Abstract
Background and Objectives
Acute otitis media (AOM) not only affects childhood quality of life (QoL), but can also affect parental QoL. We adapted a previously published questionnaire on the effect of childhood recurrent ear, nose and throat infections on parental QoL for use with AOM and used it in an observational, multicentre, prospective study of children with AOM.
Methods
The AOM-specific parental QoL questionnaire grouped 15 items into emotional, daily disturbance, total and overall parental QoL impact scores. The questionnaire was assessed using item-convergent and item-discriminant validity criteria and internal consistency reliability; and then used with parents of children aged <6 years diagnosed with AOM at 73 practices in Germany, Italy, Spain, Sweden and the UK. Bivariate analyses explored the differences in mean parental QoL impact scores by various characteristics.
Results
The questionnaire demonstrated good to excellent internal consistency reliability for the various components (Cronbach’s α 0.82–0.97). There were 1419 AOM episodes among 5882 healthy children over 1 year, of which 1063 episodes (74.9 %) among 852 children had a questionnaire. Parents reported interrupted sleep (68.4 %), worry (51.0 %), altered daily schedule (44.6 %) and less leisure time (41.5 %) with a score ≥3 (1 = least to 5 = most impact). Factors that adversely affected parental QoL included: increased parental perception of AOM severity, younger child age and multiple AOM episodes.
Conclusions
The AOM-specific parental QoL questionnaire demonstrated good performance across five European countries. Parental QoL was affected by childhood AOM proportionally to severity, number of episodes and younger child age.
Electronic supplementary material
The online version of this article (doi:10.1007/s40261-015-0319-1) contains supplementary material, which is available to authorized users.
Key Points
| We adapted and implemented a QoL questionnaire to ascertain parental QoL during an episode of childhood AOM. |
| Parents reported interrupted sleep, worry, altered daily schedule and less leisure time. |
| Parental QoL worsened with increased parental perception of AOM severity, younger child age and multiple AOM episodes. |
Introduction
Acute otitis media (AOM) affects approximately 40 % of children before 5 years of age—often with multiple episodes—and frequently leads to healthcare visits and antibiotic prescriptions [1]. AOM can cause symptoms such as otalgia, fever, headache, irritability and listlessness [2], and may lead to hearing impairment or other complications [3] that can impact negatively on children’s quality of life (QoL). Furthermore, childhood AOM also disrupts the parents’ ability to carry out daily activities, and can have a negative impact on the lives of other family members [4].
Measuring the effect of diseases on QoL can be difficult, as it is a subjective matter. However, it is an important outcome, and questionnaires to measure QoL in outcomes studies, clinical trials and routine clinical care [5] can be used to assess the benefits of preventive measures such as immunisation [6]. While most questionnaires focus on the QoL of the patient, childhood diseases can also impact on the QoL of their caregivers, leading to parental anxiety, sleep deprivation and lost work time to look after their child and/or take them to a healthcare professional.
The effects of otitis media on QoL have previously been reported, most commonly using the Otitis Media-6 (OM-6) questionnaire [5, 7–10]. This questionnaire focuses on the QoL of the child with otitis media, covering physical, functional, psychological and social aspects, with only one question on the effect on parental QoL. Caregiver QoL has been studied using the otitis media-specific Family Functioning Questionnaire [11, 12] and the Caregiver Impact Questionnaire [4, 13, 14], which also cover the effect of otitis media on other family members. The OM-6 and the Family Functioning Questionnaire have also been combined to form a telephone questionnaire to assess child and caregiver QoL during an AOM episode [6]. Recently, an AOM-specific online interview has been used to assess the burden of AOM on caregivers [15], as has the generic PedsQL Family Impact survey [16].
Although there are various otitis media-specific questionnaires for studying the effect of otitis media on QoL, they only have 1–9 questions on parental QoL [4–15, 17]. We therefore adapted a previously published questionnaire for parents of children with recurrent ear, nose and throat (ENT) infections [18] into a 15-item, AOM-specific parental questionnaire that we used to assess parental QoL during their child’s AOM episode in five European countries.
