Abstract
Comprehensive reviews of neurocognitive outcomes following mild, uncomplicated traumatic brain injury (TBI) in children have shown minimal effects on neurocognition, especially in methodologically rigorous studies. In this study, we report longitudinal (1, 6, and 12 months post injury) results in four domains of neurocognitive functioning in a large sample of children with mild TBI (n = 124, ages 8–17 at injury) relative to two demographically matched control groups (other injury: n = 94 and non-injury: n = 106). After accounting for age and parental education, significant main effects of group were observed on 7 of the 10 neurocognitive tests. However, these differences were not unique to the TBI sample but were found between both the TBI and other injury groups relative to the non-injured group, suggesting a general injury effect. Effects were primarily within the domains measuring memory, psychomotor processing speed, and language. This is the largest longitudinal study to date of neurocognitive outcomes at discrete time points in pediatric mild TBI. When controlling for pre-injury factors, there is no evidence of long-term neurocognitive impairment in this group relative to another injury control group. The importance of longitudinal analyses and use of appropriate control groups are discussed in the context of evaluating the effects of mild TBI on cognition.
Keywords: Traumatic brain injury, Child and adolescent, Cognitive, Mild TBI, Concussion, Longitudinal study
INTRODUCTION
Widely divergent results are reported on the neurocognitive outcomes following mild traumatic brain injury (TBI) in children (Kirkwood et al., 2008). Most studies report that mild TBI has minimal consequences on neurocognition in children following TBI. Reviews of the literature have consistently shown that, in general, mild TBI does not result in declines in health outcomes (Petersen, Scherwath, Fink, & Koch, 2008) or long-term deficits in cognitive or behavioral/school functioning following single, uncomplicated mild TBI (Babikian & Asarnow, 2009; Kirkwood et al., 2008; Maillard-Wermelinger et al., 2009), unlike those observed in survivors of moderate to severe injuries (Babikian & Asarnow, 2009; Fay et al., 2009). This is especially true when premorbid problems have rigorously been accounted for (Asarnow et al., 1995; Bijur, Haslum, & Golding, 1990). A comprehensive review concluded that methodologically stronger studies were generally associated with null outcomes across neurocognitive domains, but mounting variability in outcomes was noted with increasing injury severity within the “mild” range (Satz et al., 1997).
Individual studies, however, have reported select neuro-cognitive weaknesses in children following TBI. For example, one study reported deficits in visual closure in a group of young children with mild TBI (Wrightson, McGinn, & Gronwall, 1995). Problems with aspects of memory, attention, and language (specifically verbal fluency and story recall) have also been reported in children following mild TBI (Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2001; Catale, Marique, Closset, & Meulemans, 2009). A small effect for elevated hyperactivity 1–5 years post injury has been shown (Bijur et al., 1990), in addition to behavioral problems (Asarnow, Satz, Light, Lewis, & Neumann, 1991), which were obviated when pre-injury levels were controlled (Asarnow et al., 1995). Studies have also shown residual post-concussive symptoms that are differentially present in higher proportions in mild TBI groups compared to other injury groups (Barlow et al., 2010; Yeates et al., 2009).
The variability in reported outcome has been attributed to divergent definitions of mild TBI (Asarnow et al., 1991, 1995; Kirkwood et al., 2008; Lee, 2007), variations in the methodological rigor of studies accounting for premorbid problems (Asarnow et al., 1991, 1995), inclusion/exclusion criteria used for the TBI and control groups, the type of control group used (e.g., other injury or non-injury), breadth of outcome domains assessed, types of outcome measures implemented (parent report or formal neurocognitive tests), intervals between injury and follow-up assessments, retrospective versus prospective design, and the degree to which normal development is assessed (Asarnow et al., 1995). Very few studies have adequately controlled for key factors that influence outcome, notably pre-injury level of functioning. This is particularly problematic given that accidental injury in general, and noninflicted TBI in particular, is reportedly associated with several pre-existing risk factors, including psychiatric disorders such as attention deficit/hyperactivity disorder (AD/HD) (Bruce, Kirkland, & Waschbusch, 2007; Schwebel & Gaines, 2007). It is possible that the adverse effects of mild TBI on neuro-cognition found in some studies may reflect the effect of pre-injury conditions such as AD/HD and learning disabilities that are associated with poor neurocognitive function.
Neurocognitive functioning improves during the first year post-TBI. The time course of post-concussive and related symptoms has been well documented in a rigorously designed longitudinal study of mild TBI in children (Yeates, 2010; Yeates, et al., 2009). There is, however, a dearth of longitudinal data on neurocognitive functioning and degree of neurocognitive recovery at discrete time points during the first year post mild TBI in children and adolescents. The present study describes the course of neurocognitive functioning in domains sensitive to the effects of a mild TBI in a well-controlled, longitudinal model, using a large sample evaluated at tightly defined time points during the first year following a mild TBI in childhood and adolescence. We controlled for the effect of pre-injury risk factors associated with accidental injury by comparing the neurocognitive performance of children and adolescents with mild TBI to that of children with accidental orthopedic injuries. This study is one of the first studies to (1) describe longitudinal changes in cognitive functioning during the first year post injury, and (2) separate out a general injury effect from the effect of a mild TBI on cognitive functioning by comparing children with mild TBI to children with orthopedic injuries. The current study describes cognitive functioning at 1, 6, and 12 months post injury, and the course of cognitive function and recovery during the first year post injury using a longitudinal model. These results are an extension of the preliminary findings reported previously on these data that presented cross-sectional neurocognitive performance by group only at the 1-month post-injury time point (Asarnow et al., 1995).
