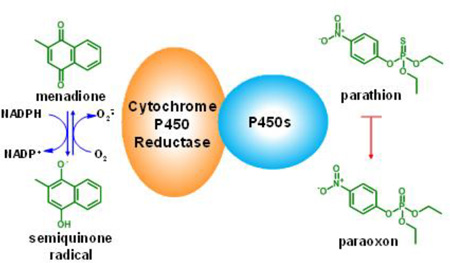
Abstract
Parathion, a widely used organophosphate insecticide, is considered a high priority chemical threat. Parathion toxicity is dependent on its metabolism by the cytochrome P450 system to paraoxon (diethyl 4-nitrophenyl phosphate), a cytotoxic metabolite. As an effective inhibitor of cholinesterases, paraoxon causes the accumulation of acetylcholine in synapses and overstimulation of nicotinic and muscarinic cholinergic receptors, leading to characteristic signs of organophosphate poisoning. Inhibition of parathion metabolism to paraoxon represents a potential approach to counter parathion toxicity. Herein, we demonstrate that menadione (methyl-1,4-naphthoquinone, vitamin K3) is a potent inhibitor of cytochrome P450-mediated metabolism of parathion. Menadione is active in redox cycling, a reaction mediated by NADPH-cytochrome P450 reductase that preferentially uses electrons from NADPH at the expense of their supply to the P450s. Using human recombinant CYP 1A2, 2B6, 3A4 and human liver microsomes, menadione was found to inhibit the formation of paraoxon from parathion. Administration of menadione bisulfite (40 mg/kg, ip) to rats also reduced parathion-induced inhibition of brain cholinesterase activity, as well as parathion-induced tremors and the progression of other signs and symptoms of parathion poisoning. These data suggest that redox cycling compounds, such as menadione, have the potential to effectively mitigate the toxicity of organophosphorus pesticides including parathion which require cytochrome P450-mediated activation.
Keywords: parathion, cytochrome P450 system, cytochrome P450 reductase, menadione, redox cycling
Graphical abstract
Introduction
There is increasing concern about exposure to toxic chemicals as a consequence of a deliberate terrorist attack, or by accident or natural disaster (Jett and Yeung, 2010). Chemicals that are readily obtainable and particularly toxic to humans include organophosphorus insecticides such as parathion, methyl parathion, azinphos-methyl, and disulfoton. Indeed, parathion, which has a rat oral LD50 of 2–13 mg/kg (Gaines, 1960), has been shown to cause severe poisoning in humans after intentional or accidental ingestion (Ferrer and Cabral, 1995). The toxicity of parathion is dependent on its metabolism by the cytochrome P450 (CYP) system to paraoxon (diethyl 4-nitrophenyl phosphate), a highly effective acetylcholinesterase (AChE) inhibitor (Neal, 1967). This leads to an accumulation of acetylcholine in synapses and overstimulation of nicotinic and muscarinic cholinergic receptors throughout the body. Ultimately, these effects elicit the classic signs of cholinergic crisis including convulsions, tremors, miosis, bradycardia, and increased secretions (Jett and Richardson, 2009).
Currently, there are two major approved treatments for organophosphate poisoning; atropine, a competitive antagonist of muscarinic acetylcholine receptors, and pralidoxime (2-PAM), which binds to organophosphate-inactivated acetylcholinesterase and regenerates the enzyme. Both of these drugs have limitations (Lotti, 1991); atropine is not a true antidote, since it only blocks the effects of cholinesterase (ChE) inhibition on muscarinic receptor hyperexcitation and does not prevent the toxicity associated with excessive stimulation of nicotinic acetylcholine receptors. Furthermore, 2-PAM, does not cross the blood-brain barrier and oxime efficacy in cases of severe organophosphate poisonings has been questioned (de Silva et al., 1992). In this regard, a recent large randomized controlled trial showed that pralidoxime did not improve, and slightly decreased, survival in patients with organophosphate insecticide poisoning (Eddleston et al., 2009); this may be the result of 2-PAM being unable to overcome the massive AChE inhibition caused by rapid conversion of parathion to the oxon. The lack of central nervous system efficacy by standard therapies is of concern because the most life-threatening symptoms following parathion intoxication (i.e., convulsions and respiratory depression) are centrally mediated. Thus, there remains a pressing need to develop new more efficacious therapies that can be used to treat parathion poisoning.
As parathion metabolism via CYP is required for toxicity, prevention of this activity may represent a viable mechanism for reducing its toxic effects and this was considered in early mechanistic studies. A variety of compounds with different mechanisms of action were shown to decrease paraoxon formation in rat liver microsomes (Neal, 1967). Additionally, pretreatment of mice with the CYP inhibitor, SKF525A, blocked parathion metabolism to paraoxon (O'Brien, 1961), and parathion-induced death (Welch and Coon, 1964). Although a number of CYP inhibitors have been described (Anders, 1971; Netter, 1980), none has been approved by the Food and Drug Administration to treat organophosphorus insecticide poisoning. For metabolic activities, CYP enzymes require NADPH cytochrome P450 reductase (CPR), which transfers reducing equivalents from NADPH to the CYPs (Riddick et al., 2013). CPR also mediates chemical redox cycling, a process by which redox active compounds are enzymatically reduced to radical anions (Wang et al., 2008; Wang et al., 2010). Under aerobic conditions, these anions reduce molecular oxygen to form superoxide anion and regenerate the uncharged parent compound. Superoxide anion rapidly dismutates to hydrogen peroxide (H2O2) (Lushchak, 2014). Menadione (2-methylnaphthalene-1,4-dione) or vitamin K3, readily redox cycles with CPR (Nishibayashi-Yamashita and Sato, 1970). In this process, menadione undergoes a one electron reduction forming an unstable semiquinone radical that reacts with oxygen to regenerate menadione. In the present studies, we tested the hypothesis that by redox cycling with CPR, menadione will divert electrons from CYP-mediated parathion metabolism, blocking the bioactivation of parathion to paraoxon and preventing toxicity.
Materials and Methods
Materials
Recombinant human acetylcholinesterase, superoxide dismutase, horseradish peroxidase, NADPH, menadione, acetylthiocholine chloride, 5,5′-dithiobis(2-nitrobenzoic acid), 7-ethoxyresorufin, tetraisopropyl pyrophosphoramide, and coumarin were purchased from Sigma (St. Louis, MO). Parathion, paraoxon, and diethylthiophosphate were from Chem Service Inc. (West Chester, PA). Amplex Red reagent was from Molecular Probes (Eugene, OR). Recombinant human CYPs (CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5 and CYP 3A7), and pooled human liver microsomes were purchased from BD Gentest (Woburn, MA). Recombinant human CPR, Vivid P450 substrates, 7-ethoxy-methyloxy-3-cyanocoumarin (EOMCC) and 7-benzyloxy-methyloxy-3-cyanocoumarin (BOMCC), 7-methoxy-4-trifluoromethyl coumarin and dibenzylfluorescein were from Life Technologies (Grand Island, NY). Glucose-6-phosphate and glucose-6-phosphate dehydrogenase were from Roche Diagnostic (Indianapolis, IN).
