Abstract
Objective
To describe the distribution of plasma high sensitivity C-reactive protein (hsCRP) and explore the relationship between hsCRP and metabolic risk factors among residents living in longevity areas of China.
Methods
268 individuals aged between 40 and 59 years and 506 individuals aged over 90 years were selected from 5 longevity areas of China to participate in a cross section longitudinal cohort study. The participants were interviewed with general health related questionnaire to collect their demographic, behavioral and lifestyle data, as well as their chronic conditions, and meanwhile their physical and biomedical parameters including waist circumference (WC), blood pressure (BP), hsCRP, plasma lipids, and fasting blood glucose (FBG) were measured.
Results
The median of hsCRP was 0.99 mg/L in the middle-aged group and 1.76 mg/L in the oldest old group. No significant gender difference was observed between the above two groups. Among the oldest old individuals, 36.56% had an hsCRP level >3.0 mg/L. The prevalence of high hsCRP was 16.79% in the middle-aged group. The results of stepwise multiple linear regression analyses showed that HDL-C was independently associated with ln (hsCRP) concentration in the middle-aged group, whereas ln (TG), HDL-C and FBG were correlated after adjustment for gender, study site, smoking, drinking, education and BMI in the oldest old group.
Conclusion
HDL-C is a stronger predictor of elevated hsCRP than other metabolic factors in the middle-aged population. For the oldest old persons, high TG, low HDL-C, and FBG predict elevated plasma hsCRP.
Keywords: High-sensitivity C-reactive protein, Blood lipids, Fasting blood glucose, Middle-aged, Oldest old individuals, Longevity, China
INTRODUCTION
Low grade systemic inflammation is common in elderly populations[1]. Advanced age generally aggravates the low-grade systemic inflammatory status[2]. High sensitivity C-reactive protein (hsCRP), an acute-phase protein, is an extremely sensitive marker of systemic inflammation. Accumulating evidence has shown that elevated CRP increases the risk of coronary heart disease events[3], hypertension[4], type 2 diabetes, metabolic syndrome[5–6], depressive symptomatology[7] and cognitive decline[8]. In a cohort of elderly men, higher CRP and lower albumin levels strongly predict both vascular and non-vascular mortality, independent of other characteristics[9].
Numerous factors may affect CRP concentrations, such as aging, lifestyle and metabolic indicators[10–11]. CRP levels were positively related with smoking status of adolescents[12] and adults[13]. Physical activity may reduce CRP concentration, which is a critical process in the pathogenesis of cardiovascular diseases[14]. Recent evidence suggests that central obesity, rather than total body fat, is a stronger contributor to elevated CRP[15] and that central adiposity may be more detrimental for women than men[16]. Lakoski et al.[17] found that hypertension was independently associated with CRP in both men and women. Low high-density lipoprotein cholesterol (HDL-C) is a predictor, among the plasma lipid parameters, for elevated plasma CRP in Taiwanese women[10]. These links between CRP concentration and most major health problems support our hypothesis that high levels of CRP are a common marker of many degenerative conditions linked to aging[18].
However, CRP levels vary among different populations[11, 19–23]. The relationship between hsCRP and the previously mentioned metabolic risk factors is primarily based on researches done in Western populations that generally focused on adults and younger elderly people. The association between hsCRP and metabolic risk factors is relatively unknown in the setting of a developing country or among the oldest old people. This study evaluated middle-aged and oldest old adults residing in longevity areas of China. It is important to understand the potential risk factors underlying elevated CRP levels among a longevity population. In this study, we examined the relationship between metabolic biomarkers and circulating CRP level in a population-based cross sectional study.
Methods
Subjects and Design
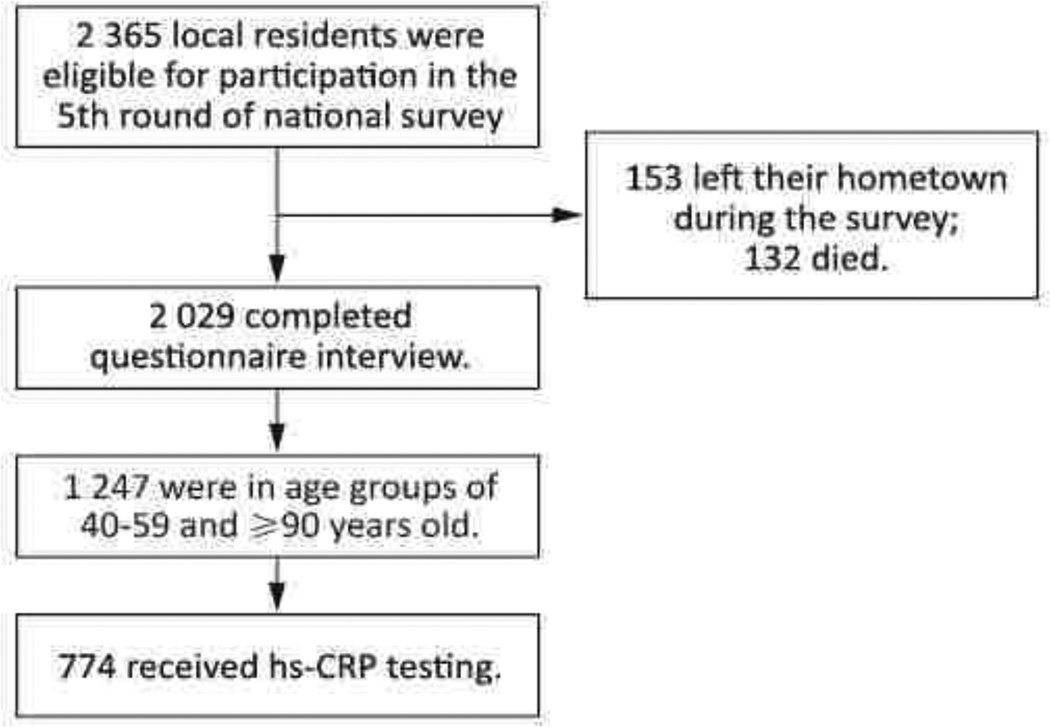
