Abstract
BACKGROUND
Lung cancer is the leading cause of cancer-related death in the United States (US) Military and worldwide, with non-small cell lung cancer (NSCLC) accounting for 87 % of cases.
OBJECTIVES
Using a US military cohort who receives equal and open access to healthcare, we sought to examine demographic, clinical features and outcomes with NSCLC.
DESIGN AND PARTICIPANTS
We conducted a retrospective cohort analysis of 4,751 patients, aged ≥ 18 years and diagnosed with a first primary NSCLC between 1 January 2003 and 31 December 2013 in the US Department of Defense (DoD) cancer registry.
MAIN MEASURES
Differences by patient and disease characteristics were compared using Chi-square and t-test. Kaplan Meier curves and Cox proportional hazards regression assessed overall survival.
RESULTS
The mean age at diagnosis was 66 years, 64 % were male, 72 % were Caucasian, 41 % were diagnosed at early stage, 77 % received treatment and 82 % had a history of tobacco use. Mean age at diagnosis was highest among Caucasians (67 years) and lowest among African Americans (AA; 62 years). Asian/Pacific Islanders (PI) were more likely to be female (p < 0.0001), have adenocarcinoma histology (p = 0.0003) and less likely to have a history of tobacco use (p < 0.0001) compared to other racial/ethnic groups. In multivariable survival analysis, older age, male gender, increasing stage, not receiving treatment, and tobacco history were associated with higher mortality risk. Untreated patients exhibited a 39 % higher mortality risk compared to treated patients (HR = 1.39; 95%CI = 1.23–1.57). Compared to Caucasian patients, Asian/PIs demonstrated a 20 % lower risk of death (HR = 0.80; 95%CI = 0.66–0.96). There was no difference in mortality risk between AAs and Hispanics compared to Caucasians.
CONCLUSION
The lack of significant outcome disparity between AAs and Caucasians and the earlier stage at diagnosis than usually seen in civilian populations suggest that equal access to healthcare may play a role in early detection and survival.
KEY WORDS: military, lung cancer, survival outcomes
INTRODUCTION
Although lung cancer is the second-most common malignancy diagnosed in the United States (US), accounting for 15 % of all new cancers, it is the leading cause of cancer deaths in both men and women.1,2 Non-small cell lung cancer (NSCLC) comprises approximately 85–90 % of all lung cancer cases, with more than half of all patients diagnosed at an advanced stage defined as stage IIIB or IV. Advanced stage disease carries an overall 5-year survival rate of less than 15 %.1–3
Stage, gender, race, and smoking are known independent prognostic factors related to survival.4–7 In the US population, survival rates for African Americans are lower than their non-African American counterparts8–10; however, studies controlling for stage, treatment, and socioeconomic status demonstrate that overall survival rates may be similar.11,12 Disparities in lung cancer outcomes have also been linked to quality healthcare access and early detection.13,14 However, comprehensive analyses of NSCLC disparities by race/ethnicity are limited in that some studies did not control for important confounding variables such as comorbidities,10,12,15 only examined white versus black race,9,16,17 did not differentiate race from ethnicity,10,18 or small cell lung cancer from NSCLC.9,19 The United States Military Healthcare System provides a unique opportunity to examine racial/ethnic disparities in prognostic factors and outcomes, controlling for all relevant confounding variables in a heterogeneous NSCLC patient population with affordable and equal access to healthcare.
Active duty military service members and military retirees (a retiree is defined as an active duty military service member with ≥ 20 years of active duty service), as well as their dependents, are eligible for care at a military treatment facility (MTF) within the military health system. Patients treated within the military health system are different from those treated at the Veterans Administration Hospitals, which covers service-connected injury patients. The MTF beneficiaries are a unique population that has no cost barriers to physician visits, laboratory or radiology tests, medications, referrals, and cancer screening and surveillance compared to the general civilian population. All active duty service members are required to undergo an annual periodic health assessment incorporating screening guidelines, smoking cessation, and lifestyle modification discussions, in addition to a mandated comprehensive health examination.
Using a military cohort who receives equal and open access to healthcare within the Department of Defense (DoD) medical system, we sought to examine demographic and clinical features of the disease, prognostic indicators including racial/ethnic disparities in outcomes, and other factors associated with NSCLC survival. The results of this study will provide further insight into disease management for both the military and civilian populations.
METHODS
Data Source
The institutional review board at Naval Medical Center, Portsmouth Virginia, approved this study. A retrospective, registry-based cohort analysis was conducted using the DoD Automated Central Tumor Registry (ACTUR). Established in 1986, ACTUR maintains data on more than 350,000 reported incident cancer cases collected from approximately one hundred military treatment facilities world-wide. Military medical facilities are required to report cancer data on all DoD beneficiaries (active duty, family members of active duty, and retirees) who were diagnosed and/or treated for cancer within an MTF. ACTUR satisfies the American College of Surgeons, Commission on Cancer requirements for a comprehensive cancer data reporting system.