Cytochrome P450 assays
The CYP activities of recombinant enzymes were determined using fluorogenic substrates as described (McLaughlin et al., 2008). Reactions were carried out at room temperature in black 96-well plates with a final volume of 100 µl containing potassium phosphate (100 mM; pH 7.4), menadione (1–100 µM) or vehicle, an NADPH regenerating system (100 µM NADPH, 10 mM glucose-6-phosphate and 0.5 unit/ml glucose-6-phosphate dehydrogenase), substrate, and recombinant CYPs (see Supplemental Table 1 for assay details). The final concentration of organic solvent in the reaction mixture was less than 1% (v/v). After a 3-min preincubation with menadione or vehicle control at room temperature, the reactions were initiated by the addition of the NADPH regenerating system. Formation of fluorescent metabolites were monitored every 30 s for 10 min using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA).
Assays for NADPH oxidation and redox cycling activity
Oxidation of NADPH in reaction mixtures was assessed by measuring decreases in absorbance at 340 nm and quantified using an extinction coefficient of 6.2 mM−1 cm−1. Reaction mixtures in a total volume of 100 µl consisted of 0.1 pmol recombinant human CPR, 0.25 mM NADPH, 1 mM EDTA, 50 mM KCl, and 50 mM potassium phosphate buffer (pH 7.0), in the presence and absence of menadione (1–100 µM).
CPR-mediated menadione redox cycling in enzyme assays was measured by the formation of superoxide anion, H2O2, and hydroxyl radicals. Superoxide anion was measured using the dihydroethidium assay as previously described (Nazarewicz et al., 2013) with modifications. Reactions were run in a total volume of 100 µl and contained 0.05 pmol recombinant human CPR, 50 mM potassium phosphate buffer, pH 7.4, 0.25 mM NADPH, menadione (0.1–10 µM) or vehicle control, and 20 µM dihydroethidium. The generation of 2-hydroxyethidium was monitored fluorometrically at 37 °C using excitation and emission wavelengths of 405 nm and 570 nm, respectively.
H2O2 was measured using either the Amplex Red assay, or by its conversion to hydroxyl radicals in the presence of ferric ion using the terephthalate assay (Wang et al., 2008; Wang et al., 2010). For Amplex Red assays, reactions were run at 37 °C in a total volume of 100 µl in 50 mM potassium phosphate buffer, pH 7.8, 0.05 pmol recombinant human CPR, 0.25 mM NADPH, 1 unit/ml HRP, menadione (0.5–100 µM) or vehicle control, and 50 µM Amplex Red reagent. The reaction was initiated by the addition of CPR and product formation analyzed fluorometrically using an excitation and emission wavelengths of 530 nm and 587 nm, respectively. To measure hydroxyl radicals, the reaction was supplemented with 1 mM terephthalate and Fe2+ (100 µM)/EDTA (110 µM) complex in place of Amplex Red and HRP. After 30 min incubation at 37 °C, the reaction was terminated by the addition of an equal volume of ice-cold methanol. After centrifugation at 12,000 g for 10 min, the supernatant was analyzed for 2-hydroxyterephthalate by reverse-phase HPLC as previously described (Mishin and Thomas, 2004).
HPLC analysis of parathion and paraoxon
Human liver microsomes (0.5 mg/ml) or recombinant CYPs (60 nM) were incubated at 37 °C in 100 mM phosphate buffer, pH 7.4, containing 50 µM tetraisopropyl pyrophosphoramide, 1.25 mM EDTA, parathion (20 µM), menadione (5–100 µM) or acetonitrile vehicle. Following a 3-min preincubation, an NADPH regenerating system (100 µM NADPH, 10 mM glucose-6-phosphate, 0.5 unit/ml glucose-6-phosphate dehydrogenase) was added to initiate the reaction. After 1–3 h, reactions were terminated by the addition of one volume of ice-cold methanol containing 0.1% phosphoric acid. Samples were centrifuged at 12,000 g for 5 min and supernatants analyzed by HPLC using a Maxsil 10 C18 column (250 × 4 mm, 5 µ; Phenomenex, Torrance, CA) with gradient elution, at a flow rate of 0.75 ml/min. The initial mobile phase of 40% methanol (buffer A) and 60% water:acetonitrile:phosphoric acid (99.49:0.5:0.01,v/v/v) (buffer B) was held for 3 min, followed by a linear gradient to 100% buffer A at 33 min, and held at this composition for an additional 2 min. Paraoxon and parathion were detected at 275 nm and eluted at 15.8 and 20.6 min, respectively. Diethylthiophosphate and p-nitrophenol were detected at 320 nm and eluted at 19.1 and 10.1 min.
Animal studies, neurobehavioral screening, metabolic profiling, and enzyme assays
Animals received humane care in compliance with the institution’s guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by the Rutgers Robert Wood Johnson Institutional Animal Care and Use Committee. Parathion (8 mg/kg) was dissolved in corn oil and administered to 3-month old female Long-Evans rats (Charles River, Malvern, PA) by oral gavage. This dose of parathion is 4 times the LD50 and produced signs and symptoms of cholinergic hyperstimulation (tremulous jaw movements and enhanced startle response) as early as 20 min after administration. Menadione bisulfite was dissolved in saline (0.9% NaCl) and administered by intraperitoneal injection (40 mg/kg) 20 min after parathion. The 20 min time point following OP exposure was chosen as this represented a time period prior to the onset of OP toxicity.
The Functional Observational Battery (FOB), developed for neurobehavioral screening, consists of home cage, open field and other behavioral evaluations, has been validated using parathion as a model organophosphate (Moser, 1995). A modified FOB in the home cage was used to assess sensorimotor behavior (startle response to a click next to the ear), tremulous jaw movements, and tremors following parathion administration. Animals were observed by 2 individuals blinded to treatment (parathion or parathion + menadione) and the FOB measurement recorded immediately prior to administration of parathion and again immediately before sacrifice. FOB endpoints for tremulous jaw movement and tremors were scored by severity on a 1–4 scale, with 1 representing no observance of these behaviors to 4 representing the response occurring during the entire observation period. Likewise, sensorimotor behavior was scored based on startle response, with a score of 1 representing a small flinch of the head and a score of 4 representing an exaggerated response in which the animal jumped back away from the sound.
