Abstract
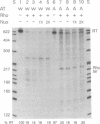
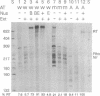
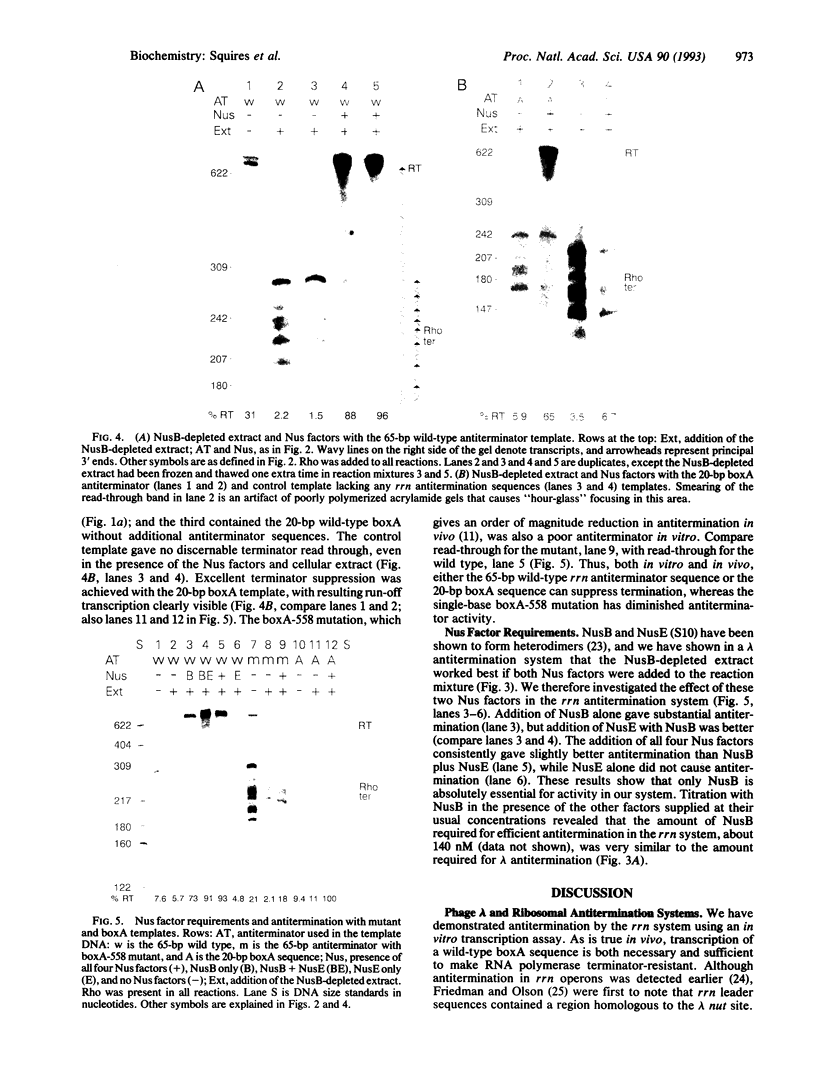
Using an in vitro transcription assay, we have successfully demonstrated read through of a Rho-dependent terminator by the ribosomal RNA antitermination system. The assay used a DNA template containing a promoter-antiterminator-terminator arrangement, RNA polymerase, termination factor Rho, antitermination factors NusA, NusB, NusE, and NusG, and a cellular extract depleted of NusB. Terminator read-through was highly efficient only in the presence of the extract and Nus factors, suggesting that an as yet uncharacterized cellular component is required for ribosomal antitermination. The NusB-depleted extract had no activity in the absence of NusB, confirming an absolute requirement for this protein in ribosomal RNA antitermination. The DNA template requirements were the same as those previously established in vivo; transcription of a wild-type boxA sequence is both necessary and sufficient to promote RNA polymerase modification into a terminator-resistant form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Squires C. L., Squires C. Evidence for antitermination in Escherichia coli RRNA transcription. J Bacteriol. 1984 Jul;159(1):260–264. doi: 10.1128/jb.159.1.260-264.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen B., Squires C. L., Li S., Squires C. Antitermination of characterized transcriptional terminators by the Escherichia coli rrnG leader region. J Mol Biol. 1990 May 5;213(1):123–134. doi: 10.1016/S0022-2836(05)80125-1. [DOI] [PubMed] [Google Scholar]
- Barik S., Ghosh B., Whalen W., Lazinski D., Das A. An antitermination protein engages the elongating transcription apparatus at a promoter-proximal recognition site. Cell. 1987 Sep 11;50(6):885–899. doi: 10.1016/0092-8674(87)90515-0. [DOI] [PubMed] [Google Scholar]
- Berg K. L., Squires C., Squires C. L. Ribosomal RNA operon anti-termination. Function of leader and spacer region box B-box A sequences and their conservation in diverse micro-organisms. J Mol Biol. 1989 Oct 5;209(3):345–358. doi: 10.1016/0022-2836(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell. 1983 Aug;34(1):143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Johnson L. L., Alessi D., Craven M. G. Transcription-dependent competition for a host factor: the function and optimal sequence of the phage lambda boxA transcription antitermination signal. Genes Dev. 1990 Dec;4(12A):2210–2222. doi: 10.1101/gad.4.12a.2210. [DOI] [PubMed] [Google Scholar]
- Goda Y., Greenblatt J. Efficient modification of E. coli RNA polymerase in vitro by the N gene transcription antitermination protein of bacteriophage lambda. Nucleic Acids Res. 1985 Apr 11;13(7):2569–2582. doi: 10.1093/nar/13.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981 May;24(2):421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Positive control of endolysin synthesis in vitro by the gene N protein of phage lambda. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3606–3610. doi: 10.1073/pnas.69.12.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz R. J., Li J., Greenblatt J. An elongation control particle containing the N gene transcriptional antitermination protein of bacteriophage lambda. Cell. 1987 Nov 20;51(4):631–641. doi: 10.1016/0092-8674(87)90132-2. [DOI] [PubMed] [Google Scholar]