METHODS
The study methodology is described in greater detail elsewhere (Asarnow et al., 1995). In brief, children and adolescents who had incurred a mild TBI were recruited from consecutive admissions to emergency rooms in the greater Los Angeles area. A control group of children with injuries other than the head (other injury group) was recruited from the same emergency rooms as the TBI sample. The children in the other injury group were matched to the mild TBI group on gender, age, ethnicity, socio-economic status, and injury severity level. A total of 14 emergency rooms in Los Angeles, Riverside, and Orange Counties participated. Parents of potential patients were contacted by telephone within the first 4 weeks following injury and were invited to participate. A third group of non-injured children (non-injury Group), also matched on gender, age, ethnicity, and socioeconomic status, were studied. This group was recruited from schools that were similar in demographic characteristics to the injured groups. All data were collected in accordance to our institutional guidelines for human subjects research. Parents consented and children and adolescents assented prior to entry into the study. Data collection was initiated in 1989 and the interviews were completed in 1997.
The Abbreviated Injury Scale (AIS) (Greenspan, McLellan, & Greig, 1985) was used as a measure of injury severity because this metric allowed for comparability across both the head injury and the other injury group. The following guidelines for the AIS scores were used for the head injury sample:
AIS 1
History of or observed presence of any two of the following: nausea, vomiting, headache, dizziness; or a diagnosis of “concussion” with any of the above or following symptoms: diplopia, ringing in the ears, or seeing stars as long as these symptoms are not treated as neurological deficits and the symptoms usually disappear in the emergency room; corresponding to an emergency room Glasgow Coma Scale (GCS) rating of 15.
AIS 2
History of or observed length of coma in emergency room for less than 1 hour; some symptoms with or without skull fracture; level of consciousness and sensorium improving; no neurological deficits; corresponding to an ER GCS rating between 13 and 14.
Inclusion criteria for the head injury group consisted of the following: (1) concussion resulting in an Abbreviated Injury Scale (AIS) score of 1 or 2; (2) no injuries above AIS level 2 at any anatomic location; (3) injury from unintentional external causes; (4) no litigation related to injury; (5) no serious injury or death of others involved in the index accident; (6) treated at 1 of 14 emergency rooms located in one of three counties within the greater Los Angeles area; (7) aged 8–17 years at the time of injury; (8) no significant preexisting central nervous system damage or serious chronic diseases (e.g., cancer, congenital malformation); (9) availability of parent/guardian consent; and (10) child residing with parent/guardian. Computed tomography scans were not available for review and, therefore, did not play a role in the selection of subjects for the study. Inclusion criteria for the other injury group included criteria 2–10 above, and injuries to an area other than the head. AIS scores of 1 or 2 for an injury to any part of the body other than the head were used as inclusion criteria for the other injury group (Greenspan et al., 1985). Specific guidelines for assigning AIS scores of 1 or 2 in the other injury sample are available separately for various anatomical regions other than the head in Greenspan et al. (1985). Children with injuries that caused restricted movement of the hands/arms or discomfort during testing were excluded. Hospitalization was not included as an exclusion criteria for either of the injury groups; only a handful (< 10) of the study participants were admitted to the hospital. Of note, original data collection included patients with AIS scores of 3 or for whom an AIS score was not identified. These subjects were dropped from the analyses conducted for this manuscript. The injured patients included in the present study had relatively mild injuries. Inclusion criteria for the non-injury group included criteria 3–10 above.
Patients were studied prospectively and assessments were conducted in the homes of the children to further avoid methodological and sampling biases typically present in studies of retrospective or clinical samples. Initial data were collected shortly after injury (at the 1 month post-injury visit) to ensure that pre-injury information, including history of learning, school, and behavior problems (e.g., attention and/or conduct problems), use of alcohol, and prior injury, would be available and least biased. Pre-injury functioning was characterized using data derived from parental interviews and questionnaires. In addition, school records were obtained and parents were instructed to complete the Child Behavior Checklist (Achenbach, 1991) to assess the child's functioning for the period roughly 6 months prior to the index incident for the injured groups.
Ten cognitive tasks were administered that fell within the following four domains of cognitive functioning shown in prior studies to be sensitive to TBI: memory (prospective, visual, and verbal memory), motor/psychomotor functioning (motor and processing speed), attention/executive functions (attention span, sustained attention, and inhibition), and general language (naming vocabulary). Table 1 lists the tasks administered for this study. Raw scores were used on all tasks with the exception of the Picture Vocabulary Test, where published norms standardized for age were used. Raw scores were used because (1) they generally provide more sensitive indices of change than scaled scores and (2) many of the tasks were developed specifically for this study and, therefore, standardized norms were not available. At the time of this study, there were far fewer commercially available neuro-cognitive measures, especially in some of the key domains of functioning sensitive to a head injury (i.e., aspects of attention, including sustained attention/concentration, prospective memory). Therefore, experimental measures were developed to help capture neurocognitive functioning in these domains. To ensure intact test properties, a study model was chosen that included (1) a well-matched non-injured control group of healthy kids, as well as (2) a very large community based normative sample (as a fourth group) that was only administered the measures at one time point, the latter of which was used to demonstrate psychometric properties of the experimental measures used. Furthermore, age at the time of assessment was modeled in the analyses to account for normal age related differences in performance in the neuro-cognitive measures. A detailed description of the tasks and the scores derived from them is contained in a previous report (Asarnow et al., 1995) and is also summarized in Table 1. The same battery of neurocognitive tests was repeated at all three time points: 1 month, 6 months, and 12 months post injury.