All rats were sacrificed by decapitation 60 min after administration of parathion. Forebrain, hindbrain and liver were collected and snap frozen in liquid nitrogen. Trunk blood was collected, allowed to clot on ice, and centrifuged at 1,500 g for serum preparation. Tissue preparation and assay of brain ChE and liver and serum carboxylesterase (Ces) activities were described previously (Richardson and Chambers, 2003; Richardson and Chambers, 2004). Assays were modified to reduce the volumes for spectrophotometric detection in 96-well plates using a plate reader. ChE activity used acetylthiocholine as the substrate and DTNB as the chromogen with detection at 412 nm. Liver and serum Ces activities were determined using p-nitrophenylvalerate as the substrate with detection at 405 nm. Changes in optical density were determined every 10 s for 5 min in a SpectraMax5 plate reader. Preliminary experiments defined conditions for incubation times and protein concentrations required for linear rates of substrate hydrolysis. Non-specific hydrolysis was determined in the presence of 10 µM eserine for ChE and 10 µM paraoxon for Ces.
For parathion metabolic profiling, brain and hepatic S9 fractions were prepared by differential centrifugation. Aliquots of plasma samples (0.3–0.5 ml) and S9 fractions from brain or liver tissues were mixed with 50 µl of internal standard (0.2 mg/ml YH439, Sigma) and extracted with 1.5 ml of ethyl acetates two times. The organic phase was removed, dried, reconstituted in 250 µl of acetonitrile and then analyzed by HPLC-UV as described.
For the preparation of liver microsomes, rat livers were washed with 0.9% NaCl and homogenized in Tris buffer (50 mM Tris-HCl, 1.15% KCl, pH 7.4) containing protease inhibitors using a Teflon homogenizer. The homogenates were centrifuged at 9,000 g at 4 °C for 20 min and the supernatants were centrifuged at 105,000 g for 90 min. The pellet was washed with EDTA buffer (1.15% KCl, 10 mM EDTA, pH 7.4) and centrifuged at 105,000 g for 30 min. The resulting pellet, containing microsomal proteins, was homogenized in 0.25 M sucrose. Microsomes from 9 rats were pooled and protein concentrations determined using the bicinchoninic acid method (BCA; Thermo Scientific, Rockford, IL) with bovine serum albumin as a standard.
Data analysis
ChE activity, P450 fluorescent metabolite formation, superoxide anion and H2O2 formation were monitored over 10–30 min, and the initial velocities analyzed using SoftMax Pro software (Molecular Devices). IC50 values were determined by the nonlinear regression method of curve fitting using Prism 5 software (GraphPad Inc., San Diego, CA). Statistical differences were determined using the Student’s t test or one-way ANOVA where appropriate. For ANOVA, if a significant F was determined, means were compared using the Student-Neuman Keuls post hoc test. A value of p < 0.05 was considered significant.
Results
Menadione redox cycling inhibits CYP-mediated reactions
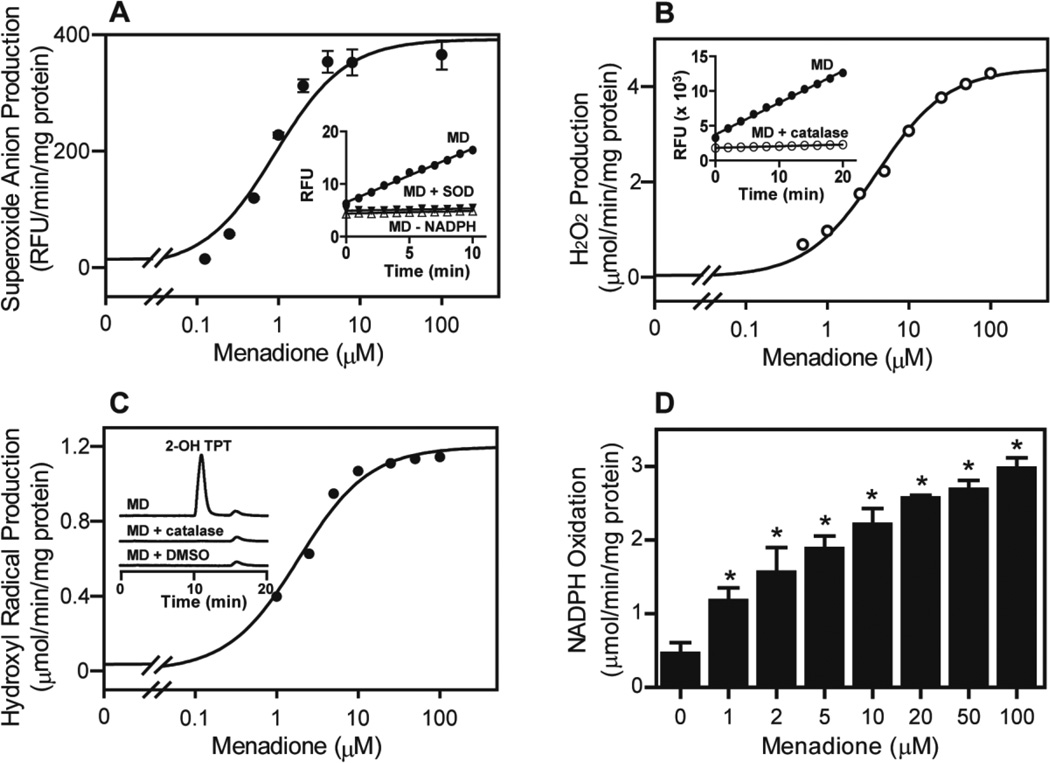
In initial experiments, menadione redox cycling by recombinant human CPR was characterized. Menadione readily generated superoxide anion, H2O2, and hydroxyl radicals in a time-and concentration-dependent manner; redox cycling by menadione was also NADPH-dependent (Fig. 1). Superoxide anion formation and the accumulation of H2O2 in enzyme assays were prevented by superoxide dismutase and catalase, respectively. Hydroxyl radical formation was efficiently suppressed by catalase and DMSO. The Km and Vmax for H2O2 formation with menadione were 4.1 µM and 4.4 µmol/min/mg CPR protein, respectively, and formation of 2-hydroxyterephthalate, a marker of hydroxyl radicals, 1.7 µM and 1.2 µmol/min/mg CPR protein, respectively. Consistent with the requirement of NADPH in the redox cycling reaction, an increase in NADPH oxidation in reaction mixes was observed following increasing concentrations of menadione (Fig. 1D).
FIG 1. Redox cycling of menadione (MD) by recombinant human NADPH cytochrome P450 reductase.
