Abstract
Background
Because acute liver failure (ALF) patients share many clinical features with severe sepsis and septic shock, identifying bacterial infection clinically in ALF patients is challenging. Procalcitonin (PCT) has proven to be a useful marker in detecting bacterial infection. We sought to determine whether PCT discriminated between presence and absence of infection in patients with ALF.
Method
Retrospective analysis of data and samples of 115 ALF patients from the United States Acute Liver Failure Study Group randomly selected from 1863 patients were classified for disease severity and ALF etiology. Twenty uninfected chronic liver disease (CLD) subjects served as controls.
Results
Procalcitonin concentrations in most samples were elevated, with median values for all ALF groups near or above a 2.0 ng/mL cut-off that generally indicates severe sepsis. While PCT concentrations increased somewhat with apparent liver injury severity, there were no differences in PCT levels between the pre-defined severity groups–non-SIRS and SIRS groups with no documented infections and Severe Sepsis and Septic Shock groups with documented infections, (p = 0.169). PCT values from CLD patients differed from all ALF groups (median CLD PCT value 0.104 ng/mL, (p ≤0.001)). Subjects with acetaminophen (APAP) toxicity, many without evidence of infection, demonstrated median PCT >2.0 ng/mL, regardless of SIRS features, while some culture positive subjects had PCT values <2.0 ng/mL.
Summary/Conclusions
While PCT appears to be a robust assay for detecting bacterial infection in the general population, there was poor discrimination between ALF patients with or without bacterial infection presumably because of the massive inflammation observed. Severe hepatocyte necrosis with inflammation results in elevated PCT levels, rendering this biomarker unreliable in the ALF setting.
Introduction
Patients with acute liver failure (ALF) have an increased risk of infection approaching 80% thus predisposing to sepsis-related multi-organ failure [1–3]. Infections have been shown to worsen hepatic encephalopathy (HE) and are one of the most common causes of multi-organ dysfunction and death in ALF [3, 4].
Signs and symptoms of ALF closely mimic those of sepsis making it difficult to accurately identify infection in this setting. The diagnosis of infection or sepsis (usually based upon a positive culture) is often delayed or not detected, even when presumptive evidence is very strong. Identification of a bacterial infection by culture requires 48–72 hours to complete and the efficacy of prophylactic antibiotics to contain an established infection remains difficult to prove. A more rapid method to identify infection is a worthwhile goal.
Procalcitonin (PCT) is a biomarker for detection of bacterial infection that has shown early promise. PCT consists of 116 amino acids and has a MW of 14.5 kDa. A precursor of calcitonin, the primary source of PCT is the parafollicular C cells of the thyroid gland. Calcitonin has a hypocalcemic and hypophosphatemic effect that inhibits bone resorption and stimulates excretion of phosphorus, calcium, and sodium by the kidney and is used as a biomarker of medullary thyroid cancer but its relation to systemic inflammation remains unclear [5–8].
In studies of patients with sepsis, pneumonia, and other types of infections, PCT has been shown to be a reliable and rapidly available indicator of bacterial infection, useful in guiding antimicrobial therapy. PCT has been examined in patients who have had a liver transplant and in patients hospitalized with chronic liver disease (cirrhosis; CLD), but there are few studies of patients with ALF. [9–14].
In this study, we sought to determine whether PCT would aid in the identification of infection in ALF patients, since distinguishing sepsis from ALF without infection has prognostic and therapeutic implications. We utilized the extensive registry and serum bank of the U. S. Acute Liver Failure Study Group (USALFSG) as well as 2 local repositories for CLD control sera, to address this question.
Materials and Methods
Study Population
This study included patients prospectively enrolled in the US Acute Liver Failure Study (established in January 1998). Additionally, data and sera from two other repositories related to CLD were accessed to provide control groups. A total of 1863 adult ALF patients were enrolled from 23 tertiary care centers within the US between Jan. 1998 and Oct. 2010. Inclusion criteria defining ALF were the presence of coagulopathy (PT > 15 seconds or INR ≥1.5 IU) and any grade of hepatic encephalopathy (HE) that had occurred within 26 weeks of the first onset of symptoms. Enrolled patients may not have any CLD or cirrhosis, with the exception of certain patients with chronic hepatitis B or Wilson’s disease [15]. Written informed consent was obtained for each subject from the next of kin because of altered mental status due to HE. All centers were in compliance with their local institutional review board requirements. After informed consent was obtained, serum samples and clinical data were collected daily for up to 7 consecutive days. Sample collection ended when one of the following events occurred: the subject was discharged, transplanted, died, or completed the 7th study day. Serum samples were stored frozen at -70° until testing.
The Chronic Liver Disease (CLDD; PI–William M. Lee, MD) and the Nucleic Acid, Serum, and Tissue Repository for the Study of Liver Diseases Database and sample repositories (NSTLD; PI–Marlyn Mayo, MD) are principal investigator-initiated studies at UT Southwestern Medical Center (UTSW) in Dallas, TX. After written informed consent was obtained, sera were obtained and frozen at -70° until testing.
