Abstract
Scope
Only a few studies analyzed the role of allium vegetables with reference to head and neck cancers (HNC), with mixed results.
We investigated the potential favorable role of garlic and onion within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium.
Methods and results
We analyzed pooled individual-level data from eight case-control studies, including 4590 cases and 7082 controls. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for associations between garlic and onion intakes and HNC risk. Compared with no or low garlic use, the ORs of HNC were 0.95 (95% CI 0.71–1.27) for intermediate and 0.74 (95% CI 0.55–0.99) for high garlic use (p for trend= 0.02). The ORs of HNC for increasing categories of onion intake were 0.91 (95% CI 0.68–1.21) for >1 to ≤3 portions per week, and 0.83 (95% CI 0.60–1.13) for >3 portions per week (p for trend= 0.02), as compared to <1 portion per week. We found an inverse association between high onion intake and laryngeal cancer risk (OR=0.69; 95% CI 0.54–0.88), but no significant association for other subsites.
Conclusions
The results of this pooled-analysis support a possible moderate inverse association between garlic and onion intake and HNC risk.
Keywords: Head and Neck Neoplasms, Allium vegetables, onion, garlic, diet
1) Introduction
Head and neck cancer (HNC) is the sixth most common cancer in the world [1]. The major recognized risk factors for HNC are tobacco and alcohol, with 5–10-fold higher risks for smokers as compared with nonsmokers [2] and for heavy drinkers as compared with abstainers or moderate drinkers [3–5]. In the International Head and Neck Cancer Epidemiology (INHANCE) database, the odds ratios (OR) for the highest alcohol drinking level (≥200 drinks-years versus 0) were 3.2 for oral cavity, 2.0 for pharyngeal and 1.8 for laryngeal cancer, and those for the highest smoking level (≥60 pack-years versus 0) were 10.1, 4.1 and 21.7, respectively [6]. The combined effect of heavy drinking and smoking led to an OR of 14.2 [7]. Thus, stopping drinking and smoking in the key factor to control HNC [8].
Selected other risk factors, including family history [9], human papillomavirus (HPV) infection for oropharyngeal cancer [10], and dietary and nutritional habits appear to play a role in the aetiology of these neoplasms [11]. The most convincing evidence on diet is a protective effect of non-starchy vegetables and fruit [12]. However, it is not yet clear which families of fruit or vegetables may exert a protective effect against HNC risk [13–16]. Previous studies reported that allium vegetables, in particular the most popular Allium sativum (garlic) and Allium cepa (onion), may have antioxidant and other anticancer properties [17, 18].
Several epidemiological investigations have suggested an inverse relation between allium vegetables intake and the risk of some cancers [19], in particular those of the digestive tract [20–23]. With reference to HNC, only a few studies analyzed the role of allium vegetables, with mixed results. Five population based case-control studies were conducted in China. Those found a possible decrease in risk of cancer at selected anatomical sites of HNC for high allium vegetable intake [24–28]. Another case-control study, conducted in Uruguay, reported an inverse association between onion consumption and laryngeal cancer risk [29]. However, the EPIC cohort study, based on 352 HNC and esophageal cancer cases, found no association [30].
The INHANCE consortium is a collaboration of epidemiological studies of HNC with the aim to improve the understanding of the causes and mechanisms of HNC [31]. To date, the consortium includes more than 30 studies conducted worldwide, in areas with different smoking and drinking prevalence, and dietary patterns. The role of diet has been investigated using data from 22 studies with more than 14,500 cases and 22,000 controls [15]. The main result was that a diet rich in fresh vegetables and fruit and poor in red and processed meat was associated with a reduced HNC risk. In particular, a high intake of allium vegetables was associated with a reduction in HNC risk by 34% (OR for the highest versus the lowest quartile of intake = 0.66; based on 8 studies).
The aim of this report is to investigate more deeply the potential favorable role of the most consumed types of allium vegetables, i.e., garlic and onion. Thus, we analyzed them separately, in consideration of their different anticancer properties [32, 33], we considered their role according to subgroups of anatomical sites, and we examined the potential confounding/modifying effect of smoking and alcohol. The INHANCE dataset represents a unique opportunity to investigate this issue, since it includes patient-level data on allium vegetables consumption on more than 4500 cases and 7000 controls.
2) Materials and methods
Studies and participants
The INHANCE consortium was established in 2004 and to date includes 35 HNC case-control studies providing data on 25,478 cases and 37,111 controls [31]. Cases included patients with histologically confirmed invasive tumors of the oral cavity, oropharynx, hypopharynx, larynx, oral cavity or pharynx not otherwise specified (NOS) or overlapping, as previously defined [34, 35]. Details on the case-control studies, harmonizing questionnaire data and data pooling methods for the INHANCE consortium have been previously described [31, 35]. All studies were performed according to the Declaration of Helsinki and were approved by the local ethics committees, according to the legislations of each country at the time of study conduction. Informed consent was obtained from all study participants.