A, Menadione generated superoxide anion by cytochrome P450 reductase (CPR). CPR was incubated with menadione and an NADPH regenerating system. Superoxide anion formation was measured by the dihydroethidium assay. Inset: Time-dependent formation of superoxide anion by 100 µM menadione. The formation of superoxide anion was dependent on NADPH and inhibited by superoxide dismutase (SOD, 50 units/ml). B, Menadione generated H2O2 by CPR. CPR was incubated with increasing concentrations of menadione and the production of H2O2 monitored using the Amplex Red assay. Inset: Time-dependent H2O2 formation by CPR in the presence of 100 µM menadione. Catalase (500 units/ml) inhibited the accumulation of H2O2. C, Menadione generated hydroxyl radicals by CPR in the presence of iron/EDTA. Hydroxyl radicals were measured using the terephthalate assay (Mishin and Thomas, 2004) and the product, 2-hydroxylterephthalate (2-OH TPT), was analyzed by HPLC. Inset shows HPLC tracings of 2-OH TPT formation in the presence of 100 µM menadione. Catalase and DMSO were effective in inhibiting the formation of hydroxyl radicals. D, Effects of menadione on NADPH oxidation. Reaction mixes consisted of CPR (200 nM), NADPH (0.25 mM), menadione (1–100 µM) or vehicle control. NADPH utilization in reaction mixes was followed by decreases in absorbance at 340 nm. Data are mean ± SE (n = 3). *Significantly different (p < 0.05) from vehicle-treated control.
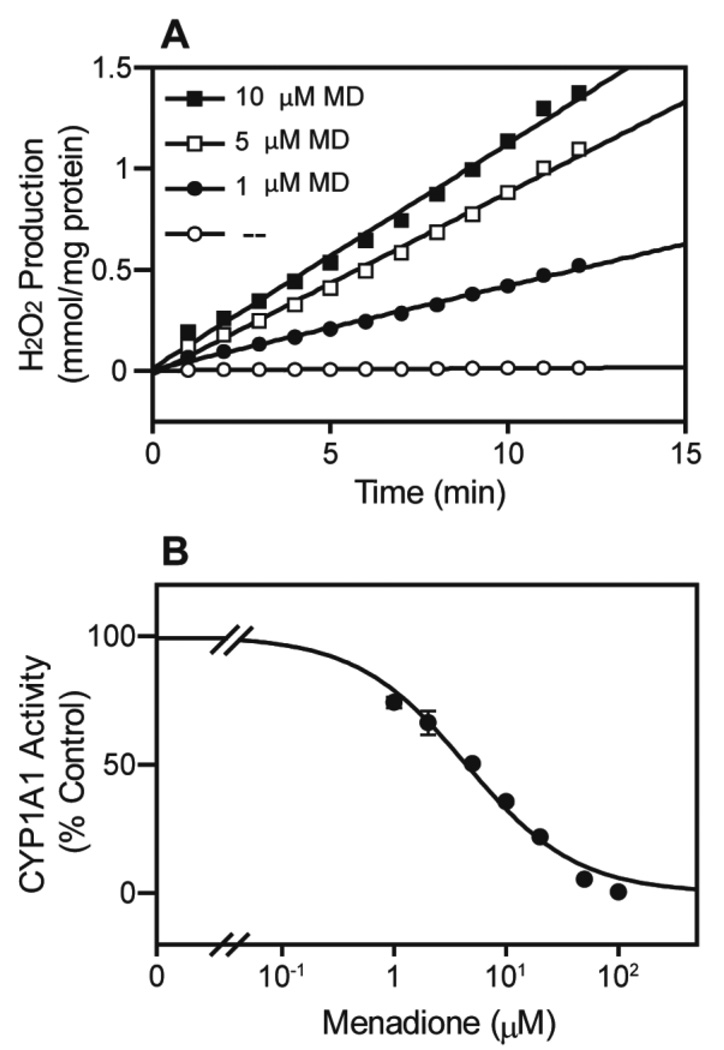
Menadione was also found to redox cycle with recombinant human CPR co-expressed with CYP1A1 (Fig. 2A). This was associated with a marked concentration-dependent inhibition of CYP1A1 metabolic activity, as measured by its 7-ethoxyresorufin-O-deethylase activity (Fig. 2B and Table 1). Similar inhibition by menadione of CYP-mediated metabolism was observed in 13 different recombinant human CYPs. Thus, menadione blocked CYP-mediated metabolism in the micromolar concentration (IC50 = 2.36–9.77 µM; Table 1). CYP2C8 was the most sensitive to menadione (IC50 = 2.36 ± 0.11 µM), while CYP3A5 was the least sensitive (IC50 = 9.77 ± 3.33 µM). Taken together, these data indicate that as a result of redox cycling, menadione inhibits CYP-mediated drug metabolism.
FIG 2. Effects of menadione on redox cycling and 7-ethoxyresorufin metabolism by recombinant human CYP1A.
Recombinant human CYP1A1 (4 pmol) was incubated with menadione (1–100 µM) and an NADPH regenerating system (100 µM NADPH, 10 mM glucose-6-phosphate and 0.5 unit/ml glucose-6-phosphate dehydrogenase) in a final volume of 100 µl. Redox cycling was measured by the formation of H2O2 (A) and was determined by the Amplex Red assay. The activity of CYP1A1 (B) was determined as 7-ethoxyresorufin O-deethylase activity with 5 µM 7-ethoxyresorufin as the substrate.
TABLE 1.
Inhibition of CYPs by Menadione.
| Recombinant CYPs1 | |||||
|---|---|---|---|---|---|
| Supersomes™ | Substrate | IC50 (µM) | Supersomes™ | Substrate | IC50 (µM) |
| CYP1A1 | 7-ethoxyresorufin | 4.39 ± 0.42 | CYP2C19 | Vivid EOMCC | 8.51 ± 0.38 |
| CYP1A2 | 7-methoxyresorufin | 4.51 ± 0.57 | CYP2D6 | Vivid EOMCC | 4.60 ± 0.87 |
| CYP1B1 | 7-ethoxyresorufin | 7.02 ± 0.53 | CYP2E1 | 7-methoxy-4-trifluoromethylcoumarin | 5.58 ± 1.22 |
| CYP2A6 | coumarin | 8.01 ± 0.62 | CYP3A4 | dibenzylfluorescein | 8.24 ± 0.71 |
| CYP2B6 | Vivid EOMCC | 5.76 ± 0.78 | CYP3A5 | Vivid BOMCC | 9.77 ± 3.33 |
| CYP2C8 | Vivid BOMCC | 2.36 ± 0.11 | CYP3A7 | Vivid BOMCC | 5.92 ± 0.81 |
| CYP2C9 | Vivid BOMCC | 8.91 ± 0.57 | |||
The activity of recombinant CYPs (BD Biosciences, San Jose, CA) was determined using different fluorogenic substrates (McLaughlin et al., 2008) in the absence and presence of increasing concentrations of menadione. The IC50‘s are the concentrations of menadione inhibiting metabolic activity by 50%. A typical reaction mixture contained 5–50 nM P450, menadione (1–100 µM), selective substrates and an NADPH regenerating system (100 µM NADPH, 10 mM glucose-6-phosphate and 0.5 unit/mL glucose-6-phosphate dehydrogenase). Data are mean ± SE, n = 3.