This retrospective analysis received Institutional Review Board (IRB) approval from the University of Texas Southwestern Medical Center (IRB#STU092010-126) and Virginia Commonwealth University (IRB#HM13517).
All study subjects were categorized into infection groups using modified definitions from the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference definitions of systemic inflammatory response syndrome (SIRS), severe sepsis, and septic shock [9, 10, 16] based upon available data from the USALFSG database. Subjects with SIRS had to have two or more of the following signs or symptoms: body temperature >38°C or <36°C, heart rate >90 bpm, respiration rate >20 breaths/min, PCO2 level <32 mm Hg, WBC >12,000 or <4,000, or bands (immature granulocytic white blood cells) >10%. Severe sepsis was defined as organ failure in the presence of sepsis (SIRS plus a documented infection defined as a positive culture (blood, trachea, wound, catheter or urine) up to 2 days prior to the blood sample). Septic shock was defined as the presence of severe persistent hypotension (mean arterial pressure <60 mmHg) despite adequate volume resuscitation and pressor therapy in the presence of sepsis [16]. Non-infected/non-SIRS (non-SIRS) samples were selected from patients who met no more than one of the SIRS criteria.
For the 1,863 patients enrolled in the ALF study, 12,492 days of demographic and bio-data were sorted into 1 of the 4 groups: non-SIRS (n = 628), SIRS (n = 1407), severe sepsis (n = 387), and septic shock (n = 31). Because the USALFSG is an observational study not specifically designed to study SIRS or infections, some data parameters noted in the 4 different SIRS categories were either not captured by the ALF CRF or were not routinely collected on all patient days (examples: cell differential, urine output, tachypnea, and cardiac index). Thus, each patient day was categorized into a SIRS category based upon available data (i.e., if a lab value was missing, it was considered to be negative for that parameter and, if present, considered positive if the parameter met positive criteria). Samples for the non-SIRS category were only selected from days that had all available data and were negative for each parameter. Once sorted into the 4 SIRS categories, 115 subjects were randomly selected, based upon sample availability and maintaining a balance between the etiologies of their acute liver failure (acetaminophen toxicity, viral hepatitis or other etiologies). Currently, 46% of all ALF cases (in both the USALFSG and the US) result from APAP toxicity, either suicidal overdoses or unintentional excessive dosing [15]. To ensure a balanced distribution of etiologies, an equal number of samples were requested for each etiology.
In addition, 10 subjects from each of the CLDD and NSTLD repositories were randomly selected as negative controls. These subjects were ambulatory patients with CLD who had provided a screening blood sample during a routine clinic visit without symptoms. Limited bio-data were available for this group. These subjects were considered to be non-SIRS and had no clinical or laboratory evidence of bacterial infection.
Immunoassay testing
The ADVIA Centaur BRAHMS PCT assay [17] is a sandwich immunoassay utilizing a monoclonal mouse antibody to fluorescein covalently linked to paramagnetic particles, a monoclonal mouse antibody to procalcitonin that is labeled with acridinium ester and a second monoclonal mouse antibody to procalcitonin labeled with fluorescein.
All testing was performed at Siemens Healthcare Diagnostics in Tarrytown, NY on a single ADVIA Centaur. Siemens Healthcare Diagnostics, Tarrytown, NY, provided all reagents required for testing.
Statistical Analyses
Descriptive statistics (median and range) are provided for all analyzed samples by group. A Kruskal-Wallis test with post hoc testing (Dunn Method) was used to determine the utility of PCT to detect infection, to examine possible measurement differences in PCT between patients with CLD and ALF, and to determine the existence of a dose affect between PCT values and severity of infection in ALF patients [18]. A Chi-square test was used as an alternative method for analyzing patient groups and infection status. PCT values were categorized into infection and non-infection groups using ROC analyses [19]. A 2-way analysis of variance was performed to examine the difference in PCT by type of culture (blood vs. all other culture types), severity categories (severe sepsis and septic shock), and the interaction of these two factors.
The median PCT values for the CLD, non-SIRS, and SIRS groups were compared to the reference range of the PCT assay. An expected reference value of <0.1 ng/mL had been previously calculated from PCT values from a population of 456 normal subjects. The 95% confidence interval for the mean concentration was 0.023 to 0.028 ng/mL [17]. SPSS version 20.0 (SPSS, Chicago, IL) and SAS version 9.2 (Cary, NC) were used for statistical analyses.
Results
Study group characteristics based upon disease severity
Available demographic and laboratory data for the 115 study subjects and control groups are shown in Tables 1 and 2. No discernible differences were apparent between the groups studied, except that the CLD group was somewhat older and the overall ALF group contained more women. Laboratory and certain clinical values differed significantly across the various study groups generally reflecting severity of illness: WBC, platelets, INR, AST, ALT, creatinine, lactate, pO2, O2 saturation, pCO2, pulse, respiration, and MAP.