Study Population and Variables
NSCLC patients were identified with a primary site code of C34.0–C34.3 or C34.8–34.9 and the World Health Organization (WHO) Classification of Tumors ICD-O-3 histology codes for squamous cell, adenocarcinoma, large cell and other non-small cell. The analysis included 4,751 military service members and their dependents, at least 18 years of age, and diagnosed with a first primary NSCLC from January 2003 to March 2013. This cohort was diagnosed prior to routine use of low-dose computed tomography (CT) scans for lung cancer screening and during this time, chest X-rays were not ordered as part of the routine health maintenance.
Demographics (age, sex, race/ethnicity, marital status), clinical characteristics (stage, histology, comorbidity), environmental/lifestyle factors (tobacco and alcohol history), military status and family history of cancer were extracted from ACTUR. Patient age at diagnosis was used as a continuous variable and also stratified into four groups: 18–54, 55–64, 65–74, and ≥ 75 years. Race/ethnicity was categorized into five mutually exclusive groups: Caucasian, African American, Hispanic, Asian/PI, and Other/Unknown. The American Joint Committee on Cancer (AJCC) classification scheme was used to classify patients into the following stage categories: early (Stage I, II and IIIA) and advanced (Stage IIIB & IV). Documented first course of therapy and associated treatment initiation date is captured in the cancer registry and was used to classify patients as “treated” or “not treated”.
Statistical Analysis
All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute Inc., Cary, North Carolina). Demographic and clinical characteristics were summarized descriptively and compared using the Chi-square test for categorical variables and analysis of variance (ANOVA) or t-tests for continuous variables to examine differences between groups. A p value < 0.05 was considered to be statistically significant.
Kaplan-Meier survival curves and corresponding log-rank tests examined unadjusted overall survival by disease stage and race/ethnicity. Since timing of treatment initiation differed between patients, the relationship between treatment and survival was evaluated using a Cox regression model with treatment as a time-dependent factor. In the time-varying Cox model, all patients belong to the “not treated” group and only switched to the “treated” group at the time of treatment receipt. Other confounders included in the Cox model were selected a priori from baseline demographic and clinical characteristics. In the survival analyses, patients were included if they survived at least 30 days after diagnosis and were excluded if they were diagnosed in 2013 (to allow for sufficient follow-up time) or if their diagnosis date was the same as their date of death. In the Cox models, follow-up was calculated beginning on the date of diagnosis up until the first occurrence of a censoring event: date of death, last contact date (if alive) or end of follow-up period (30 April 2013).
RESULTS
Demographic and Clinical Characteristics
Military service members comprised 63 % of the cohort, while their spouses accounted for 31 %. The overall mean age at diagnosis was 66 years, 64 % were male and 72 % were Caucasian. Most of the cohort were either currently using or had a history of tobacco use (82 %). Adenocarcinoma (45 %) was the most common histologic subtype. The majority of the cohort (77 %) received treatment.
There were more patients (47 %) diagnosed at advanced stage compared to early stage (41 %), and tended to be younger (mean age 65 vs. 67; p < 0.0001) and more likely to be male (66 % vs. 62 %; p < 0.0001). In the early stage cohort, 58 % had stage I disease. Compared to patients diagnosed with advanced disease, those diagnosed with earlier stage presented predominantly with adenocarcinoma (51 % vs. 42 %) and/or squamous cell histology (26 % vs. 18 %) and were more likely to receive treatment (Table 1).
Table 1.
Demographic and Clinical Characteristics by Stage at Baseline.