Menadione reduces AChE inhibition by parathion and metabolites in vitro
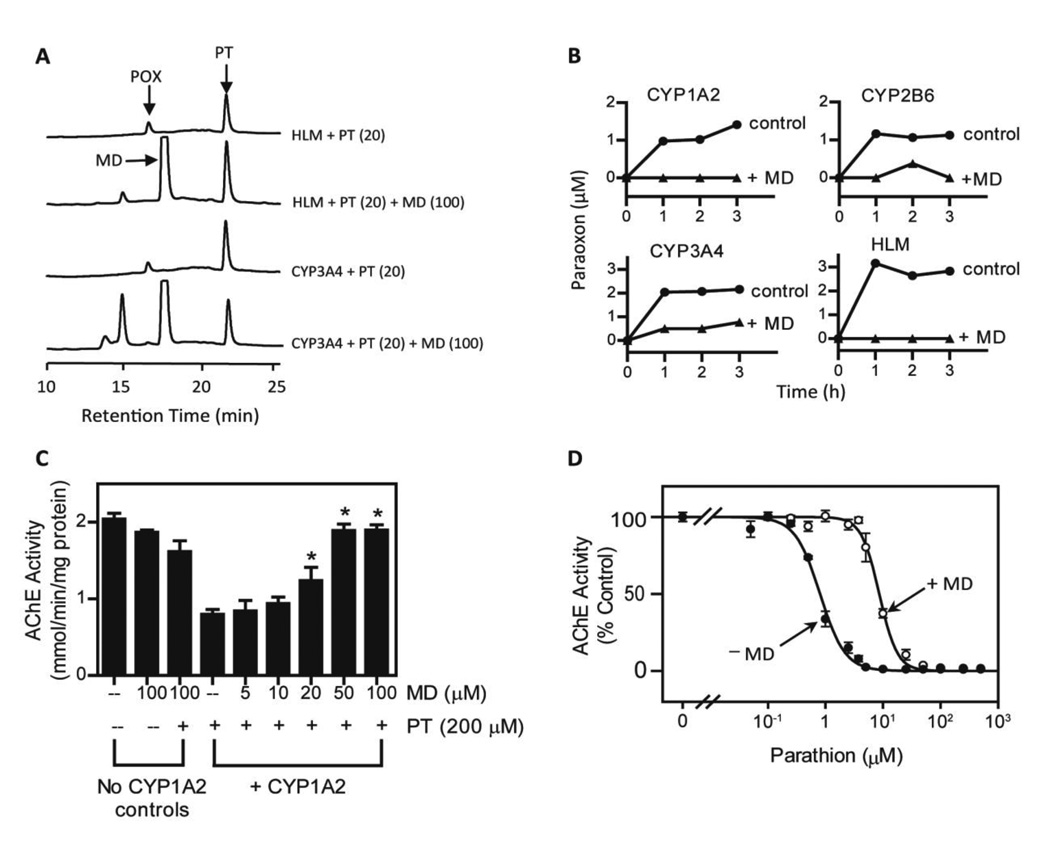
CYPs are known to metabolize parathion to its reactive intermediate, paraoxon. Since menadione was effective in inhibiting CYPs, we next determined if this redox cycling quinone could inhibit parathion metabolism. Three human recombinant CYPs (CYP1A2, 2B6, and 3A4) and human liver microsomes, all of which metabolize parathion, were found to generate paraoxon (Fig. 3A and B). A marked decrease in paraoxon formation was noted in reaction mixes containing menadione. These data are consistent with our findings that CYP1A2 mediated the transformation of parathion to a metabolite that inhibited recombinant human AChE (Fig. 3C). Menadione was found to protect against AChE dysfunction in a concentration-dependent manner (Fig. 3C); 50 µM menadione completely abolished CYP1A2 mediated activation of parathion and AChE inhibition. Menadione also reduced AChE inhibition after parathion activation by rat liver microsomes; a ten-fold increase in IC50 value was detected in microsomal incubation mixtures containing menadione (Fig. 3D, 0.80 ± 0.04 and 8.61 ± 0.38 µM without and with menadione, respectively). These results demonstrate that menadione can block CYPmediated parathion metabolism; thus preventing AChE inhibition by paraoxon.
FIG 3. Effects of menadione on CYP-mediated parathion metabolism and inhibition of acetylcholinesterase.
A, HPLC chromatograms showing parathion (PT) metabolism by CYP3A4 (6 pmol) and human liver microsomes (0.5 mg/ml) in the absence and presence of 100 µM menadione. Paraoxon (POX), menadione (MD) and parathion (PT) are shown by the arrows. Reactions contained 20 µM parathion and an NADPH regenerating system. B, Menadione inhibits the metabolism of parathion. Recombinant CYP P450’s (6 pmol) or human liver microsomes (0.5 mg/ml) were incubated at 37 °C in 100 µl reactions mixes containing 20 µM parathion and an NADPH-regeneration system in the absence and presence of 100 µM menadione. At the indicated times, paraoxon in reaction mixes was analyzed by HPLC. Controls reactions did not contain CYP1A2. C, Ability of menadione to inhibit metabolism of parathion to an inhibitor of acetylcholinesterase. CYP1A2 was incubated with parathion (200 µM), an NADPH regenerating system, and the indicated concentrations of menadione. After 10 min at 37 °C, AChE activity was measured by the Ellman assay after the addition of acetylcholinesterase (80 ng/ml), acetylthiocholine (1 mM) and 5,5′-dithiobis(2-nitrobenzoic acid) (0.33 mM) to reaction mixes. Data are mean ± SE, n = 3. *Significantly different (p < 0.05) from CYP1A2-treated with parathion. D, Effects of menadione on AChE inhibition by parathion and oxon metabolites generated by rat liver microsomes. Parathion was incubated with rat liver microsomes (0.5 mg/ml) in the absence or presence of menadione (100 µM). The reactions were initiated by the addition of NADPH (100 µM) and an NADPH regenerating system and run at 37 °C. After one hour, the reaction mixtures were supplemented with acetylcholinesterase and assayed for AChE activity using the Ellman assay.
