Abstract
The specialty of laparoscopy has evolved with the advent of new technologies over the last few years. Energy-based devices and Ultrasonic dissectors are used with a lot of factors in play-including ergonomics and economics during surgery. Here an attempt is based to review the surgical efficacy and safety of these dissectors with importance to plume production and lateral thermal damage. The factors contributing to adversities to the dissectors are also to be noted. The strategy adopted was aimed at finding relevant studies from PubMed from 1995 to 2014. The basic principle of plume production and thermal damage are studied in this review. Factors contributing to the same that can lead to adversities during laparoscopic surgeries are identified. Summarizing key points that increase lateral thermal damage and plume production amongst different ultrasonic shears and suggesting a technique to identify the right balance between the existing dissectors was possible. The RF Device and USS are both useful and widely used and are more safer than monopolar devices. RF Device is considerably slower than USS, as it cannot achieve coagulation and cutting at the same time. Although USS definitely improvises dissection and has less thermal injury than RF Device, the clinical implications in balancing dissection efficacy with hemostasis need to be investigated further. The ideal haemostatic energy-based shear device would be one with excellent hemostatic results and visual acuity while allowing none or minimal thermal energy escape at the point of application. In our current setting, a combined use of both RF and USS device usage as applied in the particular situations has potential.
Keywords: Laparoscopic, Ultrasonic dissectors, Thermal damage, Aerosol/plume, Energy devices
Introduction
Advances in energy devices have played a major role in the rapid expansion of surgery.
The specialty of laparoscopy has acquired a host of new technologies over the last few years. The techniques for hemostasis include physical modalities (compression, sutures, and endovascular staples), thermal modalities (bipolar coagulation, laser or ultrasonic dissectors), and topical sealants (e.g., fibrin glue or gelatin matrix) [1]. The advances in the evolution of energy-based haemostatic devices alone over the last few decades for tissue dissection and retraction have been with the primary aim of cutting down technical demands during minimal invasive surgery. A blend with high definition digital imaging has definitely even cut down on the surgical time. These devices allow rapid and easy dissection of tissue with ample freedom in terms of mobility and most importantly safety in haemostasis but are also prone to cause complications [2, 3]. Various factors like vessel burst pressure and seal time, lateral thermal spread, and smoke production influence their efficacy and complications [4].
Objective of this Review
The objective of this review is to compare the surgical efficacy and safety of Ultrasonic Shears with similar devices such as Radio frequency devices, to understand their potential intra-operative adversities, and to appraise a technique to increase the safety and efficacy of minimal access gynecological surgeries.
Method
This systemic review of literature was performed following the PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-Analysis) [5]. Strategy adopted was aimed at finding relevant studies from PubMed from 1995 to 2014. There appears to be paucity of literature for comparative studies with respect to the two aspects reviewed here—namely: aerosol production and lateral thermal damage. We reviewed the available reports in the literature that focused on the quantification of surgical plumes and comparative studies conducted on the thermal damage on tissues with different energy-based devices with respective to time, depth of tissue damage, and power output.
The search on PubMed showed a number of studies for laparoscopic dissectors but quantification of surgical plume had seven relevant studies; lateral thermal damage in ultrasonic dissectors had four studies.
Discussion
Analysis of Obstruction Generated by Laparoscopic Dissectors with Respect to Surgical Plume
A better understanding of plume is pivotal to use an instrument that will dissect and coagulate tissue with minimal thermal damage while allowing optimal visualization. Only recently, this area has been addressed. Digital imaging and software development permitted detection and calculation of surgical plume from a sequence of video frames.
The ultrasonic energy devices produce coagulation and transection of vessels by converting electric energy into ultrasonic vibration through a piezoelectric or magnetic transducer, which induces heat at the jaws of the instrument through friction but one of the undesirable products when energy is applied to tissue is the surgical plume or smoke [6, 7]. Although it is perceived that ultrasonic dissectors seem to generate less surgical plume than other technologies do, they still release particles from the friction of the blades and tissue adhering to the scope, producing a surgical plume [8]. The generation of a surgical plume is harmful when it persists as it decreases visualization, often requiring the surgeon to remove the scope from the surgical field and remove the obstructing aerosol particles. Compromised visualization in endoscopic surgery could be dangerous and fatal [9, 10]. There are a lot of studies on the effects on the composition of surgical smoke and its effects on the operating theater personnel [10]. The most important factor for this is better understanding of the characteristics of the surgical plume and electrophysics of thermal generation. Therefore, we initially reviewed the available reports in the literature focused on the quantification of surgical plumes.