| Characteristic | Total (N = 4751) | Early stage (N = 1954) | Advanced stage (N = 2212) | p value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age at diagnosis, n (%) | ||||
| 18–54 | 640 (13.5 %) | 220 (11.3 %) | 345 (15.6 %) | < 0.0001 |
| 55–64 | 1536 (32.3 %) | 621 (31.8 %) | 737 (33.3 %) | |
| 65–74 | 1480 (31.2 %) | 641 (32.8 %) | 661 (29.9 %) | |
| ≥ 75 | 1095 (23.0 %) | 472 (24.2 %) | 469 (21.2 %) | |
| Sex, n (%) | ||||
| Male | 3054 (64.3 %) | 1217 (62.3 %) | 1448 (65.5 %) | 0.0330 |
| Female | 1697 (35.7 %) | 737 (37.7 %) | 764 (34.5 %) | |
| Race-ethnicity, n (%) | ||||
| Caucasian | 3434 (72.3 %) | 1481 (75.8 %) | 1639 (74.1 %) | 0.4720 |
| African American | 533 (11.2 %) | 220 (11.3 %) | 264 (11.9 %) | |
| Asian/Pacific islander | 468 (9.9 %) | 189 (9.7 %) | 229 (10.4 %) | |
| Hispanic | 112 (2.4 %) | 36 (1.8 %) | 54 (2.4 %) | |
| Other/Unknown | 204 (4.3 %) | 28 (1.4 %) | 26 (1.2 %) | |
| Marital status | ||||
| Single | 204 (4.3 %) | 76 (3.9 %) | 107 (4.8 %) | 0.5962 |
| Married | 3201 (67.4 %) | 1383 (70.8 %) | 1544 (69.8 %) | |
| Separated/Divorced | 341 (7.2 %) | 143 (7.3 %) | 167 (7.5 %) | |
| Widowed | 622 (13.1 %) | 275 (14.1 %) | 300 (13.6 %) | |
| Missing/Unknown | 383 (8.1 %) | 77 (3.9 %) | 94 (4.2 %) | |
| Relationship to military, n (%) | ||||
| Self | 3004 (63.2 %) | 1227 (62.8 %) | 1455 (65.8 %) | 0.3039 |
| Spouse | 1490 (31.4 %) | 659 (33.7 %) | 677 (30.6 %) | |
| Other/Unknown | 257 (5.5 %) | 68 (3.6 %) | 80 (3.5 %) | |
| Tobacco history, n (%) | ||||
| Yes | 3871 (81.5 %) | 1688 (86.4 %) | 1887 (85.3 %) | 0.0188 |
| No | 453 (9.5 %) | 198 (10.1 %) | 209 (9.4 %) | |
| Unknown | 427 (9.0 %) | 68 (3.5 %) | 116 (5.2 %) | |
| Alcohol history, n (%) | ||||
| Yes | 2354 (49.5 %) | 1021 (52.3 %) | 1178 (53.3 %) | 0.2563 |
| No | 1611 (33.9 %) | 710 (36.3 %) | 756 (34.2 %) | |
| Unknown | 786 (16.5 %) | 223 (11.4 %) | 278 (12.6 %) | |
| Histology, n (%) | ||||
| Adenocarcinoma | 2156 (45.4 %) | 988 (50.6 %) | 923 (41.7 %) | <0.0001 |
| Squamous | 1011 (21.3 %) | 500 (25.6 %) | 389 (17.6 %) | |
| Large cell | 95 (2.0 %) | 37 (1.9 %) | 45 (2.0 %) | |
| Other, NOS | 1489 (31.3 %) | 429 (22.0 %) | 855 (38.7 %) | |
| # of Comorbidities, n (%) | ||||
| 0 | 2118 (44.6 %) | 776 (39.7 %) | 930 (42.0 %) | 0.3115 |
| 1–3 | 1371 (28.9 %) | 618 (31.5 %) | 674 (30.5 %) | |
| >3 | 1262 (26.6 %) | 560 (28.7 %) | 608 (27.5 %) | |
| Family history of cancer, n (%) | ||||
| Yes | 1790 (37.7 %) | 777 (39.8 %) | 904 (40.9 %) | 0.1646 |
| No | 1732 (36.5 %) | 775 (39.7 %) | 817 (36.9 %) | |
| Unknown | 1229 (25.9 %) | 402 (20.6 %) | 491 (22.2 %) | |
| Treatment status | ||||
| Treated | 3671 (77.3 %) | 1756 (89.9 %) | 1709 (77.3 %) | <.0001 |
| Not treated | 1080 (22.7 %) | 198 (10.1 %) | 503 (22.7 %) | |
In the stratified analysis (Table 2) by race/ethnicity, the mean age at diagnosis was highest among Caucasians (67 years) followed by Asian/PI (64 years), Hispanics (63 years), and African Americans (62 years). Asian/PIs were more likely to be female (57 %) and military spouses (51 %) compared to all other racial/ethnic groups. A history of tobacco use was highest among Caucasians (87 %) and African Americans (86 %), followed by Hispanics (74 %) and Asian/PI (65 %). Asian/PIs were also more likely to be married, have adenocarcinoma histology and were more likely to be sporadic cases (no family history) compared to the other self-described ethnic groups. Treatment rates were highest among African Americans (84 %), followed by Asian/PI (80 %), Caucasians (79 %) and lowest in Hispanics (68 %)
Table 2.