Of the 35 epidemiological studies, 10 studies had some information on garlic and/or onion intakes and thus were included in this investigation. However, two studies among the ten were excluded for the following reasons: a) a study from France (1987–1992) provided only data on leek consumption and not on garlic or onion intake [36]; b) a study from Buffalo, US, provided more than 50% missing values on garlic and onion intake [37]. Therefore, eight studies providing information on garlic and/or garlic intakes were included in this investigation, for a total of 4590 cases and 7082 controls [38–45], data version 1.5. The main characteristics of these studies are reported in Table 1. Among cases, 1040 were cancers of the oral cavity, 1703 cancers of the pharynx, 303 cancers of the oral cavity or pharynx NOS and 1544 were cancers of the larynx. In most hospital-based studies, controls were patients admitted for acute, non neoplastic diseases, not related to tobacco smoking or alcohol drinking. Questionnaires were obtained from individual studies to assess the comparability of the collected data and of the wording of interview questions among the studies. Data from individual studies were received with personal identifiers removed. Each data item was checked for illogical or missing values and inconsistencies were resolved as necessary. Food frequency questionnaires (FFQ) were designed by each individual study. More details on these FFQs were reported elsewhere [15].
TABLE 1.
Characteristics of individual studies of the International Head and Neck Cancer Epidemiology (INHANCE) consortium pooled analysis including information on allium vegetables intake
| Study namea |
Study location |
Recruitment period |
Source of controls |
Participation rate of cases and controls (%) |
Information on
|
Total oral cavity/pharynx
|
Larynx | Controls | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Garlic | Onion | Oral cavity | Oropharynx/ hypopharynx |
Oropharynx NOS |
|||||||
| Europe | |||||||||||
| Italy Multicenter [38] | Aviano, Milan, Latina | 1990–2005 | HB | >95, >95 | Yes | Yes | 209 | 502 | 90 | 460 | 2716 |
| Switzerland [42] | Lausanne | 1991–1997 | HB | >95, >95 | Yes | Yes | 138 | 247 | 7 | 124 | 883 |
| Central Europe [40] | Central Europe | 1998–2003 | HB | 96, 97 | No | Yes | 196 | 150 | 32 | 384 | 907 |
| Germany [45] | Saarland | 2001–2003 | PB | 94, - | Yes | No | 15 | 43 | 9 | 27 | 94 |
| Italy [39] | Milan | 2006–2009 | HB | >95, >95 | Yes | Yes | 66 | 58 | 18 | 229 | 755 |
| North America | |||||||||||
| USA (Seattle-Leo) [44] | Seattle | 1983–1987 | PB | 81, 75 | No | Yes | 183 | 212 | 47 | 209 | 547 |
| USA (Boston) [43] | Boston | 1999–2003 | PB | 88.7, 48.7 | Yes | Yes | 139 | 291 | 43 | 111 | 659 |
| Central America | |||||||||||
| Puerto Rico [41] | Puerto Rico | 1992–1995 | PB | 71, 83 | Yes | Yes | 94 | 200 | 57 | - | 521 |
| Total subjects | 1040 | 1703 | 303 | 1544 | 7082b | ||||||
HB: Hospital based; NOS: not otherwise specified; PB: Population based
Representative publications in which study methods are available.
The total number of controls for the analyses on laryngeal cancer was 6561, as one study was not included [41].
Among the items in the FFQ considered in this investigation, six studies collected information on garlic intake measured in terms of frequency of use [38, 39, 42, 45] or number of servings per day (or per week) [41, 43]. Information across different studies was standardized into the variable “frequency of use” scored as 1 for none or low use (corresponding to less than 0.2 servings per day), 2 for intermediate use (corresponding to 0.2–1 serving per day), and 3 for high use (corresponding to more than 1 serving per day). Information on onion intake was collected in seven studies in terms of number of servings per day (or per week) [38, 39, 41–43] or frequency of use [44, 45]. Information across different studies was standardized into the variable “number of servings per week”, by converting consumption “never or less than once a year” into 0, “less than once a month” into 0.2 portions per week, “less than once a week” into 0.5 portions per week, “1 or 2 times per week” into 1.5 portions per week, “3–5 times per week” into 4 portions per week and “every day” into 7 portions per week. For studies reporting usual portion size (small, medium, large), for which an intermediate portion corresponded to 80 grams of onion, we considered a small portion as 0.7 times an intermediate one, and a large portion as 1.3 times an intermediate one [38, 39, 42]. No study collected information on the type of garlic and onion consumed (fresh, powders or garlic supplements) and on manner of using (raw or cooked). Only a study from Puerto Rico collected information separately on use of cooked or raw onion [41].