The data were derived from the fifth round of the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The goal of CLHLS is to determine which factors, out of a large set of social, behavioral, biological, and environmental risk factors, play an important role in healthy longevity. The baseline survey was conducted in 1998, with follow-up field surveys with replacements for deceased elders conducted in respective years of 2000, 2002, 2005, and 2009. The CLHLS attempted to interview all centenarians who voluntarily agreed to participate in the study. For each centenarian, one near-by octogenarian (aged 80–89) and one near-by nonagenarian (aged 90–99) of pre-designated age and sex were interviewed. “Near-by” is loosely defined-it could be in the same sampled town or county or city. The predefined age and sex, used to identify the approximately equal numbers of male and female nonagenarians and octogenarians, were randomly determined, based on the code numbers of the centenarians. The goal was to have comparable numbers of male and female octogenarians and nonagenarians at each age from 80 to 99. The detailed study design has been described elsewhere[24]. The fifth round of CLHLS was conducted in March-June 2009 in five longevity areas of China: Xiayi county in Henan province, Zhongxiang city in Hubei province, Mayang county in Hunan province, Yongfu county in Guangxi province, and Sanshui district of Foshan city in Guangdong province. The goal of the fifth survey was designed to evaluate the health status of Chinese elderly, especially the oldest old (aged 80 and above) and centenarians compared with middle aged population, and to explore the determinants of healthy longevity. The longevity areas were formally designated by the Chinese Society of Gerontology based on 17 criteria[25]. The three key criteria are 1) more than 7 per 100 000 local residents are centenarians (aged 100 years old or above), 2) the average life expectancy of local residents is ≥3 years older than the national average, and 3) ≥1.4% of the local residents are oldest old (≥80 years old). All centenarians and older were invited to participate in the study. The sample size was expanded to those 40 years old. An equal number of their neighbors in each of the 4 age groups (40–59, 60–79, 80–99, and 100+) matched by sex and closest residence were also invited to participate. The subjects included 398 centenarians and 1 967 neighbors aged 40–99 years (Figure 1). The protocol was approved by the Ethics Committee of Peking University. All of the recruited subjects (or their proxies) gave informed consent. Considering the difficulty to match the control group of the oldest old population and the limitation of budget to biochemical analyses, we chose the middle-aged people as the control. So, this study was limited to the 774 participants whose hsCRP data were available, i.e. only the middle-aged (40–59 years old) and oldest old (90+) participants.
Figure 1.
Enrollment of study participants.
Data Collection
The study included two components: an in-house face-to-face questionnaire interview and a physical examination. The in-house interview was conducted by trained physicians or public health workers from the local centers for disease control and prevention and community hospitals. The questionnaire elicited information related to demographics, behavioral and lifestyle parameters, self-rated health status and self-reported chronic diseases. The physical exam was performed by clinicians by using standardized instruments to measure weight, waist circumference (WC), and blood pressure (BP). These clinicians also ascertained the participants’ personal and family disease history and current medications. WC was measured to the nearest 0.1 cm by using a non-elastic plastic tape at the level of the midpoint of the waist between the lower rib margin and the iliac crest. An average BP was calculated from two measurements by a mercury sphygmomanometer. The measurement was carried out on the right arm of the seated participant after the individual rested for five minutes.
Biochemical Analyses
Participants were required to fast overnight. Venous blood samples were collected in heparin anticoagulant vacuum tubes, and then centrifuged at 20 °C, 2 500 rpm for 10 min. Plasma was isolated and frozen in −20 °C, and then the samples were shipped on wet ice to the central laboratory, Capital Medical University in Beijing, where they were stored at −80 °C until analysis. Plasma lipids/lipoproteins (TC, TG, HDL-C, and LDL-C), glucose and hsCRP were measured by an Automatic Biochemistry Analyzer (Hitachi 7180, Japan) by using commercially available diagnostic kits (Roche Diagnostic, Mannheim, Germany). Plasma hsCRP was measured through an immunoturbidimetric assay. The minimal detectable concentration of hsCRP was 0.11 mg/L. The inter- and intra-plate CVs were 0.43% and 2.7% for High value QC serum and 0.41% and 3.45% for low value QC serum.