Demographic and Clinical Characteristics by Race/Ethnicity at Baseline
| Characteristic | Caucasian (N = 3434) | African American (N = 533) | Asian/Pacific Islander (N = 468) | Hispanic (N = 112) | Other/Unknown (N = 204) | p value |
|---|---|---|---|---|---|---|
| Age at diagnosis, n (%) | ||||||
| 18–54 | 408 (11.9 %) | 120 (22.5 %) | 75 (16.0 %) | 17 (15.2 %) | 20 (9.8 %) | < 0.0001 |
| 55–64 | 1061 (30.9 %) | 189 (35.5 %) | 164 (35.0 %) | 49 (43.8 %) | 73 (35.8 %) | |
| 65–74 | 1110 (32.3 %) | 130 (24.4 %) | 148 (31.6 %) | 27 (24.1 %) | 65 (31.9 %) | |
| ≥ 75 | 855 (24.9 %) | 94 (17.6 %) | 81 (17.3 %) | 19 (17.0 %) | 46 (22.5 %) | |
| Sex, n (%) | ||||||
| Male | 2256 (65.7 %) | 371 (69.6 %) | 202 (43.2 %) | 81 (72.3 %) | 144 (70.6 %) | < 0.0001 |
| Female | 1178 (34.3 %) | 162 (30.4 %) | 266 (56.8 %) | 31 (27.7 %) | 60 (29.4 %) | |
| Marital status | ||||||
| Single | 142 (4.1 %) | 32 (6.0 %) | 16 (3.4 %) | 7 (6.3 %) | 7 (3.4 %) | < 0.0001 |
| Married | 2387 (69.5 %) | 360 (67.5 %) | 344 (73.5 %) | 63 (56.3 %) | 47 (23.0 %) | |
| Separated/Divorced | 251 (7.3 %) | 56 (10.5 %) | 22 (4.7 %) | 5 (4.5 %) | 7 (3.4 %) | |
| Widowed | 482 (14.0 %) | 55 (10.3 %) | 67 (14.3 %) | 12 (10.7 %) | 6 (2.9 %) | |
| Missing/Unknown | 172 (5.0 %) | 30 (5.6 %) | 19 (4.1 %) | 25 (22.3 %) | 137 (67.2 %) | |
| Relationship to military, n (%) | ||||||
| Self | 2267 (66.0 %) | 387 (72.6 %) | 189 (40.4 %) | 80 (71.4 %) | 81 (39.7 %) | < 0.0001 |
| Spouse | 1063 (31.0 %) | 129 (24.2 %) | 237 (50.6 %) | 28 (25.0 %) | 33 (16.2 %) | |
| Other/Unknown | 104 (3.1 %) | 17 (3.2 %) | 42 (8.9 %) | 4 (3.6 %) | 90 (44.1) | |
| Tobacco history, n (%) | ||||||
| Yes | 2969 (86.5 %) | 459 (86.1 %) | 305 (65.2 %) | 83 (74.1 %) | 55 (27.0 %) | < 0.0001 |
| No | 263 (7.7 %) | 46 (8.6 %) | 125 (26.7 %) | 13 (11.6 %) | 6 (2.9 %) | |
| Unknown | 202 (5.9 %) | 28 (5.3 %) | 38 (8.1 %) | 16 (14.3 %) | 143 (70.1 %) | |
| Alcohol history, n (%) | ||||||
| Yes | 1827 (53.2 %) | 289 (54.2 %) | 162 (34.6 %) | 47 (42.0 %) | 29 (14.2 %) | < 0.0001 |
| No | 1147 (33.4 %) | 163 (30.6 %) | 242 (51.7 %) | 36 (32.1 %) | 23 (11.3 %) | |
| Unknown | 460 (13.4 %) | 81 (15.2 %) | 64 (13.7 %) | 29 (25.9 %) | 152 (74.5 %) | |
| AJCC stage, n (%) | ||||||
| I | 860 (25.0 %) | 124 (23.3 %) | 118 (25.2 %) | 19 (17.0 %) | 20 (9.8 %) | < 0.0001 |