Statistical analysis
The ORs of HNC, and the corresponding 95% confidence intervals (CIs), for different levels of garlic use (high, intermediate vs none or low) and onion intake (>3, >1 to ≤3 vs <1 portion per week) were derived using unconditional multiple logistic regression models. All models included terms for study center, age (5-year categories), gender, race/ethnicity (non-Hispanic white, black, Hispanic/Latino, other), education level (no formal education, less than junior high school, some high school, high school graduate, vocational/some college, college graduate/postgraduate; categorically), cigarette smoking (never, 1–10, 11–20, 21–30, 31–40, 41–50, >50 pack-years; categorically), duration of cigar smoking (continuously), duration of pipe smoking (continuously), alcohol drinking (nondrinkers, >0–1, >1–3, >3–8, >8–18, >18–40, >40–75, >75–115, >115–155, >155 ml per day; categorically) and body weight (quartiles; categorically). Information on ethnicity was not collected in the central Europe and Saarland studies and, since the majority of these populations is white, all study subjects were classified as non-Hispanic whites. To impute the missing values of education, multiple imputations with the PROC MI procedure in SAS was applied.
The pooled effect estimates from all studies were estimated with fixed-effects and random-effects logistic regression models [46]. We tested for heterogeneity between the study-specific ORs by conducting a likelihood ratio test comparing a model that included the product terms between each study (other than the reference study) and the variable of interest with a model without product terms, for the risk of HNC combined and for that of each anatomical subsite. We used the random-effects [46] estimates when heterogeneity was detected (p<0.10), and the fixed-effects estimates otherwise. We quantified inconsistencies across studies and their impact on the analysis by using Cochrane’s Q and the I2 statistic [47, 48]. Stratified analyses were conducted according to anatomical site (oral cavity, pharynx, oral cavity/pharynx NOS and larynx) and “a priori” selected covariates, including age (<55, ≥55 years), gender, geographic region (Europe, America), education (<high school graduate, ≥high school graduate), tobacco smoking (never users, light users defined as smokers of ≤20 pack-year equivalent, heavy users defined as >20 pack-year equivalent) and alcohol consumption (never/light to moderate drinkers defined as drinkers of <3 drinks per day of alcoholic beverages, heavy drinkers defined as ≥3 drinks per day), in order to identify possible sources of heterogeneity. We also conducted a sensitivity analysis, in which each study was excluded one at time to ensure that the magnitude of the overall estimates were not dependent on any specific study.
Analyses were performed using SAS 9.2 statistical software (SAS Institute, Cary, NC) and Stata Statistical Software: Release 11 (College Station, TX: StataCorp LP).
3) Results
The characteristics of the eight case-control studies from the INHANCE consortium included in this analysis were reported in Table 1. Five studies were conducted in Europe (65% of total cases and 75% of controls), 2 in North America (27% of total cases and 17% of controls) and 1 in Central America (8% of total cases and 8% of controls). Four studies were hospital-based and four were population-based studies.
The distribution of HNC cases and controls according to selected variables (age, gender, race/ethnicity, education cigarette smoking and alcohol drinking) is reported in Supplementary Table 1. Eighty-one percent of HNC cases were men and 95% were non-Hispanic whites. Patients with HNC were more frequently tobacco users and alcohol drinkers.
Table 2 shows the distribution of HNC cases (also by anatomical site) and controls, and the corresponding ORs and 95% CIs, according to garlic and onion intakes. Compared with no or low garlic use, on the basis of 6 studies, the ORs of HNC combined were 0.95 (95% CI 0.71–1.27) for intermediate use and 0.74 (95% CI 0.55–0.99) for high use (p for trend= 0.02). In the analyses by anatomical sites, the ORs for high garlic use were 0.77 (95% CI 0.49–1.21, p for trend= 0.29) for cancer of the oral cavity and pharynx combined, 0.84 (95% CI 0.56–1.27, p for trend=0.29) for cancer of the oral cavity, 0.62 (95% CI 0.40–0.97, p for trend= 0.07) for oro/hypopharyngeal cancer, and 1.18 (95% CI 0.60–2.30, p for trend=0.85) for cancer of oral cavity/pharynx NOS. For laryngeal cancer, the corresponding OR for high garlic use, based on 5 studies, was 0.67 (95% CI 0.42–1.06, p for trend= 0.09). Information on onion intake derived from 7 studies for HNC and oral cavity and pharyngeal cancers combined, and from 6 studies for laryngeal cancer. The ORs for increasing categories of onion intake were 0.91 (95% CI 0.68–1.21) for >1 to ≤3 portions per week, and 0.83 (95% CI 0.60–1.13) for >3 portions per week (p for trend= 0.13) for HNCs combined. The ORs for the highest versus the lowest level of onion consumption were 0.88 (95% CI 0.67–1.15) for cancer of the oral cavity and pharynx combined, 0.95 (95% CI 0.67–1.35) for oral cavity cancer, 0.84 (95% CI 0.61–1.14) for oro/hypopharyngeal cancer, and 0.84 (95% CI 0.42–1.69) for cancer of oral cavity and pharynx NOS. The corresponding OR for laryngeal cancer was 0.69 (95% CI 0.54–0.88).
TABLE 2.