Table 1.
List of tests summarized for each domain, accompanied by a brief description
| Domain | Measures |
|---|---|
| Memory | Prospective Memory Test – Subjects are required to respond to 5 tasks that approximate everyday memory situations embedded in the standard protocol (e.g., remembering to tell or give something) (Experimental). Picture Memory Test – Three groups of target pictures are presented. After each group, subjects pick out target pictures for each group from a larger “recognition” group of pictures (Experimental). Word List Memory Test – Four lists of words consisting of 10 animals are presented for free recall (Wickens, 1970). |
| Motor and Psychomotor | Symbol Digit Modalities – Adapted from the Digit-Symbol subtest of the Wechsler scales, the subject is presented with rows of blanks printed underneath nonsense symbols and asked to fill in the blanks with the number that is matched to the symbol in the key at the top of the page. The number of correct responses and errors in 90 seconds is recorded (Smith, 1968). Color Trails – Part B (Child Version) – Part B varies from the traditional Halstead version by introducing a second set of numbers that appear in contrasting colored circles. The subject is to connect the numbers in order; however, each subsequent number must be in the alternating colored circle (e.g., pink 1 to yellow 2, etc). Time to completion and number of errors are recorded. Modified from (Reitan & Wolfson, 1985). Pin Test – Subjects are required to push a pin through the holes of a metal template, puncturing a piece of paper underneath. Two 45-second intervals (one for each hand) are administered. Total hits are summed for each hand (Satz & D'Elia, 1989). |
| Attention/Concentration Inhibition | Span of Apprehension Test – Subjects are instructed to search for two predesignated target letters (T or F) which appear in 3, 5, and 10-letter arrays on a computer monitor. Subjects indicate if a T or F was present by pressing one of two response buttons. Dependent variables are the detection rates by array size. Response latencies for both correct and incorrect trials are recorded to permit analysis of speed/accuracy tradeoffs (Experimental). Stroop Test (Interference Condition) – Color names are printed but using an interfering ink (e.g., the word red is printed using blue ink, etc.), and the subject is to ignore reading the words and say the ink color that was used to print the word instead. Time to completion and number of errors made are recorded. Modified from (Golden, 1976). Degraded Stimulus Continuous Performance Test (DSCPT) – Subjects view a computer monitor on which numbers 0 through 9 are presented one per second for a short duration, and are instructed to press a response button whenever they see a 0. Number of correct detections (hits) and errors of commission (false alarms) are measured, along with response latencies for both. The sensitivity of the task is increased by randomly removing 40% of the pixels from the images (Nuechterlein, 1983). |
| Language | Peabody Picture Vocabulary Test (Revised) – Subjects are required to point to a picture corresponding to a target word that is read (Dunn & Dunn, 1981). |
Note. See Asarnow (1995) for a comprehensive description of the tests and scores presented for each of the domains above. Raw scores were used on all measures with the exception of the Peabody Picture Vocabulary Test, for which age corrected standard scores were available.
Mixed model analyses were performed for each of the 10 neurocognitive tasks using “session” (1-month, 6-month, or 12-month post-injury evaluations) as the repeated variable and “group” (TBI, other injury, and non-injury) as the between subject variable. Age (in years) and parental education (in years) were also included in the model as level 2 covariates to account for any effects these variables would have on neurocognitive performance. Also included in the model was a “group by session” interaction term. Initial analyses showed no “age by group” or “age by group by session” interactions for any of the cognitive measures and, therefore, these terms were not included in the final analyses presented to minimize the number of variables included in the model and the degrees of freedom used. Post hoc tests within the mixed model analyses using the least significant difference (LSD) method of corrections for multiple comparisons were used to determine the nature and direction of the group differences (session effects collapsed across groups and group effects collapsed across sessions). Furthermore, to identify a subset of subjects that show lingering neurocognitive problems by 12 months post-injury (for the mild TBI and other injury groups) relative to the non-injured control group, the proportion of the sample in each of the three groups that scored 1.5 or greater standard deviations below the mean of the non-injured controls on at least three or at least 4 of the 10 neurocognitive measures was calculated. All statistical analyses were conducted using the PASW Statistics 18.0 software.
RESULTS
Subject Description
Table 2 summarizes participant demographics for each group. In general, the three groups were matched well by age, with average mean age between 12 and 13 years across groups. The TBI and other injury groups were similar in gender composition (64% and 59% male, respectively), with a slightly more gender-balanced composition in the non-injury group (46% male). The ethnicity categories and parental education (both used as a measure of the socio-demographic makeup of the subjects) for all three groups and the AIS categories for the two injury groups are also presented in Table 2. Note that the AIS scores for the TBI and other injury (OI) groups were defined differently, as summarized above.
Table 2.
Demographic and clinical makeup of study participants
| TBI | Other injury | Non-injury | |
|---|---|---|---|
| N | |||
| Session 1 | 124 | 115 | 145 |
| Session 2 | 94 | 96 | 101 |
| Session 3 | 106 | 102 | 108 |
| Age, in years (SD), at initial assessment | 11.9 (2.5) | 12.8 (2.5) | 12.2 (2.5) |
| Gender (% male) | 64 | 59 | 46 |
| AIS* | |||
| 1 | 44 (35%) | 63 (55%) | N/A |
| 2 | 80 | 52 | |
| Ethnicity (%) | |||
| Black | 7 | 8 | 2 |
| Hispanic | 31 | 38 | 26 |
| White | 46 | 44 | 42 |
| Other | 10 | 4 | 6 |
| Unknown | 6 | 6 | 24 |
| Parental education | |||
| Mean in years and (SD) | 13.0 (2.8) | 12.2 (3.9) | 13.4 (3.0) |
The AIS scores for the TBI and OI group are defined differently, as indicated in the manuscript. AIS = Abbreviated Injury Scale; TBI = traumatic brain injury; OI = other injury.