The first reviewed study in 1995 identified the extent of surgical smoke from visual inspection and microscopic examination of residual particles, stains, or liquid on processed instruments. Sites that contained residual debris included junctions between insulating sheaths and activating mechanisms of laparoscopic instruments, and articulations and grooves of forceps were studied [11, 12]. This study led DesCoteaux et al. [8] in 1996 to describe the particles contained in cautery smoke produced during five laparoscopic procedures. Electron microscopic analysis and energy dispersive X-ray evaluation were used to determine particle morphology and elemental composition. The particles, distributed according to size on the seven rotating trays of the impactor, had diameters ranging from 0.05 to >25 micro m, with most being 0.1–1 μm. They concluded that surgical smoke (plume) created by electrocautery during open surgery was composed of breathable aerosol (≤4.5 μm) and possibly cellular material (≥7 μm) [8].
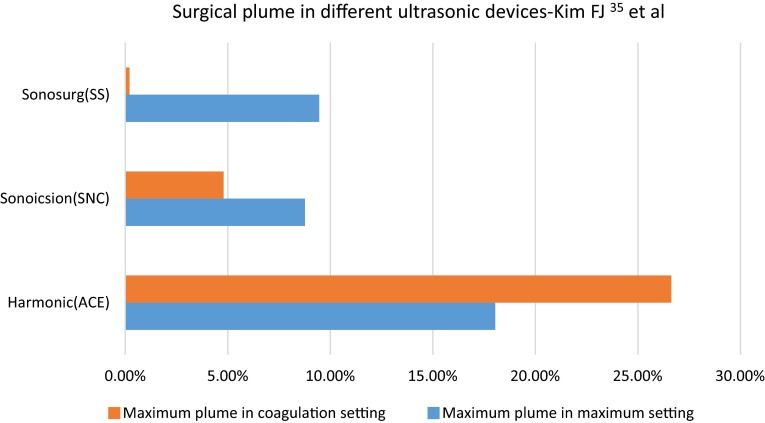
The surgical plume quantification from an ultrasonic scalpel was compared with that from electrocautery by Ott et al. [13] which described large quantities of cellular debris (>1 × 107 particles/mL) in the plume generated by the ultrasonic scalpel but had approximately one-fourth the amount of particle concentration which was further substantiated in the review exclusively on surgical smoke wherein the instrument and its effect on different types of tissue was described. Plume aerosol generated by ultrasonic dissectors can be identified up to 40 cm from the point of production and usually is composed of tissue, blood, and blood products. The surgical plume production from an ultrasonic scalpel was compared with that from electrocautery by Ott et al. [13], which described large quantities of cellular debris (>1 × 107 particles/mL) in the plume generated by the ultrasonic scalpel but had approximately one-fourth the amount of particle concentration which was further substantiated in the review exclusively on surgical smoke wherein the instrument and its effect on different types of tissue was also described [9, 13]. Fatty tissue was found to generate 17–23 times more particles than lean tissue did [13]. In an effort to quantify surgical smoke and laparoscopic visibility, Weld et al. [14] measured plume concentration from the peritoneal cavity in the light scattering equation to determine the level of reduction in visibility at surgery. Different types of dissectors and hemostatic instruments using different technologies, including electrical and ultrasonic, were reviewed. They found that the most significant influence on laparoscopic visibility was due to higher small particle concentration which remained in suspension longer, while small particle size was of secondary importance. Surgical plume generated by monopolar devices was 721 times more concentrated than were the plumes from bipolar and ultrasonic instruments [14]. Schneider et al. [15] developed another experiment to assess plume production. They develop a sealed box equipped with a light-emitting diode and a phototransistor. Infrared light transmission was assessed during the procedure, and mist formation was evaluated as the percentage of reduction in infrared transmission (Fig. 1).
Fig. 1.
Surgical plume in different ultrasonic devices
Cordless USS Improvises Agility and Handling
As an extension of this study, detection and quantification of surgical plume with ultrasonic dissectors using real-time digital quantitative technology ImageJ was pioneered by Kim et al. [16]. The investigators reported that plume generation was increased in coagulation rather than in cut mode. Moreover, they concluded that different instruments generate different amounts of obstruction of plume despite the use of the same ultrasonic technology. The ACE (Ethicon Endo-Surgery Inc, Cincinnati, Ohio) produced 5 times more plume than did Sonicision (Covidien, Mansfield, Massachusetts), while SonoSurg (Olympus USA, Center Valley, Pennsylvania) produced the least amount of plume [16]. SonoSurg generated negligible plume obstruction (0.21 % of operating field), while Sonicision obstructed 4.8 % of operating field and ACE 26.63 % [16]. Kim et al. [17] went on to identify two types of plume generations based on the blade geometry as well. Laminar flow causes minimal visual obstruction by directing the aerosol downwards and dissipates immediately, while turbulent plume is directed erratically across the cavity. It causes maximum obstruction 0.3 s after activation and clears after 2 s. Ultrasonic dissectors with straight blades have more consistent oscillations and generate more laminar flow compared with curved blades [17] (Fig. 2).