| II | 251 (7.3 %) | 34 (6.4 %) | 28 (6.0 %) | 5 (4.5 %) | 6 (2.9 %) | |
| IIIA | 370 (10.8 %) | 62 (11.6 %) | 43 (9.2 %) | 12 (10.7 %) | 2 (1.0 %) | |
| IIIB/IV | 1639 (47.7 %) | 264 (49.5 %) | 229 (48.9 %) | 54 (48.2 %) | 26 (12.7 %) | |
| Missing/Unknown | 314 (9.1 %) | 49 (9.2 %) | 50 (10.7 %) | 22 (19.6 %) | 150 (73.5 %) | |
| Histology, n (%) | ||||||
| Adenocarcinoma | 1513 (44.1 %) | 246 (46.2 %) | 258 (55.1 %) | 48 (42.9 %) | 91 (44.6 %) | 0.0003 |
| Squamous | 772 (22.5 %) | 99 (18.6 %) | 74 (15.8 %) | 15 (13.4 %) | 51 (25.0 %) | |
| Large cell | 65 (1.9 %) | 15 (2.8 %) | 8 (1.7 %) | 4 (3.6 %) | 3 (1.5 %) | |
| Other, NOS | 1084 (31.6 %) | 173 (32.5 %) | 128 (27.4 %) | 45 (40.2 %) | 59 (28.9 %) | |
| # of Comorbidities, n (%) | ||||||
| 0 | 1464 (42.6 %) | 228 (42.8 %) | 179 (38.2 %) | 68 (60.7 %) | 179 (87.7 %) | < 0.0001 |
| 1–3 | 1027 (29.9 %) | 162 (30.4 %) | 141 (30.1 %) | 27 (24.1 %) | 14 (6.9 %) | |
| > 3 | 943 (27.5 %) | 143 (26.9 %) | 148 (31.6 %) | 17 (15.2 %) | 11 (5.4 %) | |
| Family history of cancer, n (%) | ||||||
| Yes | 1439 (41.9 %) | 190 (35.6 %) | 116 (24.8 %) | 27 (24.1 %) | 18 (8.8 %) | < 0.0001 |
| No | 1226 (35.7 %) | 209 (39.2 %) | 221 (47.2 %) | 43 (38.4 %) | 33 (16.2 %) | |
| Unknown | 769 (22.4 %) | 134 (25.1 %) | 131 (28.0 %) | 42 (37.5 %) | 153 (75.0 %) | |
| Treatment status | ||||||
| Treated | 2719 (79.2 %) | 448 (84.1 %) | 376 (80.3 %) | 76 (67.9 %) | 52 (25.5) | < 0.0001 |
| Not treated | 715 (20.8 %) | 85 (15.9 %) | 92 (19.7 %) | 36 (32.1 %) | 152 (74.5) | |
Overall Survival
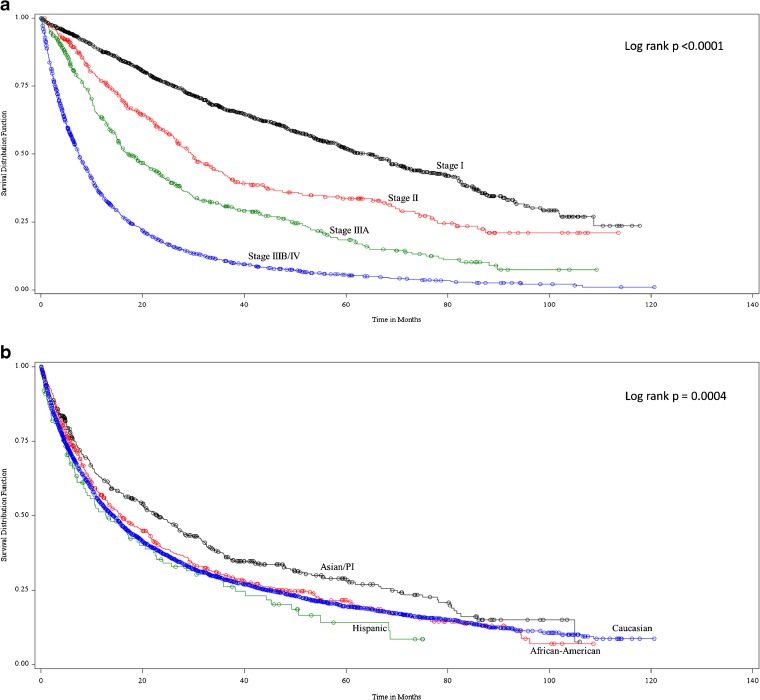
The unadjusted OS for the cohort was 14.97 months (95 % confidence interval (CI): 13.9–15.7) with significantly decreasing survival as stage increased. The unadjusted median overall survival was 64, 30, 17, and 7 months for stage I, II, IIIA, and IIIB/IV, respectively (log rank p < 0.0001; Fig. 1a). Asian/PIs had significantly higher unadjusted overall survival (Log rank p = 0.0004) compared to the other racial/ethnic groups. The unadjusted median overall survival was 13, 14, 16, and 23 months for Hispanic, Caucasian, African American and Asian/PI respectively (log rank p < 0.0004; Figure 1b)
Figure 1.
a. Unadjusted overall survival by stage. b. Unadjusted overall survival by race/ethnicity.
