Abstract
Objective/Hypothesis
Ineffective mucociliary clearance (MCC) is a common pathophysiologic process that underlies airway inflammation and infection. A dominant fluid and electrolyte secretory pathway in the nasal airways is governed by the cystic fibrosis transmembrane conductance regulator (CFTR). Decreased transepithelial Cl− transport secondary to an acquired CFTR deficiency may exacerbate respiratory epithelial dysfunction by diminishing MCC and increasing mucus viscosity. The objectives of the present study are to 1) develop a model of acquired CFTR deficiency in sinonasal epithelium using hypoxia, 2) investigate whether the polyphenol resveratrol promotes CFTR-mediated anion transport, 3) explore resveratrol mechanism of action and determine therapeutic suitability for overcoming acquired CFTR defects, and 4) test the drug in the hypoxic model of acquired CFTR deficiency in preparation for a clinical trial in human sinus disease. We hypothesize that hypoxia will induce depletion of airway surface liquid (ASL) secondary to acquired CFTR deficiency and that resveratrol will restore transepithelial Cl− secretion and recover ASL hydration.
Study Design
Basic science
Methods
Murine nasal septal (MNSE) and human sinonasal epithelial (HSNE) cultures were incubated under hypoxic conditions (1% O2,5% CO2) and transepithelial ion transport (change in short-circuit current=ΔISC) evaluated in Ussing chambers. Resveratrol was tested using primary cells and HEK293 cells expressing human CFTR by Ussing chamber and patch clamp techniques under both phosphorylating and non-phosphorylating conditions. CFTR activation was evaluated in human explants and by murine in vivo (nasal potential difference) assessment. Cellular cAMP (ELISA) and subsequent CFTR regulatory domain (R-D) phosphorylation (gel-shift assay) were also evaluated. Effects of hypoxia and resveratrol on ASL were tested using confocal laser scanning microscopy (CLSM) and micro-optical coherence tomography (μOCT).
Results
Hypoxia significantly decreased ΔISC (in μA/cm2) attributable to CFTR at 12 and 24 hours of exposure in both MNSE [13.55+/− 0.46 (12 hours);12.75+/−0.07(24 hours) vs. 19.23+/−0.18(control);p<0.05] and HSNE [19.55+/−0.56(12 hours);17.67+/− 1.13(24 hours) vs. 25.49+/−1.48(control);p<0.05]. We have shown that resveratrol (100μM) enhanced CFTR-dependent Cl− secretion in HSNE to an extent comparable to the recently FDA-approved CFTR potentiator, ivacaftor. Cl− transport across human sinonasal explants [78.42+/−1.75 vs. 1.75+/−1.5(control);p<0.05] and in vivo murine nasal epithelium [−4+/−1.8 vs. −0.8+/−1.7 mV(control);p<0.05] was also significantly increased by the drug. No increase in cellular cAMP or CFTR R-domain phosphorylation was detected. Inside out patches showed increased CFTR open probability [(NPo/N(N=channel number)] compared to controls in both MNSE [(0.329+/−0.116 vs. 0.119+/−0.059(control);p<0.05)] and HEK293 cells [(0.22+/−0.048 vs. 0.125+/−0.07(control);p<0.05). ASL thickness was decreased under hypoxic conditions when measured by CLSM [4.19+/−0.44 vs. 6.88+/−0.67(control);p<0.05]. A 30 minute apical application of resveratrol increased ASL depth in normal epithelium [8.08+/−1.68 vs. 6.11+/−0.47(control);p<0.05]. Furthermore, hypoxia-induced abnormalities of fluid and electrolyte secretion in sinonasal epithelium were restored with resveratrol treatment [5.55+/−0.74 vs. 3.13+/−0.17(control);p<0.05].
Conclusions
CFTR activation with a leading edge Cl− secretagogue such as resveratrol represents an innovative approach to overcoming acquired CFTR defects in sinus and nasal airway disease. This exciting new strategy bears further testing in non-CF individuals with CRS.
Keywords: Resveratrol, hypoxia, CFTR, cystic fibrosis, chronic rhinosinusitis, sinusitis, Ussing chamber, airway surface liquid, electrophysiology, patch clamp, ion transport, acquired CFTR deficiency
INTRODUCTION
Chronic rhinosinusitis (CRS) causes significant morbidity and detriments to quality of life for 16% of the US population and accrues an estimated aggregated cost of 8.6 billion dollars annually in healthcare expenditures.1,2,3 Patients with CRS mark worse scores for physical pain and social functioning on quality of life questionnaires than those suffering from chronic obstructive pulmonary disease, congestive heart failure or angina.2 Conventional CRS treatments are comprised of antibiotics, steroids, and surgical intervention. New therapies using safe, but effective, compounds that either augment the benefit of current treatments or target inherent properties of sinonasal mucosa are clearly needed.
Normal sinonasal mucociliary function is a critical host defense mechanism that clears the upper airways of inhaled pathogens such as bacteria, dust, and aerosols. Sinonasal respiratory epithelium is a highly-regulated barrier that is central to function of the mucociliary apparatus. Mucociliary clearance (MCC) is dependent on inherent biological properties of intact respiratory epithelium, including proper ciliary beating and the airway surface liquid (ASL). The ASL is composed of a periciliary fluid (sol) layer and a mucus (gel) layer.4 Respiratory epithelium modifies the volume of the periciliary fluid layer5 and the viscoelastic properties of the mucus in a fashion that depends on vectorial transepithelial ion transport.6,7 The active and passive transport of electrolytes [primarily sodium (Na+), chloride (Cl−), and bicarbonate (HC03−)] modulate ASL, and significant alterations in ion transport can be detrimental to the airway, as clearly exemplified by the disease cystic fibrosis (CF). CF affects approximately 30,000 persons in the U.S. (70,000 worldwide) and is the most common lethal inherited disease among caucasians.8 In CF, mutations in the gene encoding CFTR result decrease apical membrane anion conductance and cause dysregulated Na+ absorption.8 These abnormalities of fluid and electrolyte transport promote thick mucus formation and immobile exocrine secretions in multiple organ systems. In the sinuses of CF individuals, chronic stasis of inspissated mucus in combination with bacterial infection results in nearly universal inflammatory paranasal sinus disease.9-12
The CFTR is a member of the ATP binding cassette protein family and is comprised of multiple domains, including two transmembrane domains (TMs) and two nucleotide binding domains (NBDs). In addition, CFTR has a single regulatory domain (R-D) that functions in part to control CFTR activity.13 Activation of CFTR is thought to occur by a two-step process that involves 1) phosphorylation of the R-D, and 2) dimerization of the two NBDs, facilitating ATP binding and activation of the Cl− channel by inducing a conformational change in the TMs. Clinically important mutations in the CFTR gene can affect transcription, translation, post-translational modification, channel gating and function, and protein stability and turnover at the plasma membrane. The most common cause of CF is due to the deletion of phenylalanine at CFTR position 508 (F508del CFTR), which results in misfolding of the protein product in the endoplasmic reticulum and accelerated degradation by the proteosome. The absence of CFTR at the plasma membrane results in defective ion transport and the clinical manifestations of CF.13 Other mutations, such as G551D, result in adequate levels of CFTR protein at the apical cell surface, but the channels exhibit defective gating.14 The genetic mutations in individuals afflicted with CF contribute to phenotypic expression of the disease. However, recent evidence indicates that wild type CFTR processing, endocytic recycling, and function can also be markedly repressed by various environmental insults, including cigarette smoke exposure, high altitude/hypoxemia, inflammation, and infectious agents, and may be a contributing factor in CRS and other disease of MCC such as COPD.15-24 Hypoxia has been suggested to have considerable influence in the pathophysiology of chronic rhinosinusitis (CRS) among non-CF individuals.25-27 Blockage of sinus ostia may lead to hypoxia, resulting in decreased MCC, elaboration of inflammatory mediators, and bacterial colonization.27 In addition, accumulated secretions and impacted mucus lead to poor oxygen (O2) delivery, resulting in near anaerobic tissue conditions that accelerate progression of disease.28 Aaneas et al.29 used an oxygen probe to cannulate the maxillary sinuses of CF patients with CRS and found severely repressed pO2, including some individuals with complete anoxia. Hypoxia in the sinuses of CRS patients could contribute to propagation of sinus disease by creating a localized CFTR deficient environment where abnormalities of fluid and electrolyte transport persist. In either case, a strategy aimed at stimulating electrolyte secretion with drugs that target CFTR and therefore promoting mucus clearance would furnish a valuable addition to the pharmacologic armamentarium available for treatment of sinusitis.
Several small organic molecules that activate CFTR have been identified through high-throughput screening of compound libraries containing millions of discrete agents. Pharmaceutical companies have pursued CFTR potentiators for therapy of diseases of mucus clearance, including both CF and COPD. One such agent, ivacaftor (Vertex phamaceuticals), represents a novel CF therapeutic recently approved for treatment of CF patients with at least one copy of the G551D mutation.30 In this CF population, ivacaftor improves CFTR channel opening and thereby augments endpoints predictive of benefit including MCC, sweat Cl−, and lung function.31 Importantly, the same strategy being applied to activate Cl− secretion in the lower airways of CF patients is also applicable to impaired sinus and nasal mucus clearance.31
Mechanistically, ivacaftor increases channel open probability (Po) in both G511D mutated and wild type CFTR in the presence of R-D phosphorylation. This is best demonstrated in vitro following activation of cAMP/protein kinase A (PKA)-dependent pathways by drugs such as forskolin that confer phosphorylation of the R-D.32 Other compounds such as the flavonoid, genistein, increase CFTR channel Po by enhancing channel-open time and decreasing closed time, a phenomenon also observed as decreased ‘rundown’ of CFTR channels studied by membrane patch excision.33,34 Flavonoid compounds related to genistein also exhibit the capacity to activate CFTR Po,35-37 particularly after forskolin-mediated phosphorylation.38 Based on these and other functional studies, several laboratories have concluded that flavonoids may directly bind to CFTR, possibly at the interface between the two NBDs.39,40 Other molecules identified by high throughput compound library screening, such as the isoxazole UCCF-152 (3-(2-bezyloxy-phenyl)-5-chloromethyl-isoxazole), induce PKA-dependent phosphorylation of the R domain.41 In summary, multiple agents are known to activate CFTR Cl− transport by a variety of distinct mechanisms and are therefore candidates for targeting basal CFTR activity and acquired ion transport defects that underlie pathogenesis in CRS.