Menadione protects against parathion toxicity in vivo
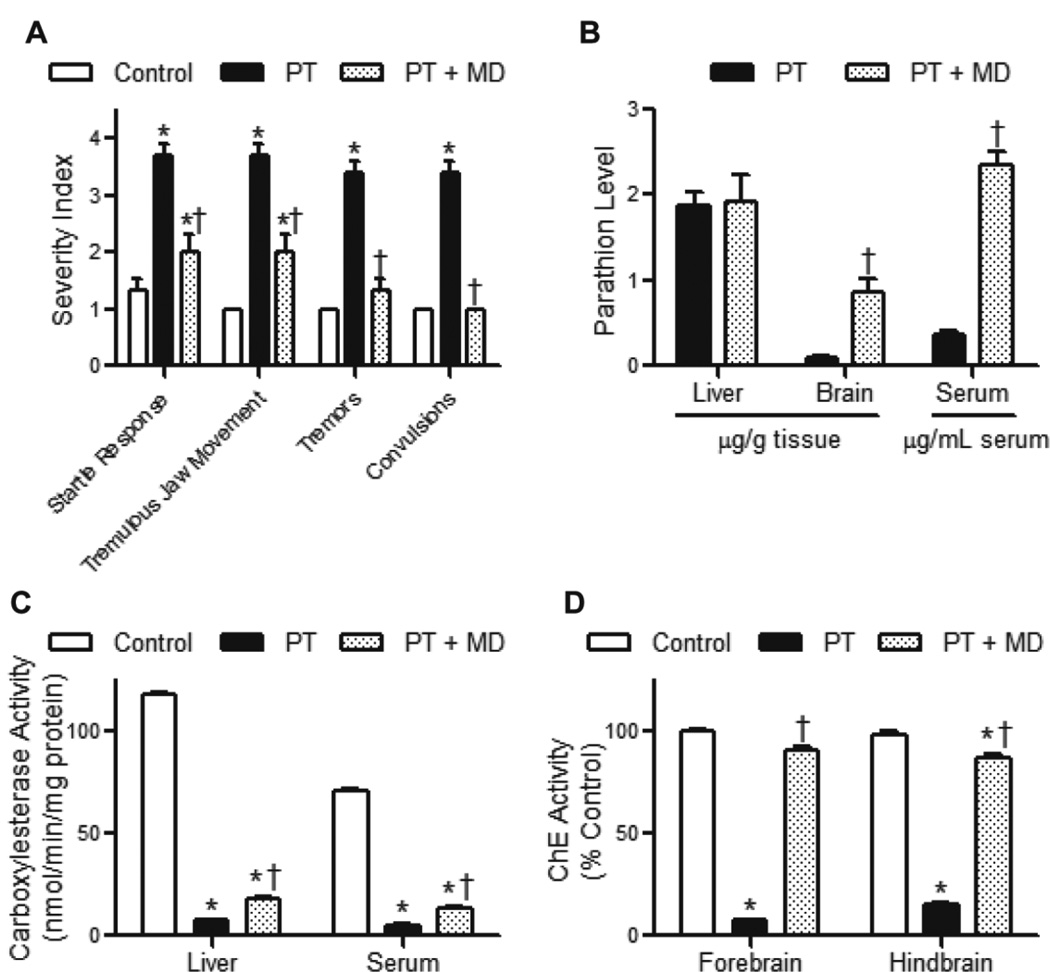
In our next set of experiments, we determined if menadione could suppress parathion toxicity. Out of 5 rats dosed with parathion, only 1 died before the end of the observation period. The surviving rats progressed through tremors to milder convulsions, but survived to 60 min following parathion administration. In contrast, all rats that received menadione 20 min after parathion administration showed a marked reduction in parathion toxicity including suppression of startle response, tremulous jaw movement, tremors and convulsions (Fig. 4A). To assess the effects of menadione on parathion metabolism in vivo, parathion and paraoxon levels were measured in serum, liver and brain. As expected, paraoxon could not be detected in any compartment because of the rapid interaction of paraoxon with a variety of macromolecules. Menadione caused a striking increase in parathion in blood without altering liver levels of parathion, demonstrating reduced conversion to paraoxon (Fig. 4B). This is supported by our findings that these animals displayed significantly reduced levels of liver and serum Ces activity (Fig. 4C). We also measured the activity of brain ChE. Following administration of parathion, forebrain and hindbrain ChE activities were inhibited by 95% and 87%, respectively (Fig. 4D). In contrast, ChE was only inhibited by 11 and 17% when rats were administered menadione 20 min after parathion exposure.
FIG 4. Effects of menadione on parathion-induced toxicity in rats.
Rats were administered parathion (8 mg/kg, oral gavage) followed 20 min later by menadione (40 mg/kg, ip). Sixty min after parathion treatment, rats were sacrificed. A, Effects of menadione on signs of parathion toxicity. Data are mean ± SE, n = 3–4. *Significantly different from control (p < 0.05) and †significantly different from parathiontreated (p < 0.05). B, Effects of menadione on parathion metabolism in liver, brain, and serum. C, Effects of menadione on carboxylesterase activity in rat livers and sera following parathion treatment. D, Effects of menadione on cholinesterase activity in rat brains following parathion treatment.
Discussion
In the microsomal P450 system, electrons are transferred from NADPH to various CYPs via CPR. However, CPR can also mediate one electron reduction of redox active quinones such as menadione. The present studies demonstrate that the supply of electrons from NADPH to menadione as a result of redox cycling via CPR occurs preferentially over their supply to the CYPs. This is based on our findings that menadione redox cycling results in inhibition of the metabolic activities of human recombinant CYPs, as well as metabolism in human liver microsomes. Because of its ability to inhibit CYP activity, menadione markedly suppressed parathion metabolism, as measured by paraoxon formation via CYP1A2, CYP2B6 and CYP3A4 and human liver microsomes. This is supported by our findings that menadione inhibited the ability of CYP1A2 to generate an inhibitor of AChE from parathion. This latter method of measuring paraoxon formation is similar to the indirect desulfuration method employed by others, and allows for an extremely sensitive measure of oxon production (Forsyth and Chambers, 1989). Our studies showing that menadione inhibits metabolism of multiple human recombinant CYPs is important as a variety of these enzymes including CYP1A2, 2B6, 2C9, 2C19, 3A4, 3A5 and 3A7 have been shown to metabolize parathion (Foxenberg et al., 2007). Thus, menadione inhibits many of the CYPs capable of metabolizing parathion to paraoxon.
Earlier studies in mice showed that alterations in the activity of CPR can also affect drug metabolism. For example, liver specific deletion or conditional hepatic knockout of CPR decreases liver CYP activity and suppresses metabolism of a number of drugs and toxins including pentobarbital and acetaminophen (Gu et al., 2003; Henderson et al., 2003; Riddick et al., 2013). In humans, CPR deficiency or mutations and polymorphisms in CPR can also alter CYP activity (Fluck and Pandey, 2011). In addition to the P450s, CPR is important in electron transfer to cytochrome b5 and enzymes important in heme catabolism and in the synthesis of sterol and bile acids (Riddick et al., 2013). Indeed, reductions in CPR in mice have been reported to alter the breakdown of heme, as well as plasma cholesterol levels, and the accumulation of triglycerides in hepatocytes (Riddick et al., 2013). The effect of quinone redox cycling on the activities of these enzymes remains to be determined.