Table 1. Demographic Data for Patients Based Upon Severity of Illness Classifications.
| Mean (± SD) or | CLD | non-SIRS | SIRS | Severe Sepsis | Septic Shock | |
|---|---|---|---|---|---|---|
| Number (%) | n = 20 | n = 30 | n = 29 | n = 40 | n = 16 | p-value |
| Age (years) | 51 (29–71) | 49 (19–86) | 37 (18–66) | 41 (17–73) | 41 (19–71) | 0.044 |
| Gender | ||||||
| Female | 10 (50) | 15 (50) | 21 (72.4) | 33 (82.5) | 12 (75) | 0.019 |
| Ethnicity* | ||||||
| Hispanic | 5 (16.7) | 5 (17.2) | 5 (12.5) | 1 (6.7) | ||
| Race** | 0.672 | |||||
| White | 14 (77.8) | 21 (66.7) | 23 (79.3) | 33 (82.5) | 14 (87.5) | |
| African American | 1 (5.6) | 5 (16.7) | 5 (17.2) | 4 (10.0) | 1 (6.2) | |
| Asian | 2 (11.1) | 2 (6.7) | 1 (3.4) | 2 (5.0) | 1 (6.2) | |
| Hawaiian | 1 (3) | |||||
| Other | 1 (5.6) | 1 (3) | 1 (2.5) | |||
| Diagnosis | ||||||
| APAP | 10 (33.3) | 10 (34.5) | 16 (40.0) | 5 (31.2) | ||
| Viral Hepatitis | 10 (50) | 10 (33.3) | 10 (34.5) | 7 (17.5) | 1 (6.2) | |
| Other | 10 (50) | 10 (33.3) | 9 (31.0) | 17 (42.5) | 10 (62.5) | |
| Outcome (TFS) | ||||||
| APAP | 6 (20) | 5 (17.2) | 12 (30.0) | 1 (6.2) | ||
| Viral Hepatitis | 4 (13.3) | 2 (6.9) | 2 (7.4) | |||
| Other | 1 (3.3) | 4 (13.8) | 5 (12.5) | 1 (6.2) | ||
| Coma Grade | ||||||
| Not Reported / 0 | 20 (100) | 4 (13.8) | 3 (7.5) | |||
| 1 | 11 (36.7) | 1 (3.4) | 4 (10.0) | |||
| 2 | 11 (36.7) | 8 (27.6) | 1 (2.5) | |||
| 3 | 4 (13.3) | 5 (17.2) | 11 (27.5) | 1 (6.2) | ||
| 4 | 4 (13.3) | 11 (37.9) | 21 (52.5) | 15 (93.8) | ||
| Culture Types | ||||||
| Blood | 6 | 5 | ||||
| Tracheal | 12 | 4 | ||||
| Urine | 5 | 3 | ||||
| Wound | 3 | 1 | ||||
| Catheter | 1 | |||||
| Multiple w Blood | 10 | 2 | ||||
| Multiple w/o Blood | 3 | 1 | ||||
| Antibiotics Used | 19 (63.3) | 23 (79.3) | 33 (82.5) | 13 (81.2) | 0.294 | |
| Prophylaxis | 15 (50) | 17 (58.6) | 19 (47.5) | 10 (62.5) | 0.673 | |
| Therapy | 7 (23) | 12 (41.4) | 22 (55.0) | 7 (43.8) | 0.069 | |
| Age of Sample (years) | 9 (1–15) | 7 (2–13) | 7 (2–13) | 6 (2–13) | 6.5 (2–11) | 0.872 |
| After Hospital Admission (days) | 2 (0–11) | 3 (0–51) | 2 (0–22) | 3 (0–16) | 0.557 |
*Ethnicity: O were reported in the CLD group; 15 of 16 were reported in the Septic Shock group
**Race: 18 of 20 reported in the CLD group / p-value reported for white vs. all other races.
Table 2. Biodata and Laboratory values for Patients Based Upon Severity of Illness Classifications.
| CLD | non-SIRS | SIRS | Severe Sepsis | Septic Shock | ||
|---|---|---|---|---|---|---|
| Median | n = 20 | n = 30 | n = 29 | n = 40 | n = 16 | p-value |
| Pulse [bpm] | 80 | 96 | 108 | 112 | <0.001 | |
| Respiration [breaths/min] | 18 | 23 | 22 | 22 | 0.005 | |
| MAP [mmHg] | 90.0 | 81.5 | 89.5 | 57.5 | 0.472* | |
| Min Temp [°C] | 36.6 | 35.9 | 36.5 | 36.0 | 0.200 | |
| Max Temp [°C] | 36.8 | 36.7 | 37.4 | 34.3 | 0.101 | |
| Laboratory values | ||||||
| PCT [ng/mL] | 0.104 | 1.57 | 2.29 | 2.51 | 5.89 | <0.001 |
| AST [IU/L] | 787 | 360 | 292 | 3069 | <0.001 | |
| ALT [IU/L] | 1894 | 775 | 750 | 1520 | 0.030 | |
| WBC [x 103 /uL] | 8.0 | 14.0 | 14.0 | 17.00 | <0.001 | |
| Platelets[x 103 /uL] | 143 | 88 | 115 | 61 | 0.002 | |
| Prothrombin time [secs] | 27.5 | 25.0 | 21.0 | 25.0 | 0.124 | |
| INR [ratio] | 3.0 | 2.0 | 2.0 | 3.0 | 0.030 | |
| Bilirubin [mg/dL] | 15.0 | 10.0 | 10.0 | 12.5 | 0.902 | |
| Glucose [mg/dL] | 122 | 103 | 110 | 145 | 0.997 | |
| Creatinine [mg/dL] | 1.00 | 2.00 | 1.00 | 3.00 | 0.003 | |
| Lactate [mg/dL] | 3.00 | 6.00 | 8.00 | 14.00 | 0.024 | |
| pO2 [mmHg] | 117 | 86 | 107 | 93 | 0.025 | |
| pCO2 [mmHg] | 34 | 27 | 27 | 25 | <0.001 | |
| O2_saturation [%] | 98 | 96 | 98 | 95 | 0.001 | |
| FiO2 [%] | 28 | 40 | 40 | 50 | 0.056 | |
| pO2/FiO2 [ratio] | 334 | 306.7 | 306.7 | 172.5 | 0.195 |
*Septic Shock was not included as MAP is defined by the category.