Key Variables and Definitions
Abdominal obesity was defined as WC values of 85 cm or greater for males and 80 cm or greater for females[26]. Hypertension was defined as systolic blood pressure (SBP) ≥140 or diastolic blood pressure (DBP) ≥90 mmHg, or receiving treatment for previously diagnosed hypertension[27]. Elevated total cholesterol (TC) was defined as≥6.22 mmol/L. Elevated triglycerides (TG) were defined as≥2.26 mmol/L. Elevated LDL cholesterol (LDL-C) was defined as ≥4.14 mmol/L. Reduced HDL cholesterol (HDL-C) was defined as <1.04 mmol/L[28]. Diabetes was defined as a fasting blood glucose (FBG) ≥7.0 mmol/L or previously diagnosed type 2 diabetes[29]. Elevated hsCRP was defined as >3.0 mg/L[30].
Smoking was classified as “yes” if the participant was a current smoker, and as “no” if the participant was a nonsmoker or former smoker. For alcohol drinking, a current drinker was categorized into “yes” while no drinking or a former drinker into “no”. Current drinker (regular drinking) was defined as drinking at least once a week on average, no matter wine, liquor or beer, and not including drinking occasionally on holidays.
Statistical Methods
The mean or median were calculated for continuous variables, and percentage was calculated for categorical variables. Covariance analyses were performed to compare continuous variables between the two age groups while adjusting for gender and study site. Cochran-Mantel-Haenszel (CMH) statistics for categorical variables were applied for the comparison by age group while adjusting for study site. As the distribution of hsCRP levels was highly skewed, they were expressed as the geometric mean, median and interquartile range. HsCRP and TG were natural log-transformed for all other analyses. Partial Spearman correlation coefficients, adjusted for gender and study site, were calculated for ln (hsCRP) with variables of metabolic risk factors, such as WC, BMI, BP, TC, TG, HDL-C, and FBG. Stepwise multiple linear regression analyses were performed in the two age groups to determine whether the metabolic factors were associated with ln (hsCRP) level. The criterion for inclusion or exclusion in the model was a p-value of 0.10 or less. Data management and statistical analyses were performed with the SAS statistical package Version 9.1 (SAS Institute, Cary, NC, USA). Statistical tests were 2-sided, and a P value<0.05 was considered statistically significant.
Results
Characteristics of Study Participants
A total of 774 participants were included in the analysis, including 268 in the middle-aged group and 506 in the oldest-old group. The average ages of the participants in these two groups were 50.5 and 97.4 years, respectively. The middle-age participants were more likely to smoke and drink alcohol than the oldest-old participants; they had significantly higher values of WC, TG, TC, and LDL, but lower SBP levels than the oldest-old (P<0.001). There were no statistical differences with respect to DBP, FBG, HDL-C, and prevalence of chronic diseases in these two age groups (Table 1).
Table 1.
Comparison of Characteristics among Middle Aged and Oldest-old Residents in Chinese Longevity Areas
| Characteristics | Middle-aged (40–59) (n=268) |
Oldest-old (90+) (n=506) |
Statistical Value |
P Value |
|---|---|---|---|---|
| Age (years)* | 50.5(5.7) | 97.4 (5.0) | ||
| Education? | ||||
| Illiterate | 83 (31.0) | 484 (95.7) | ||
| Primary School | 46 (17.2) | 11 (2.2) | ||
| Junior high school and above | 139(51.8) | 8 (1.6) | 384.3086 | <0.0001 |
| Not answer | 3 (0.6) | |||
| Family Income yuan/year* | 23494.3 (26395.4) | 23638.8 (30655.5) | 0.01 | 0.9116 |
| WC (cm)* | 83.3 (11.7) | 75.5 (11.5) | 67.07 | <0.0001 |
| SBP (mmHg)* | 128.5 (19.1) | 139.2 (26.8) | 51.42 | <0.0001 |
| DBP (mmHg)* | 81.2 (12.9) | 79.4 (14.8) | 0.02 | 0.8855 |
| FBG (mmol/L)* | 5.60 (2.31) | 5.47 (1.79) | 0.86 | 0.3551 |
| TG (mmol/L)* | 1.79 (2.02) | 1.14 (0.51) | 46.71 | <0.0001 |
| TC (mmol/L)* | 4.54 (1.08) | 4.08 (1.07) | 32.29 | <0.0001 |
| LDL-C (mmol/L)* | 2.51 (0.76) | 2.17 (0.80) | 32.93 | <0.0001 |
| HDL-C (mmol/L)* | 1.31 (0.40) | 1.31 (0.32) | 0.00 | 0.9864 |
| Current smoker? | 111 (41.4) | 136 (26.9) | 16.6623 | <0.0001 |
| Alcohol drinker ? | 107 (39.9) | 122 (24.1) | 20.7305 | <0.0001 |
Note.
Data listed as mean±SD deviation for continuous variables, were tested for differences by age group by using covariance analysis after adjustment for study site.
Data listed as n (%) for categorical variables were tested by CMH statistics by age group after adjustment for study site.
The Distribution of hsCRP and Prevalence of High hsCRP
The median hsCRP was 0.99 mg/L in the middle-aged group and 1.76 mg/L in oldest-old group. Oldest-old participants had a significantly higher hsCRP level than middle-aged people (ln (hsCRP): mean 2.12 in oldest-old vs. 1.89 in middle-aged group) after adjustment for study site and gender. No significant difference in the hsCRP concentrations was observed between men and women in both age groups (Table 2).
Table 2.