Fig. 2.
Cordless USS improvises agility and handling
In the comparative study [16] among different ultrasonic dissectors, the SonoSurg produced minimal obstruction during activation. The ACE generated the most plume, with approximately five times more plume than the Sonicision. The differences between all the instruments in the coagulation setting were significant (p\0.001). The average plume with respect to time was calculated with 95 % confidence intervals. Likewise, the ACE generated the most plume obstruction of the three devices, whereas the SonoSurg had the least plume during coagulation activation. There was less difference between the devices in the cutting mode. The Sonicision and SonoSurg produced the least amount of obstruction. Deviation of the ACE from the Sonicision and SonoSurg was significant (p\0.05). There was a statistically significant difference in mean dissection speed and production of plume where Sonicision was resultantly shown to be faster in the tested tissue [16] (Table 1).
Table 1.
Table for coagulation obstruction by surgical plume between different hemostatic technologies [16]
| Average coagulation obstruction | Monopolar | Ace | Sonicision | SonoSurg | p value |
|---|---|---|---|---|---|
| Maximum obstruction (%) | 721 × 26.63 | 26.63 ± 3.70 | 4.80 ± 0.86 | 0.21 ± 0.07 | <0.001 |
| Range (%) | 8.12–73.50 | 0.24–19.83 | 0.06–1.05 | ||
| Average cutting obstruction | |||||
| Maximum obstruction (%) | 12.65 ± 0.97 | 8.76 ± 1.49 | 9.46 ± 1.36 | 0.026 | |
| Range (%) | 9.07–18.15 | 4.32–17.41 | 5.68–22.12 |
The clinical significance of these findings is that obstruction of the surgical field by plume production may increase operating time to clean the scope, potential increase risk (e.g., bleeding) for patients if decrease in visibility occurs during crucial phase of the operation, and subjective increase in the level of surgical team’s frustration [11, 16].
To summarize, factors that increase plume production are to be borne in mind preoperatively to ensure safety and efficacy:
Nature of tissue-thick flaps and fat increases plume production,
Coagulative process than cutting increases plume.
Curved blades that cause turbulent plume flow increase plume.
Choice of instrument—monoplar devices, more plume.
It may be concluded that ultrasonic devices could produce smaller amounts of plume and, subsequently, less visual obstruction than do monopolar energy devices [16].
Thermal Damage in Laparoscopy—An In-depth Review
Electrosurgery has facilitated the development of advanced laparoscopic surgery by allowing rapid division of structures which are vascular, while maintaining the goal of hemostasis [18, 19]. Various devices have been introduced in clinical practice to achieve safe and faster hemostatic operative field. However, there is evidence that these advanced and electronically controlled devices may lead to inadvertent damage to nearby structures through the lateral spread of thermal energy, and this could result in delayed injuries to surrounding structures [19–22]. The earlier instruments used which are primarily Electrosurgical in nature were found to be relatively unsafe in abdominal surgery since their comparative lateral thermal spread possibly caused damage to vital structures [7, 20, 23]. Monopolar electrosurgery is associated with well-known risks that can cause substantial thermal injury to surrounding tissues. Minimizing thermal damage to surrounding tissues without compromising safety and tissue integrity is of importance in surgery. Ultrasonic instruments are tried to be safer than traditional diathermy [7, 20, 24–26]. The Ultrasonic shears incorporate piezoelectric transducers that induce a vibration frequency at the functional tip and transduces a lower amount of energy to the tissue than high-frequency current or laser techniques, resulting in reduced lateral thermal damage and penetration depth owing to lower temperatures [7, 20, 27]. Ultrasonic energy controls bleeding through the process of coaptive coagulation [4]. The present review of literature attempts to investigate the degree of lateral thermal injury on the surrounding tissues and their efficacy of ultrasonic shears with different energy devices (Fig. 3).
Fig. 3.
