Abstract
Background
Duffy (FY) blood group genotyping is important in transfusion medicine because Duffy alloantibodies are associated with delayed hemolytic transfusion reactions and hemolytic disease of the fetus and newborn. In this study, FY allele frequencies in Thai blood donors were determined by in-house PCR with sequence-specific primers (PCR-SSP), and the probability of obtaining compatible blood for alloimmunized patients was assessed.
Methods
Five hundred blood samples from Thai blood donors of the National Blood Centre, Thai Red Cross Society, were included. Only 200 samples were tested with anti-Fya and anti-Fyb using the gel technique. All 500 samples and four samples from a Guinea family with the Fy(a-b-) phenotype were genotyped by using PCR-SSP. Additionally, the probability of obtaining antigen-negative red blood cells (RBCs) for alloimmunized patients was calculated according to the estimated FY allele frequencies.
Results
The FY phenotyping and genotyping results were in 100% concordance. The allele frequencies of FY*A and FY*B in 500 central Thais were 0.962 (962/1,000) and 0.038 (38/1,000), respectively. Although the Fy(a-b-) phenotype was not observed in this study, FY*BES/FY*BES was identified by PCR-SSP in the Guinea family and was confirmed by DNA sequencing.
Conclusions
Our results confirm the high frequency of the FY*A allele in the Thai population, similar to that of Asian populations. At least 500 Thai blood donors are needed to obtain two units of antigen-negative RBCs for the Fy(a-b+) phenotype.
Keywords: Duffy blood group, Genotyping, PCR-SSP, Allele frequencies, Thais
INTRODUCTION
The Duffy (FY) blood group system (The International Society of Blood Transfusion [ISBT] 008) was first reported in 1950, when anti-Fya was observed in a hemophiliac patient with a serious hemolytic transfusion reaction following multiple blood transfusions [1]. One year later, anti-Fyb was discovered in a case of hemolytic disease of the fetus and newborn (HDFN) [2]. Generally, four Duffy phenotypes, Fy(a+b-), Fy(a-b+), Fy(a+b+), and Fy(a-b-), are defined according to the antibodies anti-Fya and anti-Fyb. A high frequency of the Fya phenotype has been described among Asian populations, ranging from 87.4% to 99.9% [3,4,5,6]. Although the Fy(a-b-) phenotype is commonly found in African populations, it is extremely rare in Asian populations [3,4,5,6,7]. After alloimmunization by transfusion or during a pregnancy, anti-Fy3 can be detected, making it difficult to find compatible blood donors when a patient requires blood transfusions [7].
On the basis of a previous study in Thailand, the most common alloantibodies found in multitransfused patients are those in the Rh system (42.2%), followed by the MNS system (31.9%), and the Kidd system (10.5%) [8]. In the Duffy system, anti-Fyb is frequently found in combination with Rh and other antibodies (2.6%), whereas anti-Fya is found as a single antibody (0.1%) [8]. Moreover, a positive direct antiglobulin test (DAT) is often found in patients with repeated transfusions; thus, Duffy DNA-based genotyping can be used as an alternative to conventional phenotyping in clinical situations, e.g., for finding matched blood for alloimmunized patients, assessing the risk of HDFN, and studying anthropology and disease associations [9,10].
The FY gene has three major alleles, FY*A, FY*B, and FY*ES (ES, erythrocyte silent), and is located on chromosome 1 at position q22-q23. The FY*A and FY*B polymorphism is caused by a missense point mutation at c.125G>A, resulting in a p.Gly42Asp substitution, which encodes the Fya and Fyb antigens [6,11,12]. In addition, a single mutation in a GATA motif in the FY*ES promoter at c.-33T>C causes a non-expression antigen in FY-negative individuals [11,12,13]. Current DNA technology for FY blood group genotyping enables the detection of FY alleles. Various PCR-based methods including allele-specific PCR (AS-PCR), PCR-restriction fragment length polymorphism (PCR-RFLP), PCR with sequence-specific primer (PCR-SSP) as single or multiplex assays, real-time quantitative PCR, high-resolution melting analysis, and DNA microarray hybridization have been used for blood group genotyping [11,12,14,15,16,17,18]. Although the Duffy blood group phenotypes in Thai blood donors have been studied [5,19], the FY allele frequencies in this group have not been investigated to date. In this study, the FY allele frequencies in Thai blood donors were determined by in-house PCR-SSP, and the probability of obtaining compatible blood for alloimmunized patients was assessed.