Previous studies have shown that one h pretreatment of rats with the CYP inhibitor, SKF525A, significantly increased plasma levels of parathion, providing experimental evidence that CYP inhibition can alter its pharmacokinetics (Hurh et al., 2000). We found that treatment of rats with menadione 20 min after parathion caused significant increases in serum and brain parathion levels. These data indicate that by altering CYP-mediated parathion metabolism, menadione modifies parathion pharmacokinetics. Importantly, we also found that there were no significant differences in liver concentration of parathion in the presence or absence of menadione, demonstrating that changes in serum parathion levels in menadione treated rats are not due to altered disposition or first pass transport of parathion to the liver. That menadione causes a reduction in paraoxon formation is further supported by our findings of reduced inhibition of liver Ces activity. In the liver, Ces enzymes are primarily microsomal in origin (Crow et al., 2007), where they stoichiometrically bind organophosphate oxons, including paraoxon (Moser, 1995; Pond et al., 1998; Karanth and Pope, 2000). The fact that menadione reduced the inhibition of liver Ces by parathion is notable because liver Ces represents a close in situ measurement of oxon formation by the CYPs. The most conclusive evidence of menadione’s inhibitory effects on parathion toxicity comes from our observation that menadione reduced the inhibition of brain ChE by parathion to less than 20%. These data suggest that menadione could be effective in inhibiting parathion intoxication and indeed, this is what we observed in vivo. Thus, menadione readily inhibited the onset of a cholinergic crisis, as measured by the startle response, tremulous jaw movements, tremors and convulsions.
A number of experimental compounds have been examined in an attempt to modify the pharmacokinetics or neurotoxicity of organophosphate insecticides, including parathion. Several compounds that cause transcriptional regulation of detoxication genes, such as chlorcyclizine, phenobarbital, beta-napthoflavone and polychlorinated biphenyls have been shown to reduce parathion toxicity (Welch and Coon, 1964; Chambers and Chambers, 1990; Carr et al., 2002). However, these compounds typically require several days pre-treatment in order to exert protective effects, likely because of the requirement for the synthesis of new proteins. In our studies, administration of menadione 20 min after the onset of cholinergic crisis reduced or prevented the occurrence of signs and symptoms of parathion intoxication. The principal signs of neurotoxicity are related to inhibition of AChE and overstimulation of cholinergic receptors, which give rise to a classic cholinergic crisis characterized by increased salivation, lacrimation, urination, and defecation. Typically, these signs of toxicity are observed between 30 min and 2 h following parathion depending on the route of administration (Chambers and Chambers, 1990; Moser, 1995). Here, we observed the first signs of cholinergic toxicity approximately 20 min after parathion administration. This progressed from tremulous jaw movements to tremors and convulsions by the time of sacrifice, 40 min later. This timing is earlier than described previously by Moser (Moser, 1995), and is likely due to our use of female rats, which are more sensitive to parathion (Gaines, 1969).
It is important to note that in the present studies, only one time point (60 min) was used to evaluate the effects of menadione on signs of parathion toxicity in the rat model. At this time, the conversion of parathion to paraoxon was inhibited. However, it is not clear if protection can be extended to later time points. Since blood and brain levels of parathion in the menadione-treated rats were increased, it is possible that the toxic responses could simply have been delayed by menadione, and not eliminated altogether. Further studies are needed at later time points to confirm that menadione is protective. Alternatively, multiple doses of menadione may be needed to further delay or eliminate parathion toxicity. In any event, even a delay in parathion toxicity in humans may be beneficial as this may increase the time available to treat parathion poisoning with other treatments and/or drugs.
Previous studies incorporating pre-treatments with putative experimental CYP inhibitors and some drugs that are purported to inhibit CYP have shown mixed results regarding prevention of parathion toxicity. For example, a 4 day pre-treatment with piperonyl butoxide, a commonly used insecticide that inhibits P450, reduced lethality of parathion in mice (Welch and Coon, 1964). In contrast, piperonyl butoxide had no effect on brain ChE inhibition induced by parathion in rats (Chambers and Chambers, 1990). The H2 histamine receptor blocker cimetidine, which inhibits some CYP activities, was found to enhance paraoxon formation and parathion toxicity (Agyeman and Sultatos, 1998). Thus, the effects of menadione on parathion metabolism appear to be unique. Our findings that menadione can reduce or prevent cholinergic toxicity indicates that it could be a promising agent to counter organophosporus poisoning in humans. It should be noted that a number of quinones redox cycle with CPR (Bus and Gibson, 1982; Rooseboom et al., 2004; Klotz et al., 2014) and these are also likely to inhibit activation of parathion, as well as toxicity. Given the concern about exposure to toxic chemicals such as parathion as a consequence of a terrorist attack, or by accident, the fact that menadione is an FDA approved drug should facilitate its movement through the regulatory process.
Supplementary Material
Research Highlights.