The physics of thermal damage with laparoscopic dissectors
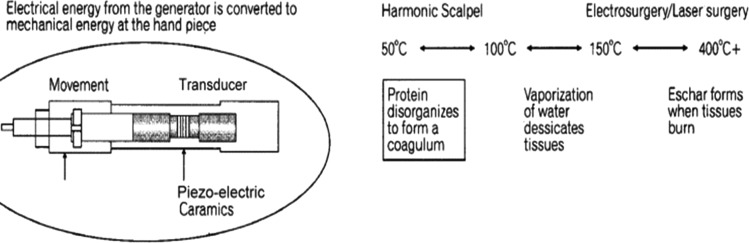
The cutting mechanisms of the ultrasonic shear devices are different from that observed with electro surgery or laser surgery. The first mechanism is cavitational cutting and fragmentation. As the blade tip vibrates, it produces large transient pressure changes, which causes cellular water to vaporize at low temperature, rupturing cells, leading to very precise cutting and dissection. The second mechanism for cutting by Ultrasonic shears is the actual power cutting offered by a relatively large blade vibrating 55,500 times per second. The blade edge cuts tissue by stretching it beyond its elastic limit and on a more microscopic level, by breaking molecular bands. The heat generated from friction of tissue is typically <80 °C. This minimizes tissue charring, desiccation, and the zone of thermal injury [1, 4, 6, 8]. Disadvantages of this technology are the formation of aerosolized fatty droplets from the tissue being treated, which can significantly interfere with visualization through a laparoscope [9, 16, 20].
Ultrasonic shears and Radiofrequency devices have been used in laparoscopic and open surgery for several years and have changed the way we operate. Both technologically advanced, they have been suggested to have excellent results and minimal lateral thermal injury. [6, 7, 18, 20, 24, 26]. Laparoscopic ultrasonic instruments obtain the same cutting and coagulation objectives but in different manners. Ultrasonic shears control bleeding by tamponating the vessel and sealing it with a protein coagulum at temperatures ranging from 50 to 100 °C. They work at lower temperatures than electrosurgical devices, since it denatures proteins by mechanically breaking the hydrogen bounds in protein molecules when the blade vibrates at 55.5 kHz, thus generating much less heat from tissue friction [7, 20, 22]. The review below compared all the energy-based laparoscopic devices and the degree of thermal damage with respect to time, power output, and tissue depth (Fig. 4).
Fig. 4.
Far less mist production with thin flaps vs thick flaps
Perko et al. [20] studied the effects of the Harmonic Scalpel from varying durations of it's application on tissues: a single 5-s application, a single 10-s application, and a regimen of two sequential 5-s applications. Using light microscopy and morphometric imaging analysis, the width of tissue lateral thermal damage was measured from the point of Harmonic Scalpel incision. The team showed lateral thermal damage over a mean width of 0.0522 ± 0.0097 mm after a 5-s Harmonic Scalpel application, a damage width of 0.1544 ± 0.0419 mm after a 10-s application, and a damage width of 0.1020 ± 0.0430 mm after a 5-s application followed by 5 s of inactivity and another 5 s of activity. The findings lead to the conclusion that tissue lateral thermal damage after Harmonic Scalpel application at standard output power is greater when a longer sustained period of application is used. Lateral thermal damage also is greater if the Harmonic Scalpel application time is continuous rather than of the same total duration with a brief midpoint interruption [20].
In 2009, the European Surgical Research also confirmed the previous studies by Pogorelic [7] and team that coagulation necrosis is bigger if the usage is continuous rather than if it is disconnected/reconnected. The findings mostly on porcine and rat abdominal walls have led to the conclusion that tissue lateral thermal damage after Ultrasonic shears application at standard output power is greater when a longer sustained period of application is used. Lateral thermal damage also is greater if the Ultrasonic shears' application time is continuous rather than of the same total duration with a brief midpoint interruption [7].
The LigaSure vessel sealing system is a bipolar feedback-controlled sealing system that effectively seals vessels up to 7 mm in diameter with a minimal thermal spread. The device applies a precise amount of mechanical pressure and radiofrequency energy to tissue, causing fusion of the opposing layers by creating a seal of denatured collagen, which can then be transected [28, 29]. The superiority of LigaSure over bipolar electrocautery is that the tissue fusion is created by the denaturation of proteins, thus forming a true seal rather than creating a proximal thrombus. Its lateral thermal spread is reported to be less than 1 mm [18]. After recruiting 100 patients for a comparative study of Monopolar diathermy, Harmonic scalpel and LigaSure at the department of surgery in Croatia, Družijanić [30] and team with the help of light microscopy and morphometric imaging analysis measured the width of tissue lateral thermal damage from the point of the peritoneal incision. After a peritoneal incision, the mean lateral thermal damages of monopolar diathermy, Harmonic scalpel (output power 3), Harmonic scalpel (output power 5), and LigaSure were 215.79, 90.42, 127.48, and 144.18 μm, respectively as shown in Table 2. The conclusion of this study showed that the degree of lateral thermal spread varied by instrument type, power setting, and application time. LigaSure and Harmonic scalpel were the safest and most efficient methods of tissue coagulation. Monopolar diathermy resulted in the greatest degree of thermal damage in tissues [30].