HsCRP Concentrations (mg/L) in Middle-aged and Oldest-old Groups
| n | Geometric mean |
Median (IQR) | Ln (hsCRP) (mean±SD) |
|
|---|---|---|---|---|
| Middle-aged group | 268 | 1.08 | 0.99 (0.52–1.98) | 1.89 (0.33)* |
| Male | 146 | 1.13 | 1.08 (0.55–1.83) | 1.89 (0.31)? |
| Female | 122 | 1.03 | 0.92 (0.45–2.07) | 1.90 (0.35)? |
| Oldest-old group | 506 | 1.96 | 1.76 (0.68–4.52) | 2.12 (0.54)* |
| Male | 107 | 2.52 | 2.33 (0.94–5.14) | 2.20 (0.58)? |
| Female | 399 | 1.84 | 1.55 (0.65–4.16) | 2.10 (0.52)? |
Note. SD, standard deviation.
P value<0.0001, was calculated by comparing ln (hsCRP) between the middle-aged group and the oldest-old group, after adjustment for study site and gender.
P value=0.7202, was calculated by comparing ln (hsCRP) between males and females in the middle-aged group, after adjustment for study site.
P value=0.0869, was calculated by comparing ln (hsCRP) between males and females in the oldest-old group, after adjustment for study site.
More oldest-old participants (36.6%) had the hsCRP level >3.0 mg/L than middle-aged participants (16.8%) after adjusting for study site and sex (P<0.001). The difference was also observed between the two age groups among males (43.0% vs. 14.4%) and females (34.8% vs. 19.7%) (Table 3).
Table 3.
Comparison of the Percentage of High hsCRP Level in Middle-aged and Oldest-old Groups
| Male |
Female |
Total |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Middle-aged group | 21 | 14.38 | 24 | 19.67 | 45 | 16.79 |
| Oldest-old group | 46 | 42.99 | 139 | 34.84 | 185 | 36.56 |
| χ2 | 24.7839 | 9.8552 | 32.9597 | |||
| P value | <.0001 | 0.0017 | <.0001 | |||
Note. The criterion of high hsCRP was >3.0 mg/L. P value was calculated by comparing the middle-aged group with the oldest-old group, after adjustment for study site and gender.
We also analyzed the relationship between elevated hsCRP and different chronic conditions (Table 4). 25.9% of the middle-aged population with high FBG had an hsCRP concentration >3.0 mg/L. That percentage is significantly higher than in people with normal FBG (14.3%) (P<0.05).
Table 4.
The Percentage of Elevated hsCRP in different Chronic Conditions in Middle-aged and Oldest-old Groups
| Abdominal Obesity |
Hypertension |
High TG |
Low HDLc |
High FBG |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P Value | Yes | No | P Value | Yes | No | P Value | Yes | No | P Value | Yes | No | P Value | |
| Middle-aged group | |||||||||||||||
| Normal hsCRP | 82.43 | 84.17 | 0.5085 | 79.80 | 85.21 | 0.1619 | 80.43 | 83.78 | 0.5815 | 73.21 | 85.85 | 0.0719 | 74.14 | 85.71 | 0.0464* |
| Elevated hsCRP | 17.57 | 15.83 | 20.20 | 14.79 | 19.57 | 16.22 | 26.79 | 14.15 | 25.86 | 14.29 | |||||
| Oldest-old group | |||||||||||||||
| Normal hsCRP | 64.24 | 63.10 | 0.5427 | 64.85 | 61.50 | 0.5294 | 80.95 | 62.68 | 0.0899 | 58.42 | 64.69 | 0.123 | 60.19 | 64.32 | 0.3956 |
| Elevated hsCRP | 35.76 | 36.90 | 35.15 | 38.50 | 19.05 | 37.32 | 41.58 | 35.31 | 39.81 | 35.68 | |||||
Note.
P<0.05.
The Relationship between hsCRP Level and Metabolic Factors we examined the relationship between ln (hsCRP) and metabolic factors while adjusting for gender and study site (Table 5). Among the oldest-old persons, ln (hsCRP) was negatively correlated with HDL-C (P<0.05). The strongest correlation was observed between ln (hsCRP) and WC in the middle-aged group (r=0.23, P<0.0001).
Table 5.
Spearman Partial Correlation Coefficients between ln (hsCRP), WC, SBP, DBP, TC, ln (TG), HDL-C, and FBG
| Middle-aged group | Ln (hsCRP) | WC | SBP | DBP | TC | Ln(TG) | HDL-C |
|---|---|---|---|---|---|---|---|
| WC | 0.23* | ||||||
| SBP | 0.12* | 0.22* | |||||
| DBP | 0.03 | 0.25* | 0.73* | ||||
| TC | 0.01 | 0.22* | 0.16* | 0.16* | |||
| Ln(TG) | 0.15* | 0.32* | 0.21* | 0.18* | 0.33* | ||
| HDL-C | −0.18* | −0.21* | −0.03 | −0.01 | 0.35* | −0.42* | |
| FBG | 0.07 | 0.19* | 0.19* | 0.10 | 0.35* | 0.37* | −0.04 |
| Oldest-old group | |||||||
| WC | 0.00 | ||||||
| SBP | −0.04 | 0.20* | |||||
| DBP | −0.06 | 0.15* | 0.65* | ||||
| TC | −0.03 | 0.04 | 0.11* | 0.18* | |||
| Ln(TG) | −0.01 | 0.09 | 0.05 | 0.07 | 0.36* | ||
| HDL-C | −0.12* | −0.10* | 0.13* | 0.13* | 0.54* | −0.17* | |
| FBG | 0.08 | 0.04 | −0.01 | −0.07 | 0.10* | 0.13* | 0.04 |
Note. The data were adjusted for gender, age and study site.