METHODS
1. Subjects
Peripheral venous blood was collected in EDTA tubes from 500 unrelated, healthy Thai blood donors at the National Blood Centre, Thai Red Cross Society, Bangkok, Thailand from May to July 2014, and the study was performed until December 2014. The donors were from central Thailand and their ages ranged from 19 to 58 yr. Informed consent was obtained from each subject. This study was approved by the Committee on Human Rights Related to Research Involving Human Subjects, Thammasat University, Pathumtani, Thailand. Genomic DNA was extracted from all samples by using the Genomic DNA Extraction Kit (REAL Genomics, RBC Bioscience, Taipei, Taiwan) and was stored at -20℃ until use.
In addition, four DNA samples from a family of Guinea origin with Fy(a-b-) phenotypes consisting of a mother, father, and twins were included to confirm the serological testing results. All samples obtained from this family were subjected to FY phenotyping at the National Blood Centre, Thai Red Cross Society, Bangkok, Thailand.
2. DNA standards
Nine DNA samples with known phenotypes confirmed by DNA sequencing, including three Fy(a+b-), three Fy(a-b+), and three Fy(a+b+) phenotypes, were used as controls. Moreover, two DNA samples from individuals with Fy(a-b-) phenotypes of FY*BES (c.-33C) were also included.
3. Duffy blood group phenotyping using the gel technique
A 1% RBC suspension in Diluent-II (Bio-Rad, Morat, Switzerland) was prepared. Fifty microliters of RBC suspension and 50 µL of anti-Fya and/or anti-Fyb were added to the appropriate microtube with the ID-Card "Diaclon anti-Fya" and/or "Diaclon anti-Fyb" (Bio-Rad). The ID-card was incubated for 15 min at 37℃ and was centrifuged for 10 min in the ID-centrifuge (Dia-Med AG, Morat, Switzerland). The results were read and recorded according to the manufacturer's instructions. A total of 200 blood samples from Thai blood donors were tested by FY phenotyping.
4. Duffy blood group genotyping by PCR-SSP
Duffy blood group was genotyped was performed by using the PCR-SSP technique, following previously described methods [11] with some modifications. Individual FY genotyping tests included four sets of PCR reaction mixtures. For each PCR reaction, 1 µL of genomic DNA (50 ng/µL) was amplified in a total volume of 20 µL by using 1 µL of forward primers for the FY promoter region polymorphism (GATA-AB-F/FY-AB-F) and 1 µL of reverse primer for the FY*A and FY*B polymorphism (FY-A-R/FY-B-R). Sequences of the primer combinations used in the four primer mixtures and the allele detected by each mixture are shown in Table 1. In addition, co-amplification of the human growth hormone gene (HGH) using 1 µL of the HGH-F primer and 1 µL of the HGH-R primer was run as an internal control. The PCR was performed with 10 µL of the PCR reaction mixture (OnePCR Plus, GeneDirex, New Taipei City, Taiwan) and 5 µL of sterile distilled water in a G-STORM GS1 thermal cycler (Gene Technologies Ltd., Somerset, UK).