In the multivariable survival analysis, stage was the predominant predictor of mortality risk (Table 3). As stage increased, mortality risk also significantly increased up to threefold, adjusting for all other variables in the model. There was no significant difference in mortality risk between Caucasians and African Americans, and Caucasians and Hispanics. However, compared to Caucasian patients, Asian/PI patients demonstrated a significant 20 % lower risk of death (HR = 0.80; 95%CI = 0.66–0.96). Untreated patients had a 39 % increased risk of death compared to treated patients regardless of disease stage. Stratifying the model by stage showed a slightly higher mortality risk increase among the untreated advanced stage patients than in the untreated early stage patients. Risk of death also significantly increased with increasing age, in patients with greater than three comorbidities, and among those with a history of tobacco use.
Table 3.
Multivariate Analysis of Overall Mortality Risk
| Characteristics | Total (N = 2105) | Early stage (N = 587) | Advanced stage (N = 1220) | ||||
|---|---|---|---|---|---|---|---|
| N | HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | |
| Treatment status | |||||||
| Treated | 1320 | ref | ref | ref | |||
| Not treated | 785 | 1.39 | 1.23–1.57 | 1.37 | 1.04–1.80 | 1.46 | 1.26–1.69 |
| Age at diagnosis | |||||||
| 18–54 | 324 | ref | ref | ref | |||
| 55–64 | 684 | 1.04 | 0.88–1.23 | 0.80 | 0.50–1.25 | 1.11 | 0.91–1.34 |
| 65–74 | 606 | 1.23 | 1.04–1.46 | 1.06 | 0.67–1.68 | 1.21 | 0.99–1.48 |
| ≥ 75 | 491 | 1.20 | 0.99–1.44 | 1.17 | 0.72–1.88 | 1.20 | 0.95–1.50 |
| Race | |||||||
| Caucasian (ref) | 1513 | ref | ref | ref | |||
| African American | 223 | 0.90 | 0.77–1.07 | 0.95 | 0.66–1.38 | 0.87 | 0.72–1.08 |
| Asian/Pacific islander | 221 | 0.80 | 0.66–0.96 | 0.67 | 0.41–1.09 | 0.84 | 0.68–1.06 |
| Hispanic | 52 | 1.16 | 0.83–1.63 | 2.64 | 1.06–6.62 | 0.99 | 0.64–1.53 |
| Other/Unknown | 96 | 0.88 | 0.58–1.32 | 1.04 | 0.22–4.96 | 1.08 | 0.56–2.07 |
| Sex | |||||||
| Male | 1359 | ref | ref | ref | |||
| Female | 746 | 0.89 | 0.79–1.00 | 0.75 | 0.56–0.99 | 0.88 | 0.76–1.01 |
| Stage | |||||||
| I | 293 | ref | |||||
| II | 115 | 1.21 | 0.89–1.64 | ||||
| IIIA | 179 | 1.65 | 1.28–2.12 | ||||
| IIIB/IV | 1220 | 2.64 | 2.17–3.22 | ||||
| Missing/Unknown | 298 | 1.73 | 1.35–2.22 | ||||
| Histology | |||||||
| Squamous | 442 | ref | ref | ref | |||
| Adenocarcinoma | 869 | 0.96 | 0.83–1.11 | 0.60 | 0.44–0.82 | 1.02 | 0.85–1.22 |
| Large cell | 41 | 0.95 | 0.65–1.39 | 0.62 | 0.22–1.75 | 1.00 | 0.63–1.58 |
| Other | 753 | 1.15 | 1.00–1.32 | 0.82 | 0.61–1.10 | 1.19 | 1.00–1.42 |
| # of Comorbidities | |||||||
| 0 | 994 | ref | ref | ref | |||
| 1–3 | 591 | 1.08 | 0.95–1.22 | 1.02 | 0.77–1.37 | 1.08 | 0.93–1.25 |
| > 3 | 520 | 1.36 | 1.19–1.54 | 1.39 | 1.03–1.87 | 1.37 | 1.17–1.61 |
| Tobacco history | |||||||
| No | 205 | ref | ref | ref | |||
| Yes | 1687 | 1.27 | 1.04–1.55 | 1.16 | 0.72–1.85 | 1.37 | 1.08–1.74 |
| Unknown | 213 | 1.08 | 0.78–1.51 | 1.02 | 0.45–2.31 | 1.17 | 0.78–1.77 |
Model also includes family history, marital status, region, and alcohol history
DISCUSSION
This DoD cohort of U.S. military service members and their dependents who are cared for at a MTF provides a unique opportunity to evaluate both prognostic factors and outcomes. The equal and open access to health care offers no cost barriers to receive comprehensive evaluations as compared to the general civilian sector, and requires annual exams in the active duty service population. In this cohort, treatment, age, race, gender, stage, histology, number of comorbidities and tobacco history were all prognostic variables related to survival as would be expected based on many previous epidemiologic studies to date.20–22 However, several striking differences in this population compared to the general civilian population were revealed.