Within the group of 115 ALF patients examined, all 56 with severe sepsis or septic shock had demonstrated positive bacterial cultures (Table 1), and the CLD, non-SIRS, and SIRS groups had no reported positive cultures on or prior to the study day. Blood stream infections (with or without other positive cultures) were present in 23 of the 56, with the majority of the remaining infections being tracheal, urinary tract or multiple without blood stream infection (n = 16, 8 and 4 respectively).
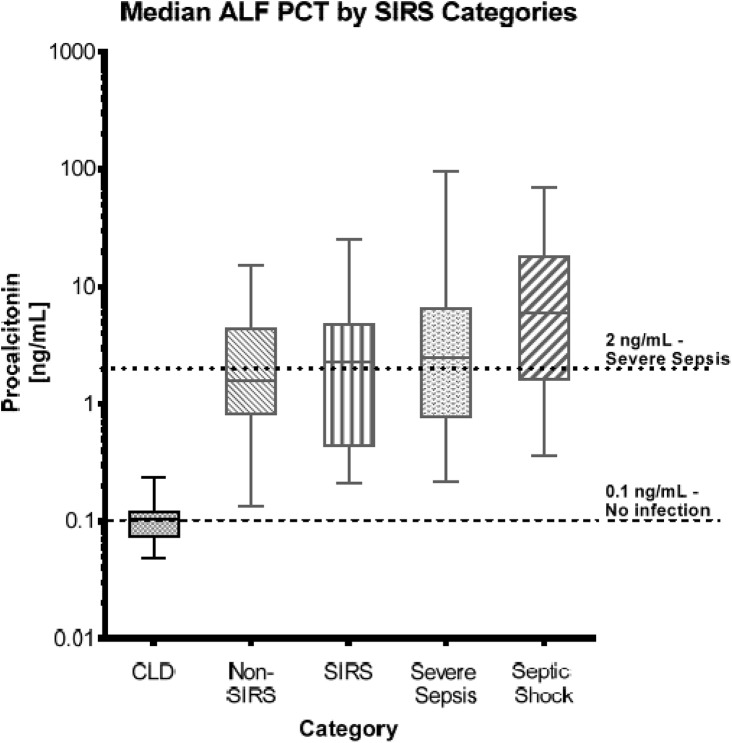
Fig 1 and Table 2 present the median results for the PCT assay across all categories. There was a significant difference between the CLD, non-SIRS and SIRS groups with a Chi-square = 43.68 (df = 2, p-value <0.001) as well as between the CLD group and either the non-SIRS or the SIRS groups (both p-values <0.001). However, there was no significant difference between the non-SIRS and SIRS groups (p-value >0.05). When PCT results for CLD, non-SIRS, and SIRS groups combined were compared to the documented reference value of the PCT assay (<0.1 ng/mL—(17)), the median PCT value (0.97 ng/mL) was significantly higher than the reference value (p <0.001). Results for the CLD group (median = 0.104 ng/mL) considered separately did not differ from the published reference range (p = 0.985), while results from each of the other two groups (non-SIRS–median = 1.57 ng/mL and SIRS–median = 2.29 ng/mL) were significantly higher than the reference and the CLD median value (p <0.001 for both).
Fig 1. Median PCT Values by Category.
This graph represents the median PCT values for the five patient severity groups. The lower dashed horizontal line represents the 0.1 ng/mL PCT cut-off value indicative of no infection. The upper dotted horizontal line represents the 2.0 ng/mL PCT cut-off value indicative of severe sepsis.
When PCT concentrations were compared across all categories, there was a significant difference between the CLD group and the four ALF categories (Chi-square = 53.98, p-value <0.001). Post hoc pairwise testing indicated that the median PCT of the CLD group was significantly different from each of the ALF categories (p<0.001). However, there were no significant differences observed (p = 0.169) between the four ALF categories themselves (non-SIRS, SIRS, severe sepsis and septic shock). When we compared the PCT values between ALF subjects without a documented infection (non-SIRS and SIRS) to those with a documented infection (severe sepsis and septic shock), there was no difference between the 2 groups (p = 0.082).