Resveratrol is an organic polyphenol found in many plants and vegetables, including the skins of red fruits, trees, some flowering plants, and peanuts.42,43 Flavonoids are also plant-based compounds that possess the common flavone ring structure (2-phenyl-γ-benzopyrone).38 Resveratrol lacks the central ring of the common three ring flavone backbone, but is otherwise structurally similar to flavonoids as a polyphenolic molecule. Broadly recognized for its immune-modulator activity attributable to inhibition of NF-κB signaling,44 resveratrol markedly reduces inflammatory pathways mediated by granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8 (or the murine homologue KC), inducible nitric oxide synthase (iNOS), and COX-2.44 However, utility as a therapeutic agent for acquired CFTR dysfunction in sinus disease has not been previously investigated.
The objectives of the current study were to 1) use oxygen restriction to create a model of acquired CFTR deficiency in sinonasal epithelium; 2) investigate whether the polyphenol resveratrol stimulates CFTR-mediated anion transport in vitro, ex vivo, and in vivo; 3) explore the mechanistic action of resveratrol on CFTR function and activity in sinonasal epithelium to determine therapeutic suitability for acquired CFTR defects in human sinus disease; and 4) test the drug in the hypoxic model of acquired CFTR deficiency in preparation for a clinical trial of mucociliary activators in human sinus disease. We hypothesize that hypoxia induces depletion of ASL secondary to alterations in transepithelial ion transport in a similar fashion to CF disease and that the impact of the environmental hypoxic insult can be ameliorated through the application of resveratrol.
METHODS
Institutional Animal Care and Use Committee and Institutional Review Board approval were obtained prior to initiation of the study. Written informed consent was obtained from each participant on a document approved by the Institutional Review Board.
Tissue Culture
Primary sinonasal epithelial cells (from humans, mice, and pigs) were cultured at an air-liquid interface according to previously established protocols.45-53 All murine nasal septal epithelial (MNSE) cells were obtained from congenic C57/BL6 wild type and CFTR−/− mice. Primary human sinonasal epithelial (HSNE) cells were prepared and cultured on collagen coated Costar 6.5-mm-diameter permeable filter supports (Corning, Lowell, MA) submerged in culture media. Differentiation and ciliogenesis occurred in all cultures within 10 to 14 days. All experiments were performed only when cell monolayers were fully differentiated with widespread ciliogenesis and transepithelial resistances (Rt) > 300 Ω. cm2. A recombinant human CFTR-expressing HEK293 cell line (“D060”) was also employed for patch clamp analysis.54
For hypoxia experiments, cells were incubated in a sealed modular incubator chamber at 37°C with an environment comprised of a 1% O2, 5% CO2 (the remainder consisting of nitrogen) or a physiologic environment (21% O2, 5% CO2).
Electrophysiology
Ussing Chamber Short Circuit (ISC) Measurements
To investigate pharmacologic manipulation of vectorial ion transport, a large array of electrophysiology assays were employed. Human sinonasal epithelial explants were removed from patients undergoing endoscopic surgery for pituitary tumor, benign sinonasal tumor or cerebrospinal fluid leak repair. Specimens were immediately stored in ice cold DMEM/F-12 media (Invitrogen, USA) and transferred to the laboratory within 15 minutes. A thin layer of epithelial tissue was dissected from the surgical specimen, placed on a slider with an open area of 0.031 – 0.71cm2, and mounted between Ussing-type hemichambers (Easy Mount, Physiologic Instruments Inc. CA. USA) to evaluate resveratrol activity ex vivo. Transwell inserts containing monolayers as well as tissues were arranged in Ussing chambers (VCC 600; Physiologic Instruments Inc. CA. USA) and continuously analyzed under short circuit conditions following fluid resistance compensation using automatic voltage clamps. Bath solutions for the transwell filters were warmed to 37°C, and each sample continuously gas lifted with 95%O2-5%CO2 or 94%N2/1%O2/5% CO2 mixture for hypoxia experiments. The bath solution contained (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose with pH 7.3 - 7.4. Chemicals were acquired from Sigma (St. Louis, MO). Each solution was formulated as 1000x stock and used at 1x in the Ussing chamber. All experiments were performed with low Cl− (6 mM) in the mucosal bath. Pharmacologic preparations were the following: amiloride (100μM), ivacaftor (10 μM), forskolin (20 μM), INH-172 (10 μM). Resveratrol (trans isoform) was utilized at a range of doses to establish a working concentration. Amiloride blocks epithelial sodium (Na+) channels, and supports the notion that variation in short-circuit current (ΔISC) from subsequent manipulation is due to effects on Cl− channel activity. Forskolin stimulates the cystic fibrosis transmembrane conductance regulator via cAMP-mediated/PKA-dependent phosphorylation of the R-D as described above. INH-172 is a selective inhibitor of CFTR and aids in determining the degree of contribution of CFTR to the ISC under experimental conditions. Corresponding DMSO (vehicle) control solutions for resveratrol and ivacaftor were studied in parallel. The ISC was assessed at one current measurement per second. By convention, a positive deflection in ISC was defined as the net movement of anions in the serosal to mucosal direction.
Single Channel Studies
Conventional patch clamp techniques were adapted to record single channel currents by cell-attached and inside-out configuration using MNSE and HEK293 cells expressing WT human CFTR. Recording pipettes were constructed from borosilicate glass capillaries (Warner Instruments, Hamden CT) using a Narishige PP83 microelectrode puller and fire polished with a PP90 microforge (Narishige, Tokyo). The pipettes were partially filled with a solution containing (in mmol/l) 150 CsCl, 1 CaCl2, 1 MgCl2 and 10 HEPES (pH 7.2) with tip resistances of 6-8 MΩ. All experiments were performed at room temperature (20-22°C). Single channel currents were obtained using an Axopatch 200B patch clamp amplifier (Axon Instruments (AI), Molecular Devices, USA) with voltage commands and data acquisition controlled by Clampex software (pClamp 10, Axon Instruments) and digitized (Digidata 1440A interface, AI) at a sampling frequency of 1 kHz. MNSE or HEK293 cells seeded on glass coverslips were mounted in a flow through chamber. To acquire seals, bath solutions contained (in mmol/l) 140 NaCl, 4.0 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 glucose, 10 HEPES, pH 7.4. For inside out patches, bath solution was comprised of (in mmol/l) 150 CsCl, 5 EDTA and 10 HEPES pH 7.4 designed to minimize channel rundown. Resveratrol was used at a working concentration of 100 μM. Vehicle controls were included in all studies [DMSO (0.02%)]. Confirmation that stimulated currents were due to CFTR activity was achieved using the inhibitor INH-172 at 10 μM. Single channel recordings were analyzed using pClamp 10 software (AI). Vcom = command potential. Tracings were filtered post acquisition at 500 Hz.
Nasal Potential Difference Assay
A 3-Step protocol was used, as described previously.55 Animals were anesthetized with ketamine (200 mg/kg), acepromazine (0.6 mg/kg), and xylazine (30 mg/kg) by intraperitoneal injection prior to testing. First, nasal cavities of anesthetized mice (C57/BL6) were perfused sequentially with 1) Ringer's solution containing 140mM NaCl, 5mM KCl, 1mM MgCl2, 2mM CaCl2, 10mM HEPES, and amiloride 50μM (pH 7.3); 2) amiloride + a low-Cl−-containing solution (NMDG, 6 mM Cl−, pH 7.3); and 3) amiloride + low-Cl−-containing solution + resveratrol, forskolin 20 mM, or DMSO control. Because of the continuous presence of amiloride (50μM) and the complete replacement of Na+ with a membrane-impermeant cation (140 mM) NMDG in the perfusion solution), hyperpolarization in this setting reflects Cl− secretion rather than Na+ absorption. Each condition was studied for 5 to 10 minutes until a steady signal was achieved. Activity of the test solution was measured from the stable baseline to the highest point of hyperpolarization. All traces were interpreted in a blinded fashion.
CFTR R-D Phosphorylation and cAMP Levels
Because activation of CFTR-mediated anion transport requires PKA-dependent phosphorylation of the R-D, an ELISA-based detection kit (Cayman Chemicals, Ann Arbor, MI) was used to measure stimulation of cellular cAMP by resveratrol in MNSE cultures, as previously described.56,57 To confirm absence of a cAMP/PKA dependent mechanism, polyclonal NIH-3T3 cells expressing HA-tagged R-domain were treated with resveratrol for 20 minutes, and compared to forskolin (20 μM) × 5 minutes as a positive control and DMSO as negative control. Following lysis, equal amounts (50 μg) of total cell lysate were electrophoresed through a 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and immunoblotted with antibody to the HA tag (Covance, Cumberland, VA). Phosphorylation of the R-domain was visualized as a 2-4 kD shift in migration, as previously described.56
Airway Surface Liquid Depth
Confocal Laser Scanning Microscopy
Cells were washed 3 times with PBS followed by administration of 100 μM CellTracker™ Green BODIPY® (Invitrogen, Cat#: C2102) to the basolateral medium and 10mg/ml Texas Red® (Invitrogen, Cat #: D-1863) in FC-70 (Flourinert FC-70, Fisher, Cat #: NC 9062226) to the apical side 2 hours before confocal visualization. Cells incubated at 1% O2/5% CO2 were compared to physiologic O2 (air control). Resveratrol, resveratrol + CFTR(inh)-172 (10 μM) or vehicle (DMSO) control were added to apical (in 30 μl volume) chambers and imaging performed with a Zeiss LSM 710 Confocal Microscope using Zeiss Plan Apo 20x .8 na dry Objective in 1 micron steps. ASL depth was measured in the orthogonal (head on X-Z) image view. Two regions of interest were analyzed for each monolayer and average ASL depth was measured for 5 equally distributed locations in each region.
Statistical analysis
Statistical analyses were conducted using Excel 2010 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized paired and unpaired Student t tests, the Mann-Whitney rank sum test, or the analysis of variance followed by Tukey-Kramer multiple comparison test as appropriate.