Table 1. Primer sequences for FY blood group genotyping.
| Allele | Primer | Primer sequence (5'→3') | Product size (bp) | Final concentration (µM) |
|---|---|---|---|---|
| FY*AES | GATA-AB-F | CTCATTAGTCCTTGGCTCTTAC | 711 | 0.5 |
| FY-A-R | AGCTGCTTCCAGGTTGGCAC | |||
| FY*BES | GATA-AB-F | CTCATTAGTCCTTGGCTCTTAC | 711 | 0.5 |
| FY-B-R | AGCTGCTTCCAGGTTGGCAT | |||
| FY*A | FY-AB-F | CTCATTAGTCCTTGGCTCTTAT | 711 | 0.5 |
| FY-A-R | AGCTGCTTCCAGGTTGGCAC | |||
| FY*B | FY-AB-F | CTCATTAGTCCTTGGCTCTTAT | 711 | 0.5 |
| FY-B-R | AGCTGCTTCCAGGTTGGCAT | |||
| HGH | HGH-F | TGCCTTCCCAACCATTCCCTTA | 434 | 0.5 |
| HGH-R | CCACTCACGGATTTCTGTTGTGTTTC |
Abbreviations: HGH, human growth hormone; ES, erythrocyte silent.
PCR was performed by using the conditions described below. The initial denaturation was performed at 94℃ for 2 min. The cycle parameters were as follows: 10 cycles of 10 sec at 94℃ and 60 sec at 69℃, then 20 cycles of 30 sec at 94℃, 60 sec at 62℃, and 30 sec at 72℃. The last step was a final extension for 5 min at 72℃. PCR products were electrophoresed at 100 volts with a 1.5% agarose gel using 1× Tris borate ethylenediaminetetraacetate (TBE) buffer. Products were visualized under a blue-light transilluminator. The PCR product sizes of the FY*AES, FY*BES, FY*A, and FY*B alleles were each 711 bp, whereas that of the internal control (i.e., the HGH gene) was 434 bp.
Known DNA samples for the Fy(a+b-), Fy(a-b+), Fy(a+b+), and Fy(a-b-) phenotypes were used as controls. In total, 500 DNA samples obtained from Thai blood donors and four DNA samples from the Guinea family were tested.
5. DNA sequencing
To validate our in-house PCR-SSP technique for FY blood group genotyping, genomic DNA samples of eight genotyped blood donors (three, FY*A/FY*A; three, FY*A/FY*B; and two FY*B/FY*B) were sequenced. Additionally, four DNA samples obtained from the Guinea family were sequenced to confirm the FY phenotype results. A fragment of 931 bp containing both polymorphisms (c.125G>A and c.-33T>C) was obtained by PCR amplification of genomic DNA using the forward primer 5'-GTGTAGTCCCAACCAGCCAA-3' and reverse primer 5'-AGGATACCCAGGACACTGGT-3'. The PCR program consisted of one cycle of 95℃ for 5 min, followed by 30 cycles at 95℃ for 30 sec, 65℃ for 40 sec, and 72℃ for 30 sec, with a final extension at 72℃ for 5 min.
6. Statistical analysis
Gene frequencies were calculated by the gene-counting method. The chi-square (χ2) test was used to evaluate whether the observed genotype frequencies were in agreement with the expected frequencies under Hardy-Weinberg equilibrium. The χ2 test of homogeneity was used to determine the difference between Thai and other Asian populations. A P value of equal to or less than 0.05 was considered statistically significant. In addition, the probability of successfully obtaining antigen-negative RBCs for alloimmunized patients was calculated by dividing the number of antigen-negative units desired by the incidence of antigen-negative individuals in the donor population [20].
RESULTS
The distribution of Duffy blood group phenotypes among 200 Thai blood donors was examined. Fy(a+b-) was the most common phenotype (177/200, 88.5%), followed by Fy(a+b+) (22/200, 11.0%), and Fy(a-b+) (1/200, 0.5%); and Fy(a-b-) was not found. Five hundred DNA samples included 200 samples with known phenotypes and an additional 300 samples that were genotyped for FY alleles by in-house PCR-SSP. The DNA controls were tested with four sets of primer combinations and the results were in agreement. According to the PCR-SSP results, FY*A/FY*A and FY*B/FY*B samples were positive with only the set of FY-AB-F and FY-A-R primers and the set of FY-AB-F and FY-B-R primers, respectively. In addition, FY*A/FY*B samples were positive with both sets of primers.
