MR, Ng JC. A review of animal models for the study of arsenic carcinogenesis. Toxicology letters. 2002;133:17–31. doi: 10.1016/s0378-4274(02)00086-3. [DOI] [PubMed] [Google Scholar]
- Wang YL, Faiola F, Xu M, Pan S, Martinez E. Human ATAC Is a GCN5/PCAF-containing acetylase complex with a novel NC2-like histone fold module that interacts with the TATA-binding protein. The Journal of biological chemistry. 2008;283:33808–33815. doi: 10.1074/jbc.M806936200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behavioral neuroscience. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Zarazua S, Rios R, Delgado JM, Santoyo ME, Ortiz-Perez D, Jimenez-Capdeville ME. Decreased arginine methylation and myelin alterations in arsenic exposed rats. Neurotoxicology. 2010;31:94–100. doi: 10.1016/j.neuro.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94:1936–1937. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To establish validity as a control for immunoblotting, H3 levels were assessed in PD70 control and arsenic-exposed male and female offspring in both tissue types. DAE does not alter the protein expression levels of total histone protein 3 (at 17 kDa) in the (A) dentate gyrus or the (B) frontal cortex of either sex and can be used to normalize other histone modifications localized to histone 3. Additionally, H3 cleavage can occur in embryonic stem cells in mouse and other species; thus, we assessed if arsenic impacted levels of the 17 kDa and 15 kDa fragments of H3. Arsenic exposure has no impact on cleavage of H3 in the dentate gyrus (C) nor frontal cortex (D). Representative immunoblots showing cleavage products of H3 in both brain regions are below graphs. DG = dentate gyrus, FC = frontal cortex
Separate antibody validation studies were performed for each antibody used in the immunoblotting analysis. As most histone antibodies provide information for modifications present at the 17 kDa band for H3, all the histone modifications were assessed on separate gels (i.e. one gel for H3K9me3, one gel for H3K9ac, etc). A representative image depicts the ladder (left, in red with molecular weights) and H3K9me3 (yellow) and H3 (green) in the male frontal cortex to demonstrate typical cross-reactivity of antibody binding; Co = control samples; As = developmental arsenic exposure samples.