Distribution of cases of head and neck cancers (HNC) combined and by anatomical site, and of controls, and corresponding odds ratiosa (OR) and 95% confidence intervals (CI), according to garlic and onion intake. INHANCE Consortium
| Garlic useb OR (95% CI) |
Onion intakec (portions/week) OR (95% CI) |
|||||
|---|---|---|---|---|---|---|
| No/Low | Moderate | High | <1 | >1 – ≤3 | > 3 | |
|
| ||||||
| HNC combined | 1d | 0.95 (0.71–1.27) | 0.74 (0.55–0.99) | 1d | 0.91 (0.68–1.21) | 0.83 (0.60–1.13) |
| Cases:Controls | 1293:2325 | 1061:1888 | 207:530 | 2010:3660 | 482:764 | 920:1347 |
| p for trend | 0.02 | 0.13 | ||||
| p for heterogeneity between studies | 0.02 | <0.01 | ||||
| Total oral cavity and pharynx cancer | 1d | 0.91(0.74–1.12) | 0.77 (0.49–1.21) | 1d | 0.82 (0.56–1.19) | 0.88 (0.67–1.15) |
| Cases:Controls | 871:2325 | 732:1888 | 132:530 | 1221:3660 | 270:764 | 679:1347 |
| p for trend | 0.29 | 0.15 | ||||
| p for heterogeneity between studies | 0.05 | <0.01 | ||||
| Oral cavity | 1d | 0.99 (0.76–1.27) | 0.84 (0.56–1.27) | 1d | 0.91 (0.58–1.43) | 0.95 (0.67–1.35) |
| Cases:Controls | 248:2325 | 199:1888 | 41:530 | 376:3660 | 94:764 | 261:1347 |
| p for trend | 0.29 | 0.58 | ||||
| p for heterogeneity between studies | 0.31 | 0.07 | ||||
| Oropharynx/hypopharynx | 1d | 0.92 (0.71–1.19) | 0.62 (0.40–0.97) | 1d | 0.77 (0.53–1.11) | 0.84 (0.61–1.14) |
| Cases:Controls | 555:2325 | 459:1888 | 75:530 | 740:3660 | 151:764 | 355:1347 |
| p for trend | 0.07 | 0.10 | ||||
| p for heterogeneity between studies | 0.04 | 0.02 | ||||
| Oral cavity/pharynx NOS | 1d | 0.94 (0.60–1.45) | 1.18 (0.60–2.30) | 1d | 1.30 (0.59–2.89) | 0.84 (0.42–1.69) |
| Cases:Controls | 68:2325 | 74:1888 | 16:530 | 105:3660 | 25:764 | 63:1347 |
| p for trend | 0.85 | 0.10 | ||||
| p for heterogeneity between studies | 0.20 | 0.03 | ||||
| Larynx | 1d | 1.07 (0.56–2.05) | 0.67 (0.42–1.06) | 1d | 1.15 (0.88–1.49) | 0.69 (0.54–0.88) |
| Cases:Controls | 422:2235 | 328:1522 | 75:502 | 789:3556 | 212:716 | 241:1015 |
| p for trend | 0.09 | 0.04 | ||||
| p for heterogeneity between studies | <0.01 | 0.15 | ||||
ORs from random effects estimates when significant heterogeneity (p<0.10) was detected and from the fixed-effects estimates otherwise. Adjusted for age, gender, race/ethnicity, education, study, cigarette smoking (pack-years), duration of cigar smoking, duration of pipe smoking, alcohol intake and body weight. The sum does not add up to the total because of some missing information
Includes 6 studies for HN and OP cancers [38, 39, 41–43, 45] and 5 studies for laryngeal cancer [38, 39, 42, 43, 45]
Reference category.
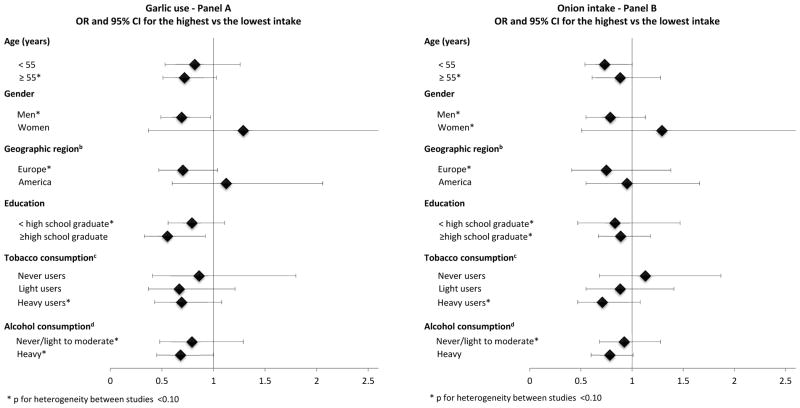
Stratified-analyses are presented in Figure 1. Panel A shows stratified results for high garlic use and Panel B those for high onion consumption. No significant heterogeneity was found across strata of age, gender, geographical region, education, tobacco and alcohol consumption. However, an apparently stronger inverse association emerged in men for high garlic use (p for heterogeneity between strata p=0.34), and in heavy tobacco users (i.e., smokers of >20 pack-year equivalent) for high onion intake (p=0.38).
Figure 1.