RESULTS
Hypoxic primary sinonasal epithelial cultures are a valid in vitro model of acquired CFTR deficiency
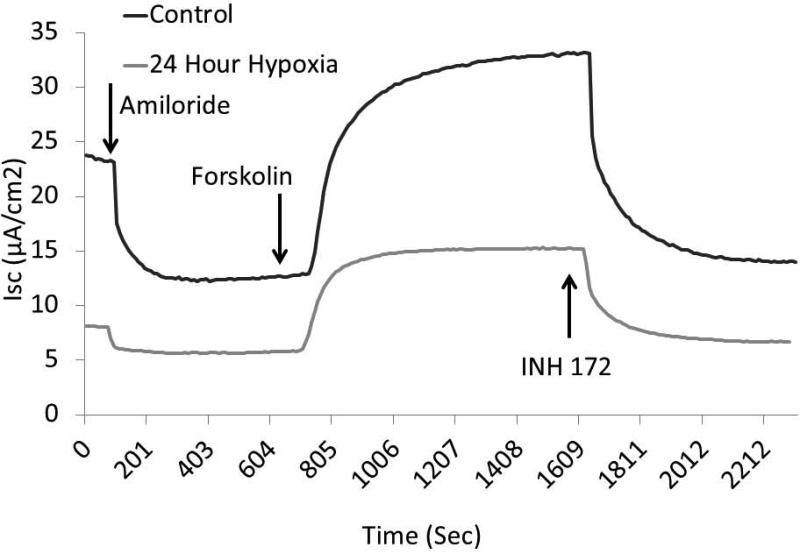
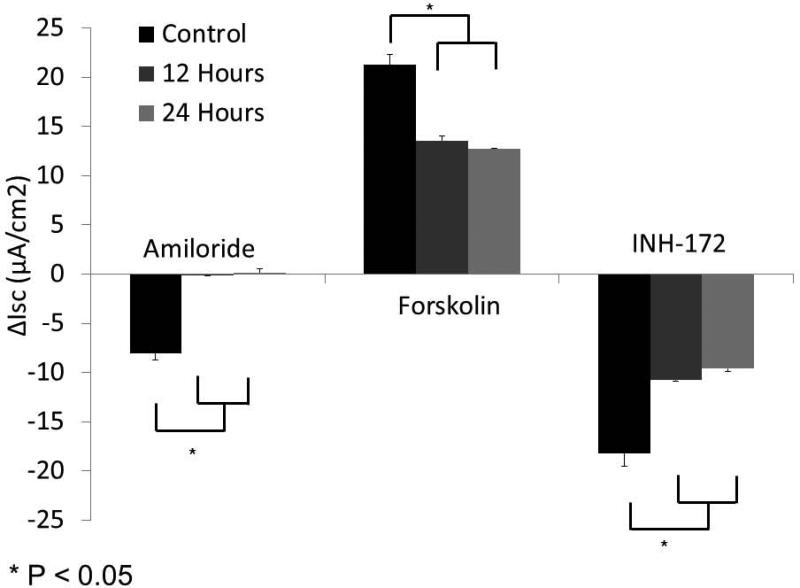
In order to establish and confirm the validity of hypoxia-induced acquired CFTR deficiency, MNSE and HSNE cultures were incubated for 12 and 24 hours at 1% O2 and evaluated in Ussing chambers using pharmacologic manipulation. The change in short-circuit current (ΔISC(μA/cm2) attributable to CFTR (forskolin-stimulated transport) was significantly decreased at 12 and 24 hours in MNSE (Figure 1A, 1B) incubated in an oxygen-restricted environment [13.55+/− 0.46 (12 hours); 12.75+/−0.07 (24 hours) vs. 19.23+/−0.18 (control); p<0.05)]. Inhibition with the specific CFTR inhibitor, INH-172, was also markedly decreased [−10.79+/− 0.10 (12 hours); −9.57+/−0.28 (24 hours) vs. −17.19+/−1.80 (control); p<0.05)] indicating overall deficiency of CFTR-dependent anion transport after hypoxia. Of note, ISC attributable to epithelial Na+ channel (ENaC) transport as measured by amiloride blockade was nearly absent by 12 hours [−0.16+/−0.01 vs. −7.76+/−0.80; p<0.05].
Figure 1. Hypoxia induces acquired defects in transepithelial ion transport in murine nasal epithelia.
MNSE cells grown on transwell permeable supports and incubated in 1% O2 or physiologic O2 (control, 21%) for 12 or 24 hours were mounted in Ussing chambers under short-circuit conditions and sequentially exposed to amiloride (100μM), forskolin (20 μM), and INH-172 (10 μM). Representative tracings (at 24 hours) are shown in (A). By convention, a positive deflection in the tracing represents movement of anion in the serosal to mucosal direction. Summary data is represented in (B). Under these conditions, change in short-circuit current (ΔISC) was significantly decreased at all time points with amiloride (used as a means to monitor epithelial sodium channels) and forskolin (a pharmacologic activator of CFTR). CFTR blockade by INH-72 was also significantly decreased compared to control (n ≥ 6 per condition). (Adapted from 63)
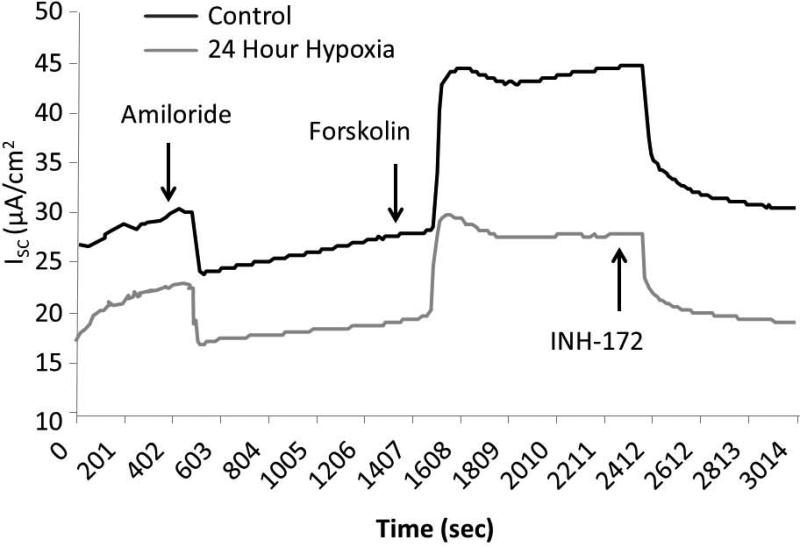
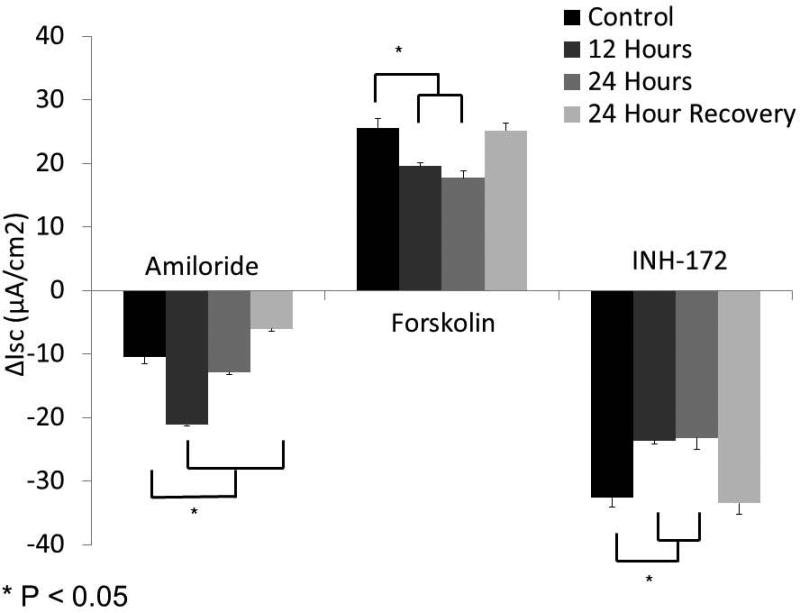
Similar to MNSE, forskolin-stimulated ΔISC in HSNE (Figure 2A, 2B) was sensitive to hypoxic stress, and demonstrated significant reductions in CFTR-mediated Cl− transport [19.55+/−0.56 (12 hours); 17.67+/−1.13 (24 hours) vs. 25.49+/−1.48 (control); p<0.05)]. Decreased ΔISC following INH-172 pharmacologic blockade verified a decreased contribution of CFTR to the ISC [−23.67+/−0.05 (12 hours); −23.21+/−1.86 (24 hours) vs. −32.66+/−1.15 (control); p<0.05)]. Sodium absorption in HSNE (amiloride blockade) appeared to be resistant to hypoxia and, in fact, was significantly increased at 12 hours [−21.13+/−0.27 (12 hours) vs. −10.45+/−1.05 (24 hours); p <0.05] and returned to baseline by 24 hours (−12.89+/−0.37). HSNE also demonstrated recovery of Cl− transport following 24 hours in physiologic O2 (21%) (25.12+/−1.24). However, Na+ absorption was significantly inhibited following return to atmospheric O2 environment (−6.05+/−0.33).
Figure 2. HSNE exhibits a CF-like ion transport phenotype.
Hypoxic (1%) HSNE cells were studied in Ussing chambers under identical conditions to MNSE plus the addition of a 24 hour recovery period under physiologic O2 (21%). Representative tracings at 24 hours (A) show stable amiloride-sensitive ISC, but diminished CFTR-mediated ISC (forskolin). Summary data (B) reveal a relative increase in ΔISC attributable to sodium blockade with amiloride at 12 hours that returns to baseline at 24 hours. Forskolin-stimulated ΔISC was sensitive to hypoxic stress, and demonstrated significant reduction in CFTR-mediated Cl− transport (n ≥ 6 per condition). Decreased ΔISC from INH-172 blockade verified the contribution of CFTR to the ISC. HSNE demonstrated significant recovery of Cl− transport following 24 hours in a physiologic O2 (21%) environment. (Adapted from 63)
Hypoxia depletes airway surface liquid
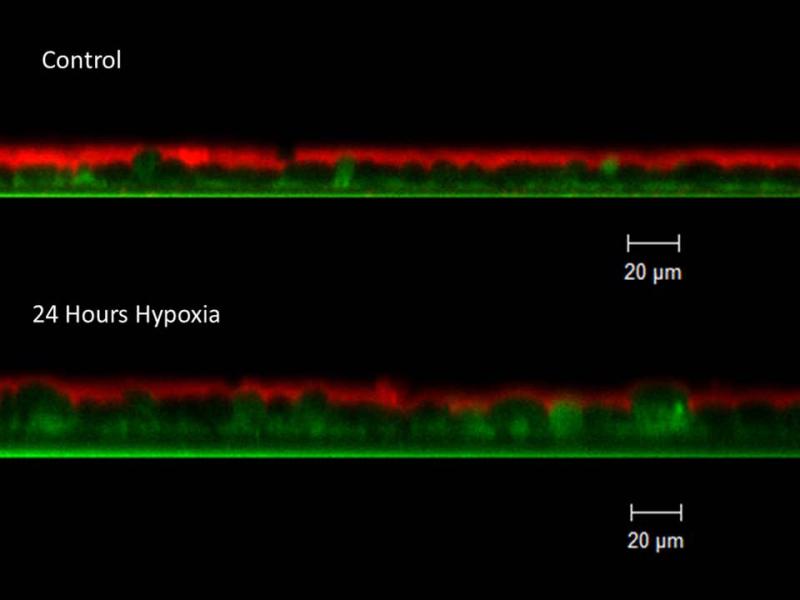
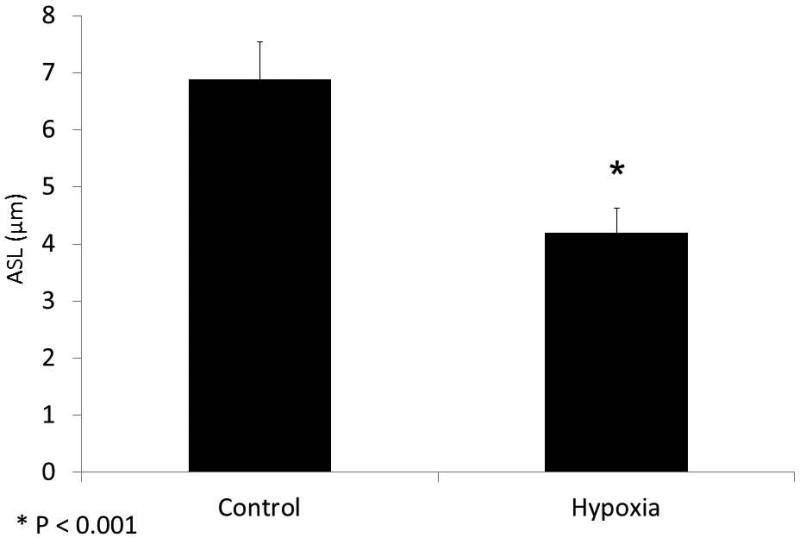
To assess whether the ion transport deficiencies observed in the Ussing chamber would confer abnormalities of ASL, hypoxic MNSE were evaluated at 24 hours by CLSM and the μOCT techniques. ASL depth was decreased under hypoxic conditions when measured by CLSM (in μm:4.19+/−0.44,hypoxia;6.88+/−0.67,n≥5 per condition, control; p<0.05) providing direct evidence that altered ion transport in this culture model leads to ASL depletion.