Table 2.
Comparison of thermal damage at different power settings among different laparoscopic shears
| Group comparison | Mean value A (μm) | Mean value B (μm) | p value |
|---|---|---|---|
| Monopolar diathermy vs. Harmonic scalpel (output power 3) | 215.79 | 90.42 | <0.001 |
| Monopolar diathermy vs. Harmonic scalpel (output power 5) | 215.79 | 127.48 | <0.001 |
| Monopolar diathermy v. LigaSure | 215.79 | 144.18 | <0.001 |
| Harmonic scalpel (output power 3) vs. Harmonic scalpel (output power 5) | 90.42 | 127.48 | 0.001 |
| Harmonic scalpel (output power 3) vs. LigaSure | 90.42 | 144.18 | <0.001 |
| Harmonic scalpel (output power 5) vs. LigaSure | 127.48 | 144.18 | 0.39 |
| Harmonic scalpel (output power 5)vs. Sonosurg | 90.49 | 117.6 | <0.001 |
A more extensive and prospective study to understand lateral thermal damage caused by different dissectors was undertaken by Lamberton et al. [31] in 2008, where a comparison of four laparoscopic vessel ligation devices (two bipolar sealing devices [LigaSure V (LS) and Gyrus PK (GP)], an ultrasonic device [Harmonic Scalpel ACE (HS)] and a novel device using nanotechnology [EnSeal PTC (ES)]) with the study endpoints including lateral thermal spread, time to seal, burst pressure, smoke production and subjective (blinded review of video clips) and objective (measured using an aerosol monitor) effect upon visibility. The HS demonstrated the least thermal spread. The LS (10.0 s) and GP (11.1 s) had the fastest sealing times (p < 0.001 for both) when compared to ES (19.2 s) and HS (14.3 s) (p < 0.01 for all).The LS has the highest burst pressure and fastest sealing time and was the highest rated overall. The HS produced the lowest thermal spread and smoke but had the lowest mean burst pressure. The GP had the highest smoke production and variable burst pressures. Despite employing nanotechnology, the ES device was the slowest [31].
In a study by Kim [32] and team who have done extensive work on ultrasonic laparoscopic dissectors, the Harmonic ACE (ACE), Covidien Sonicision (SNC), and Olympus SonoSurg (SS) and thermal damage were studied. Temperature was measured with an infrared thermal imager, and the histological examination after temperatures reaching 60 C was analyzed. The results were as follows: The ACE, Sonicision, and SonoSurg had emissivity measurements of 0.49 ± 0.01, 0.40 ± 0.00, and 0.39 ± 0.01, respectively. Maximum cutting temperatures were: ACE = 191.1°, SNC = 227.1°, and SNS 184.8° * (*p < 0.001). Maximum coagulation temperatures did not differ significantly among devices (p = 0.490). The cooling times to reach 60 °C after activation were 35.7 s (ACE), 38.7 s (SNC), and 27.4 s* (SS) (*p < 0.001). The cooling times of passive jaws to reach 60 °C after activation were 25.4 s* (ACE), 5.7 s (SNC), and 15.4 s (SS) (*p < 0.001).This study showed that Sonicision improves cutting by getting the blade hotter, while the SonoSurg has more precise coagulation effects by heating slower. Emissivity values varied among instruments, providing equally varied results. Although a different blade geometry is apparent between instruments, the jaws are also designed differently between the generations of instruments [16, 32, 33] (Table 3).
Table 3.