Methods
Quality-of-Life (QoL) Questionnaire Adaptation and Validation
An AOM-specific self-administered questionnaire was developed based on the parental QoL questionnaire for recurrent ENT infections in their children (PAR-ENT-QoL) [18]. The original French version of the questionnaire had been validated using multitrait analysis (for construct validity) and Cronbach’s α (for internal consistency reliability), but the English version included in the paper by Berdeaux et al. [18] was not validated. A diagrammatic overview of the adaptation process is provided in Online Resource 1. Berdeaux et al.’s ENT-specific non-validated English-language QoL questionnaire [18] was altered to be specific for AOM by a review panel (one with experience in clinical and outcomes research; one QoL expert; a medical writer; and a medical doctor). The team also improved the English in the questionnaire to ensure easy and correct interpretation; and this was tested on a UK native-speaking mother with a child with a history of multiple otitis media. The questionnaire was reviewed (for ease of understanding, relevance and completeness) by five non-medically educated volunteers (three mothers and two fathers; four UK native speakers and one UK-speaking non-native; four with a child <5 years and one with an older child but very frequent otitis media since an early age). Feedback was consolidated and discussed by all participants. All comments and suggestions for each question of the QoL questionnaire, as well as additional questions, were considered. Proposals were shared, clarified and motivated. A consensus was easily obtained for all questions, and the questionnaire was adapted accordingly. The resultant adapted UK QoL questionnaire was then reviewed by the core team to ensure the original concepts were retained. The complete questionnaire can be found in Online Resource 2. Fourteen items were grouped into three impact scores: emotional [question (Q)1–6, 13, 14], daily disturbance (Q7–12) and total impact score (Q1–14). A fifteenth item (Q15) assessed overall parental QoL.
In order to be used in the respective countries, the final English questionnaire was translated and adapted for cross-cultural differences in order to be used in Germany, Italy, Spain and Sweden. This was performed by experienced, qualified translators in their native language at TransPerfect Translations (Belgium) according to the Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes Measures [19] by applying dual forward translation, reconciliation of the dual forward translation, back translation, and reconciliation of the back translation with the forward translation. When there were differences between the dual forward translations, the most appropriate and/or more widely used terms were chosen. The finalised versions were sent to three laymen in each country. Each respondent reviewed the instrument and participated in a face-to-face interview so that insight could be gained regarding the general impression of the instrument, clarity of the instructions, comprehensibility and appropriateness of each question and the corresponding response choices. All of the final country-specific questionnaires were submitted as part of the EU AOM study and approved by the respective ethics committees.
The internal consistency of the scores was measured using Cronbach’s α. As per Berdeaux et al. [18], Cronbach’s α ≥0.7 was considered reasonable, >0.8 good and >0.9 excellent. Spearman correlations were calculated between: items and items (to reveal any redundancy); items and scales (to reveal any item that could possibly belong to a different scale than the one targeted); and items and the overall QoL score (Q15) (to check the homogeneity consistency of the overall QoL score).
QoL Questionnaire Implementation
Study Design
An epidemiological, multicentre, observational study was conducted to determine the QoL of the parents of children aged <6 years with AOM from 73 medical practices in Germany, Italy, Spain, Sweden and the UK between July 2008 and January 2009 (GSK study identifier: EPI-STREP-111288). This study was part of a larger prospective cohort study to estimate the burden of AOM, including incidence, symptoms and complications, results of which have been reported separately [20].
In Germany, Italy, Spain and the UK, most children were enrolled during a medical visit, but they could also be recruited via mailings or telephone calls. In Sweden, all children were recruited via mailings based on population registers [20]. To be eligible, children had to: be aged <6 years; have medical records at the investigational site for the previous year (or from age 1 month for those aged <1 year); have parental written informed consent; and be expected to be available for a 1-year prospective follow-up. The only exclusion criterion was any sign/symptom of AOM or upper respiratory tract infection at the time of enrolment. All children who met the inclusion/exclusion criteria were included apart from 12 with a lack of follow-up contact information [20]. Demographic data at the first visit and clinical data at each visit were collected by the doctor. A new episode of AOM was defined as AOM after a 30-day symptom-free interval since the resolution of a previous AOM episode. At each visit for a new episode of AOM, parents received a questionnaire to assess their QoL changes associated with diagnosed AOM. Parents were asked to complete the questionnaire and return it within 14 days after their first doctor visit for a new episode of AOM. Parents also received an AOM Faces Scale questionnaire [21], to assess their perceived severity of their child’s AOM (1 = least to 7 = most severe).