Subjects in severe sepsis and septic shock categories were examined based upon types of cultures: blood cultures vs. all other culture types. There were no significant differences observed for any of the 3 interactions: type of culture (p = 0.224), severity categories (p = 0.309), or the interaction between these two factors (p = 0.241). There was also no difference observed between the median PCT levels for subjects with positive blood cultures and those with positive cultures from other sources.
Study group characteristics based upon PCT cut-off value
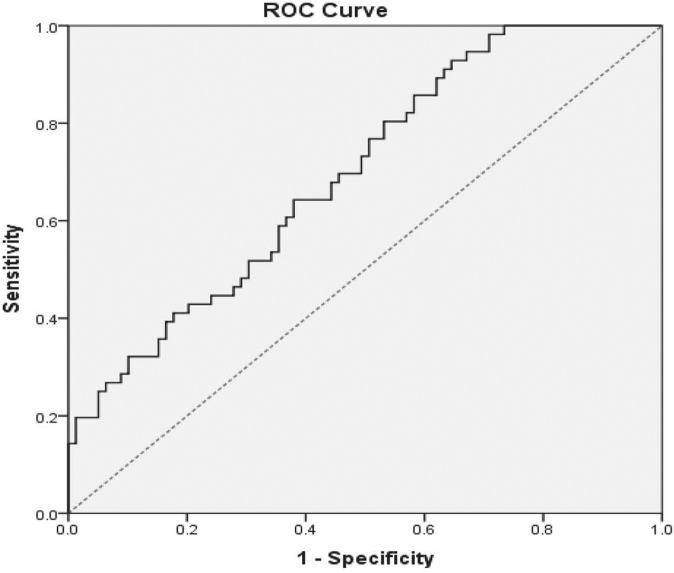
In previous studies, values of PCT above 2.0 ng/mL were considered to be associated with bacterial infection. While our non-SIRS and SIRS categories lacked evidence of infection, both demonstrated median PCT values near or slightly above the 2.0 ng/mL cut-off, 1.57 and 2.29 respectively. Given the uncertainty concerning what a reasonable cut-off value for infection should be for this population, we used a receiver operator characteristic (ROC) analysis, in which the optimal area under the curve (AUC) was equal to 0.697 (95% CI: 0.609–0.784), with a cut-off value of 1.62 ng/mL, somewhat below the 2.0 ng/mL level (Fig 2). The cut-off value determined from this set of data was made using a combination of criteria: 1) the point on the curve that was at the greatest distance (0.186) from the 0.5 reference line at a 45° angle to the line, 2) accuracy, 3) sensitivity (0.643), 4) specificity (0.620), and 5) likelihood ratio (1.693). We used this 1.62 ng/mL cut-off for subsequent analyses.
Fig 2. Receiver Operator Curve for the Detection of Infection in ALF and ALI patients Using PCT.
The ROC analysis resulted in an AUC of 0.697 with a sensitivity of 64.3% and a specificity of 62.0% for the use of PCT in the detection of infection this population of ALF patients.
When additional biochemical data ALF between subjects with a PCT that was <1.62 ng/mL and from those with a PCT that was ≥1.62 ng/mL were compared, platelet, creatinine, AST, ALT, and bilirubin showed significant differences between the theoretically ‘infected’ (above the cut-off) and ‘non-infected’ (below the cut-off) groups (Table 3). Of interest, 85.4% of all APAP subjects had values above the 1.62 ng/mL level while only 21 of 41 (51.2%) of the APAP subjects had a documented bacterial infection. In the non-APAP subjects, 43.2% had values above 1.62 ng/mL with documented bacterial infection in 35 of 74 (47.3%) of the subjects.
Table 3. Demographic and Biodata Results Based Upon the Calculated Infection Cut-off Value of 1.62 ng/mL.
| Median or Number (%) | PCTs < 1.62 ng/mL n = 48 | PCT ≥ 1.62 ng/mL n = 67 | p-value |
|---|---|---|---|
| ALF Etiology | |||
| APAP | 6 (12.5) | 35 (52.2) | |
| Viral hepatitis | 17 (34.7) | 11 (16.4) | |
| Other | 25 (51.0) | 21 (31.3) | |
| Outcome (TFS) | |||
| APAP | 3 (6.2) | 21 (31.3) | |
| Viral Hepatitis | 5 (10.4) | 3 (4.4) | |
| Other | 5 (10.4) | 6 (9.0) | |
| Procalcitonin [ng/mL] | 0.6904 | 5.325 | <0.001 |
| WBC [x 103 /uL] | 13.0 | 13.0 | 0.740 |
| Platelets [x 103 /uL] | 135 | 92 | 0.019 |
| INR [ratio] | 2.0 | 2.0 | 0.786 |
| Glucose [mg/dL] | 115.5 | 120.0 | 0.787 |
| Creatinine [mg/dL] | 1.0 | 2.0 | <0.001 |
| AST [IU/L] | 337 | 913 | 0.06 |
| ALT [IU/L] | 684 | 1759 | 0.07 |
| Lactate [mg/dL] | 3.5 | 6.0 | 0.230 |
| Bilirubin [mg/dL] | 19.0 | 7.0 | <0.001 |
| pO2 [mmHg] | 92.5 | 103.0 | 0.707 |
| pCO2 [mmHg] | 29.0 | 27.0 | 0.108 |
| Pulse [bpm] | 84 | 68 | 0.084 |
| Respiration [breaths/min] | 17 | 13 | 0.094 |
| Min Temp [°C] | 36.0 | 37.0 | 0.298 |
| Max Temp [°C] | 37.0 | 37.0 | 0.298 |
| Coma Grade | |||
| 0 / Not reported | 3 | 4 | |
| 1 | 7 | 9 | |
| 2 | 12 | 8 | |
| 3 | 9 | 12 | |
| 4 | 18 | 33 |
Samples were resorted based upon the cut-off value of 1.62 ng/mL and demographic and biodata were re-examined.