Resveratrol is a robust CFTR-dependent Cl− Secretagogue
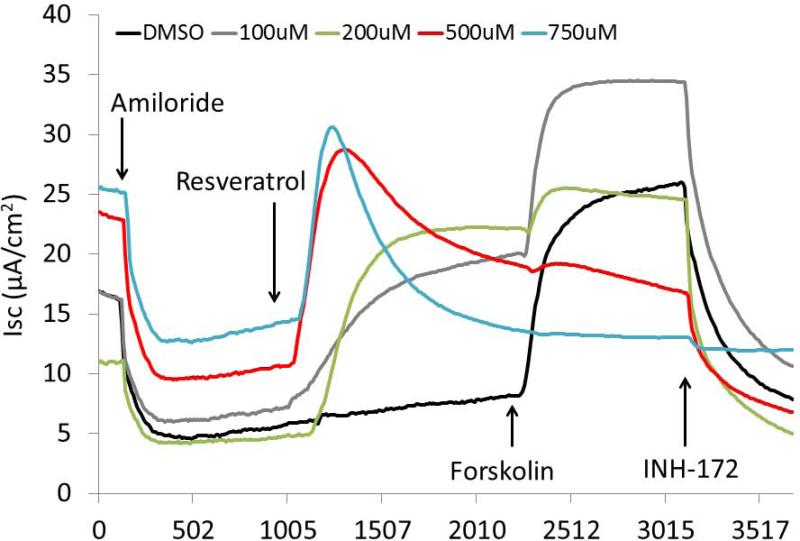
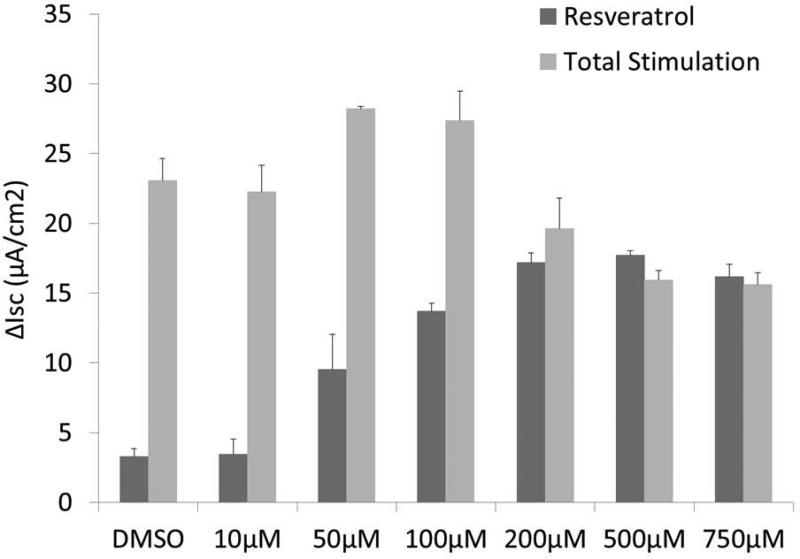
Following confirmation of our model of hypoxia-induced CFTR deficiency, a comprehensive evaluation of resveratrol was implemented to assess its utility as a Cl− secretagogue in sinonasal epithelium. Dose-dependent CFTR-mediated anion transport (ΔISC) was analyzed using MNSE cultures in Ussing chambers. Resveratrol activated CFTR-mediated Cl− conductance in a dose dependent fashion up to 500 μM, but demonstrated modest inhibitory effects on total CFTR activation with post-treatment forskolin (100 nM) at concentrations > 100 μM (Figure 4A and 4B). Thus, 100 μM resveratrol [(ΔISC, 13.51+/−0.77 vs. 4.4+/−0.66(control);p<0.05] was considered an optimal dose for subsequent studies.
Figure 4. Resveratrol stimulates CFTR-mediated Cl− Transport in vitro.
Representative Ussing chamber tracings reveals resveratrol activation of Cl− transport in a dose-dependent fashion (A). Note the negative deflection after a peak effect with subsequent inhibition of forskolin (100 nM) response at higher concentrations of resveratrol. Summary data (B) of resveratrol stimulation vs. total stimulation [resveratrol + forskolin (20 μM))] of transepithelial Cl− secretion (n ≥ 3 per condition). Resveratrol concentrations > 100 uM result in modestly decreased total stimulation. (C) Ussing chamber tracings of CFTR−/− MNSE monolayers exposed to resveratrol (100 μM) following amiloride (100 nM) blockade shows no measurable response, indicating Cl− secretagogue activity is dependent on the presence of CFTR. (Adapted from 64)
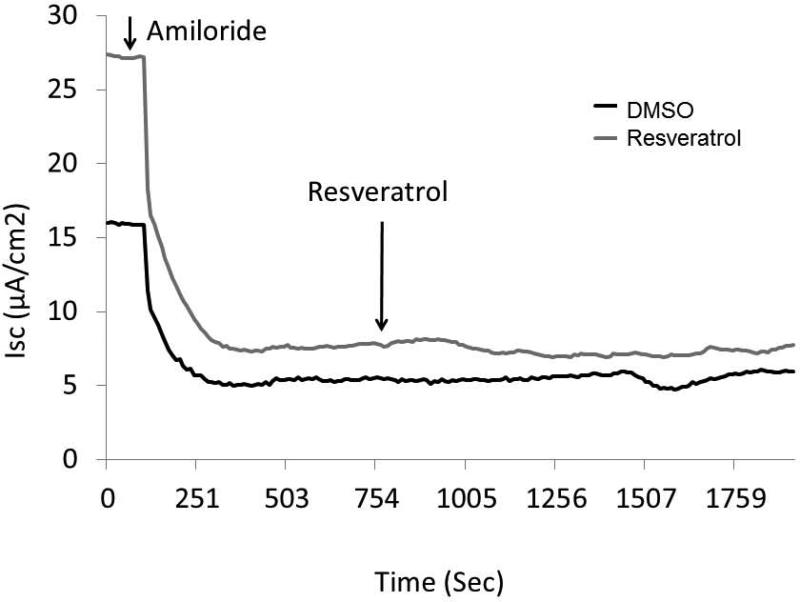
CFTR−/− knockout MNSE on the same genetic background were used to confirm that resveratrol-stimulated ΔISC is mediated through a CFTR-dependent mechanism (Figure 4C). Following application of amiloride, resveratrol (100μM) resulted in no measurable transepithelial secretion and, thus, no contribution from alternative Cl− transport pathways (i.e. calcium-activated Cl− channels) was implicated.