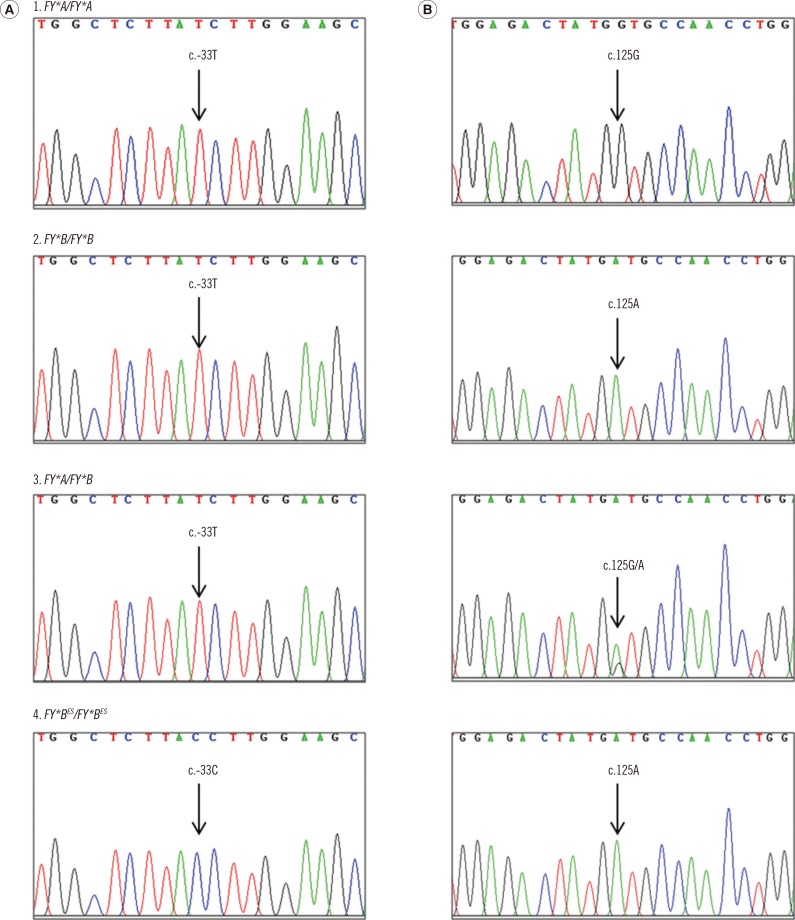
The FY genotyping results for the 200 samples with known phenotypes were in 100% concordance with the phenotyping results obtained using the gel technique. Moreover, PCR-SSP was used to determine the FY genotype of 300 DNA samples. FY*A/FY*A was the most common allele (464/500), followed by FY*A/FY*B (34/500) and FY*B/FY*B (2/500); the FY*AES and FY*BES alleles were not observed in this study. Additionally, the PCR-SSP technique was validated by using eight randomly chosen DNA samples by DNA sequencing and the results were in agreement (Fig. 1).
Fig. 1. Electropherograms of the FY gene at the GATA box motif of the promoter region, single nucleotide polymorphism (SNP) c.-33T>C (column A) and the FY*A and FY*B polymorphism region, SNP c.125G>A (column B). DNA sequences of the FY*A homozygote (1), the FY*B homozygote (2), the FY*A/FY*B heterozygote (3), and the FY*BES homozygote (4) are amplified in both regions.
The FY genotypes of the 500 Thai blood donors determined in this study were consistent with Hardy-Weinberg equilibrium (χ2=1.1133, P=0.736). The allele frequencies of FY*A and FY*B in the central Thailand population were 0.962 (962/1,000) and 0.038 (38/1,000), respectively. Duffy blood group allele frequencies in the Thai population and other Asian populations were compared. The observed allele frequencies were similar to those found in Chinese populations [17,21] and were significantly different from those of Japanese, Korean, and Malaysian populations (Table 2) [14,22,23].