Odds ratios (OR)a and 95% confidence intervals (CI), according to highest intake of garlic (Panel A) and onion (Panel B) versus the lowest one in strata of selected covariates. INHANCE Consortium
aORs from random effects estimates when significant heterogeneity (p<0.10) was detected and the fixed-effects estimates otherwise. Adjusted for age, gender, race/ethnicity, education, study, cigarette smoking (pack-years), duration of cigar smoking, duration of pipe smoking, alcohol intake and body weight. The reference category was the lowest intake of garlic (i.e., no/low use) or onion (<1 portion/week) in each stratum.
bEurope included two studies from Italy [38, 39], one from Switzerland [42], one from Germany [45], and one from Central Europe [40]. America included two studies from USA [43, 44] and one from Puerto Rico [41].
cLight tobacco users were smokers of ≤20 pack-year equivalent (combination of pack-years of cigarettes and equivalent amount of cigars or pipe). Heavy tobacco users were smokers of >20 pack-year equivalent.
dNever/Light to moderate drinkers were drinkers of <3 drinks per day of alcoholic beverages and heavy drinkers were those consuming ≥3 drinks per day.
4) Discussion
In this pooled analysis of 8 case-control studies included in the INHANCE consortium, there was an inverse relation between garlic consumption and HNC risk. The pattern of risk was similar for onion, though the inverse association was significant for larynx only. In particular, high garlic use was associated with a significant risk reduction of overall HNC and oro/hypopharyngeal cancers by 26% and 38%, respectively. Further, consuming more than 3 portions of onion per week was associated with a significant reduction of laryngeal cancer risk by more than 30%.
The combination of garlic and onion is difficult to interpret, given the different measures used to collect information on the two intakes in various studies. We therefore presented details of the two separate items only in the present analysis. A previous publication related to food and HNC in the INHANCE database, based on 8 studies and about 4000 cases [15], tried to derive a summary measure of all allium vegetables. The ORs for subsequent quartiles were 0.98, 0.86 and 0.66, and the test for trend was significant. Thus, the early analysis is broadly consistent with the present more detailed one.
The inverse association between allium vegetables and the risk HNC may be related to various components of garlic and onion. Allium vegetables contain many chemicals with potential antioxidant and anticancer activity, including organosulfur compounds, flavonoids, minerals and vitamins [49]. In particular, garlic contains around 33 sulfur compounds. When its clove’s membrane is disrupted, alliin is metabolize to produce allicin (diallyl thiosulfinate), which is further metabolized to diallyl sulfide (DAS), diallyl disulfide, diallyl trisulfide (DATS), and other compounds such as ajoene. Onions contain several sulfoxides, mainly S-propenylcysteine sulfoxide, S-propylcysteine sulfoxide and S-methylcysteine sulfoxide [50]. Many experimental studies have suggested a protective effect of organosulfur compounds in ‘in vitro’ and ‘in vivo’ models against different types of cancer [50], and cancer chemopreventive effects may be related to various biological mechanisms, including modulation of metabolizing enzymes, and regulation of cell proliferation and apoptosis [17]. In particular for HNC, S-allylcysteine, a water-soluble organosulfur compound present in garlic, significantly suppressed 7,12-dimethylbenz[a]anthracene-induced buccal pouch carcinogenesis in hamsters by modulating lipid peroxidation and enhancing the level of reduced glutathione (GSH), glutathione peroxidase (GPx) and glutathione S-transferase (GST)[51]. N-acetylcysteine, another organosulfur compound, tested in two human tongue squamous carcinoma cell lines and in a human oral squamous cell carcinoma xenograft mouse model, inhibited cell proliferation and induced apoptosis by the regulation of Epidermal Growth Factor Receptor (EGFR)/Akt/HBP1signaling pathway [52]. Flavonoids, present in garlic and onions, may also contribute to the anticancer effects through their antioxidant activity. In particular, flavonoids have been inversely related with laryngeal cancer risk [53]. The anticancer effect of quercetin, a very abundant flavonol in onions, was also evaluated in ‘in vitro’ studies. In particular, the growth of a human laryngeal cancer cell line (Hep-2) was significantly inhibited by quercetin probably through apoptosis [54], as members of the MAPK pathways seem to be involved. Support for a real inverse association between garlic and HNC and between onion and cancer of the larynx comes from the significant inverse dose-risk relation, and from the consistent relation reported across strata of potential confounders and effect modifiers.
Our data of a significant inverse relation of onion intake with laryngeal cancer risk are in agreement with those of an Uruguayan case-control study not included in the INHANCE consortium, which found an OR of 0.50 (95% CI 0.31–0.80) for an increment of onion consumption of 3.4 g per day [29].
Among the strengths of the study are the uniquely large sample size, the involvement of many populations with different dietary and lifestyle habits, particularly alcohol drinking and diet, and the proper allowance for the major confounding factors, including tobacco smoking and alcohol drinking. In a sensitivity analysis, exclusion of each study from the pooled-analysis did not materially change the summary estimates, indicating that the results were not driven by any single study. Recall of dietary intake has been shown satisfactorily reproducible in most of studies [15], and should not be different on the basis of the disease status or among various types of controls, as allium vegetables is not commonly known to affect HNC risk.