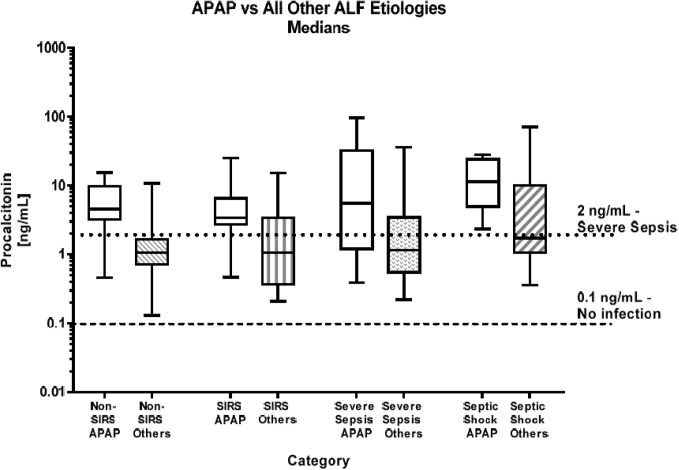
When PCT results were examined based solely upon etiology, median values for subjects with APAP toxicity were typically much higher, between 3.0 and 6.6 times higher than the results observed for the same severity category for subjects with non-APAP etiologies (Fig 3).
Fig 3. Median APAP vs All Other Etiologies PCT values by Category.
This graph represents the median PCT values for the four ALF patient severity groups sorted by etiologies: APAP and All Others etiologies (combined viral and other) with the lower dashed line indicative of no infection (0.1 ng/mL) and the upper dotted line indicative of severe sepsis (2.0 ng/mL).
Discussion
In this study of ALF patients selected for increasing severity of SIRS features and for the presence of bacterial infection, PCT measurement disclosed markedly elevated values but did not provide any direct correlation to presence or absence of infection. Determining the presence with certainty of systemic infection in those with advance SIRS features is problematic in a retrospective study; we assumed that the presence of advanced SIRS features plus positive cultures was the best surrogate for systemic infection and this may not be the case in every instance. However, virtually all ALF subjects displayed PCT values well above both the 0.1 ng/mL reference value and the median value observed in the CLD controls, regardless of any evidence for infection, particularly high values being observed in a large group that demonstrated no evidence for infection. The differences in PCT levels between severity categories among ALF patients were not significant due apparently to elevated levels in the absence of infection. Thus, both the non-SIRS and SIRS categories that would be expected to show low or normal PCT levels demonstrated PCT levels approximating those that have been observed previously in other studies during active bacterial infection in a non-ALF setting.
Using a cut-off value determined by the ROC analysis, the presumed uninfected categories (non-SIRS and SIRS) included 31 patients with elevated PCTs (≥1.62 ng/mL). Of these subjects, 13 demonstrated signs of infection (bacterial or fungal, n = 5 each) 1 or more days after the PCT sample was collected or other evidence of infection upon closer examination of the each subject’s case report form (CRF), including pancreatitis, tooth abscess or ventilator acquired pneumonia with infiltrate on x-ray. Subjects with fungal infections were classified as culture negative since values of PCT in yeast and fungal infections have been observed to be lower than those seen with most bacterial infections [20].
The median PCT values in the severe sepsis and septic shock categories (3.46 ng/mL and 589 ng/mL, respectively), were both well above the 1.62 ng/mL calculated cut-off and 2.0 ng/mL literature cut-off for severe sepsis [10], but these were not significantly different from the median PCT values in non-SIRS and SIRS patients without documented infection. Of interest, 17 of 40 severe sepsis subjects and 3 of 16 septic shock subjects had PCT values below the 1.62 ng/mL cut-off. Prophylactic antibiotics might provide a partial explanation for lower PCT values during a documented infection but, overall, the decreased results remain unexplained. Full bacterial culture reports were not collected as part of the USALFSG study nor did the study case report forms capture information related to quantity of bacterial growth or antibiotic sensitivities that might have helped determine some the differences among the various severity groups.