Comparison of emissivity and maximum cutting temperature in different ultrasonic shears
| Type of ultrasonic dissector | Emissivity | Max. cutting temperature |
|---|---|---|
| Harmonic (ACE) | 0.49 ± 0.01 | 191.10 |
| Sonicision (SNC) | 0.40 ± 0.00 | 227.1 |
| Sonosurg (SS) | 0.39 ± 0.01 | 184.8 |
Maximum coagulation temperatures did not differ significantly among devices [32] (p = 0.490)
Sutton [34] and team in a comparative study of monopolar and bipolar diathermy, the Harmonic scalpel and Ligasure recorded temperatures generated in the tissues adjacent and 1 cm away. After a 5-s application at the highest power setting, temperatures recorded at the tips of monopolar and bipolar diathermy, Harmonic Scalpel, and Ligasure instruments were 78.9(4.1), 41.9(2.2), 47.6(2.5), and 44.2(2.6) °C respectively. Temperatures at the instrument tips after use for 15 s remained above 42 °C for 55, 25, 15, and 15 s respectively. Applying monopolar diathermy (10 s at 40 W) resulted in a temperature recording of 59.2(2.2) °C in tissues 1 cm away from the tip of the instrument. The degree of lateral thermal spread varied with instrument type, power setting, and application time. Monopolar diathermy resulted in the highest temperatures and the greatest degree of thermal spread in tissues [34].
In our review of most studies, thermal injury of the surrounding tissue was much more evident after monopolar diathermy than after Radiofrequency devices or even than ultrasonic shears [18, 24, 25, 34, 35]. Ultrasonic energy delivered through a harmonic scalpel has been shown to be safe and to produce minimal damage to the surrounding tissues but high-power and prolonged ultrasonic dissection may result in considerable heat production and collateral tissue damage, especially when the activation time exceeds 10 s [4, 7, 20]. Harmonic scalpel application times of more than 5 s presented a risk of lateral thermal damage, especially near sensitive tissues or organs such as the common bile duct or ureter. The findings of these studies should suggest that after 5 s of application, a 5-s pause should be made, followed by an additional 5 s if necessary thereby clears the field of aerosol and minimizes thermal spread [7, 20].
Lateral thermal damage produced by the Harmonic scalpel at an output power of 5 was greater than that at an output power of 3 [7, 20, 36]. At the highest power setting, we found slightly less thermal injury caused by the Harmonic scalpel (127.48 μm) than LigaSure (144.18 μm), but that difference was nonsignificant. Certain studies, e.g., Diamantis et al.; Sutton et al. [26, 28] in their studies, found that LigaSure (197.79 μm) might cause less thermal injury than Harmonic scalpel (205.61 μm) but that difference was not significant. Sartori and colleagues [18] found that the only real advantage between LigaSure and Harmonic scalpel was a shorter duration of surgery with the Harmonic scalpel, probably owing to its ability to coagulate and cut at the same time.
To summarize, Minimizing thermal damage to surrounding tissues without compromising safety and tissue integrity is of importance in surgery, and it is to be borne in our minds that the degree of lateral thermal spread depends on the following factors:
Appropriate method of usage
Type of instrument
The power settings—higher output-higher damage
Duration of application—a 5 s application followed by a 5 s pause and then application minimizes thermal damage
Final outcome The ideal haemostatic energy-based shear device would be one with excellent hemostatic results and visual acuity while allowing none or minimal thermal energy escape at the point of application. Our findings suggest that RF Device and USS are both useful and widely used hemostatic and dissecting devices. They are much safer and more effective than the monopolar diathermy. RF Device is considerably slower than USS, as it cannot achieve coagulation and cutting at the same time. Although USS definitely improvises dissection and has less thermal injury than RF Device, the clinical implications in balancing dissection efficacy with hemostasis need to be investigated further.
Conclusion
The future might hold promise for the combination of beneficial capabilities into one device. In our current setting, a combined use of both RF and USS device usage as applied in the particular situations has potential.
Dr. Rajesh Devassy
is a Consultant Advanced Laparoscopic Surgeon at Dubai London Clinic & Specialty Hospital, Jumeira, Dubai, UAE and Director of Gem Advanced Minimal Access Surgery Training Centre, University Hospital for Gynecology, Oldenburg, Germany.
Compliance with Ethical Standards
Conflict of interest
None declared.
Footnotes
Rajesh Devassy is a Consultant Advanced Laparoscopic Surgeon in Dubai London Clinic & Specialty Hospital, Dubai, UAE and Director of Gem Advanced Minimal Access Surgery Training Centre, University Hospital for Gynecology, Oldenburg, Germany; Sreelatha Gopalakrishnan is Specialists in Ob/Gynecologist at Dubai London Specialty Hospital, Jumeira, Dubai, UAE.