Table 2. FY*A and FY*B allele frequencies in Asian populations.
| Population | Number | FY*A | FY*B | Method | P value |
|---|---|---|---|---|---|
| Thais (present study) | 500 | 0.962 | 0.038 | PCR-SSP | - |
| Brazilian Japanese descendants [14] | 209 | 0.785 | 0.215 | PCR-RFLP | < 0.001 |
| Jiangsu Chinese [21] | 146 | 0.938 | 0.062 | PCR-SSP | 0.114 |
| Dalian Chinese [17] | 120 | 0.933 | 0.067 | PCR-ASP | 0.075 |
| Korean [22] | 107 | 0.921 | 0.079 | PCR-RFLP | 0.014 |
| Malaysian [23] | 60 | 0.908 | 0.092 | PCR-ASP | 0.013 |
Abbreviations: SSP, sequence-specific primers; RFLP, restriction fragment length polymorphism; ASP, allele-specific primer.
Four blood samples from the Guinea family, with Fy(a-b-) phenotypes, were genotyped by using PCR-SSP and confirmed by DNA sequencing. Using PCR-SSP, positive results were obtained for all four samples using only the set of GATA-AB-F and FY-B-R primers, similar to the DNA control samples with Fy(a-b-) phenotypes of FY*BES/FY*BES (c.-33C). In addition, DNA sequencing results for the Guinea family also confirmed a point mutation in the GATA promoter region of FY*BES.
Using PCR-SSP for FY genotyping of 500 samples, the predicted phenotypes for each blood sample were summarized; FY*A/FY*A was Fy(a+b-) (92.8%), FY*A/FY*B was Fy(a+b+) (6.8%), and FY*B/FY*B was Fy(a-b+) (0.4%). Even though the FY*AES and FY*BES alleles were not detected in this study, the presence of FY*AES/FY*AES, FY*BES/FY*BES, and FY*AES/FY*BES can be predicted to be associated with the Fy(a-b-) phenotype.
In this study, only two Fy(a-b+) samples were found among 500 Thai blood donors. For a patient with the Fy(a-b+) phenotype who has anti-Fya and requires a blood transfusion, the probability of successfully providing antigen-negative RBCs from individuals with the Fy(a-b+) phenotype among Thai blood donors for crossmatching was calculated. In this case, when two units are required, testing at least 500 random units should yield two Fy(a-b+) units [2/0.004]. If this patient has multiple antibodies, such as anti-Fya and anti-E, 755 random units [2/0.004×0.662] would need to be tested to find two compatible units [5,20].
DISCUSSION
In this study, the Duffy blood group phenotypes and the predicted phenotypes in 200 and 300 Thai blood donors confirmed that the Fy(a+b-) phenotype was the most common, followed by Fy(a+b+) and Fy(a-b+), similar to other studies in Thai populations [5,19,24]. Fy(a-b-), a rare phenotype, was not observed. Routinely, FY blood group phenotyping requires an indirect antiglobulin phase; hence, it is difficult to type RBCs of multitransfused patients on the basis of a positive direct antiglobulin test.
After FY blood group genotyping using in-house PCR-SSP, the genotyping results, including FY*A, FY*B, FY*AES, and FY*BES allele detection, were computed for all four predicted phenotypes. The validated in-house PCR-SSP genotyping results were confirmed by DNA sequencing. Moreover, the fidelity of the genotyping technique was high based on the 100% concordance between the genotyping and phenotyping results.
As previously reported in multitransfused Thai patients, anti-Fyb was frequently found together with other blood group antibodies [8]; as a consequence, the difficulty in finding compatible RBCs depends on the prevalence of antibody combinations. Although anti-Fy3 has not been reported in Thai patients, we included FY*AES and FY*BES allele detection in our PCR-SSP analysis. Owing to the large population of multi-ethnic residents in Bangkok, a higher number of individuals with rare blood phenotypes in donors and patients could be found.