Upon examining results according to etiology, subjects with APAP toxicity demonstrated much higher PCT levels than any other etiologies. It seems likely that the elevated PCT values in these subjects are not indicative of infection. Rather, APAP toxicity unleashes an inflammatory response, with macrophage activation, release of cytokines and pro-inflammatory regulators including TNF-alpha that may secondarily contribute to liver injury and the appearance of a septic milieu [21, 22]. While the exact origin and pathogenic pathway of PCT stimulation is still unknown, it has been suggested that PCT is produced by neuroendocrine cells in the liver while other data suggest that it is released by macrophages [23]. It is also possible that increases in PCT may be initiated by TNF-alpha and IL-1b [24, 25]. It appears that in the acute liver failure setting PCT is increased due in large part to the massive inflammatory response to liver injury, particularly in the case of acetaminophen toxicity. While we did see higher PCT levels in patients with bacterial infections, if PCT is to be useful in this population, a better understanding of the mechanism of PCT production will need to be ascertained.
Prior studies examining PCT values in the acute liver failure setting have been limited. Studies in patients with chronic liver disease (cirrhosis) [26–29], liver metastasis of non-liver primary solid tumors [23], and other acute and chronic liver diseases have been performed [30]. While baseline PCT values increased as the severity of the underlying liver disease increased, an elevated PCT was indicative of bacterial infection. A previous study of PCT use in ALF patients due to APAP toxicity also showed increased PCT values in subjects without evidence of bacterial infection [14], similar to our findings.
We considered whether the use of older retrospective samples from the ALFSG repository might affect results, since long-term sample storage may decrease observed PCT levels. Schuetz, et al. studied two sets of samples that were stored at -80°C for 4.8 to 5.5 years and 3.3 to 4.6 years [31]. The PCTs for each set decreased by 11.4% and 13.5% respectively. Samples used in this study had been stored an average of approximately 6.75 years at -80°C. While it is assumed that the length of time in storage might have decreased the PCT values obtained, the exact amount of decrease is unknown and an estimate of the extent of the effect cannot be calculated. However, since each sample group had similarly aged samples, this would appear to negate a specific effect on the overall study results (Table 1).
Conclusion
Procalcitonin detects bacterial infection and sepsis in the general population, but appears to be a more general marker of moderate to severe inflammation. As such, it has limited value as a single marker to discriminate between the presence or absence of bacterial infection in the setting of acute liver failure but may be of value in combination with other markers. PCT elevations appeared to be roughly proportional to the degree of hepatic necrosis. A prospective study with a larger sample size employing prospective determination of infection and standardized antibiotic usage might provide additional information regarding the relationship between PCT and severe liver injury, particularly APAP toxicity.
Supporting Information
(XLSX)
Acknowledgments
Members and institutions participating in the Acute Liver Failure Study Group 1998–2011 are as follow: William M. Lee, M.D. (Principal Investigator), The University of Texas Southwestern Medical Center, Dallas, TX; Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Timothy Davern, M.D., University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco, CA), Oren Fix, M.D., University of California, San Francisco; Michael Schilsky, M.D., Mount Sinai School of Medicine, New York, NY (current address: Yale University, New Haven, CT); Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwyn Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, Jody A. Balko, Ph.D., the University of Texas Southwestern Statistics and Data Management Group included Joan Reisch, PhD, Linda Hynan, PhD, Janet P. Smith, Joe W. Webster, and Mechelle Murry, and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Catherine Dillon, Holly Battenhouse and Tomoko Goddard.
Abbreviations
- ALF
acute liver failure
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- AUC
area under the receiver operator characteristic curve
- CLD
chronic liver disease
- CRF
case report form
- HBV
hepatitis B virus
- HE
hepatic encephalopathy
- INR
International Normalized Ratio of prothrombin time
- LLD
lower limit of detection
- LT
liver transplantation
- MAP
mean arterial pressure
- PCT
procalcitonin
- PT
prothrombin time
- ROC
receiver operator characteristic curve
- SIRS
systemic inflammatory response syndrome
- TFS
transplant-free survival
- WBC
white blood cell count
Data Availability
All relevant data are contained within the supporting information file.