There was significant heterogeneity across studies. Potential sources of heterogeneity include differences among populations in dietary correlates, patterns of smoking and alcohol drinking, and other covariates of HNC risk, and the way of consumption and cooking of garlic and onion in various countries.
An important limitation of this analysis included the paucity of information on the modalities of garlic and onion consumption (in particular no distinction between raw and cooked allium vegetables was made in studies, except for a study from Puerto Rico [41]), as there may be differences in the composition, concentration, and activities of bioactive compounds according to the modalities of cooking [55, 56]. The modalities of consumption (raw or cooked) and the cooking methods (boiled, fried and temperature of oven heating) of allium vegetables are responsible of their bioavailability and the activity of their bioactive components. Indeed, it was reported a reduction of about 30% in quercetin content by boiling onions, and boiled allium vegetables lose much of their antioxidant activity. In particular, cooking garlic may inactivate the enzyme alliinase, which is responsible for converting allicin to allicin, which in turn yields all other bioactive compounds. [57].
With reference to confounding, we were able to control for several risk factors for HNC. In particular, we paid attention to adjust for alcohol and tobacco, which are also the major risk factors for HNC [2–5], inversely related to high vegetables and fruit intake [58]. No information was available in the INHANCE data version 1.5 on HPV – a recognized risk facror for oropharyngeal cancer – and total energy intake. We adjusted for body weight to address this limitation because weight would reflect the energy intake in middle-aged adults [59]. In addition, the risk estimates did not materially change after adjustment for vegetable and fruit intake, which has been favorably related to HNC and may correlate with allium vegetables intake. For instance, in the Mediterranean diet, onion and garlic are often eaten or cooked in combination with other foods, such as tomato sauces for pasta, various vegetables and raw olive oil and tomatoes in salads, and some studies reported a synergistic favorable effect of garlic and tomatoes on cancer [60, 61]. We cannot however exclude some reverse causation as the presence of preneoplastic lesions, mainly in the oral cavity/pharynx, or symptoms of these diseases may have led to a reduction of allium vegetables intake among cases.
The results of this pooled-analysis of case-control studies support the hypothesis of a moderate inverse association between allium vegetables intake and the risk of HNC, and provide a quantitative estimate of the effect, even if some bias, confounding, and reverse causality cannot be excluded. In conclusion, although the most effective strategy for HNC prevention involves alcohol and tobacco control, selected dietary advices, including a vegetable rich diet, specifically with a high allium vegetable intake, may reduce the occurrence of this neoplasm with high incidence and low survival.
Supplementary Material
Acknowledgments
The authors would like to thank I. Garimoldi for her editorial assistance and all of the participants who took part in this research for providing us very insightful and constructive comments, which helped to improve this manuscript.
The INHANCE core data pooling was supported by NIH grants (NCI R03CA113157 & NIDCR R03DE016611). The individual studies were supported by the following grants: Italy Multicenter study: Italian Ministry of Health, General Directorate of European and International Relations; Swiss study: Swiss study: Swiss League against Cancer and the Swiss Research against Cancer/Oncosuisse [KFS-700, OCS-1633]; Central Europe study: Central Europe study: World Cancer Research Fund and the European Commission INCO-COPERNICUS Program [Contract No. IC15- CT98-0332]; German – Saaland study: Ministry of Science, Research and Arts Baden-Wurttemberg; Milan study: Italian Association for Research on Cancer (AIRC); Seattle-LEO study: NIH[R01CA030022]; Boston study: NIH [R01CA078609, R01CA100679]; Puerto Rico study: jointly funded by National Institutes of Health (NCI) US and NIDCR intramural programs. The work of CG was supported by Fondazione Veronesi. FT was supported by a fellowship from the Italian Foundation of Cancer Research (FIRC).
Abbreviations
- CI
Confidence Interval
- HNC
Head and Neck Cancers
- HPV
Human PapillomaVirus
- INHANCE
International Head and Neck Cancer Epidemiology
- NOS
Not Otherwise Specified
- OR
Odds Ratio
Footnotes
CLV, PB, MH, Y-C A L and CG designed the research. ZFZ, DS, PB, EF, JL, DM, PR, OS, NS-D, TLV, KK, MMC, FL, RBH, MPP, CB, HB conducted the research. CG, FT and VG analyzed data. CG, FT, AT, CP, CLV wrote the paper.
The authors declare no conflict of interest.
5) REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, et al., editors. ARC Cancer Base No. 10. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- 2.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turati F, Garavello W, Tramacere I, Pelucchi C, et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers: results from subgroup analyses. Alcohol Alcohol. 2013;48:107–118. doi: 10.1093/alcalc/ags100. [DOI] [PubMed] [Google Scholar]
- 5.Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 6.Lubin JH, Gaudet MM, Olshan AF, Kelsey K, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol. 2010;171:1250–1261. doi: 10.1093/aje/kwq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashibe M, Brennan P, Chuang SC, Boccia S, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev. 2008;17:340–344. doi: 10.1097/CEJ.0b013e3282f75e91. [DOI] [PubMed] [Google Scholar]
- 9.Negri E, Boffetta P, Berthiller J, Castellsague X, et al. Family history of cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer. 2009;124:394–401. doi: 10.1002/ijc.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 11.Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54:111–142. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 12.World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. [Google Scholar]
- 13.Bradshaw PT, Siega-Riz AM, Campbell M, Weissler MC, et al. Associations between dietary patterns and head and neck cancer: the Carolina head and neck cancer epidemiology study. Am J Epidemiol. 2012;175:1225–1233. doi: 10.1093/aje/kwr468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bravi F, Edefonti V, Randi G, Ferraroni M, et al. Dietary patterns and upper aerodigestive tract cancers: an overview and review. Ann Oncol. 2012;23:3024–3039. doi: 10.1093/annonc/mds197. [DOI] [PubMed] [Google Scholar]
- 15.Chuang SC, Jenab M, Heck JE, Bosetti C, et al. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23:69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009;45:461–467. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan K. Antioxidant potential of spices and their active constituents. Crit Rev Food Sci Nutr. 2014;54:352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- 19.Galeone C, Pelucchi C, Levi F, Negri E, et al. Onion and garlic use and human cancer. Am J Clin Nutr. 2006;84:1027–1032. doi: 10.1093/ajcn/84.5.1027. [DOI] [PubMed] [Google Scholar]
- 20.Guercio V, Galeone C, Turati F, La Vecchia C. Gastric cancer and allium vegetable intake: a critical review of the experimental and epidemiologic evidence. Nutr Cancer. 2014;66:757–773. doi: 10.1080/01635581.2014.904911. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Zhuang W, Hu W, Liu GJ, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–89. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: A case-control study and meta-analysis. Mol Nutr Food Res. 2015;59:171–179. doi: 10.1002/mnfr.201400496. [DOI] [PubMed] [Google Scholar]
- 23.Turati F, Guercio V, Pelucchi C, La Vecchia C, Galeone C. Colorectal cancer and adenomatous polyps in relation to allium vegetables intake: a meta-analysis of observational studies. Mol Nutr Food Res. 2014;58:1907–1914. doi: 10.1002/mnfr.201400169. [DOI] [PubMed] [Google Scholar]
- 24.Liu YT, Dai JJ, Xu CH, Lu YK, et al. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. Cancer Causes Control. 2012;23:589–599. doi: 10.1007/s10552-012-9923-z. [DOI] [PubMed] [Google Scholar]
- 25.Zheng W, Blot WJ, Shu XO, Diamond EL, et al. A population-based case-control study of cancers of the nasal cavity and paranasal sinuses in Shanghai. Int J Cancer. 1992;52:557–561. doi: 10.1002/ijc.2910520410. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W, Blot WJ, Shu XO, Diamond EL, et al. Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol Biomarkers Prev. 1992;1:441–448. [PubMed] [Google Scholar]
- 27.Zheng W, Blot WJ, Shu XO, Gao YT, et al. Diet and other risk factors for laryngeal cancer in Shanghai, China. Am J Epidemiol. 1992;136:178–191. doi: 10.1093/oxfordjournals.aje.a116484. [DOI] [PubMed] [Google Scholar]
- 28.Zheng W, Shu XO, Ji BT, Gao YT. Diet and other risk factors for cancer of the salivary glands:a population-based case-control study. Int J Cancer. 1996;67:194–198. doi: 10.1002/(SICI)1097-0215(19960717)67:2<194::AID-IJC8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.De Stefani E, Boffetta P, Oreggia F, Brennan P, et al. Plant foods and risk of laryngeal cancer: A case-control study in Uruguay. Int J Cancer. 2000;87:129–132. doi: 10.1002/1097-0215(20000701)87:1<129::aid-ijc19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Boeing H, Dietrich T, Hoffmann K, Pischon T, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006;17:957–969. doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 31.Conway DI, Hashibe M, Boffetta P, Wunsch-Filho V, et al. Enhancing epidemiologic research on head and neck cancer: INHANCE - The international head and neck cancer epidemiology consortium. Oral Oncol. 2009;45:743–746. doi: 10.1016/j.oraloncology.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Curcic MG, Stankovic MS, Radojevic ID, Stefanovic OD, et al. Biological effects, total phenolic content and flavonoid concentrations of fragrant yellow onion (Allium flavum L.) Med Chem. 2012;8:46–51. doi: 10.2174/157340612799278441. [DOI] [PubMed] [Google Scholar]