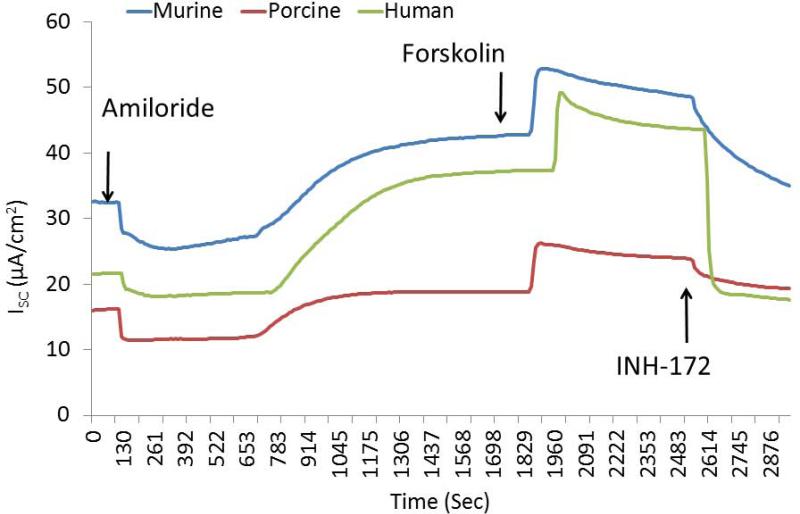
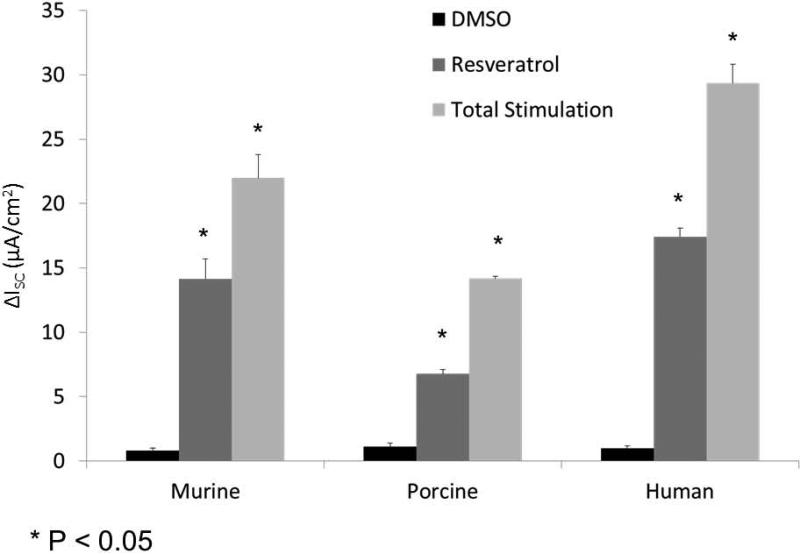
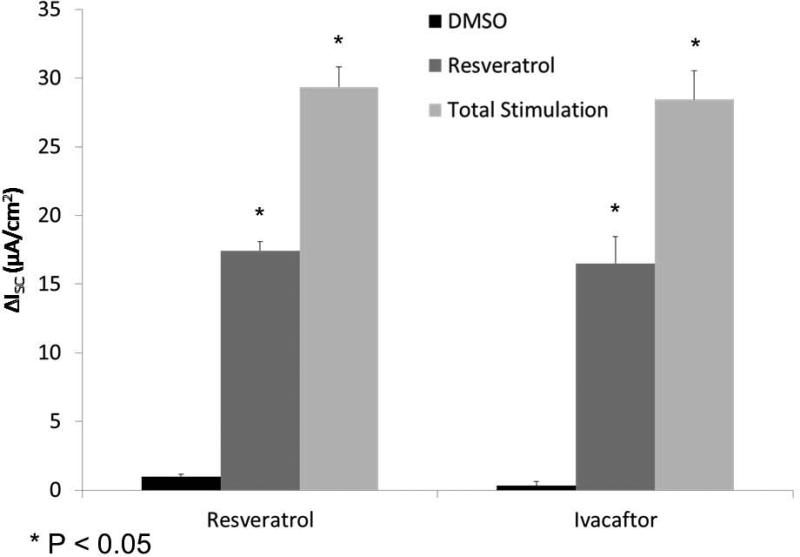
Activities in culture derived from other mammalian species (including human) were next evaluated. Murine [(ΔISC, 14.2+/−1.5 vs. 0.8+/−0.2(control)], human [(17.4 +/− 0.7 vs. 1.0 +/−0.2(control)], and porcine [(6.8 +/− 0.3 vs. 1.1 +/−0.3(control)] sinonasal airway epithelial cells exhibited significant CFTR-mediated anion secretion following application of the drug (p<0.05) (Figure 5A and 5B). Resveratrol activated between 50 and 70% of total CFTR-dependent ISC as judged by addition of saturating (20 μM) forskolin (murine 22.0+/−1.8, human 29.4+/−1.5, and porcine 14.2 +/− 0.2). The drug produced equivalent levels of CFTR-mediated anion transport when compared to ivacaftor (16.5+/−1.9 at an optimal concentration of 10 μM) in human sinonasal epithelial cultures (Figure 5C).
Figure 5. Resveratrol activates CFTR-mediated Cl− secretion in primary sinonasal epithelial cell cultures from several mammalian species.
(A) Representative Ussing chamber tracings show resveratrol (100 μM) activation of Cl− transport in murine, porcine, and human sinonasal epithelial cell cultures. (B) Graphic representation of resveratrol stimulation vs. total stimulation [resveratrol (100 μM) + forskolin (20 uM)] for transepithelial Cl− transport in these cells. Significant stimulation (p<0.05) of Cl− transport was demonstrated in primary sinonasal cultures as compared to untreated vehicle controls (DMSO) in all species. Total stimulation (addition of 20 μM forskolin) was also significantly greater than resveratrol alone (p<0.05). Resveratrol (as a percentage of total stimulation) consistently activated 50 to 70% of total CFTR-mediated anion transport. (C) Resveratrol activates wild type CFTR-mediated anion transport to the same magnitude as ivacaftor (10 μM) in human sinonasal epithelial cultures. (Adapted from 54)
Resveratrol activates human sinonasal CFTR-mediated Cl− transport ex vivo
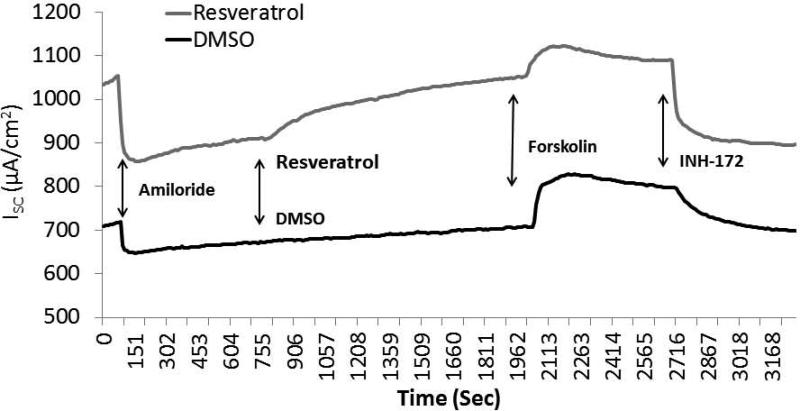
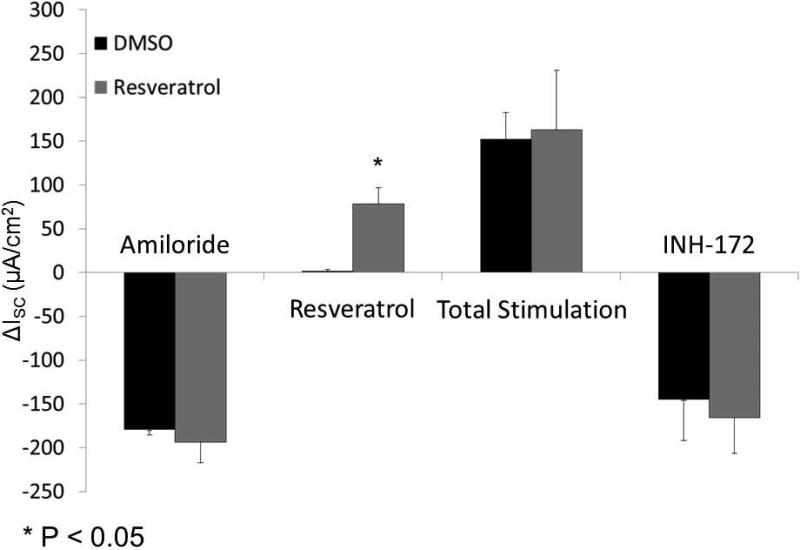
Once in vitro evaluation was confirmed, activity in freshly excised sinus tissue from individuals undergoing endoscopic sinus surgery was performed (n=5; paired samples) (Figure 6A and 6B). Resveratrol briskly stimulated CFTR-mediated Cl− transport in human sinus explants [78.42 +/− 1.75 vs. 1.75 +/− 1.5 (control), p<0.05)]. Amiloride-sensitive ISC (−193.5 +/− 23.5 vs. 179.6 +/− 5.7 (control)) representing epithelial sodium channel activity, total stimulated ISC with forskolin (resveratrol + forskolin 163.0 +/− 67.9 vs. control + forskolin 152.4 +/− 30.0), and CFTR inhibition with INH-172 (−165.7 +/− 40.9 vs. 145.2 +/− 46.8) were equivalent between groups, indicating that physiologic ion transport was intact among resveratrol treated and untreated mucosal samples.
Figure 6. Resveratrol activates CFTR-mediated Cl− secretion in human sinonasal mucosa ex vivo.
Representative Ussing chamber tracings of full thickness human sinonasal tissue explants demonstrating robust activation of CFTR-dependent Cl− secretion with 100 μM resveratrol (A). Ussing chamber summary data evaluating paired sinus mucosal samples from 5 individuals, and demonstrating significant activation (p<0.05) of ex vivo CFTR-dependent Cl− transport (B). (Adapted from 54)
Resveratrol stimulates CFTR-dependent Cl− secretion across in vivo murine nasal epithelium
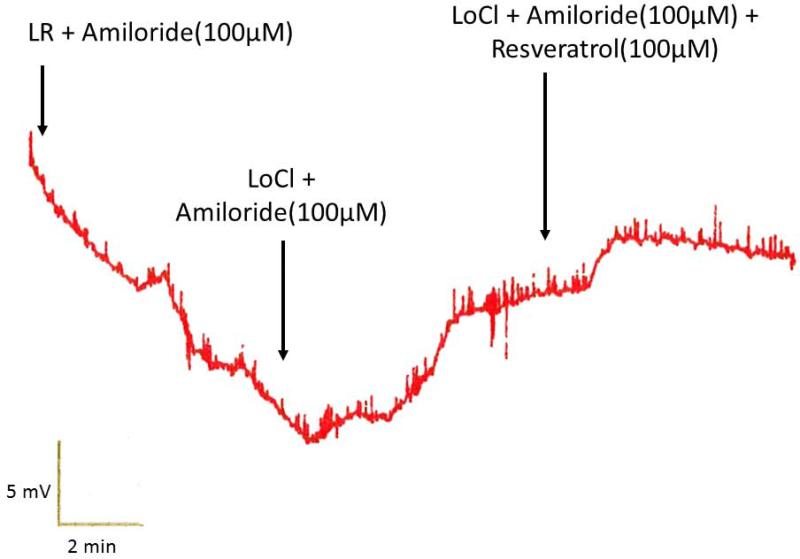
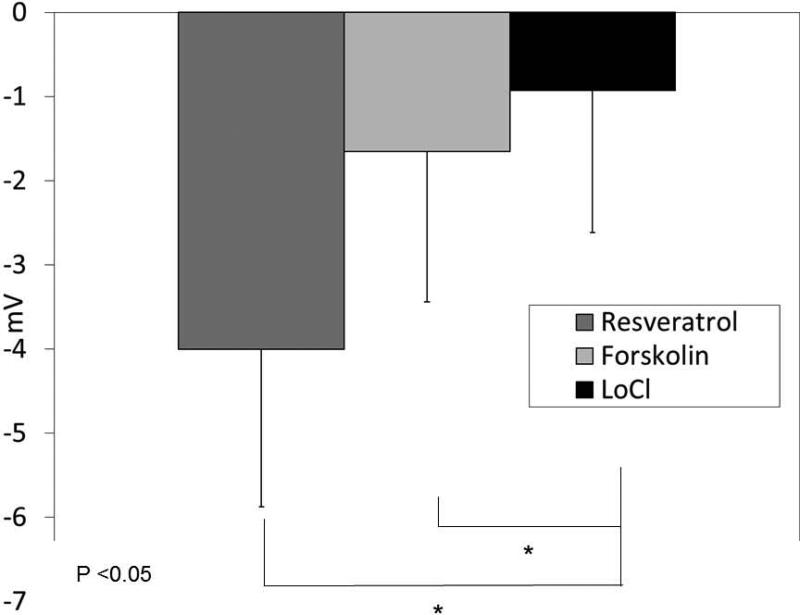
To establish resveratrol's capabilities to activate Cl− transport in nasal epithelium in vivo, the murine nasal potential difference assay was utilized. Mice perfused with resveratrol (100 uM) in the presence of amiloride (100 μM) and a Cl− secretory gradient demonstrated significant hyperpolarization (−4 ± 1.87 mV) compared with continued depolarization seen in mice perfused with vehicle and identical conditions [−0.93 ± 1.69 mV, p<0.01). Activity was increased compared to the positive control (forskolin, −1.65 +/− 1.78 mV), although this was not statistically significant (Figure 7A and 7B).