Funding Statement
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, W. M. Lee, Principal Investigator [grant U-01 DK58369]. Additional support was provided by Siemens Healthcare Diagnostics, Tarrytown, NY. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology, 2000; 32(4): 734–739. [DOI] [PubMed] [Google Scholar]
- 2. Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. Timing and aetiology of bacterial infections in a liver intensive care unit. J Hosp Infect, 2003; 53(2): 144–146. [DOI] [PubMed] [Google Scholar]
- 3. Stravitz RT. Critical management decisions in patients with acute liver failure. Chest, 2008; 134: 1092–1102. 10.1378/chest.08-1071 [DOI] [PubMed] [Google Scholar]
- 4. Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology, 2003; 125(3): 755–764. [DOI] [PubMed] [Google Scholar]
- 5. Becker KL, Nylen ES, White JC, Muller B, Snider RH, Jr. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: A journey from calcitonin back to its precursors. J Clin Endocrinol Metab, 2004; 89(4): 1512–1525. [DOI] [PubMed] [Google Scholar]
- 6. Bracq S, Machairas M, Clement B, Pidoux E, Andreoletti M, Mokhtar MS, et al. Calcitonin gene expression in normal human liver. FEBS Letters, 1993; 331(1,2): 15–18. [DOI] [PubMed] [Google Scholar]
- 7. Carrol ED, Thomson APJ, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents, 2002; 20: 1–9. [DOI] [PubMed] [Google Scholar]
- 8. Muller B, Becker KL. (2001). Procalcitonin: How a hormone became a marker and mediator of sepsis. Swiss Medi Wkly, 2001; 131(41–42): 595–602. [DOI] [PubMed] [Google Scholar]
- 9. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med, 2000; 26: S148–S152. 10.1007/BF02900728 [DOI] [PubMed] [Google Scholar]
- 10. Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. American Journal of Respiratory Critical Care Medicine, 2001; 164: 396–402. [DOI] [PubMed] [Google Scholar]
- 11. Brunkhorst FM, Eberhard OK, Brunkhorst R. Discrimination of infectious and noninfectious causes of early acute respiratory distress syndrome by procalcitonin. Crit Care Med, 1999; 27(10): 2172–2176. [DOI] [PubMed] [Google Scholar]
- 12. Luyt C, Guerin V, Combes A, Trouillet J, Ayed SB, Bernard M, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med, 2005; 171: 48–53. [DOI] [PubMed] [Google Scholar]
- 13. Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schuttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med, 1998; 24: 680–684. [DOI] [PubMed] [Google Scholar]
- 14. Jackson N, Batouche S, Sherwood R, Wendon J. Serial plasma procalcitonin levels in patients requiring admission to liver ITU with paracetamol induced acute liver failure. Abstract Brit J Anaesth, 2000; 84 (5): 92P. [Google Scholar]
- 15. Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology, 2008; 47: 1401–1415. 10.1002/hep.22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med, 2003; 31(4): 1250–1256. [DOI] [PubMed] [Google Scholar]
- 17.Siemens Healthcare Diagnostics, Ltd. Advia centaur and advia centaur XP systems—procalcitonin (PCT). Frimley, Camberley, UK; 2010. Rev B.
- 18. Elliot AC, Hynan LS. A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Programs Biomed, 2011; 102(1): 75–80. 10.1016/j.cmpb.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 19. Riffenburgh RH. Statistics in Medicine. Second Edition New York, NY, Elsevier, 2006. [Google Scholar]
- 20. Nakamura A, Wada S, Takeda T, Nobori T. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock, 2009; 31(6): 586–591. 10.1097/SHK.0b013e31819716fa [DOI] [PubMed] [Google Scholar]
- 21. Dragomir A-C, Laskin JD, Laskin DL. Macrophage activation by factors released from acetaminophen-injured hepatocytes: Potential role of HMGB-1. Toxicol Appl Pharmacol, 2011; 253: 170–177. 10.1016/j.taap.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev, 2012; 44(1): 88–106. 10.3109/03602532.2011.602688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matzaraki V, Alexandraki KI, Venetsanou K, Piperi C, Myrianthefs P, Malamos N, et al. Evaluation of serum procalcitonin and interleukin-6 levels as markers of liver metastasis. Clin Biochem, 2007; 40: 336–342. [DOI] [PubMed] [Google Scholar]
- 24. Whang KT, Vath SD, Nylen ES, Muller B, Li Q, Tamarkin L, et al. Procalcitonin and proinflammatory cytokine interactions in sepsis. Shock, 1999; 12(4): 268–273. [DOI] [PubMed] [Google Scholar]
- 25. Domenech VS, Nylen ES, White JC, Snider RH Jr, Becker KL, Landmann R, et al. Calcitonin gene-related peptide expression in sepsis: Postulation of microbial infection-specific response elements within the calcitonin I gene promoter. J Investig Med, 2001; 49(6): 514–521. [DOI] [PubMed] [Google Scholar]
- 26. Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med, 2005; 146(6): 347–351. [DOI] [PubMed] [Google Scholar]
- 27. Connert S, Stremmel W, Eising C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Abstract Z Gastroenterol, 2003; 41(2): 165–170. [DOI] [PubMed] [Google Scholar]
- 28. Spahr L, Morard I, Hadengue A, Vadas L, Pugin J. Procalcitonin is not an accurate marker of spontaneous bacterial peritonitis in patients with cirrhosis. Hepatogastroenterology, 2001; 48(38): 502–505. [PubMed] [Google Scholar]
- 29. Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Tardy B, et al. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: Diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med, 2000; 26: 1082–1086. [DOI] [PubMed] [Google Scholar]
- 30. Elefsiniotis IS, Skounakis M, Vezali E, Pantazis KD, Petrocheilou A, Pirounaki M, et al. Clinical significance of serum procalcitonin levels in patients with acute or chronic liver disease. Eur J Gastroenterol Hepatol, 2006; 18(5): 525–530. [DOI] [PubMed] [Google Scholar]
- 31. Schuetz P, Christ-Crain M, Huber AR, Muller B. Long-term stability of procalcitonin in frozen samples and comparison of kryptor and VIDAS automated immunoassays. Clin Biochem, 2010; 43(3): 341–344. 10.1016/j.clinbiochem.2009.08.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are contained within the supporting information file.