P < 0.05.
Association between hsCRP and Metabolic Factors
In the stepwise multiple linear regression models, we considered age, SBP, ln (TG), HDL-C, and FBG as potential correlates of hsCRP. HsCRP and TG were natural log-transformed (Table 6).
Table 6.
Multiple Linear Regression of ln (hsCRP) with Relevant Factors, in the Middle-aged and Oldest-old Groups Separately
| Variable | β | SE | Standardized β |
P Value | Model R2 (%) |
|
|---|---|---|---|---|---|---|
| Middle-aged group, n=218 | ||||||
| Model 1* | HDL-C | −0.117 | 0.053 | −0.141 | 0.0287 | 3.22 |
| Oldest-old group, n=417 | ||||||
| Model 1* | Ln(TG) | −1.351 | 0.555 | −0.111 | 0.0152 | |
| HDL-C | −0.206 | 0.080 | −0.122 | 0.0097 | ||
| FBG | 0.036 | 0.014 | 0.119 | 0.0088 | 4.22 | |
| Model 2? | HDL-C | −0.226 | 0.094 | −0.128 | 0.0172 | |
| FBG | 0.039 | 0.018 | 0.116 | 0.0260 | 3.45 | |
Note.
Model 1 was adjusted for gender, study site, smoking, drinking, and education.
Model 2 was adjusted for gender, study site, smoking, drinking, education, and BMI.
In Model 1, the confounding factors included gender, study site, smoking behavior, alcohol consumption and education. HDL-C was independently associated with ln (hsCRP) levels in the middle-aged group, whereas ln (TG), HDL-C and FBG were associated with the ln (hsCRP) in the oldest-old group. BMI and SBP were not included in Model 1, according to the significance level (P<0.10). We further adjusted BMI in Model 2 due to known associations of BMI with lipid parameters and FBG that could confound the relationship of hsCRP with ln (TG), HDL-C, and FBG. The results show that HDL-C and FBG were associated with ln (hsCRP) for both age groups, but the coefficient for ln (TG) was not significant in the oldest-old group.
DISCUSSION
Oldest-old Chinese have a higher hsCRP Level than Middle-aged Persons Residing in Longevity Areas the present study demonstrated that hsCRP was significantly higher with advancing age. However, no significant gender-related differences in hsCRP levels were observed in the middle-aged or oldest-old groups. The results are in agreement with Cartier et al.[31], who observed a significant positive relation between age and CRP among Canadian men aged 18–72 years (r=0.36). CRP levels also vary among different populations[11, 19–23]. Absolute values, however, may not be comparable since measurements were made with different methods. In Koreans aged 18–64 years, the median CRP values were 0.6 mg/L for males and 0.4 mg/L for females[10]. The lowest hsCRP levels were reported in Japanese with the average age of 52, the medians were 0.28 mg/L for males and 0.20 mg/L for females[20].
There were also several studies that give the distribution of hsCRP among Chinese populations. Ye et al.[19] found that the median CRP level was 0.68 mg/L among the study population aged 50–70, and no gender differences were observed. Yen et al.[21] also observed that the median of CRP was 1.5 mg/L among 8 374 healthy Chinese men aged 20–80 in Taiwan, and this distribution of serum CRP levels was similar to Western studies. The geometric mean of CRP concentrations was 2.58 mg/L in men and 2.75 mg/L in women in a large Chinese population study (aged 50–85)[32]. Ford et al.,[22–23] reported that the median of CRP concentration was 2.7 mg/L and 1.6 mg/L among adult U.S. women and men (age≥20 yr). Although the hsCRP level in our study cannot be compared directly with other studies, in general, the participants in our study had a trend of higher hsCRP levels than Koreans and Japanese, but lower than Americans.
A high prevalence of elevated hsCRP could indicate a state of chronic inflammation in the population. Wen et al.[33] reported that 19.0% had an hsCRP concentration >3 mg/L among 4 940 rural Chinese persons aged 30–97. Michos et al.[34] found the adjusted prevalence of high CRP among individuals with a normal lipid profile was 28.6%. The prevalence of high CRP increased with age and was higher among women and Mexican-American participants. In the same study, the prevalence of high CRP was associated with increasing BMI, with a prevalence of 31.4%–35.1% for overweight people and 52.5%–60.9% for obese participants. In this study, one third of the oldest-old in China appeared to have chronic inflammation. We also found that the percentage of elevated hsCRP in the population with high FBG was significantly higher than in people with normal FBG (25.86% vs. 14.29%, P<0.05) in the middle-aged group. Furthermore, oldest-old participants with high TG had a lower prevalence of high hsCRP than the subjects with normal TG (19.1% vs. 37.3%), but that was not significantly different (P<0.10). So, we supposed that there would be different relationship between hsCRP and metabolic factors in middle-aged and oldest-old groups and further analysis of the data would be needed on this point.