Menadione redox cylces with cytochrome P450 reductase and generates reactive oxygen species
Redox cycling inhibits cytochrome P450-mediated parathion metabolism
Short term administration of menadione inhibits parathion toxicity by inhibiting paraoxon formation
Acknowledgments
Funding Information
This work was supported in part by the National Institutes of Health [NS079249, NS072097, ES007148, AR005073, ES005022, ES004738 and CA132624].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agyeman AA, Sultatos LG. The actions of the H2-blocker cimetidine on the toxicity and biotransformation of the phosphorothioate insecticide parathion. Toxicology. 1998;128(3):207–218. doi: 10.1016/s0300-483x(98)00082-1. [DOI] [PubMed] [Google Scholar]
- Anders MW. Enhancement and inhibition of drug metabolism. Annu. Rev. Pharmacol. 1971;11:37–56. doi: 10.1146/annurev.pa.11.040171.000345. [DOI] [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Mechanisms of superoxide radical-mediated toxicity. J. Toxicol. Clin. Toxicol. 1982;19(6–7):689–697. doi: 10.3109/15563658208990398. [DOI] [PubMed] [Google Scholar]
- Carr RL, Richardson JR, Guarisco JA, Kachroo A, Chambers JE, Couch TA, Durunna GC, Meek EC. Effects of PCB exposure on the toxic impact of organophosphorus insecticides. Toxicol. Sci. 2002;67(2):311–321. doi: 10.1093/toxsci/67.2.311. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Chambers HW. Time course of inhibition of acetylcholinesterase and aliesterases following parathion and paraoxon exposures in rats. Toxicol. Appl. Pharmacol. 1990;103(3):420–429. doi: 10.1016/0041-008x(90)90315-l. [DOI] [PubMed] [Google Scholar]
- Crow JA, Borazjani A, Potter PM, Ross MK. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol. 2007;221(1):1–12. doi: 10.1016/j.taap.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva HJ, Wijewickrema R, Senanayake N. Does pralidoxime affect outcome of management in acute organophosphorus poisoning? Lancet. 1992;339(8802):1136–1138. doi: 10.1016/0140-6736(92)90733-j. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS Med. 2009;6:e1000104. doi: 10.1371/journal.pmed.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A, Cabral R. Recent epidemics of poisoning by pesticides. Toxicol. Lett. 1995;82–83:55–63. doi: 10.1016/0378-4274(95)03468-4. [DOI] [PubMed] [Google Scholar]
- Flück CE, Pandey AV. Clinical and biochemical consequences of p450 oxidoreductase deficiency. Endocr. Dev. 2011;20:63–79. doi: 10.1159/000321221. [DOI] [PubMed] [Google Scholar]
- Forsyth CS, Chambers JE. Activation and degradation of the phosphorothionate insecticides parathion and EPN by rat brain. Biochem. Pharmacol. 1989;38(10):1597–1603. doi: 10.1016/0006-2952(89)90307-9. [DOI] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab. Dispos. 2007;35(2):189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- Gaines TB. The acute toxicity of pesticides to rats. Toxicol. Appl. Pharmacol. 1960;2:88–99. doi: 10.1016/0041-008x(60)90074-0. [DOI] [PubMed] [Google Scholar]
- Gaines TB. Acute toxicity of pesticides. Toxicol. Appl. Pharmacol. 1969;14(3):515–534. doi: 10.1016/0041-008x(69)90013-1. [DOI] [PubMed] [Google Scholar]
- Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, Zhang L, Ding X. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J. Biol. Chem. 2003;278(28):25895–25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Otto DM, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J. Biol. Chem. 2003;278(15):13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- Hurh E, Lee E, Lee A, Kim Y, Kim S, Lee M. Effects of enzyme inducers or inhibitors on the pharmacokinetics of intravenous parathion in rats. Biopharm. Drug Dispos. 2000;21(5):193–204. doi: 10.1002/1099-081x(200007)21:5<193::aid-bdd229>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Jett DA, Richardson JR. In: Neurotoxic Pesticides, in Clinical Neurotoxicology: Syndromes, Substances, and Environments. Dobbs M, editor. San Diego, CA: Elsevier; 2009. pp. 491–499. [Google Scholar]
- Jett DA, Yeung DT. The CounterACT research network: basic mechanisms and practical applications. Proc. Am. Thorac. Soc. 2010;7(4):254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol. Sci. 2000;58(2):282–289. doi: 10.1093/toxsci/58.2.282. [DOI] [PubMed] [Google Scholar]
- Klotz LO, Hou X, Jacob C. 1,4-naphthoquinones: from oxidative damage to cellular and intercellular signaling. Molecules. 2014;19(9):14902–14918. doi: 10.3390/molecules190914902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti M. Treatment of acute organophosphate poisoning. Med. J. Aust. 1991;154(1):51–55. doi: 10.5694/j.1326-5377.1991.tb112852.x. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224C:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- McLaughlin LA, Dickmann LJ, Wolf CR, Henderson CJ. Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug Metab. Dispos. 2008;36(7):1322–1331. doi: 10.1124/dmd.108.021261. [DOI] [PubMed] [Google Scholar]
- Mishin VM, Thomas PE. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem. Pharmacol. 2004;68(4):747–752. doi: 10.1016/j.bcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Moser VC. Comparisons of the acute effects of cholinesterase inhibitors using a neurobehavioral screening battery in rats. Neurotoxicol. Teratol. 1995;17(6):617–625. doi: 10.1016/0892-0362(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Nazarewicz RR, Bikineyeva A, Dikalov SI. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J. Biomol. Screen. 2013;18(4):498–503. doi: 10.1177/1087057112468765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal RA. Studies of the enzymic mechanism of the metabolism of diethyl 4-nitrophenyl phosphorothionate (parathion) by rat liver microsomes. Biochem. J. 1967;105(1):289–297. doi: 10.1042/bj1050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter KJ. Inhibition of oxidative drug metabolism in microsomes. Pharmacol. Ther. 1980;10(3):515–535. doi: 10.1016/0163-7258(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Nishibayashi-Yamashita H, Sato R. Vitamin K3-dependent NADPH oxidase of liver microsomes. Purification, properties, and identity with microsomal NADPH-cytochrome c reductase. J. Biochem. 1970;67(2):199–210. doi: 10.1093/oxfordjournals.jbchem.a129243. [DOI] [PubMed] [Google Scholar]
- O'Brien RD. The effect of SKF 525A (2-diethylaminoethyl 2:2-diphenylvalerate hydrochloride) on organophosphate metabolism in insects and mammals. Biochem. J. 1961;79:229–235. doi: 10.1042/bj0790229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond AL, Chambers HW, Coyne CP, Chambers JE. Purification of two rat hepatic proteins with A-esterase activity toward chlorpyrifos-oxon and paraoxon. J. Pharmacol. Exp. Ther. 1998;286(3):1404–1411. [PubMed] [Google Scholar]
- Richardson J, Chambers J. Effects of gestational exposure to chlorpyrifos on postnatal central and peripheral cholinergic neurochemistry. J. Toxicol. Environ. Health A. 2003;66(3):275–289. doi: 10.1080/15287390306369. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Chambers JE. Neurochemical effects of repeated gestational exposure to chlorpyrifos in developing rats. Toxicol. Sci. 2004;77(1):83–90. doi: 10.1093/toxsci/kfh014. [DOI] [PubMed] [Google Scholar]
- Riddick DS, Ding X, Wolf CR, Porter TD, Pandey AV, Zhang QY, Gu J, Finn RD, Ronseaux S, McLaughlin LA, Henderson CJ, Zou L, Fluck CE. NADPH-cytochrome P450 oxidoreductase: roles in physiology, pharmacology, and toxicology. Drug Metab. Dispos. 2013;41(1):12–23. doi: 10.1124/dmd.112.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004;56(1):53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity. Free Radic. Biol. Med. 2008;44(6):1169–1179. doi: 10.1016/j.freeradbiomed.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD. Distinct roles of cytochrome P450 reductase in mitomycin C redox cycling and cytotoxicity. Mol. Cancer Ther. 2010;9(6):1852–1863. doi: 10.1158/1535-7163.MCT-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM, Coon JM. Studies on the effect of chlorcyclizine and other drugs on the toxicity of several organophosphate anticholinesterases. J. Pharmacol. Exp. Ther. 1964;143:192–198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.