As this was a non-interventional study, no specific guidelines for AOM diagnosis were applied, but diagnoses were classified according to the level of certainty based on patient charts and recorded symptoms or tests. Three levels of diagnostic certainty of AOM were used in this study:
Physician-diagnosed AOM: AOM diagnosed by a physician and documented in the medical file or other source document.
Physician-confirmed AOM: physician-diagnosed AOM plus documented visual appearance of the tympanic membrane, i.e., redness, bulging, loss of light reflex or presence of acute middle-ear effusion (shown by otoscopy or tympanometry) and ≥2 of the following documented signs or symptoms: otalgia, ear discharge, hearing loss, lethargy, irritability, anorexia, vomiting, diarrhoea, fever (axillary temperature ≥38.0 °C, rectal temperature ≥38.5 °C) or analgesic/antipyretic therapy for preceding fever.
Laboratory-confirmed AOM: physician-diagnosed or -confirmed AOM plus positive result of bacteria in middle ear fluid (after spontaneous perforation or tympanocentesis).
The study was conducted according to Good Clinical Practice, the Declaration of Helsinki and the local rules and regulations of each country. The protocol was submitted to the regulatory agencies in accordance with local regulatory requirements. The Institutional Review Boards/Independent Ethics Committees approved the protocol according to the local laws/customs of each participating country.
Statistical Analysis
The sample size of 6250 children was chosen to assure robust data on recurrent AOM cases based on the assumption of 0.25 AOM episodes/child/year of which ~15 % are recurrent. Descriptive statistics were performed for demographic and clinical variables. Responses from the questionnaire were measured using a five-point Likert scale ranging from 1 (least impact) to 5 (most impact). Mean scores (scale 1–5) for each question were calculated. After imputing missing data for each patient (using the mean of the non-missing items of each score, provided responses were given for ≥50 % of the items in that score), scores were combined to give an emotional impact score [mean of questions (Q)1–6, 13, 14], a daily disturbance impact score (mean of Q7–12), and a total impact score (mean of emotional and daily disturbance impact scores). Mean impact scores for all patients were linearly transformed from a scale of 1–5 to a scale of 0–100 [i.e., (mean – 1) × (100 – 0)/(5 – 1)], such that 0 was the best state and 100 the worst. Bivariate analyses (cross-tabulation) explored the differences in the mean parental QoL impact scores by country, level of diagnostic certainty, parental perception of AOM severity, child age, sibling status and number of AOM episodes. The p values for the differences in mean scores by these various characteristics were calculated using the Kruskall Wallis test (except for country and number of episodes). A p value of <0.05 was considered to be significant. All statistical analyses were performed using SAS version 9.1.
Results
QoL Questionnaire Assessment
The internal consistency reliability of the questionnaire was good for the total impact score (Cronbach’s α = 0.82) and excellent for the emotional (0.95) and daily disturbance (0.97) scores.
The Spearman item–item correlation coefficients were all <0.8 (range 0.16–0.72), indicating no redundancy of questions (Table 1). The corrected Spearman item–scale correlations were all >0.40 for correlations between each item and its own scale(s), showing moderate–strong correlation; each item had a better correlation with its own scale than with the other scale (Table 2), revealing no outliers.
Table 1.