- Houman F., Diaz-Torres M. R., Wright A. Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell. 1990 Sep 21;62(6):1153–1163. doi: 10.1016/0092-8674(90)90392-r. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W. Rho-dependent transcription termination at lambda R1 requires upstream sequences. J Biol Chem. 1985 Jan 10;260(1):574–584. [PubMed] [Google Scholar]
- Li J., Horwitz R., McCracken S., Greenblatt J. NusG, a new Escherichia coli elongation factor involved in transcriptional antitermination by the N protein of phage lambda. J Biol Chem. 1992 Mar 25;267(9):6012–6019. [PubMed] [Google Scholar]
- Li S. C., Squires C. L., Squires C. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell. 1984 Oct;38(3):851–860. doi: 10.1016/0092-8674(84)90280-0. [DOI] [PubMed] [Google Scholar]
- Mason S. W., Greenblatt J. Assembly of transcription elongation complexes containing the N protein of phage lambda and the Escherichia coli elongation factors NusA, NusB, NusG, and S10. Genes Dev. 1991 Aug;5(8):1504–1512. doi: 10.1101/gad.5.8.1504. [DOI] [PubMed] [Google Scholar]
- Mason S. W., Li J., Greenblatt J. Direct interaction between two Escherichia coli transcription antitermination factors, NusB and ribosomal protein S10. J Mol Biol. 1992 Jan 5;223(1):55–66. doi: 10.1016/0022-2836(92)90715-v. [DOI] [PubMed] [Google Scholar]
- Mason S. W., Li J., Greenblatt J. Host factor requirements for processive antitermination of transcription and suppression of pausing by the N protein of bacteriophage lambda. J Biol Chem. 1992 Sep 25;267(27):19418–19426. [PubMed] [Google Scholar]
- Morgan E. A. Antitermination mechanisms in rRNA operons of Escherichia coli. J Bacteriol. 1986 Oct;168(1):1–5. doi: 10.1128/jb.168.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A. Insertions of Tn 10 into an E. coli ribosomal RNA operon are incompletely polar. Cell. 1980 Aug;21(1):257–265. doi: 10.1016/0092-8674(80)90133-6. [DOI] [PubMed] [Google Scholar]
- Nodwell J. R., Greenblatt J. The nut site of bacteriophage lambda is made of RNA and is bound by transcription antitermination factors on the surface of RNA polymerase. Genes Dev. 1991 Nov;5(11):2141–2151. doi: 10.1101/gad.5.11.2141. [DOI] [PubMed] [Google Scholar]
- Sharrock R. A., Gourse R. L., Nomura M. Defective antitermination of rRNA transcription and derepression of rRNA and tRNA synthesis in the nusB5 mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5275–5279. doi: 10.1073/pnas.82.16.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopta M., Carthew R. W., Greenblatt J. Isolation of three proteins that bind to mammalian RNA polymerase II. J Biol Chem. 1985 Aug 25;260(18):10353–10360. [PubMed] [Google Scholar]
- Sullivan S. L., Gottesman M. E. Requirement for E. coli NusG protein in factor-dependent transcription termination. Cell. 1992 Mar 6;68(5):989–994. doi: 10.1016/0092-8674(92)90041-a. [DOI] [PubMed] [Google Scholar]
- Swindle J., Zylicz M., Georgopoulos C., Li J., Greenblatt J. Purification and properties of the NusB protein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10229–10235. [PubMed] [Google Scholar]
- Whalen W. A., Das A. Action of an RNA site at a distance: role of the nut genetic signal in transcription antitermination by phage-lambda N gene product. New Biol. 1990 Nov;2(11):975–991. [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell W. S., Roberts J. W. The phage lambda gene Q transcription antiterminator binds DNA in the late gene promoter as it modifies RNA polymerase. Cell. 1992 Jun 26;69(7):1181–1189. doi: 10.1016/0092-8674(92)90639-t. [DOI] [PubMed] [Google Scholar]