Groups were comparable at each time point on the demographic variables of gender, age, parental education, and ethnicity. Although some attrition was noted (between 17 and 25% for the two injury groups and up 30% to for the non-injury group) across the three time points, most of the subjects participated in all three longitudinal evaluations. Among the demographic variables (age, ethnicity, gender, and parent education), there were no significant differences between those with and without complete data for the TBI and other injury groups. In the non-injured control group, the sample without complete data was older (mean age, 14.5 vs. 12.0 years; p = .001). Furthermore, the OI group with complete data had slightly higher AIS scores than the group with incomplete data (mean, AIS 1.51 vs. 1.25; p = .025). With regard to the neurocognitive measures, in the TBI group, those with complete data showed better performance on only one of the 10 neurocognitive measures than those with incomplete data. In the other injury group, there were no group differences between those with and without complete data on any of the 10 cognitive measures. In the non-injured control group, there were group differences on 5 of the 10 cognitive measures, with those with complete data performing better than those with incomplete data on all five.
Table 3 lists the most common neurological findings for the head injury sample, including length of unconsciousness, disorientation, hours to lucidity, length of post-traumatic amnesia, and number of post-concussive symptoms, including headache, dizziness, nausea, blurry vision, and ringing in the ears. To determine whether there were significant associations between injury severity indicators (AIS, length of post-traumatic amnesia, length of loss of consciousness, and total number of post-concussive symptoms) and the 10 neurocognitive measures in the mild TBI group, Spearman correlation coefficients were computed for Session 1 outcomes. Relatively few statistically significant correlations were noted, including AIS with both Symbol Digit Modalities (r = .231; p = .010) and Stroop Test (r = −.180; p = .047), as well as total number of post-concussive symptoms with both Span of Apprehension Test (r = .329; p = .003) and Picture Memory (r = .228; p = .039). In the OI group, AIS was statistically significantly correlated with Picture Memory (r = −2.206; p = .029). In the few instances where there were statistically significant correlations, these correlations were relatively small with small effect sizes, suggesting that there was relatively little variability in injury severity (at least with the parameters used to define severity in this study) to the extent necessary to explain large amounts of variance in outcome.
Table 3.
Perentage of the mild TBI sample with neurologic symptoms
| Classification | Proportion of sample (%) | |
|---|---|---|
| Length of unconsciousness | None | 54 |
| <10 min | 43 | |
| 11-60 min | 3 | |
| Length of post-traumatic amnesia | None | 45 |
| <10 min | 26 | |
| 11-60 min | 7 | |
| 1-3 hours | 5 | |
| 3-24 hours | 10 | |
| >24 hours | 7 | |
| Hours to lucidity* | <10 min | 49 |
| 11-60 min | 13 | |
| 1-3 hours | 23 | |
| 3-24 hours | 15 | |
| Disoriented | Yes | 68 |
| No | 32 | |
| Post-concussive symptoms** (total #) | 0 | 2 |
| 1 | 16 | |
| 2 | 20 | |
| 3 | 24 | |
| 4 | 27 | |
| 5 | 11 |
Lucidity: during an interview, parents were asked how long after the accident did it take for their child to know who he/she was, where he/she was, recognized other people, and knew date/time.
Post-concussive symptoms include headache, dizziness, nausea, blurry vision, and ringing in the ears.
Memory
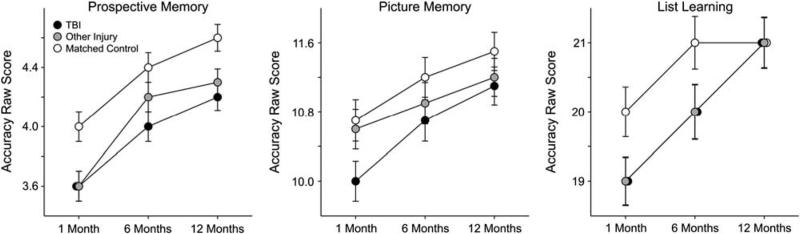
The tasks included in this domain were Prospective Memory, Picture Memory, and List Learning (Figure 1). There were no “session × group” interactions for any of the three measures. The covariate of age was significant for all three measures while the covariate of parental education was significant for the Picture Memory and Learning tests only. Significant session and group main effects were observed for all three measures of memory (see Table 4). LSD post hoc analyses were used to determine the nature of the group differences, collapsed across all three sessions. Both the TBI and the other injury groups scored more poorly than the non-injured control group on the Prospective Memory and the List Learning tests, while only the TBI group performed more poorly than the non-injured control group on the Picture Memory test. Effect sizes suggested that the group differences for the Picture Memory and List Learning tasks were relatively small, but the effect size for the difference between the TBI and non-injured control group on the Prospective Memory test approached a moderate level (Hedge's g = .434) (see Table 5 for means, standard errors, and effect sizes associated with these group differences).
Fig. 1.
Estimated marginal means of memory scores by group across three longitudinal time points post injury derived from mixed model analyses.
Table 4.