Figure 7. Murine nasal potential difference measurements demonstrate activation of Cl− Transport in vivo.
(A) Mice underwent a standardized nasal potential difference (NPD) protocol with the addition of 100 uM resveratrol (or vehicle control) in the final perfusion solution (20 μg/mL in Cl− free gluconate solution and amiloride (100 μM)). A representative tracing is shown from C57 mice stimulated with resveratrol in the final perfusate. (B) Resveratrol perfusion resulted in a −4 mV mean NPD polarization that was significantly different than in mice receiving vehicle alone (p<0.05). (Adapted from 64)
CFTR is activated in the absence of R-D phosphorylation
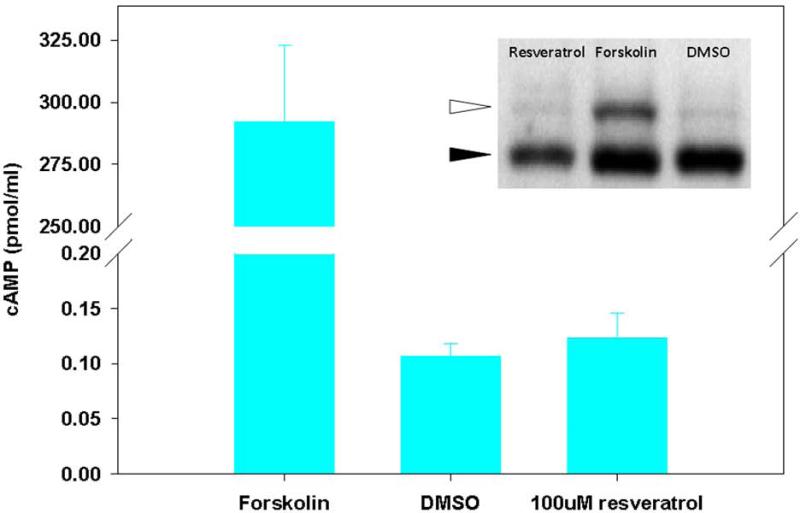
PKA/cAMP-dependent phosphorylation of the CFTR R-D is a critical step in channel activation, but it has been previously demonstrated that flavonoid compounds (e.g. genistein58) activate CFTR through a mechanism independent of PKA/cAMP-dependent phosphorylation. To examine whether resveratrol might activate CFTR via PKA-dependent pathways, cAMP levels and R-D phosphorylation were evaluated. Unlike the cAMP agonist forskolin, resveratrol had no measurable effect on the phosphorylation-dependent mobility shift of recombinant CFTR R-D and did not elevate cellular cAMP (Figure 8).
Figure 8. Resveratrol does not increase cellular cAMP or phosphorylate the CFTR R-domain.
Cellular cAMP was minimal in the presence of resveratrol and not significantly changed when compared to DMSO control. The adenylate cyclase agonist forskolin was used as a positive control. Polyclonal NIH-3T3 cells expressing HA-tagged R-domain were treated with resveratrol for 20 minutes, and compared to forskolin (20 μM) or DMSO control in an assay of R-D phosphorylation (inset). Phosphorylation results in a 2-4 kD shift in migration (white arrow). (Adapted from 64)
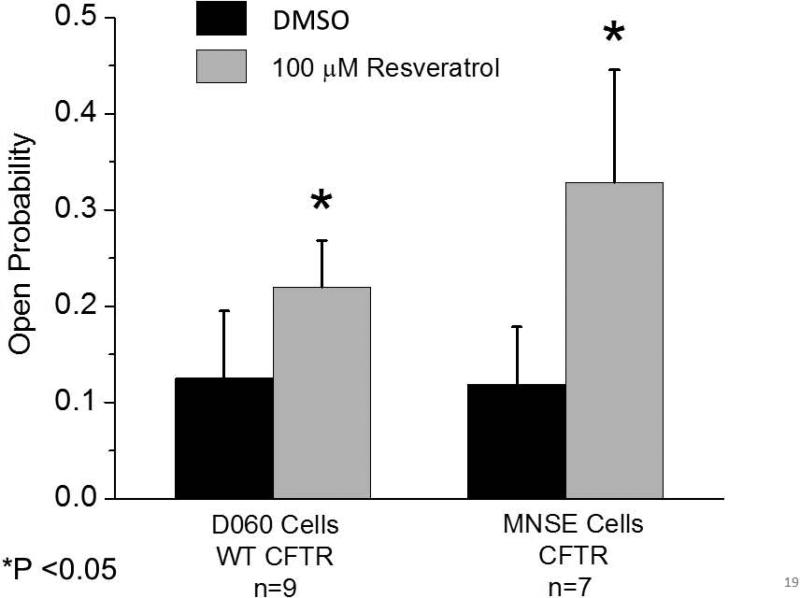
Resveratrol increases CFTR open probability
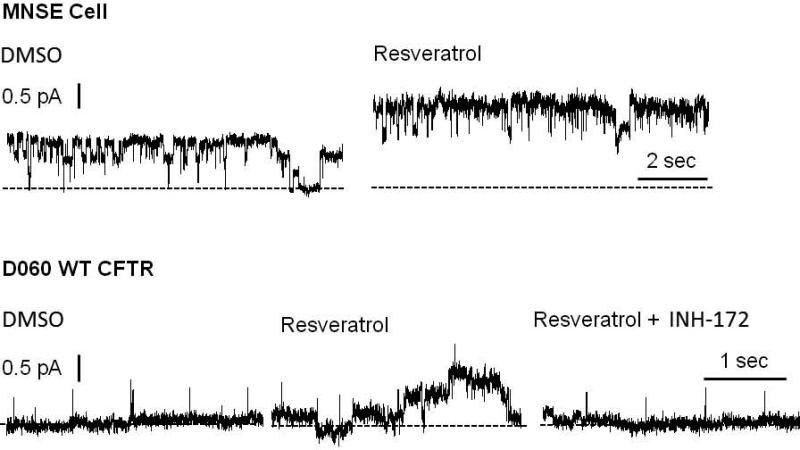
Once we recognized that resveratrol did not activate CFTR via PKA-dependent pathways, patch clamp techniques were required to evaluate impact on CFTR channel open time. Segments of the apical plasma membrane sealed within the tip of a patch pipette were evaluated after application of test compounds to the intracellular surface in the excised, inside-out patch clamp configuration. In patches from apical membranes of MNSE cells, resveratrol stimulated an ~8 pS chloride channel consistent with CFTR potentiation in the absence of ATP and PKA. These experiments are the first to characterize single channel CFTR in freshly isolated/low passage number sinonasal epithelia. Patches were excised and spontaneous activity recorded at −Vcom = +50 mV. Channel function was enhanced within seconds of administration of 100 μM resveratrol (Figure 9A, Upper Tracing). This observation was validated in D060/HEK293 cells expressing exogenous human WT-CFTR (Figure 9A, Lower Tracing). Under cell-free conditions, an increase in channel Po (rather than new channel insertion) was determined to account for CFTR activation. Complete inhibition of stimulated current by INH-172 further supports the finding that CFTR activity is amplified by resveratrol.
Figure 9. Resveratrol stimulates single CFTR channel activity.
(A) Under non-phosphorylating conditions, application of resveratrol enhanced channel activity in both MNSE (upper tracing) and D060/HEK293 cells expressing exogenous WT human CFTR (bottom tracing). Patches were excised and spontaneous channel function recorded at – Vcom = +50 mV. Activity was stimulated within seconds of application of 100 μM resveratrol. Similar enhancement was observed in human CFTR-expressing D060 cells and abrogated by addition of CFTR(inh)172. Dotted lines indicate zero current level. (B) Open probability (NPo/N where N represents channel number) was calculated from single channel recordings under control conditions and after perfusion with 100 μM resveratrol obtained in similar experiments to those displayed in (A). Data are presented as mean ± SEM. Data points were compared with control using paired Student's t test (*P<0.05). (Adapted from 54)
Open probability (NPo/N where N represents channel number) was calculated from single channel recordings following perfusion with 100 μM resveratrol and under control conditions. Open probability was significantly augmented following the application of resveratrol in MNSE cells (0.329 ± 0.116 vs. 0.119 ± 0.059 for control, p<0.05). This result was confirmed in D060/HEK293 cells expressing human WT-CFTR, where open probability was significantly increased compared to control (0.22 ± 0.048 vs. 0.125±0.07, p<0.05) (Figure 9B).
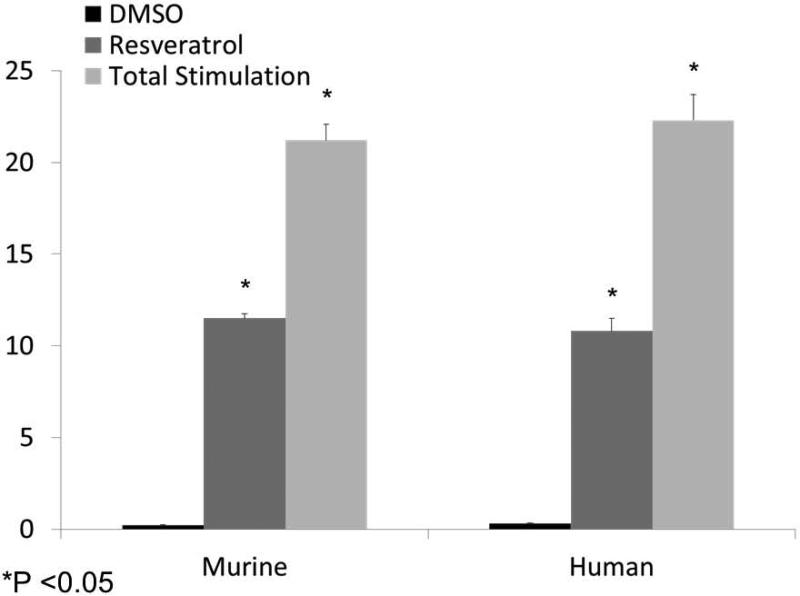
Resveratrol activates transepithelial Cl− secretion in hypoxic cells and reverses hypoxia-induced reduction of ASL depth
Following comprehensive evaluation of the Cl− secretagogue activity of resveratrol and its underlying mechanism of action, we next investigated the ability of the agent to compensate for the acquired defects in CFTR-mediated anion secretion demonstrated in hypoxic sinonasal epithelium. After 24 hours incubation in a hypoxic environment, MNSE and HSNE filters were evaluated in the Ussing chamber for the ability of resveratrol to stimulate CFTR-mediated anion transport. The drug significantly increased CFTR-dependent Cl− transport in MNSE [11.51+/−0.23 vs. 0.2+/− 0.05(control);p<0.05] and HSNE [10.8+/−0.7 vs. 0.3+/−0.05 (control); p<0.05] when compared to control vehicle (Figure 10). As a percentage of the maximal CFTR stimulus induced by forskolin (20 μM) in murine (21.2+/−0.89) and human (22.3+/−1.4) monolayers, resveratrol activated 54.3% and 48.4% of total ISC, respectively.