Spearman item–item correlation coefficientsa
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (ES) | 1.00 | 0.59 | 0.45 | 0.38 | 0.38 | 0.40 | 0.34 | 0.33 | 0.30 | 0.36 | 0.29 | 0.16 | 0.41 | 0.29 |
| Q2 (ES) | 0.59 | 1.00 | 0.69 | 0.57 | 0.61 | 0.55 | 0.49 | 0.43 | 0.43 | 0.49 | 0.45 | 0.27 | 0.46 | 0.32 |
| Q3 (ES) | 0.45 | 0.69 | 1.00 | 0.67 | 0.65 | 0.59 | 0.47 | 0.38 | 0.33 | 0.44 | 0.43 | 0.28 | 0.45 | 0.34 |
| Q4 (ES) | 0.38 | 0.57 | 0.67 | 1.00 | 0.72 | 0.53 | 0.43 | 0.38 | 0.38 | 0.43 | 0.44 | 0.33 | 0.51 | 0.32 |
| Q5 (ES) | 0.38 | 0.61 | 0.65 | 0.72 | 1.00 | 0.59 | 0.47 | 0.42 | 0.42 | 0.51 | 0.46 | 0.33 | 0.47 | 0.32 |
| Q6 (ES) | 0.40 | 0.55 | 0.59 | 0.53 | 0.59 | 1.00 | 0.47 | 0.40 | 0.38 | 0.48 | 0.45 | 0.32 | 0.44 | 0.44 |
| Q7 (DDS) | 0.34 | 0.49 | 0.47 | 0.43 | 0.47 | 0.47 | 1.00 | 0.59 | 0.55 | 0.58 | 0.51 | 0.32 | 0.42 | 0.41 |
| Q8 (DDS) | 0.33 | 0.43 | 0.38 | 0.38 | 0.42 | 0.40 | 0.59 | 1.00 | 0.69 | 0.67 | 0.55 | 0.31 | 0.36 | 0.37 |
| Q9 (DDS) | 0.30 | 0.43 | 0.33 | 0.38 | 0.42 | 0.38 | 0.55 | 0.69 | 1.00 | 0.70 | 0.63 | 0.36 | 0.35 | 0.38 |
| Q10 (DDS) | 0.36 | 0.49 | 0.44 | 0.43 | 0.51 | 0.48 | 0.58 | 0.67 | 0.70 | 1.00 | 0.62 | 0.33 | 0.40 | 0.39 |
| Q11 (DDS) | 0.29 | 0.45 | 0.43 | 0.44 | 0.46 | 0.45 | 0.51 | 0.55 | 0.63 | 0.62 | 1.00 | 0.37 | 0.39 | 0.36 |
| Q12 (DDS) | 0.16 | 0.27 | 0.28 | 0.33 | 0.33 | 0.32 | 0.32 | 0.31 | 0.36 | 0.33 | 0.37 | 1.00 | 0.30 | 0.28 |
| Q13 (ES) | 0.41 | 0.46 | 0.45 | 0.51 | 0.47 | 0.44 | 0.42 | 0.36 | 0.35 | 0.40 | 0.39 | 0.30 | 1.00 | 0.35 |
| Q14 (ES) | 0.29 | 0.32 | 0.34 | 0.32 | 0.32 | 0.44 | 0.41 | 0.37 | 0.38 | 0.39 | 0.36 | 0.28 | 0.35 | 1.00 |
DDS daily disturbance scale, ES emotional scale, Q question
aAll item–item correlation coefficients between different items are <0.8, indicating no redundancy
Table 2.
Spearman item–scale correlation coefficientsa
| ES | DDS | TS | Overall QoL | |
|---|---|---|---|---|
| Q1 (ES) | 0.65 | 0.38 | 0.58 | 0.36 |
| Q2 (ES) | 0.81 | 0.55 | 0.75 | 0.55 |
| Q3 (ES) | 0.80 | 0.49 | 0.72 | 0.53 |
| Q4 (ES) | 0.76 | 0.50 | 0.70 | 0.51 |
| Q5 (ES) | 0.77 | 0.55 | 0.73 | 0.54 |
| Q6 (ES) | 0.76 | 0.52 | 0.71 | 0.53 |
| Q7 (DDS) | 0.58 | 0.75 | 0.72 | 0.58 |
| Q8 (DDS) | 0.51 | 0.83 | 0.72 | 0.57 |
| Q9 (DDS) | 0.50 | 0.86 | 0.73 | 0.55 |
| Q10 (DDS) | 0.58 | 0.85 | 0.77 | 0.58 |
| Q11 (DDS) | 0.53 | 0.78 | 0.71 | 0.53 |
| Q12 (DDS) | 0.36 | 0.53 | 0.48 | 0.31 |
| Q13 (ES) | 0.67 | 0.46 | 0.62 | 0.49 |
| Q14 (ES) | 0.60 | 0.46 | 0.59 | 0.47 |
| Q15 (Overall QoL) | 0.66 | 0.67 | 0.72 | 1.00 |
Bold numbers are all >0.40, showing correlation between each question and its relevant scale(s)
DDS daily disturbance scale, ES emotional scale, Q question, QoL quality of life, TS total score
aCorrected for overlap. <0.4 = weak correlation; 0.4 to <0.7 = moderate correlation; ≥0.7 = strong correlation
The homogeneity and satisfactory consistency of overall QoL (Q15) was assessed using the Spearman correlation coefficients between this and each of Q1–14. Most correlations were moderate, with the best correlations for Q7 (time for other family members) and Q10 (activities affected) (Table 2).