Results of the mixed model analyses
| Age | Parental education | Group | Session | Group × Session | |
|---|---|---|---|---|---|
| Prospective Memory | 60.286* | 3.484 | 10.176* | 34.958* | .293 |
| Picture Memory | 9.158* | 5.007* | 3.323* | 10.265* | .232 |
| List Learning | 241.040* | 65.345* | 3.698* | 19.686* | .587 |
| Pin Test | 396.746* | 3.044 | 2.352 | 10.697* | .371 |
| Color Trails | 373.328* | 64.559* | 6.089* | 26.147* | .324 |
| Symbol Digit Modalities | 806.076* | 60.703* | 6.222* | 34.590* | .075 |
| Span Test | 83.416* | 8.802* | 3.024* | 60.019* | .092 |
| Stroop Test | 710.253* | 22.725* | 2.339 | 53.816* | .078 |
| Continuous Performance Test | 113.861* | 8.015* | 2.456 | 6.929* | 1.362 |
| Picture Vocabulary | .395 | 208.204* | 7.131* | 6.044* | .219 |
Note. F values derived from Mixed Model analyses.
significant at p < .05.
Table 5.
List of all statistically significant post-hoc (LSD) pairwise group comparisons collapsed across sessions
| TBI vs. Control | OI vs. Control | TBI vs. OI | ||||
|---|---|---|---|---|---|---|
| Pic. Memory | 10.6 (.1) | 11.1 (.1) | ||||
| g = .254 | ||||||
| Pros. Memory | 4.0 (.06) | 4.3 (.06) | 4.1 (.06) | 4.3 (.06) | ||
| g = .434 | g = .310 | |||||
| List Learning | 20 (.21) | 21 (.21) | 20 (.22) | 21 (.21) | ||
| g = .242 | g = .222 | |||||
| S-D Modalities | 46.5 (.6) | 49.3 (.6) | 47.0 (.6) | 49.3 (.6) | ||
| g = .328 | g = .267 | |||||
| Color Trails | 38.5 (.8) | 34.9 (.8) | 37.5 (.8) | 34.9 (.8) | ||
| g = .335 | g = .243 | |||||
| PPVT | 102 (.97) | 105 (.97) | 101 (.97) | 105 (.97) | ||
| g = .280 | g = .355 | |||||
| Span Test | 55.8 (.3) | 54.7 (.3) | ||||
| g = .243 | ||||||
Estimated marginal means (and associated standard errors) and effect sizes (Hedge's g) for all statistically significant (p < .05) Pairwise Comparisons for Groups Collapsed Across Session derived from mixed model analyses. LSD = least significant difference; TBI = traumatic brain injury; OI = other injury; PPVT = Peabody Picture Vocabulary Test-Revised.
Motor and Psychomotor Processing
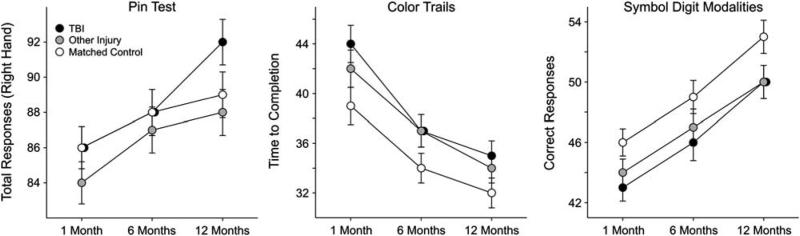
The measures included in this domain were the Pin Test, Color Trails (Part 2), and the Symbol Digits Modalities Test (Figure 2). There were no “session × group” interactions for any of the three measures. The covariate of age was significant for all three measures while the covariate of parental education was significant for the Color Trails and Symbol Digit Modalities tests only. Significant session effects were observed for all three measures and significant group main effects were observed for the Color Trails and Symbol Digit Modalities tests only (see Table 4). LSD post hoc analyses were used to determine the nature of the group differences, collapsed across all three sessions. Both the TBI and the other injury groups performed more poorly than the non-injured control group on the Symbol Digit Modalities and Color Trails tests. Of note, effect sizes associated with these group differences were relatively small (see Table 5).
Fig. 2.
Estimated marginal means of motor and psychomotor processing scores by group across three longitudinal time points post injury derived from mixed model analyses.
Attention/Concentration and Inhibition
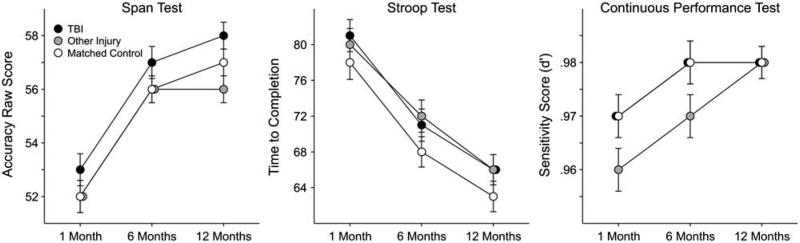
The measures included in this domain were the Span of Apprehension Test, Stroop Test (Interference Condition), and the Continuous Performance Test Sensitivity Score (d’) (Figure 3). There were no “session × group” interactions for any of the three measures. The covariates of age and parental education, as well as the main effect of session, were statistically significant for all three measures. However, a statistically significant group main effect was observed only for the Span Test (see Table 4). LSD post hoc analyses were used to determine the nature of the group differences, collapsed across all three sessions. The TBI group performed slightly better than the other injury group on the Span Test, with a relatively small associated effect size (see Table 5).
Fig. 3.
Estimated marginal means of attention/concentration and inhibition scores by group across three longitudinal time points post injury.