In the current analysis, racial disparity between African Americans and Caucasians was not demonstrated. Numerous studies have described the phenomenon of racial/ethnic disparities in lung cancer survival in the general US population.23–25 African Americans with lung cancer have lower survival rates than non-African Americans. Although stage is arguably the most powerful predictor of survival in lung cancer patients, even in early stage disease, African Americans have inferior outcomes than their non-African American counterparts.24 Racial/ethnic differences in genetics, tumor histology, and access to care may explain the variation in survival.10,24,26
The unadjusted median overall survival rates for Caucasians and African Americans were similar (14 vs. 16 months), and the lack of significant outcome disparity was confirmed in the multivariable survival model. Our findings are consistent with a previous study performed at the Walter Reed Medical Center documenting a lack of difference in survival between African Americans and Caucasians.27 Taken together, these data support the theory that poorer outcomes seen in civilian African Americans are more likely related to quality healthcare access rather than tumor biology.10,26
Moreover, our cohort was diagnosed at a younger age and with a higher proportion of early stage disease; specifically, stage I. This differs from the general US civilian population, where the average age at diagnosis is 70 years with a small percentage (17 %) diagnosed at the localized stage.23 The younger age and earlier stage at diagnosis in our cohort may be due to the routine health screening and required annual health examination for all active duty service members, which spans at least 20 years in length. The active health surveillance in this population facilitates an environment where disease may be discovered earlier.
Our cohort was diagnosed prior to routine use of low dose CT scanning for lung cancer screening, making the younger age and earlier stage at diagnosis in our cohort versus the civilian population even more compelling. The implementation of screening guidelines per the National Lung Cancer Screening Trials (NLST) as part of the mandatory annual health examination, combined with the higher smoking rates among military members, will likely have an even greater impact on early detection rates within the military healthcare system. This unique model of open access health care provided to this DoD population coupled with data from the NLST suggests that greater support for lung cancer screening improves outcomes.28
Not only was male gender found to be an independent unfavorable prognostic factor in our cohort, this finding holds true for the general US population.29 It has been postulated that the gender difference in outcomes is influenced by the high proportion of adenocarcinoma among females.29 The incidence of adenocarcinoma among females was 54 % in the present study; however, we also found a lower likelihood of tobacco use compared to males, which may account for the improved prognosis of females in our cohort.
The discovery of the epidermal growth factor receptor (EGFR) mutations in NSCLC and the exploitation of these mutations to direct treatment strategies has been a breakthrough for advanced stage patients. Patients with these mutations are more often Asian, female, and never or light smokers with adenocarcinoma histology.30 While approximately 10 % of Caucasians exhibit these mutations, upwards of 30–50 % of Asians harbor them.31 EGFR mutations predict increased sensitivity to EGFR directed therapies, as well as standard chemotherapies.30–32 In this study, Asian/PIs were more likely to be female and less likely to have a history of tobacco use. One could postulate that the high proportion of Asian/PI patients in our study possess the EGFR mutation and contribute to the higher survival compared to other racial/ethnic groups. However, information on molecular markers and their association with outcomes was not available for our population.
Use of the DoD data for this type of analysis has several strengths, including the large sample size and diverse geographic representation of NSCLC patients in the United States military. The distinct characteristics of equal access to affordable care, emphasis on health maintenance and screening, and lack of socioeconomic restrictions make the active duty military service members, their dependents, and retirees an influential group to study. However, given that this study was based on a population of patients serving in the military and their dependents, these results may not be generalizable to the US civilian population covered under other insurance. Outside of the unique nature of the population served, our study is also limited by the lack of data regarding performance score and molecular markers. These factors may influence the observed outcomes and as such, our survival model may have residual confounding. Nonetheless, our findings remain compelling and further investigation into how performance score and molecular markers may impact outcomes in this cohort is warranted.
In conclusion, our DoD registry-based analysis suggests a lack of survival disparity between African Americans and Caucasians, as well as the discovery of disease at an earlier stage and younger age compared to the general civilian population. These findings underscore the importance of equal and open access to healthcare, and imply that breaking down socioeconomic obstacles to care may positively influence outcomes in patients with NSCLC.
Acknowledgments
The authors would like to thank Elizabeth Butts at the DoD Cancer Registry and Surveillance Program, Nicholas Sicignano at the Navy Marine Corps Public Health Center and Tod Downen at Health Research Tx for their assistance with data acquisition and decoding. The authors would also like to acknowledge Larry Leon, Ph.D. for thoughtful review and recommendations on the statistical analyses.
Conflict of Interest
This study was funded by Genentech, Inc. through a contract with Q.D. Research, Inc. and with the Department of Defense. Dr. Reyes and Mr. Goertz are employees of Genentech and shareholders of Roche. Dr. Satram-Hoang and Mr. Gunuganti work for Q.D. Research in a research and consulting capacity. All other authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2010. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf [accessed February 26, 2015].