Correlations between HDL-C, TG, and FBG
In this study, a statistically significant positive correlation existed between hsCRP and ln (TG) (r=0.15) and a negative correlation existed between hsCRP and HDL-C (r=−0.18) in the middle-aged group. Our findings are in line with observations from the following two studies. Lim et al[35] demonstrated that the CRP level was significantly correlated with TG (r=0.14) and HDL-C (r=−0.10) in 9 773 Koreans aged 40–69. Significant inverse associations were observed between HDL-C (r=−0.22) in Indian women aged 35–80[36].
Plasma HDL-C showed a stronger negative association with plasma hsCRP levels than TG in the oldest-old group (standardized β coefficient −0.122 vs. −0.111) after adjustment for smoking, drinking and education. Furthermore, after adjustment for gender, study site, smoking, drinking, education and BMI, the relationship between hsCRP and TG disappeared. Tsai et al.[10] reported that hsCRP appeared to have a stronger negative correlation with HDL-C than with other lipid parameters examined among Taiwanese women aged 65 and older. However, the opposite results were found in a study of Korean adults. Ln (TG) was independently and positively associated with ln (CRP) levels in Korean females aged 18–64 (β coefficient= 0.217)[11]. Yen et al.[21] also observed a significant positive correlation between CRP and TG (β coefficient =0.00188) in a study of Chinese men aged 20–80. We further analyzed and found the prevalence of high TG was 4.3%, which was lower than the results from a national sample survey in 2002 (14.8%[37]). TG level could reflect the nutritional status. Our oldest-old participants lived to a ripe old age with an average age of 97.4 years. Although they were long-lived, their health and nutrition status was not good. On the other hand, the oldest-old participants in our study had a low TG level, but a high hsCRP level. So, we inferred that TG was not a sensitive factor for reflection of the health outcome in oldest-old people. This may explain why some of our results differ from previous researches, and therefore, further study is needed to evaluate the inflammatory effect of blood lipid markers among the oldest-old persons.
We also found that FBG was a predictor of elevated hsCRP after adjustment for confounders in the oldest-old group, but not in the middle-aged group. In our study, FBG levels were not measured at the local sites, but were stored at −80 °C until being assayed in the central laboratory in Beijing. Whether or not that process would influence the results is not clear. But the result of our study supported the theory related to diabetes and inflammations. Some previous studies have demonstrated that CRP levels increased in subjects with diabetes or impaired glucose tolerance[5, 38–39]. Pitsavos et al.[5] reported a positive association between CRP and diabetes in a population without cardiovascular disease. Ãoban et al.[39] suggested that the levels of serum hsCRP were correlated with fasting glucose in type 2 diabetes and impaired fasting glucose groups (P<0.05). Fukuhara et al.[40] found that the hemoglobin A1c (HbA1c) level was significantly and independently associated with the CRP level. Based on the above analysis, we found that hsCRP had much closer relationship with metabolic factors in the oldest-old group than in the middle-aged group. We could strengthen the use of hsCRP to assess the health status of the oldest-old people. And further study is needed to evaluate the relationship between hsCRP and health outcomes, such as coronary artery disease and diabetes in the oldest-old people.
Relationship with Waist Circumference and Blood Pressure
Several studies have found that obesity may be a key independent contributor to chronic systemic inflammation[1, 14–15, 41]. The present study suggested that there was a significant and positive correlation between hsCRP and WC in the middle-aged group (r=0.23, P<0.0001), rather than in the oldest-old group (r=0.00). There were age-related differences in the association between hsCRP and WC in this population. The average WC was 79.2 and 75.8 in males and females aged 90–99 yrs, and 79.4 and 76.0 in males and females aged 100 yrs[42]. Since the oldest-old individuals usually had a slim body, the relationship between hsCRP and WC could not be found in this population. Furthermore, in the multiple linear regression analysis, WC was not statistically significant enough to be included in the model for the middle-aged sample (P=0.6228). There was no statistically significant difference in the percentage of high hsCRP between the participants with abdominal obesity and those with normal WC in the two age groups. Tsai et al.[10] reported a marginal association between WC and CRP among elderly Taiwanese women aged 65 and older. Waist-to-hip ratio (WHR) is a better indicator of body fat than WC or BMI in reflecting the elevated plasma CRP in elderly Taiwanese[10]. On the other hand, Lapice et al.[43] reported that abdominal adiposity was associated with hsCRP, independent of age and BMI among healthy non-obese persons. HsCRP values and the proportion of people with elevated hsCRP were significantly higher in those with abdominal adiposity than in control subjects. Choi et al.[11] found that abdominal obesity was the strongest predictor of CRP levels among Korean adults aged 18–64. Excess adipose tissue releases inflammatory cytokines which increase CRP levels, and fat accumulation in the liver also stimulates hepatic cytokine production, leading to further enhanced CRP level[44]. Thus, it appears that the relationship between hsCRP and abdominal obesity is inconsistent between the middle-aged and oldest-old groups.