Language
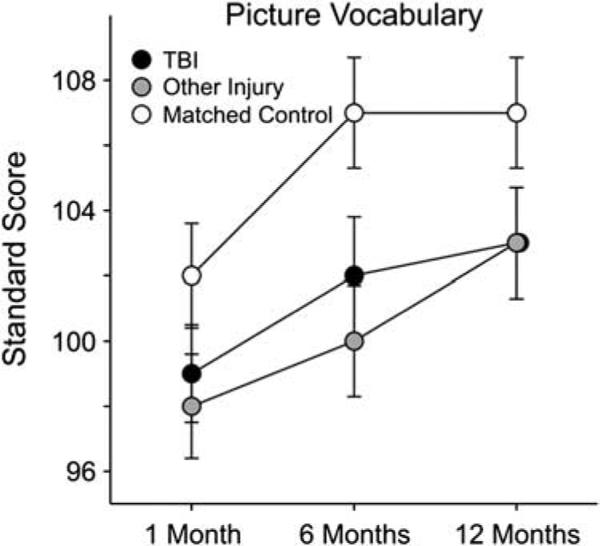
The Peabody Picture Vocabulary Test-Revised (PPVT-R) was used as a receptive vocabulary test and to estimate general level of intellectual functioning (Figure 4). There was no “session × group” interaction on this measure and no covariate effect of age, since age corrected standard scores were used in the analyses. However, there were main effects for the covariate parental education, as well as main effects for group and session (see Table 4). LSD post hoc analyses were used to determine the nature of the group effects observed, collapsed across all three sessions. Both the TBI and other injury groups performed more poorly than the non-injured control group (see Table 5).
Fig. 4.
Estimated marginal means of language scores by group across three longitudinal time points post injury.
Subset with Lingering Neurocognitive Deficits at 1-Year Post Injury
Almost a third of both the TBI (29%) and the other injury (32%) groups scored 1.5 standard deviations or more below the mean of the non-injured control group at 12 months post injury on at least 3 of the 10 measures. In contrast, only 18% of the non-injured control group scored 1.5 standard deviations below its mean on three or more measures. This difference in proportions was statistically significant using one tailed statistics (Z = 1.74; p = .04 for the TBI vs. the non-injured control group and Z = 2.19; p = .01 for the OI vs. the non-injured control group). When the cutoff was raised to four or more of the 10 tests, 14% of the TBI group, 17% of the other injury group, and 10% of the non-injured control group scored 1.5 standard deviations or more below the non-injured control group's mean, although these differences in proportions were not statistically significant.
DISCUSSION
Meta-analyses of neurocognitive outcomes have shown that, in general, adults with mild TBI experience few neurocognitive sequelae following injury, and that existing problems diminish by approximately 3 months post injury (Belanger & Vanderploeg, 2005; Schretlen & Shapiro, 2003), especially in studies of unselected samples (Belanger & Vanderploeg, 2005). In a meta-analysis of neurocognitive outcomes in children, very few or small effects were noted in select neurocognitive domains following a mild TBI, with these effects substantially diminishing with studies at later time points post injury (Babikian & Asarnow, 2009). The latter review and previous reviews (Satz et al., 1997), however, have highlighted the lack of methodological rigor, particularly the failure to control for pre-injury level of functioning, in most published studies and documented the paucity of longitudinal specific neurocognitive functioning at discrete time points post injury following a mild TBI in childhood. The current study is one of the largest studies of a sample of children with mild TBI in which, (1) neurocognitive functioning was studied at discrete time points post injury to describe the impairment and course of recovery present during the first year post mild TBI, and (2) for which the effect of pre-injury risk factors for accidental injuries were controlled.
In the memory domain, after accounting for age and parental education, statistically significant group effects were identified, with post hoc analyses revealing that these differences were largely driven by both the injury groups (TBI and other injury) scoring lower than the non-injured control group on two of the three memory measures (Prospective Memory and List Learning). The TBI group, but not the other injury group, performed more poorly than the non-injured control group on the third memory measure, the Picture Memory test. In the psychomotor and processing speed domain, after accounting for age and parental education, there were no group differences in fine motor speed/dexterity (Pin Test). However, significant group effects were noted for the Color Trails and the Symbol Digit Modalities tasks, where both the TBI and the other injury group performed more poorly than the non-injured control group. In the attention and executive functioning domain, after accounting for age and parental education, a main group effect was only noted for the Span Test with the TBI group taking a longer time to complete the task than the other injury group. Finally, on a language measure (picture vocabulary), after accounting for age and parental education, significant group effects were noted, with both injury groups (TBI and other injury) performing more poorly than the non-injured control group, although all mean PPVT-R scores were within the “average” range.
As a group, the mild TBI children showed poorer performance than the non-injured control group on 6 of the 10 neurocognitive measures. However, when present, these differences were generally associated with small effect sizes in all cases with the exception of one effect size approaching the moderate range (i.e., Prospective Memory between the TBI group and non-injured control comparison). Furthermore, in five of six instances where group differences were observed between the TBI and the non-injured control group, the other injury group also performed more poorly than the non-injured control group suggesting that these effects were a general injury effect and unlikely due to a brain injury. Nonetheless, almost a third of both the mild TBI and the other injury subjects performed 1.5 standard deviations or more lower than the non-injured control group's mean on at least 3 of the 10 neurocognitive measures, with only 18% of the non-injured control group showing a similar pattern. This difference in proportions was statistically significant. These findings show that despite the small group differences at 1 year post injury, a relatively large proportion compared to the non-injured control group of the TBI and other injury groups show problems on several measures.