Figure 10. Resveratrol restores transepithelial Cl− transport in hypoxic cells.
MNSE and HSNE monolayers were subjected to 24 hours of hypoxia and the effect of resveratrol was evaluated in Ussing chambers. Resveratrol significantly increased ISC compared to vehicle controls in hypoxic murine (11.51+/−0.23 vs.0.2+/−0.05 (control);p<0.05) and human (10.8+/−0.7 vs. 0.3+/−0.05(control);p<0.05) monolayers; maximal CFTR stimulus induced by forskolin indicates resveratrol activated ISC to 54.3% and 48.4% in murine (21.2+/−0.89) and human (22.3+/−1.4) monolayers, respectively.
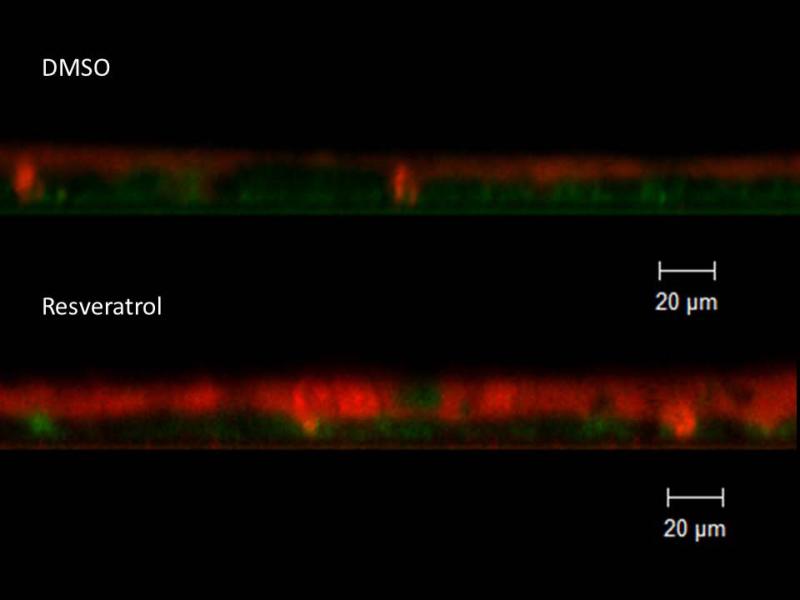
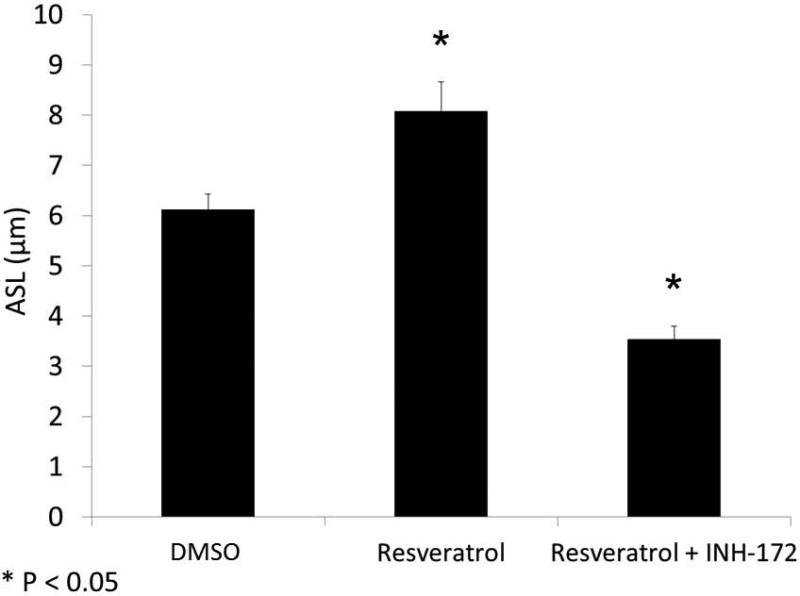
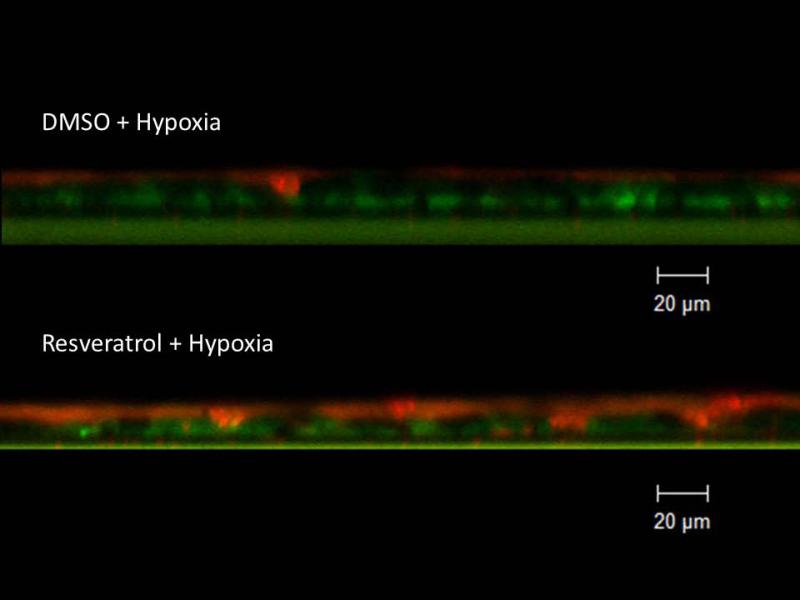
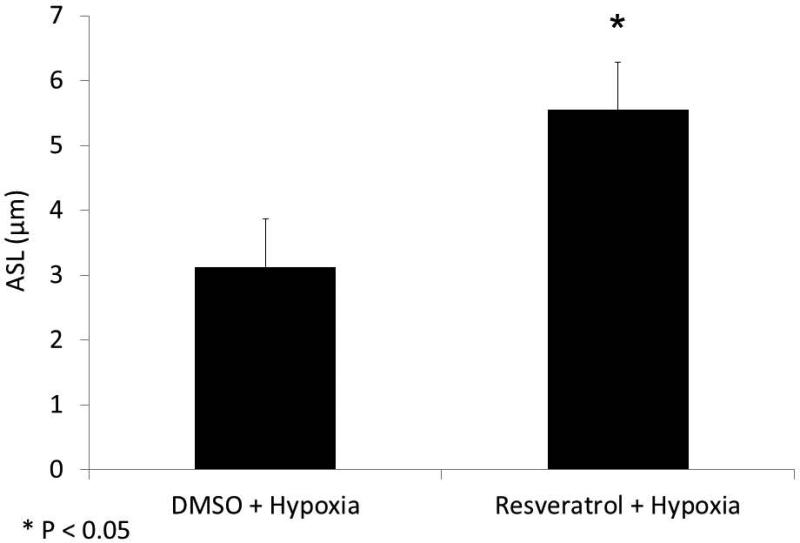
With evidence that resveratrol activates CFTR-mediated anion transport in cells that are deficient in the ability to secrete transepithelial Cl− due to acquired CFTR deficiency, we next assessed whether resveratrol mitigated the depleted ASL demonstrated in oxygen-restricted cultures. First, we established that Cl− secretagogue activity attributable to resveratrol would translate to hydration of ASL in epithelium compared to corresponding DMSO vehicle control in a normal oxygen environment. Resveratrol increased ASL depth in MNSE cultures after a 30 minute apical application (in μm: 8.08+/−1.68 vs. 6.11+/−0.47, DMSO control,p<0.05, n ≥ 5 per condition) (Figure 11) indicating a robust treatment effect resulting from stimulation of apical Cl− secretion and enhanced CFTR channel Po. ASL hydration was significantly diminished by addition of the INH-172 (3.54±0.34,p<0.05). INH-172 also suppresses constitutively activated CFTR, accounting for the overall decrease in ASL depth. The effects of drug were then measured on hypoxic MNSE and compared to the control solution. Hypoxia-induced abnormalities of fluid and electrolyte secretion in sinonasal epithelium were improved by a 30 minute application of resveratrol following 24 hours of hypoxia (5.55+/−0.74 vs. 3.13+/−0.17, n≥5 per condition,p<0.05) providing confirmation that the compound mitigates effects of acquired CFTR deficiency on depleted ASL (Figure 12).
Figure 11. Resveratrol hydrates ASL.
Resveratrol significantly increased ASL depth (in μm) in murine nasal septal epithelial cultures (8.08+/−1.68 vs. 6.11+/−0.47, control, *p<0.05). Addition of CFTR(inh)-172 inhibited resveratrol-dependent ASL accumulation (3.54+/−0.34, *p<0.05) (A). Confocal images demonstrate treatment effect from stimulation of apical Cl− secretion and enhanced CFTR channel Po (Red-ASL; Green-Cell marker) (B). (Adapted from 54)
Figure 12. Resveratrol restores hypoxia-depleted ASL.
Because apical fluid is applied with resveratrol treatment, hypoxia controls require the corresponding control (DMSO) vehicle. Resveratrol treatment significantly mitigated hypoxia-induced reductions in ASL depth (3.13+/−0.17 μm (DMSO + hypoxia) vs. 5.55+/−0.74 μm (resveratrol + hypoxia) p < 0.05), reaching 91% of the ASL depth found in normal MNSE monolayers incubated with DMSO (6.11+/−0.47, from Figure 11).