In the present study, SBP (r=0.12) but not DBP (r=0.03) was correlated with hsCRP levels in the middle-aged group. This result is similar to a study conducted on subjects in Japan aged 30 and older[3]. We found that neither SBP nor DBP was related to hsCRP levels in the oldest-old group. Different age-related associations between hsCRP and BP appeared in this population. Tamakoshi et al.[45] reported that SBP (r=0.12), DBP (r=0.11) and fasting glucose (r=0.088) were positively correlated with serum CRP in Japanese adult men.
Limitations
There were several limitations in our study. Firstly, the hsCRP assay was only performed in the middle-aged and oldest-old groups. So, we cannot describe the whole age distribution of hsCRP among Chinese persons residing in longevity areas. Secondly, our study was cross-sectional, and hence was unable to establish a causal relationship between hsCRP and metabolic risk factors. Further analyses, as a part of the longitudinal study, can be conducted in the next round of our survey in 2012. Thirdly, study specimens were stored on wet ice during shipment from the study site to the central lab in Beijing where fasting blood glucose was measured, and the storage and shipping procedures may have affected the accuracy of the results.
CONCLUSION
Our study showed that the oldest-old Chinese had a higher prevalence of high hsCRP than middle-aged individuals, after adjustment for study site and gender. Based on these results, one third of the oldest-old population in China exhibit evidence of chronic inflammation. The study suggests that age differences exist in the association of hsCRP with metabolic risk factors. HDL-C is a stronger predictor for elevated hsCRP than other metabolic factors in the middle-aged group. For the oldest-old people, high TG, low HDL-C, and high FBG predict elevated plasma CRP.
ACKNOWLEDGEMENTS
Authors of this work are grateful for support of the National Natural Science Foundation of China (70533010) and NIH grant # 5R24 TW 007988, the Fogarty International Clinical Research Scholars Support Center at Vanderbilt-AAMC.
REFERENCES
- 1.Zuliani G, Volpato S, Galvani M, et al. Elevated C-reactive protein levels and metabolic syndrome in the elderly: The role of central obesity data from the InChianti study. Atherosclerosis. 2009;203(2):626–632. doi: 10.1016/j.atherosclerosis.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima K. Elderly people with low body weight may have subtle low-grade inflammation. Obesity. 2009;17(4):803–808. doi: 10.1038/oby.2008.596. [DOI] [PubMed] [Google Scholar]
- 3.Arima H, Kubo M, Yonemoto K, et al. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28(7):1385–1391. doi: 10.1161/ATVBAHA.107.157164. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 5.Pitsavos C, Tampourlou M, Panagiotakos DB, et al. Association Between Low-Grade Systemic Inflammation and Type 2 Diabetes Mellitus Among Men and Women from the ATTICA Study. Rev Diabet Stud. 2007;4(2):98–104. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laaksonen DE, Niskanen L, Nyyssnen K, et al. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47(8):1403–1410. doi: 10.1007/s00125-004-1472-x. [DOI] [PubMed] [Google Scholar]
- 7.Hamer M. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: the English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009;34(7):1050–1055. doi: 10.1016/j.psyneuen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Komulainen P, Lakka TA, Kivipelto M, et al. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Ageing. 2007;36(4):443–448. doi: 10.1093/ageing/afm051. [DOI] [PubMed] [Google Scholar]
- 9.Clarke R, Emberson JR, Breeze E, et al. Biomarkers of inflammation predict both vascular and non-vascular mortality in older men. Eur Heart J. 2008;29(6):800–809. doi: 10.1093/eurheartj/ehn049. [DOI] [PubMed] [Google Scholar]
- 10.Tsai HJ, Tsai AC. The association of plasma C-reactive protein levels with anthropometric and lipid parameters in elderly Taiwanese. Asia Pac J Clin Nutr. 2008;17(4):651–656. [PubMed] [Google Scholar]
- 11.Choi EY, Park EH, Cheong YS, et al. Association of C-reactive protein with the metabolic risk factors among young and middle-aged Koreans. Metabolism. 2006;55(3):415–421. doi: 10.1016/j.metabol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.O'Loughlin J, Lambert M, Karp I, et al. Association between cigarette smoking and C-reactive protein in a representative, population-based sample of adolescents. Nicotine Tob Res. 2008;10(3):525–532. doi: 10.1080/14622200801901997. [DOI] [PubMed] [Google Scholar]
- 13.Chien KL, Hsu HC, Chen MF, et al. Association of C-reactive Protein, Smoking and Metabolic Syndrome Among the Health Check-up Population. Acta Cardiol Sin. 2005;21:98–104. [Google Scholar]
- 14.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Perry CD, Alekel DL, Ritland LM, et al. Centrally located body fat is related to inflammatory markers in healthy postmenopausal women. Menopause. 2008;15(4):619–628. doi: 10.1097/gme.0b013e318159f1a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentine RJ, Vieira VJ, Woods JA, Evans EM. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause. 2009;16(1):84–89. doi: 10.1097/gme.0b013e31817fcb8f. [DOI] [PubMed] [Google Scholar]