- 3.Surveillance Epidemiology and End Results (SEER) Stat bite: lung cancer stage at diagnosis in the United States, 1995–2001. J Natl Cancer Inst. 2005;97:1805. doi: 10.1093/jnci/dji454. [DOI] [PubMed] [Google Scholar]
- 4.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Lung carcinoma symptoms–an independent predictor of survival and an important mediator of African-American disparity in survival. Cancer. 2004;101:1655–63. doi: 10.1002/cncr.20547. [DOI] [PubMed] [Google Scholar]
- 5.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125:27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Ebbert JO, Williams BA, Sun Z, et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer. 2005;47:165–72. doi: 10.1016/j.lungcan.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 8.Fry WA, Menck HR, Winchester DP. The national cancer data base report on lung cancer. Cancer. 1996;77:1947–55. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Ries LA. Influence of extent of disease, histology, and demographic factors on lung cancer survival in the SEER population-based data. Semin Surg Oncol. 1994;10:21–30. doi: 10.1002/ssu.2980100106. [DOI] [PubMed] [Google Scholar]
- 10.Blackstock AW, Herndon JE, 2nd, Paskett ED, et al. Outcomes among African-American/non-African-American patients with advanced non-small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Natl Cancer Inst. 2002;94:284–90. doi: 10.1093/jnci/94.4.284. [DOI] [PubMed] [Google Scholar]
- 11.Blackstock AW, Herndon JE, 2nd, Paskett ED, et al. Similar outcomes between African American and non-African American patients with extensive-stage small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:407–12. doi: 10.1200/JCO.2005.02.1436. [DOI] [PubMed] [Google Scholar]
- 12.Bryant AS, Cerfolio RJ. Impact of race on outcomes of patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:711–5. doi: 10.1097/JTO.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 13.McDavid K, Tucker TC, Sloggett A, Coleman MP. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163:2135–44. doi: 10.1001/archinte.163.18.2135. [DOI] [PubMed] [Google Scholar]
- 14.Fergusson RJ, Thomson CS, Brewster DH, et al. Lung cancer: the importance of seeing a respiratory physician. Eur Respir J. 2003;21:606–10. doi: 10.1183/09031936.03.00060803. [DOI] [PubMed] [Google Scholar]
- 15.Fesinmeyer MD, Goulart B, Blough DK, Buchwald D, Ramsey SD. Lung cancer histology, stage, treatment, and survival in American Indians and Alaska Natives and whites. Cancer. 2010;116:4810–6. doi: 10.1002/cncr.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy D, Xia R, Liu CC, Cormier JN, Nurgalieva Z, Du XL. Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients. Cancer. 2009;115:4807–18. doi: 10.1002/cncr.24521. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Enewold L, Zahm SH, et al. Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiol Biomarkers Prev. 2012;21:1841–7. doi: 10.1158/1055-9965.EPI-12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed AM, Toonkel R, Glassberg MK, et al. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: an analysis of the survival, epidemiology, and end results database. Cancer. 2012;118:4495–501. doi: 10.1002/cncr.26686. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116:2437–47. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 20.Sonobe M, Date H, Wada H, et al. Prognostic factors after complete resection of pN2 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146:788–95. doi: 10.1016/j.jtcvs.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Brunelli A, Salati M, Refai M, et al. Development of a patient-centered aggregate score to predict survival after lung resection for non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146:385–90 e1-2. doi: 10.1016/j.jtcvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–7. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 23.Howlader N NA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Available at: http://seer.cancer.gov/csr/1975_2009_pops09/ [accessed February 26, 2015].
- 24.Wang SJ, Fuller CD, Thomas CR., Jr Ethnic disparities in conditional survival of patients with non-small cell lung cancer. J Thorac Oncol. 2007;2:180–90. doi: 10.1097/JTO.0b013e318031cd4e. [DOI] [PubMed] [Google Scholar]
- 25.Gadgeel SM, Severson RK, Kau Y, Graff J, Weiss LK, Kalemkerian GP. Impact of race in lung cancer: analysis of temporal trends from a surveillance, epidemiology, and end results database. Chest. 2001;120:55–63. doi: 10.1378/chest.120.1.55. [DOI] [PubMed] [Google Scholar]
- 26.Jack RH, Gulliford MC, Ferguson J, Moller H. Geographical inequalities in lung cancer management and survival in South East England: evidence of variation in access to oncology services? Br J Cancer. 2003;88:1025–31. doi: 10.1038/sj.bjc.6600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 28.National Lung Screening Trial Research T. Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–91. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 30.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 31.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 32.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]