DISCUSSION
The data presented in the current study suggests that a low oxygen environment profoundly affects normal ion transport physiology in both MNSE and HSNE and leads to acquired defects in CFTR-mediated transport. While stimulation of transepithelial Cl− transport would be expected to hydrate the ASL, increased Na+ absorption (as demonstrated in CF) reduces water content leading to decreased clearance of dehydrated and, eventually, impacted mucus.13 Notably, ENaC channels exhibited a time dependent blockade as detected by amiloride-dependent ISC in MNSE. Thus, both Na+ absorption and Cl− secretion are reduced in the hypoxic murine model; an indication that the overall influence of hypoxia on the ASL could be mitigated by opposing effects on epithelial Na+ and Cl− transport (with a net effect of diminished ASL depth as measured in our experiments). HSNE exhibited a marked reduction in forskolin-stimulated ISC over 24 hours of low oxygen exposure. In contrast to the murine situation, however, Na+ absorption increased during the first 12 hours (as measured by amiloride blockade of ENaC). The finding that HSNE develops a globally decreased transepithelial Cl− secretion and increased Na+ absorption in response to hypoxic conditions indicates that in human sinonasal mucosa, the detrimental effects on ion that impact ASL hydration (Cl− and HCO3− secretion through CFTR; Na+ absorption through ENaC) are compounded by tissue hypoxia. Thus, the ion transport properties are consistent with CF airway pathophysiology (decreased Cl− secretion and enhanced Na+ absorption) resulting in dehydration of the ASL and, ultimately, disrupted mucociliary transport.
CFTR activation has been shown to confer excellent therapeutic benefit in CF, a disease characterized by defective MCC. The drug ivacaftor has recently been FDA approved for CF patients with at least one copy of the G551D mutation, but the agent is very expensive (approximately $300,000/year in the U.S.).59 Recent studies have also shown that ivacaftor augments ASL depth, accelerates MCC, and pharmacologically reverses acquired CFTR dysfunction due to cigarette smoke exposure.20 Given this precident, resveratrol represents an excellent candidate for treatment of acquired Cl− transport defects based on an established safety profile,60 activity equivalent to ivacaftor with regard to wild type human CFTR stimulation, and well described anti-inflammatory benefits. In addition, the working concentration of resveratrol was 100 μM, a drug concentration routinely achievable in topically applied, superperfused, or aerosolized solutions in human subjects in vivo.61,62 Single channel patch clamp analysis indicates the mechanism underlying resveratrol activity is CFTR channel potentiation; channel open probability increases with drug application as demonstrated in both wild type MNSE cells and a recombinant human CFTR expressing cell line. Resveratrol's conserved activity across several mammalian species also provides a means to study MCC activation in animal models [unlike ivacaftor, which has no effect on murine or porcine CFTR in primary nasal airway epithelium (data not shown)].31
Because resveratrol is a robust CFTR channel potentiator in sinonasal epithelia, we hypothesized that the drug could ameliorate acquired CFTR dysfunction conferred by hypoxic incubation. The compound effectively stimulated transepithelial anion secretion in hypoxic epithelial cells and increased ASL thickness in both control and hypoxic monolayers, indicating that 1) the increase in hydration is most likely attributable to potentiating effects on CFTR channels and 2) acquired CFTR dysfunction represents a therapeutic target that can be addressed with resveratrol and/or other Cl− secretagogues. Activation that is independent of PKA provides additional evidence of direct CFTR binding of the compound and the potentiating effects of the drug represent an optimal approach to directly stimulate CFTR, fluid and electrolyte transport, and ASL hydration. Since acquired defects of CFTR in chronic diseases such as sinusitis can result in both decreased channel expression and increased turnover in nasal airway epithelium,63 potentiating CFTR channels already situated in the plasma membrane could serve as an effective strategy for reversing ASL dehydration in individuals with CRS. .
CONCLUSION
Hypoxia-induced acquired CFTR dysfunction can be ameliorated by resveratrol potentiation of CFTR channels, resulting in improved epithelial function. CFTR activation with Cl− secretagogues such as resveratrol represents an innovative approach to overcoming acquired CFTR defects in sinus and nasal airway disease. Additional studies to define the role of CFTR activation by resveratrol are indicated in CRS and could result in novel and improved therapeutic strategies for the disease.
Figure 3. ASL depth is decreased in hypoxic MNSE.
Because CFTR secretes Cl− ions to regulate ASL depth, an important determinant of normal MCC, we evaluated whether hypoxic incubation for 24 hours would induce ASL depletion in MNSE by confocal 28 microscopy (Panel A represents confocal images) Incubation in 1% O2 decreased ASL depth compared with control monolayers (4.19+/−0.44 (hypoxia) vs. 6.88+/−0.67 μm (21% O2 control); p< 0.05) (B).
ACKNOWLEDGEMENTS
Shaoyan Zhang, Ph.D. – procurement of data; Daniel Skinner B.S. – procurement of data; Carmel McNicholas, Ph.D. – expertise in patch clamp technique; John C. Kappes, M.D. – generous gift of D060 cells; Eric J. Sorscher, M.D. – mentorship.
Funding: NIH/NHLBI (1K08HL107142-05) and NIH/NIDDK (5P30DK072482-04)
Footnotes
Presented at the Combined Otolaryngology Spring Meetings, Boston, Ma. 2015, Winner of the Edmund Prince Fowler Award.
Conflict of Interest: Bradford Woodworth, MD is an inventor on a patent pending regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c)) in the United States Patent and Trademark Office.
REFERENCES
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 2.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–109. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MP, Fishman P, Short SO, Sullivan SD, Yueh B, Weymuller EA., Jr. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg. 2002;127:367–376. doi: 10.1067/mhn.2002.129815. [DOI] [PubMed] [Google Scholar]
- 4.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 5.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274:L258–263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JE, Wong LB, Yeates DB. Bidirectional transepithelial water transport: measurement and governing mechanisms. Biophys J. 1999;76:869–877. doi: 10.1016/S0006-3495(99)77250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowles M, Gatzy J, Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983;71:1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014;20:623–631. doi: 10.1097/MCP.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013;27:473–478. doi: 10.2500/ajra.2013.27.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27:387–395. doi: 10.2500/ajra.2013.27.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virgin F, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for severe, recalcitrant cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy. 2012:26. doi: 10.2500/ajra.2012.26.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 14.Cobb BR, Ruiz F, King CM, et al. A(2) adenosine receptors regulate CFTR through PKA and PLA(2). Am J Physiol Lung Cell Mol Physiol. 2002;282:L12–25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- 15.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 16.MacEachran DP, Stanton BA, O'Toole GA. Cif is negatively regulated by the TetR family repressor CifR. Infect Immun. 2008;76:3197–3206. doi: 10.1128/IAI.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacEachran DP, Ye S, Bomberger JM, et al. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2007;75:3902–3912. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bebok Z, Varga K, Hicks JK, et al. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP- mediated Cl- secretion in airway epithelia. J Biol Chem. 2002;277:43041–43049. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 19.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 20.Sloane PA, Shastry S, Wilhelm A, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 22.Bomberger JM, Ye S, Maceachran DP, et al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011;7:e1001325. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122:1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baroody FM. Mucociliary transport in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:103–119. [PubMed] [Google Scholar]
- 26.Pahl A, Szelenyi S, Brune K. Hypoxia induced chemokine expression in nasal epithelial cells: development of an in vitro model for chronic rhinosinusitis. ALTEX. 2006;23:59–63. [PubMed] [Google Scholar]
- 27.Steinke JW, Woodard CR, Borish L. Role of hypoxia in inflammatory upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:16–20. doi: 10.1097/ACI.0b013e3282f3f488. [DOI] [PubMed] [Google Scholar]
- 28.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aanaes K, Rickelt LF, Johansen HK, et al. Decreased mucosal oxygen tension in the maxillary sinuses in patients with cystic fibrosis. J Cyst Fibros. 2011;10:114–120. doi: 10.1016/j.jcf.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- 33.Luo J, Zhu T, Evagelidis A, Pato MD, Hanrahan JW. Role of protein phosphatases in the activation of CFTR (ABCC7) by genistein and bromotetramisole. Am J Physiol Cell Physiol. 2000;279:C108–119. doi: 10.1152/ajpcell.2000.279.1.C108. [DOI] [PubMed] [Google Scholar]
- 34.Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- 35.Springsteel MF, Galietta LJ, Ma T, et al. Benzoflavone activators of the cystic fibrosis transmembrane conductance regulator: towards a pharmacophore model for the nucleotide-binding domain. Bioorg Med Chem. 2003;11:4113–4120. doi: 10.1016/s0968-0896(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 36.Galietta LJ, Springsteel MF, Eda M, et al. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 37.Caci E, Folli C, Zegarra-Moran O, et al. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol Lung Cell Mol Physiol. 2003;285:L180–188. doi: 10.1152/ajplung.00351.2002. [DOI] [PubMed] [Google Scholar]
- 38.Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- 39.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melin P, Thoreau V, Norez C, Bilan F, Kitzis A, Becq F. The cystic fibrosis mutation G1349D within the signature motif LSHGH of NBD2 abolishes the activation of CFTR chloride channels by genistein. Biochem Pharmacol. 2004;67:2187–2196. doi: 10.1016/j.bcp.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Pyle LC, Ehrhardt A, Mitchell LH, et al. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am J Physiol Lung Cell Mol Physiol. 2011;301:L587–597. doi: 10.1152/ajplung.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti- asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 44.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF- induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 45.Woodworth BA, Antunes MB, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23:149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 47.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:235–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 48.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 49.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol. 2007;21:533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 50.Dean N, Ranganath NK, Jones B, et al. Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis. Int Forum Allergy Rhinol. 2014;4:565–570. doi: 10.1002/alr.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:e104090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazrak A, Jurkuvenaite A, Ness EC, et al. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am J Respir Cell Mol Biol. 2014;50:953–962. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon GM, Frederick C, Zhang S, et al. IP-10 Is a Potential Biomarker of Cystic Fibrosis Acute Pulmonary Exacerbations. PLoS One. 2013;8:e72398. doi: 10.1371/journal.pone.0072398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PLoS One. 2013;8:e81589. doi: 10.1371/journal.pone.0081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 56.Pyle LC, Fulton JC, Sloane PA, et al. Activation of CFTR by the Flavonoid Quercetin: Potential Use as a Biomarker of {Delta}F508 CFTR Rescue. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moran O, Zegarra-Moran O. A quantitative description of the activation and inhibition of CFTR by potentiators: Genistein. FEBS Lett. 2005;579:3979–3983. doi: 10.1016/j.febslet.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khanna D, Sethi G, Ahn KS, et al. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Noone PG, Hamblett N, Accurso F, et al. Safety of aerosolized INS 365 in patients with mild to moderate cystic fibrosis: results of a phase I multi-center study. Pediatr Pulmonol. 2001;32:122–128. doi: 10.1002/ppul.1098. [DOI] [PubMed] [Google Scholar]
- 62.Solomon GM, Konstan MW, Wilschanski M, et al. An international randomized multicenter comparison of nasal potential difference techniques. Chest. 2010;138:919–928. doi: 10.1378/chest.10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope. 2011;121:1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope. 2011;121:1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]