- 17.Lakoski SG, Cushman M, Palmas W, et al. The relationship between blood pressure and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2005;46(10):1869–1874. doi: 10.1016/j.jacc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 18.Gurven M, Kaplan H, Winking J, et al. Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci. 2008;63(2):196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Yu Z, Li H, et al. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007;49(17):1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 20.Oda E, Kawai R. Tentative cut point of high-sensitivity C-reactive protein for a component of metabolic syndrome in Japanese. Circ J. 2009;73(4):755–759. doi: 10.1253/circj.cj-08-0848. [DOI] [PubMed] [Google Scholar]
- 21.Yen ML, Yang CY, Yen BL, et al. Increased high sensitivity C-reactive protein and neutrophil count are related to increased standard cardiovascular risk factors in healthy Chinese men. Int J Cardiol. 2006;110(2):191–198. doi: 10.1016/j.ijcard.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Giles WH, Myers GL, et al. Population distribution of high-sensitivity C-reactive protein among US men: findings from National Health and Nutrition Examination Survey 1999–2000. Clin Chem. 2003;49(4):686–690. doi: 10.1373/49.4.686. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Mokdad AH, et al. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50(3):574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 24.Gu D. General data assessment of the Chinese longitudinal healthy longevity survey in 2002. In: Zeng Y, Poston D, Smith J, Vlosky DA, Gu D, editors. Healthy longevity in China: demographic, socioeconomic, and psychological dimensions. Dordrecht, Netherlands: Springer; 2008. pp. 39–59. [Google Scholar]
- 25.Criteria for “longevity areas”. Chinese Society of Gerontology (online) Available at: http://www.zgcsx.net/content.asp?id =751. [Google Scholar]
- 26.National guidelines for overweight and obesity control and prevention of adult. Beijing: People’s Medical Publishing House; 2006. Ministry of Health of People’s Republic of China. [Google Scholar]
- 27.National guideline for hypertension control and prevention (revision version 2005) Beijing: People’s Medical Publishing house; 2006. [Google Scholar]
- 28.National guideline for dyslipidemia control and prevention in adult. Beijing: People’s Medical Publishing House; 2007. [Google Scholar]
- 29.Sub-association of diabetes, Chinese medical association. Beijing: National guideline for type 2 diabetes; 2007. [Google Scholar]
- 30.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the Am Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 31.Cartier A. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58(10):1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Lao XQ, Thomas GN, Jiang CQ, et al. C-reactive protein and the metabolic syndrome in older Chinese: Guangzhou Biobank Cohort Study. Atherosclerosis. 2007;194(2):483–489. doi: 10.1016/j.atherosclerosis.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 33.Wen J, Liang Y, Wang F, et al. Association of C-reactive protein and metabolic syndrome in a rural Chinese population. Clin Biochem. 2009;42(10–11):976–983. doi: 10.1016/j.clinbiochem.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Michos ED, Blumenthal RS. Prevalence of low low-density lipoprotein cholesterol with elevated high sensitivity C-reactive protein in the U.S.: implications of the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) study. J Am Coll Cardiol. 2009;53(11):931–935. doi: 10.1016/j.jacc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Lim S, Lee HK, Kimm KC, et al. C-reactive protein level as an independent risk factor of metabolic syndrome in the Korean population. CRP as risk factor of metabolic syndrome. Diabetes Res Clin Pract. 2005;70(2):126–133. doi: 10.1016/j.diabres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Ganguli D, Das N, Saha I, et al. Association between inflammatory markers and cardiovascular risk factors in women from Kolkata, W.B, India. Arq Bras Cardiol. 2011;96(1):38–46. doi: 10.1590/s0066-782x2010005000165. [DOI] [PubMed] [Google Scholar]
- 37.Zhao WH. 2002 Report on Lipid of National Nutrion and Health Survey. Beijing: People’s Medical Publishing House; 2008. [Google Scholar]
- 38.Doi Y, Kiyohara Y, Kubo MT, et al. Relationship between C-reactive protein and glucose levels in community-dwelling subjects without diabetes: the Hisayama Study. Diabetes Care. 2005;28(5):1211–1213. doi: 10.2337/diacare.28.5.1211. [DOI] [PubMed] [Google Scholar]
- 39.Ãoban E, Sari R. The Levels of Serum High Sensitivity C-reactive Protein in Subjects with Impaired Fasting Glucose. Turkish Journal of Endocrinology and Metabolism. 2003;4:165–168. [Google Scholar]
- 40.Fukuhara M, Matsumura K, Wakisaka M, et al. Hyperglycemia promotes microinflammation as evaluated by C-reactive protein in the very elderly. Intern Med. 2007;46(5):207–212. doi: 10.2169/internalmedicine.46.1868. [DOI] [PubMed] [Google Scholar]
- 41.Santos AC, Lopes C, Guimarães JT, et al. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int J Obes. 2005;29(12):1452–1456. doi: 10.1038/sj.ijo.0803035. [DOI] [PubMed] [Google Scholar]
- 42.Shi XM, Yin ZX, Qian HZ, et al. A study on chronic diseases and other related health indicators of centenarians in longevity areas in China. Chin J Prev Med. 2010;44(2):101–107. [PubMed] [Google Scholar]
- 43.Lapice E, Maione S, Patti L, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32(9):1734–1736. doi: 10.2337/dc09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G1–G5. doi: 10.1152/ajpgi.00384.2001. [DOI] [PubMed] [Google Scholar]
- 45.Tamakoshi K, Yatsuya H, Kondo T, et al. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int J Obes Relat Metab Disord. 2003;27(4):443–449. doi: 10.1038/sj.ijo.0802260. [DOI] [PubMed] [Google Scholar]