Neurocognitive domains that participants showed problems on in the analyses described above were those measuring various memory abilities, language, and rapid psychomotor tests, with these vulnerabilities found not to be unique only to the TBI group but to the other injury group as well. In fact, for the most part, the other injury and mild TBI group appeared to perform very similarly across many of the measures. Without another injury group, the differences observed between the mild TBI and non-injured control group would have been erroneously attributed to a brain injury. However, the findings from the other injury control group suggest that there is a general injury effect—perhaps due to psychosocial factors associated with an injury, as suggested in the TBI literature (Yeates et al., 1997)—and/or that children who are prone to injuries tend to have subtle, likely undiagnosed, neurocognitive weaknesses that probably antedate the injury, and, perhaps, make them more susceptible to succumbing to an injury. This point is especially well illustrated by the somewhat surprising findings from the Picture Vocabulary test. Crystallized language skills are typically relatively resistant to a brain injury (Cattell, 1963; Russell, 1980), particularly with milder injuries. The group differences noted between the non-injury group and both the mild TBI and other injury group in language skills is most likely indicative of the pre-injury level of functioning in the two injury groups and may reflect the findings from epidemiological studies, suggesting that children who incur accidental injuries frequently come from families with lower education backgrounds or socio-economic status or have a higher rate of premorbid learning issues (Durkin, Davidson, Kuhn, O'Connor, & Barlow, 1994; Kogan, Overpeck, & Fingerhut, 1995; Ramsay et al., 2003). These findings underscore the importance of controlling for pre-injury risk factors. Without including the other injury group, one would have erroneously concluded that mild TBI adversely affects many aspects of neurocognitive functioning, including language, psychomotor processing, and memory. Controlling for pre-injury level of functioning when evaluating cognitive outcomes is especially important in mild TBI where (1) the cognitive deficits are relatively subtle and/or transient, and (2) deficits in the cognitive outcomes assessed in children with TBI, such as problems with attention, are frequently found in the general pediatric population.
This study also highlights the importance of longitudinal analyses of outcomes in studies of TBI, especially in children whose brains are in a rapid stage of development and new skills are acquired. Comparing the development of children with a head injury over time with that of a carefully matched non-injury group allows us to describe the course of cognitive recovery following a mild TBI by controlling for normal cognitive development. Furthermore, it is important to note that the sample in this study represented the milder end of a traditionally defined (using GCS) mild TBI sample. It is likely that the results reported in this study would have varied somewhat if a broader definition of mild TBI was used.
In the current study, the vast majority of children with mild TBI did not differ from the non-injured control group. There was, however, a small subset of children with mild TBI in this study who showed persistent neurocognitive problems and post-concussive type symptoms. These symptoms can result in functional morbidity and are, therefore, of substantial clinical concern. This is particularly true in the context of evidence indicating that mild TBI and resulting post-concussive symptoms result in considerable burden on the family and contribute to parental distress (Ganesalingam et al., 2008). We are currently in the process of analyzing data from the current sample of pediatric mild TBI patients to identify pre-injury factors and injury characteristics that predict which children with mild TBI will develop persisting neurocognitive and/or post-concussive type symptoms.
There were several limitations associated with this study that are important to acknowledge. There were no data collected on eligible subjects who chose not to participate, therefore potentially contributing to a bias in the sample included in the current analyses. We also did not have GCS scores or neuroimaging data to better characterize the nature and/or severity of the injury. Our injury severity variable (AIS) was very limited in identifying a range of acute injury severity within the mild TBI group that could potentially explain the poor outcomes in a small subset of the TBI group. Also, because only AIS scores of 1 or 2 were included in the analyses presented in the manuscript, it is important to note that the sample in this study was likely comprised of a relatively mild sample that did not include “complicated” cases. Furthermore, while the participants in the non-injury control group who had complete data did not differ from the participants with incomplete data with regard to demographic characteristics, on three of the six neurocognitive measures where the TBI group performed more poorly than the non-injury controls at time 3, the non-injured controls with missing data had poorer scores at time 1 compared to non-injured controls with complete data. This raises the possibility that the differences between the TBI and non-injured control groups at time 3 might have been reduced on these three tests (PPVT, List Learning, and Color Trails) had the entire non-injury control group completed all three assessments. Finally, we chose to focus on neurocognitive outcomes in this manuscript. It is possible that despite the null findings on neurocognitive effects that are unique to the mild TBI group, that there are potentially other morbidities related to a mild TBI that are burdensome on mental health and other health care and negatively impact the quality of life of children, adolescents, thereby their families, who incur a mild TBI. Furthermore, by focusing on neurocognitive outcomes, we also heavily relied on neurocognitive measures that were experimental at the time of the data collection and, therefore, may not have adequate psychometric properties.
ACKNOWLEDGMENTS
NINDS R01NS026801 Neurobehavioral Sequelae of Mild Brain Injury in Children; NICHD R01HD061504 Reconnection of Neural Networks and Cognitive Recovery After Pediatric TBI; NINDS 1F32NS053169 Neuroimaging and Recovery After Pediatric Brain
Footnotes
There are no conflicts of interest to disclose.