References
- 1.Lallouf LB, Beri A, Klinger CH, et al. Practical hints for hemostasis in laparoscopic surgery. Minim Invasive Ther Allied Technol. 2007;16(1):45–51. doi: 10.1080/13645700601157984. [DOI] [PubMed] [Google Scholar]
- 2.da Silva RD, Sehrt D, Molina WR, et al. Significance of surgical plume obstruction during laparoscopy. JSLS. 2014;18(3):69. doi: 10.4293/JSLS.2014.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Den Boer KT, de Jong T, Dankelman J, et al. Problems with laparoscopic instruments: opinion of experts. J Laparoendosc Adv Surg Tech A. 2001;11(3):149–155. doi: 10.1089/10926420152389297. [DOI] [PubMed] [Google Scholar]
- 4.Emam TA, Cuschieri A. How safe is high-power ultrasonic dissection. Ann Surg. 2003;237:186–191. doi: 10.1097/01.SLA.0000048454.11276.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21:339. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimino W, Bond L. Physics of ultrasonic surgery using tissue fragmentation: part I. Ultrasound Med Biol. 1996;1:89–100. doi: 10.1016/0301-5629(95)02021-7. [DOI] [PubMed] [Google Scholar]
- 7.Pogorelić Z, Perko Z, Družijanić N, et al. How to prevent lateral thermal damage to tissue using the harmonic scalpel: experimental study on pig small intestine and abdominal wall. Eur Surg Res. 2009;43:235–240. doi: 10.1159/000226219. [DOI] [PubMed] [Google Scholar]
- 8.DesCoteaux J, Picard P, Poulin E, et al. Preliminary study of electrocautery smoke particles produced in vitro and during laparoscopic procedures. Surgical Endoscopy. 1996;10:152. doi: 10.1007/BF00188362. [DOI] [PubMed] [Google Scholar]
- 9.Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17(6):979–987. doi: 10.1007/s00464-002-8584-5. [DOI] [PubMed] [Google Scholar]
- 10.Matthew MD, Stevens E, Mark Taylor S, et al. The air that we breathe’: assessment of laser and electrosurgical dissection devices on operating theater air quality. J Otolaryngol Head Neck Surg. 2014;43(1):39. [Google Scholar]
- 11.Ball K. Survey of physicians attitudes toward surgical smoke. Can Oper Room Nurs J. 1995;13(4):18–21. [PubMed] [Google Scholar]
- 12.Hoglan M. Potential hazards from electrosurgery plume–recommendations for surgical smoke evacuation. Can Oper Room Nurs J. 1995;13(4):10–16. [PubMed] [Google Scholar]
- 13.Ott DE, Moss E, Martinez K. Aerosol exposure from an ultrasonically activated (Harmonic) device. J Am Assoc Gynecol Laparosc. 1998;5(1):29–32. doi: 10.1016/S1074-3804(98)80007-8. [DOI] [PubMed] [Google Scholar]
- 14.Weld KJ, Dryer S, Ames CD, et al. Analysis of surgical smoke produced by various energy-based instruments and effect on laparoscopic visibility. J Endourol. 2007;21(3):347–351. doi: 10.1089/end.2006.9994. [DOI] [PubMed] [Google Scholar]
- 15.Schneider A, Doundoulakis E, Can S, et al. Evaluation of mist production and tissue dissection efficiency using different types of ultrasound shears. Surg Endosc. 2009;23(12):2822–2826. doi: 10.1007/s00464-009-0512-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim FJ, Sehrt D, Pompeo A, et al. Comparison of surgical plume among laparoscopic ultrasonic dissectors using a real-time digital quantitative technology. Surg Endosc. 2012;26:3408–3412. doi: 10.1007/s00464-012-2351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim FJ, Sehrt D, Pompeo A, et al. Laminar and turbulent surgical plume characteristics generated from curved- and straight-blade laparoscopicultrasonic dissectors. Surg Endosc. 2014;28(5):1674–1677. doi: 10.1007/s00464-013-3369-6. [DOI] [PubMed] [Google Scholar]
- 18.Sartori PV, De Fina S, Colombo G, et al. Ligasure versus Ultracision in thyroid surgery: a prospective randomized study. Langenbecks Arch Surg. 2008;393:655–658. doi: 10.1007/s00423-008-0386-3. [DOI] [PubMed] [Google Scholar]
- 19.Gözen AS, Teber D, Rassweiler JJ. Principles and initial experience of a new device for dissection and hemostasis. Minim Invasive Ther Allied Technol. 2007;16:58–65. doi: 10.1080/13645700701191537. [DOI] [PubMed] [Google Scholar]