QoL Questionnaire Implementation
Study Population
Over 1 year, there were 1419 episodes of AOM—mean ± standard deviation (SD) duration 7 ± 8 days (n = 411)—among the 5882 included healthy children (cumulative incidence 24.1 %). The parents of 852 children who developed at least one new episode of AOM filled out a questionnaire. Of the 852 children, 57.3 % had received at least one dose of pneumococcal conjugate vaccine, ranging from 30.4 % in Sweden to 83.7 % in Spain. Baseline demographics for the 852 children are shown in Table 3.
Table 3.
Demographic and acute otitis media (AOM) episode characteristics of the children with ≥1 quality-of-life (QoL) questionnaire available
| Characteristic | Germany | Italy | Spain | Sweden | UK | Overall |
|---|---|---|---|---|---|---|
| Number of children | 176 | 169 | 215 | 194 | 98 | 852 |
| Male, n (%) | 86 (48.9) | 86 (50.9) | 109 (50.7) | 108 (55.7) | 56 (57.1) | 445 (52.2) |
| Age, months, median (range) | 24 (1–66) | 34 (0–71) | 25 (0–69) | 24.5 (1–68) | 22.5 (1–71) | 25 (0–71) |
| Visits due to AOM | 277 | 227 | 487 | 278 | 138 | 1407 |
| Episodes of AOM with a questionnaire, n (%) | ||||||
| 1 per child | 140 (79.5) | 143 (84.6) | 159 (74.0) | 166 (85.6) | 79 (80.6) | 687 (80.6) |
| 2 per child | 27 (15.3) | 23 (13.6) | 43 (20.0) | 20 (10.3) | 16 (16.3) | 129 (15.1) |
| 3–5 per child | 9 (5.1) | 3 (1.8) | 13 (6.0) | 8 (4.1) | 3 (3.1) | 36 (4.2) |
| AOM episodes with QoL questionnaire/all AOM episodes, n/N (%) | 225/323 (69.7) | 198/240 (82.5) | 287/374 (76.7) | 231/302 (76.5) | 122/180 (67.8) | 1063/1419 (74.9) |
| Level of AOM diagnostic certainty, n (%) | ||||||
| Physician-diagnosed | 136 (60.4) | 116 (58.6) | 200 (69.7) | 145 (62.8) | 99 (81.1) | 696 (65.5) |
| Physician-confirmed | 82 (36.4) | 82 (41.4) | 82 (28.6) | 82 (35.5) | 21 (17.2) | 349 (32.8) |
| Laboratory-confirmed | 7 (3.1) | 0 | 5 (1.7) | 4 (1.7) | 2 (1.6) | 18 (1.7) |
| Signs/symptomsa, n (%) | ||||||
| Ear pain/otalgia | 158 (70.2) | 172 (86.9) | 185 (64.5) | 149 (64.5) | 59 (48.8) | 723 (68.1) |
| Redness of tympanic membrane | 158 (70.2) | 138 (69.7) | 201 (70.0) | 172 (74.5) | 50 (41.3) | 719 (67.7) |
| Fever | 105 (46.7) | 96 (48.5) | 140 (48.8) | 112 (48.5) | 42 (34.7) | 495 (46.6) |
| Bulging of tympanic membrane | 72 (32.0) | 72 (36.4) | 94 (32.8) | 128 (55.4) | 15 (12.4) | 381 (35.9) |
| Loss of light reflex | 111 (49.3) | 70 (35.4) | 110 (38.3) | 5 (2.2) | 2 (1.7) | 298 (28.1) |
| Presence of acute middle-ear effusion | 75 (33.3) | 55 (27.8) | 52 (18.1) | 7 (3.0) | 4 (3.3) | 193 (18.2) |
| Ear discharge | 28 (12.4) | 27 (13.6) | 45 (15.7) | 59 (25.5) | 26 (21.5) | 185 (17.4) |
| Ear tugging | 22 (9.8) | 24 (12.1) | 9 (3.1) | 17 (7.4) | 25 (20.7) | 97 (9.1) |
| Hearing loss | 7 (3.1) | 9 (4.5) | 8 (2.8) | 1 (0.4) | 2 (1.7) | 27 (2.5) |
| Other | 50 (22.2) | 24 (12.1) | 103 (35.9) | 31 (13.4) | 47 (38.8) | 255 (24.0) |
| Perforation of the tympanic membrane, n (%) | 13 (5.8) | 5 (2.5) | 12 (4.2) | 40 (17.3) | 3 (2.5) | 73 (6.9) |
| Antibiotic prescription, n (%) | 174 (77.3) | 182 (91.9) | 252 (87.8) | 224 (97.0) | 114 (93.4) | 946 (89.0) |
AOM acute otitis media, UK United Kingdom, QoL quality of life
aData missing for one UK episode
A total of 19.4 % of the 852 children had >1 episode of AOM, resulting in 1063/1419 episodes (74.9 %) with a completed questionnaire (ranging from 67.8 % in the UK to 82.5 % in Italy). Of these questionnaires, 92.5 % were complete (ranging from 88.4 % in Italy to 96.7 % in the UK), 6.3 % had one item missing and 1.2 % had ≥2 items missing. A total of 1060 episodes (99.7 %) had a questionnaire with computable scores (i.e., responses for ≥50 % of the items in each score). The mother completed the questionnaire in 92.0 % of the cases; the father in 8.0 %.