REFERENCES
- Achenbach TM. Child behavior checklist. Achenbach System of Empirically Based Assessment; Burlington, VT: 1991. [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Outcome from mild head injury in young children: A prospective study. Journal of Clinical and Experimental Neuro-psychology. 2001;23(6):705–717. doi: 10.1076/jcen.23.6.705.1015. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Satz P, Light R, Lewis R, Neumann E. Behavior problems and adaptive functioning in children with mild and severe closed head injury. Journal of Pediatric Psychology. 1991;16(5):543–555. doi: 10.1093/jpepsy/16.5.543. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Satz P, Light R, Zaucha K, Lewis R, McCleary C. The UCLA study of mild closed head injury in children and adolescents. In: Broman S, Michel ME, editors. Traumatic brain injury in children. Oxford University Press; New York: 1995. pp. 117–146. [Google Scholar]
- Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374–e381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. Journal of the International Neuropsychological Society. 2005;11(4):345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- Bijur PE, Haslum M, Golding J. Cognitive and behavioral sequelae of mild head injury in children. Pediatrics. 1990;86(3):337–344. [PubMed] [Google Scholar]
- Bruce B, Kirkland S, Waschbusch D. The relationship between childhood behaviour disorders and unintentional injury events. Paediatric Child Health. 2007;12(9):749–754. doi: 10.1093/pch/12.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catale C, Marique P, Closset A, Meulemans T. Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. Journal of Clinical and Experimental Neuropsychology. 2009;31(3):331–338. doi: 10.1080/13803390802134616. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The theory of fluid and crystallized intelligence: A critical experiment. Journal of Educational Psychology. 1963;54(1):1–22. doi: 10.1037/h0024654. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test—revised manual. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Durkin MS, Davidson LL, Kuhn L, O'Connor P, Barlow B. Low-income neighborhoods and the risk of severe pediatric injury: A small-area analysis in northern Manhattan. American Journal of Public Health. 1994;84(4):587–592. doi: 10.2105/ajph.84.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Wade SL, Drotar D, Stancin T, Taylor HG. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23(3):271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesalingam K, Yeates KO, Ginn MS, Taylor HG, Dietrich A, Nuss K, Wright M. Family burden and parental distress following mild traumatic brain injury in children and its relationship to post-concussive symptoms. Journal of Pediatric Psychology. 2008;33(6):621–629. doi: 10.1093/jpepsy/jsm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JC. Identification of brain disorders by the Stroop Color and Word Test. Journal of Clinical Psychology. 1976;32:654–658. doi: 10.1002/1097-4679(197607)32:3<654::aid-jclp2270320336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Greenspan L, McLellan BA, Greig H. Abbreviated Injury Scale and Injury Severity Score: A scoring chart. Journal of Trauma. 1985;25(1):60–64. doi: 10.1097/00005373-198501000-00010. [DOI] [PubMed] [Google Scholar]
- Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: A neuropsychological review from injury through recovery. Clinical Neuropsychology. 2008;22(5):769–800. doi: 10.1080/13854040701543700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Overpeck MD, Fingerhut LA. Medically attended nonfatal injuries among preschool-age children: National estimates. American Journal of Preventive Medicine. 1995;11(2):99–104. [PubMed] [Google Scholar]
- Lee LK. Controversies in the sequelae of pediatric mild traumatic brain injury. Pediatric Emergency Care. 2007;23(8):580–583. doi: 10.1097/PEC.0b013e31813444ea. quiz 584–586. [DOI] [PubMed] [Google Scholar]
- Maillard-Wermelinger A, Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Wright M. Mild traumatic brain injury and executive functions in school-aged children. Developmental Neurorehabilitation. 2009;12(5):330–341. doi: 10.3109/17518420903087251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. Journal of Clinical and Abnormal Psychology. 1983;92:4. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Petersen C, Scherwath A, Fink J, Koch U. Health-related quality of life and psychosocial consequences after mild traumatic brain injury in children and adolescents. Brain Injury. 2008;22(3):215–221. doi: 10.1080/02699050801935245. [DOI] [PubMed] [Google Scholar]
- Ramsay LJ, Moreton G, Gorman DR, Blake E, Goh D, Elton RA, Beattie TF. Unintentional home injury in preschool-aged children: Looking for the key–An exploration of the inter-relationship and relative importance of potential risk factors. Public Health. 2003;117(6):404–411. doi: 10.1016/S0033-3506(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Russell EW. Fluid and crystallized intelligence: Effects of diffuse brain damage on the WAIS. Perceptual Motor Skills. 1980;51(1):121–122. doi: 10.2466/pms.1980.51.1.121. [DOI] [PubMed] [Google Scholar]
- Satz P, D'Elia L. The Pin Test Professional Manual. Psychological Assessment Resources; Odessa, FL: 1989. [Google Scholar]
- Satz P, Zaucha K, McCleary C, Light R, Asarnow R, Becker D. Mild head injury in children and adolescents: A review of studies (1970-1995). Psychological Bulletin. 1997;122(2):107–131. doi: 10.1037/0033-2909.122.2.107. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. International Review of Psychiatry. 2003;15(4):341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- Schwebel DC, Gaines J. Pediatric unintentional injury: Behavioral risk factors and implications for prevention. Journal of Developmental and Behavioral Pediatrics. 2007;28(3):245–254. doi: 10.1097/01.DBP.0000268561.80204.2a. [DOI] [PubMed] [Google Scholar]
- Smith A. The Symbol Digit Modalities test: A neuropsychologic test for learning and other cerebral disorders. Journal of Learning Disorders. 1968;3:83–91. [Google Scholar]
- Wickens D. Encoding categorized words: An empirical approach to memory. Psychological Review. 1970;77:1–15. [Google Scholar]
- Wrightson P, McGinn V, Gronwall D. Mild head injury in preschool children: Evidence that it can be associated with a persisting cognitive defect. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;59(4):375–380. doi: 10.1136/jnnp.59.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO. Mild traumatic brain injury and postconcussive symptoms in children and adolescents. Journal of the International Neuropsychological Society. 2010;16(6):953–960. doi: 10.1017/S1355617710000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, Schatschneider C. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3(6):617–630. [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Nuss K, Jones BL. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123(3):735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]