- 33.Trio PZ, You S, He X, He J, et al. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014;5:833–844. doi: 10.1039/c3fo60479a. [DOI] [PubMed] [Google Scholar]
- 34.Galeone C, Tavani A, Pelucchi C, Turati F, et al. Coffee and tea intake and risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2010;19:1723–1736. doi: 10.1158/1055-9965.EPI-10-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashibe M, Brennan P, Benhamou S, Castellsague X, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 36.Benhamou S, Tuimala J, Bouchardy C, Dayer P, et al. DNA repair gene XRCC2 and XRCC3 polymorphisms and susceptibility to cancers of the upper aerodigestive tract. Int J Cancer. 2004;112:901–904. doi: 10.1002/ijc.20474. [DOI] [PubMed] [Google Scholar]
- 37.Jayaprakash V, Rigual NR, Moysich KB, Loree TR, et al. Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg. 2006;132:1231–1236. doi: 10.1001/archotol.132.11.1231. [DOI] [PubMed] [Google Scholar]
- 38.Bosetti C, Gallus S, Trichopoulou A, Talamini R, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12:1091–1094. [PubMed] [Google Scholar]
- 39.Bravi F, Bosetti C, Filomeno M, Levi F, et al. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109:2904–2910. doi: 10.1038/bjc.2013.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashibe M, Boffetta P, Zaridze D, Shangina O, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006;15:696–703. doi: 10.1158/1055-9965.EPI-05-0710. [DOI] [PubMed] [Google Scholar]
- 41.Hayes RB, Bravo-Otero E, Kleinman DV, Brown LM, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 42.Levi F, Pasche C, La Vecchia C, Lucchini F, et al. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77:705–709. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Peters ES, McClean MD, Liu M, Eisen EA, et al. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol Biomarkers Prev. 2005;14:476–482. doi: 10.1158/1055-9965.EPI-04-0431. [DOI] [PubMed] [Google Scholar]
- 44.Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2:305–312. [PubMed] [Google Scholar]
- 45.Twardella D, Loew M, Rothenbacher D, Stegmaier C, et al. The diagnosis of a smoking-related disease is a prominent trigger for smoking cessation in a retrospective cohort study. J Clin Epidemiol. 2006;59:82–89. doi: 10.1016/j.jclinepi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. J Nutr. 2007;137:2264–2269. doi: 10.1093/jn/137.10.2264. [DOI] [PubMed] [Google Scholar]
- 50.Guercio V, Galeone C, Turati F, La Vecchia C. Gastric Cancer and Allium Vegetable Intake: A Critical Review of the Experimental and Epidemiologic Evidence. Nutr Cancer. 2014:1–17. doi: 10.1080/01635581.2014.904911. [DOI] [PubMed] [Google Scholar]
- 51.Balasenthil S, Nagini S. Inhibition of 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis by S-allylcysteine. Oral Oncol. 2000;36:382–386. doi: 10.1016/s1368-8375(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 52.Lee MF, Chan CY, Hung HC, Chou IT, et al. N-acetylcysteine (NAC) inhibits cell growth by mediating the EGFR/Akt/HMG box-containing protein 1 (HBP1) signaling pathway in invasive oral cancer. Oral Oncol. 2013;49:129–135. doi: 10.1016/j.oraloncology.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Garavello W, Rossi M, McLaughlin JK, Bosetti C, et al. Flavonoids and laryngeal cancer risk in Italy. Ann Oncol. 2007;18:1104–1109. doi: 10.1093/annonc/mdm078. [DOI] [PubMed] [Google Scholar]
- 54.Liu R, Wang J. Effects of quercetin on human laryngeal cancer Hep-2 cells. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22:169–171. [PubMed] [Google Scholar]
- 55.Ioku K, Aoyama Y, Tokuno A, Terao J, et al. Various cooking methods and the flavonoid content in onion. J Nutr Sci Vitaminol (Tokyo) 2001;47:78–83. doi: 10.3177/jnsv.47.78. [DOI] [PubMed] [Google Scholar]
- 56.Shin JH, Ryu JH, Kang MJ, Hwang CR, et al. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264. 7 macrophages. Food Chem Toxicol. 2013;58:545–551. doi: 10.1016/j.fct.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Jastrzebski Z, Leontowicz H, Leontowicz M, Namiesnik J, et al. The bioactivity of processed garlic (Allium sativum L.) as shown in vitro and in vivo studies on rats. Food Chem Toxicol. 2007;45:1626–1633. doi: 10.1016/j.fct.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 58.La Vecchia C, Negri E, Franceschi S, Parazzini F, Decarli A. Differences in dietary intake with smoking, alcohol, and education. Nutr Cancer. 1992;17:297–304. doi: 10.1080/01635589209514199. [DOI] [PubMed] [Google Scholar]
- 59.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 60.Bhuvaneswari V, Abraham SK, Nagini S. Combinatorial antigenotoxic and anticarcinogenic effects of tomato and garlic through modulation of xenobiotic-metabolizing enzymes during hamster buccal pouch carcinogenesis. Nutrition. 2005;21:726–731. doi: 10.1016/j.nut.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Sengupta A, Ghosh S, Das S. Modulatory influence of garlic and tomato on cyclooxygenase-2 activity, cell proliferation and apoptosis during azoxymethane induced colon carcinogenesis in rat. Cancer Lett. 2004;208:127–136. doi: 10.1016/j.canlet.2003.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.