- 20.Perko Z, Pogorelić Z, Bilan K, et al. Lateral thermal damage to rat abdominal wall after harmonic scalpel application. Surg Endosc. 2006;20:322–324. doi: 10.1007/s00464-005-0089-6. [DOI] [PubMed] [Google Scholar]
- 21.Kadesky KM, Schopf B, Blair GK. Proximity injury by the ultrasonically activated scalpel during dissection. J Pediatr Surg. 1997;32:878–879. doi: 10.1016/S0022-3468(97)90641-2. [DOI] [PubMed] [Google Scholar]
- 22.Amaral JF. Depth of thermal injury: ultracisionally activated scalpel vs. electrosurgery. Surg Endosc. 1995;9:226. [Google Scholar]
- 23.Humes DJ, Ahmed I, Lobo DN. The pedicle effect and direct coupling: delayed thermal injuries to the bile duct after laparoscopic cholecystectomy. Arch Surg. 2010;145:96–98. doi: 10.1001/archsurg.2009.236. [DOI] [PubMed] [Google Scholar]
- 24.Diamantis T, Kontos M, Arvelakis A, et al. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, Ultracision, and Ligasure. Surg Today. 2006;36:908–913. doi: 10.1007/s00595-006-3254-1. [DOI] [PubMed] [Google Scholar]
- 25.Diamantis T, Gialikaris S, Kontos M, et al. Comparison of safety and efficacy of ultrasonic and bipolar thermal energy: an experimental study. Surg Laparosc Endosc Percutan Tech. 2008;18:384–390. doi: 10.1097/SLE.0b013e31816f85c9. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb WL, Hope WW, Schmelzer TM, et al. Comparison of blood vessel sealing among new electrosurgical and ultrasonic devices. Surg Endosc. 2009;23:90–96. doi: 10.1007/s00464-008-9932-x. [DOI] [PubMed] [Google Scholar]
- 27.Druzijanić N, Perko Z, Kraljević D, et al. Harmonic scalpel in transanal microsurgery. Hepatogastroenterology. 2008;55:356–358. [PubMed] [Google Scholar]
- 28.Smulders JF, de Hingh IH, Stavast J, et al. Exploring new technologies to facilitate laparoscopic surgery: creating intestinal anastomoses without sutures or staples, using a radio-frequency-energy-driven bipolar fusion device. Surg Endosc. 2007;21:2105–2109. doi: 10.1007/s00464-007-9330-9. [DOI] [PubMed] [Google Scholar]
- 29.Elemen L, Yazir Y, Tugay M, et al. LigaSure compared with ligatures and endoclips in experimental appendectomy: how safe is it? Pediatr Surg Int. 2010;26:539–545. doi: 10.1007/s00383-010-2557-x. [DOI] [PubMed] [Google Scholar]
- 30.Družijanić N, Pogorelić Z, Perko Z, et al. Comparison of lateral thermal damage of the human peritoneum using monopolar diathermy, Harmonic scalpel and LigaSure. Can J Surg. 2012;55(5):317–321. doi: 10.1503/cjs.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamberton GR, Hsi RS, Jin DH, Lindler TU, et al. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22:2307–2312. doi: 10.1089/end.2008.9715. [DOI] [PubMed] [Google Scholar]
- 32.Kim FJ, Sehrt D, da Silva RD, et al. Evaluation of emissivity and temperature profile of laparoscopic ultrasonic devices (blades and passive jaws) Surg Endosc. 2015;29(5):1179–1184. doi: 10.1007/s00464-014-3787-0. [DOI] [PubMed] [Google Scholar]
- 33.Kim FJ, Chammas MF, Jr, Gewehr E, et al. Temperature safety profile of laparoscopic devices: harmonic ACE (ACE), Ligasure V (LV), and plasma trisector (PT) Surg Endosc. 2008;22:1464–1469. doi: 10.1007/s00464-007-9650-9. [DOI] [PubMed] [Google Scholar]
- 34.Sutton PA, Awad S, Perkins AC, et al. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel and the Ligasure. Br J Surg. 2010;97:428–433. doi: 10.1002/bjs.6901. [DOI] [PubMed] [Google Scholar]
- 35.Kwok A, Nevell D, Ferrier A, et al. Comparison of tissue injury between laparosonic coagulating shears and electrosurgical scissors in the sheep model. J Am Assoc Gynecol Laparosc. 2001;8:378–384. doi: 10.1016/S1074-3804(05)60334-9. [DOI] [PubMed] [Google Scholar]
- 36.Emam TA, Cuschieri A. How safe is high-power ultrasonic dissection. Ann Surg. 2003;237:186–191. doi: 10.1097/01.SLA.0000048454.11276.62. [DOI] [PMC free article] [PubMed] [Google Scholar]