In a previous study, not only limited phenotype matching (D, C, c, E, e, and K), but also extended phenotype matching of S, Fya, and Jkb was suggested for transfusion in patients with sickle cell disease [25]. In Thailand, the prevalence of thalassemia patients requiring repeated blood transfusions is high; therefore, the implementation of extended FY genotyping for both FY*A and FY*B in donors and patients would be beneficial for the prevention of alloimmunization. In our examination of the Guinea family, we also confirmed that the Fy(a-b-) phenotype was associated with homozygous FY*BES alleles by PCR-SSP and DNA sequencing in all samples. Hence, the probability of finding extended-matched donors in Thai populations is unlikely for Fy(a-b-) patients. In this case, autologous or family-related donations are recommended. In addition, the in-house PCR-SSP technique is simple, convenient, and reproducible. Interestingly, it could reduce the mutagenic and toxic effects of ethidium bromide to visualize fragments. Owing to the remarkably rare observation of the FY*X allele in Asian populations [10,12], the in-house PCR-SSP does not include the detection of this allele.
This is the first report of FY blood group allele frequencies in the Thai population. The FY*A allele was the most common and its frequency was similar to that observed in Chinese populations [17,21]; but it was statistically significantly different (P<0.05) from that in Korean, Japanese, and Malaysian populations [14,22,23]. A recent study compared the Kidd blood group allele frequencies in two Thai blood donors [26], and found that Kidd blood group allele frequencies in Thai individuals were statistically different from those of Japanese individuals, but similar to those of Chinese individuals [21]. Therefore, the analysis of various RBC gene divergences among populations could provide insight into national history, human origins, and migration patterns.
In conclusion, our study confirms a high frequency of the FY*A allele in the Thai population. At least 500 Thai blood donors are needed to obtain two units of antigen RBCs for the Fy(a-b+) phenotype.
Acknowledgments
This work was supported by grants from the Thammasat University Fund and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Cutbush M. The Duffy blood group system. Heredity (Edinb) 1950;4:383–389. doi: 10.1038/hdy.1950.31. [DOI] [PubMed] [Google Scholar]
- 2.Ikin EW, Mourant AE, Pettenkofer HJ, Blumenthal G. Discovery of the expected haemagglutinin, anti-Fyb. Nature. 1951;168:1077–1078. doi: 10.1038/1681077b0. [DOI] [PubMed] [Google Scholar]
- 3.Makroo RN, Bhatia A, Gupta R, Phillip J. Prevalence of Rh, Duffy, Kell, Kidd & MNSs blood group antigens in the Indian blood donor population. Indian J Med Res. 2013;137:521–526. [PMC free article] [PubMed] [Google Scholar]
- 4.Lin-Chu M, Broadberry RE, Chang FJ. The distribution of blood group antigens and alloantibodies among Chinese in Taiwan. Transfusion. 1988;28:350–352. doi: 10.1046/j.1537-2995.1988.28488265265.x. [DOI] [PubMed] [Google Scholar]
- 5.Bejrachandra S, Nathalang O, Saipin J, Kuvanont S, Wichitchinda K, Vongpattranon A. Distribution of the blood group systems in Thai blood donors determined by the gel test. Siriraj Hosp Gaz. 2002;54:403–409. [Google Scholar]
- 6.Reid M, Lomas-Francis C, et al., editors. The blood group antigen factsbook. 3rd ed. Amsterdam: Elsevier Academic Press; 2012. [Google Scholar]
- 7.Daniels G. Duffy blood group system. In: Daniels G, editor. Human blood groups. 2nd ed. Malden, MA: Blackwell Science; 2002. pp. 324–341. [Google Scholar]
- 8.Kupatawintu P, Emthip M, Sungnoon D, O-vataga P, Manakul V, Limtamaporn S, et al. Unexpected antibodies of patients' bloods samples sent for testing at NBC.TRCS. (in Thai) J Hematol Transfus Med. 2010;20:255–256. http://www.tci-thaijo.org/index.php/JHematolTransfusMed/article/viewFile/4371/3812. [Google Scholar]