Acute Otitis Media (AOM) Episode Characteristics
Signs/symptoms of AOM were documented by physicians during 99.2 % of episodes, mainly ear pain/otalgia (68.1 %) and redness of the tympanic membrane (67.7 %) (Table 3). There were 1407 physician visits; most episodes resulted in a prescription for antibiotics (89.0 %). Perforation of the tympanic membrane was reported in 6.9 % of episodes, other complications in 0.4 % and hospitalisation in 0.4 %.
Parental QoL Results
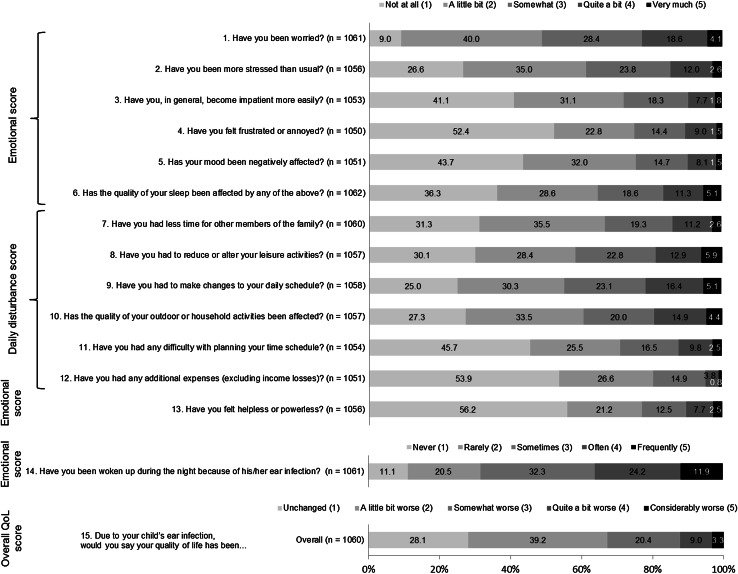
The distribution of emotional, daily disturbance, overall impact scores and their items as per the parents’ responses are shown in Fig. 1. The highest impact scores of ≥3 on a scale of 1–5 were observed for parental interrupted sleep (68.4 %), worry (51.0 %), having to alter their daily schedule (44.6 %) and less time for leisure activities (41.5 %). The highest mean ± SD impact score (3.1 ± 1.2) was observed for parents being woken during the night because of the child’s ear infection. Overall, 71.9 % of parents reported worsening (score 2–5) of their overall QoL, with 32.6 % reporting somewhat–considerable worsening (score 3–5) (Fig. 1).
Fig. 1.
Proportions of responses for each quality-of-life (QoL) questionnaire item and mean ± standard deviation (SD) parental QoL impact scores
Differences in mean impact scores (emotional, daily disturbance, total and overall QoL) by country, AOM diagnostic certainty, parental perception of AOM severity, child age, sibling status and number of AOM episodes are shown in Fig. 2. By country, parents in Italy reported the least impact of AOM on their QoL (Fig. 2a).
Fig. 2.