- 9.Meny GM. The Duffy blood group system: a review. Immunohematology. 2010;26:51–56. [PubMed] [Google Scholar]
- 10.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson ML, Hansson C, Avent ND, Akesson IE, Green CA, Daniels GL. A clinically applicable method for determining the three major alleles at the Duffy (FY) blood group locus using polymerase chain reaction with allele-specific primers. Transfusion. 1998;38:168–173. doi: 10.1046/j.1537-2995.1998.38298193099.x. [DOI] [PubMed] [Google Scholar]
- 12.Sellami MH, Kaabi H, Midouni B, Dridi A, Mojaat N, Boukef MK, et al. Duffy blood group system genotyping in an urban Tunisian population. Ann Hum Biol. 2008;35:406–415. doi: 10.1080/03014460802082127. [DOI] [PubMed] [Google Scholar]
- 13.Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 14.Flôres MA, Visentainer JE, Guelsin GA, Fracasso Ade S, de Melo FC, Hashimoto MN, et al. Rh, Kell, Duffy, Kidd and Diego blood group system polymorphism in Brazilian Japanese descendants. Transfus Apher Sci. 2014;50:123–128. doi: 10.1016/j.transci.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Jungbauer C, Hobel CM, Schwartz DW, Mayr WR. High-throughput multiplex PCR genotyping for 35 red blood cell antigens in blood donors. Vox Sang. 2012;102:234–242. doi: 10.1111/j.1423-0410.2011.01542.x. [DOI] [PubMed] [Google Scholar]
- 16.Beiboer SH, Wieringa-Jelsma T, Maaskant-Van Wijk PA, van der Schoot CE, van Zwieten R, Roos D, et al. Rapid genotyping of blood group antigens by multiplex polymerase chain reaction and DNA microarray hybridization. Transfusion. 2005;45:667–679. doi: 10.1111/j.1537-2995.2005.04319.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Liu M, An W, Liang X, Yu W, Piao F. A new method for analyzing the Duffy blood group genotype by TaqMan minor groove binding probes. J Clin Lab Anal. 2015;29:203–207. doi: 10.1002/jcla.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka M, Takahahi J, Hirayama F, Tani Y. High-resolution melting analysis for genotyping Duffy, Kidd and Diego blood group antigens. Leg Med (Tokyo) 2011;13:1–6. doi: 10.1016/j.legalmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Fongsarun J, Nuchprayoon I, Yod-in S, Kupatawintu P, Kidprasirt C. Blood groups in Thai blood donors. (in Thai) Thai J Hematol Transfus Med. 2002;12:277–286. http://www.tsh.or.th/journal_detail2.php?id=63. [Google Scholar]
- 20.Trudell KS. Detection and identification of antibodies. In: Harmening DM, editor. Modern blood banking and transfusion practices. 5th ed. Philadelphia: F.A. Davis Company; 2005. p. 257. [Google Scholar]
- 21.Liu Z, Zeng R, Chen Q, Li M, Shi GY, Wei P, et al. Genotyping for Kidd, Kell, Duffy, Scianna, and RHCE blood group antigens polymorphisms in Jiangsu Chinese Han. Chin Med J. 2012;125:1076–1081. [PubMed] [Google Scholar]
- 22.Lim CS, Kim YK, Lee KN. The Duffy blood group genotypes in Asian populations. Korean J Blood Transfus. 2007;18:145–151. [Google Scholar]
- 23.De Silva JR, Lau YL, Fong MY. Genotyping of the Duffy blood group among Plasmodium knowlesi-infected patients in Malaysia. PLoS One. 2014;9:e108951. doi: 10.1371/journal.pone.0108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandanayingyong D, Sasaki TT, Greenwalt TJ. Blood groups of Thais. Transfusion. 1967;7:269–276. doi: 10.1111/j.1537-2995.1967.tb05516.x. [DOI] [PubMed] [Google Scholar]
- 25.Castro O, Sandler SG, Houston-Yu P, Rana S. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: theoretical and practical implications. Transfusion. 2002;42:684–690. doi: 10.1046/j.1537-2995.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 26.Sudkaew A, Intharanut K, Leetrakool N, Nathalang O. Kidd blood group allele frequencies in Thai blood donors. Clin Lab. 2014;60:1401–1403. doi: 10.7754/clin.lab.2013.130806. [DOI] [PubMed] [Google Scholar]