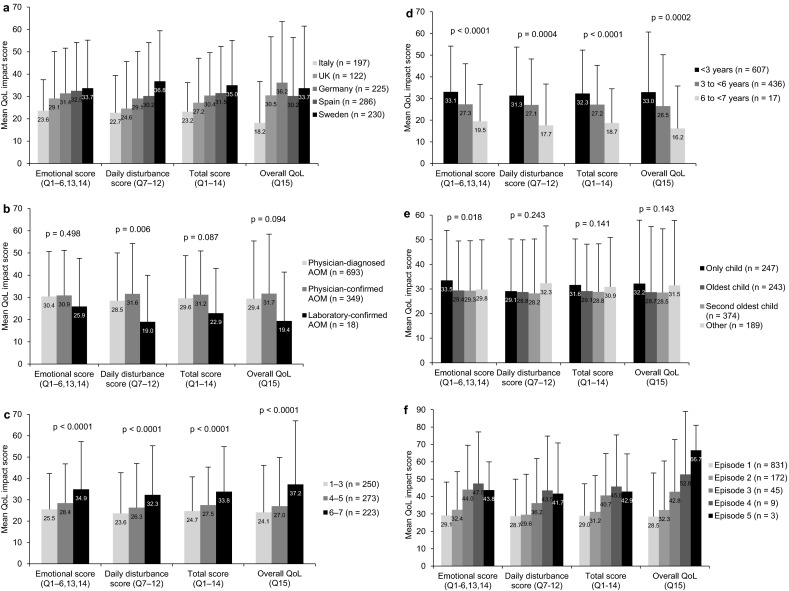
Mean ± standard deviation (SD) parental quality-of-life (QoL) impact scores (0 = best to 100 = worst) by a country, b level of diagnostic certainty, c parental AOM Faces Scale score [21] (1 = least severe to 7 = most severe; not used in Sweden), d child age at the time of the AOM episode, e sibling status and f number of episodes of AOM. p values: Kruskall Wallis test was used for across-group comparisons. AOM acute otitis media, Q question
By certainty of diagnosis, mean impact scores for the 349 episodes of physician-confirmed AOM were somewhat higher than for the 693 episodes of physician-diagnosed AOM, while the least impact of AOM on parental QoL was observed for 18 children with laboratory-confirmed AOM (Fig. 2b). By country, impact scores were all higher for physician-confirmed than physician-diagnosed AOM, except in the UK, where the trend was reversed for the emotional, daily disturbance and total impact scores.
By parental perception of AOM severity, based on the AOM Faces Scale [21] (which was not used in Sweden), parental QoL impact scores increased significantly (p < 0.0001) with increasing perception of severity (Fig. 2c). Similar trends were seen in Spain and the UK. In Germany, impact scores were highest for Faces Scale scores of 6–7 but similar for 1–3 and 4–5; in Italy, they varied very little with the Faces Scale.
By child age, mean parental QoL impact scores increased significantly with decreasing age (Fig. 2d), although it should be noted that there were only 17 children in the 6- to <7-year age group. Impact scores were considerably higher for parents of children aged <3 versus 3 to <6 years in Spain, Sweden and the UK, but similar for both age groups in Germany and Italy.
By sibling status, the mean emotional impact score was significantly higher for children with no siblings than for those with ≥1 sibling(s), but the other impact scores did not vary significantly (Fig. 2e). In Italy and the UK, impact scores were highest for parents of only children, while in Germany, they were highest for parents whose oldest child was ill; there were no notable trends by sibling status in Spain or Sweden.
For increasing numbers of AOM episodes, all of the mean scores increased from one up to four episodes; mean scores for the three children with five episodes were generally similar to those with four episodes (Fig. 2f). Impact scores mainly increased from one to two episodes of AOM in Germany, Sweden and Italy, but were generally similar or slightly decreased from one to two episodes in the UK and Spain; there were too few children per country with three or more episodes to make comparisons.
Discussion
QoL Questionnaire
Most otitis media QoL studies have examined the effect of otitis media predominantly on the child’s QoL [5, 7–10, 12, 17, 22, 23], the effect of tympanostomy tube insertion mainly on the child’s QoL [24–29] or the effects of chronic otitis media and/or surgery on the QoL of adults with otitis media [30–34]. Although various otitis media-specific questionnaires have been used to assess the effect of childhood AOM on parental QoL, these have contained a maximum of nine parental questions [4, 6, 8, 13–15, 35]. In comparison, our questionnaire contained 15 questions on parental QoL, potentially allowing a somewhat more detailed assessment of parental QoL affected by childhood AOM.
We found good correlations between each item and its impact scores (total plus either emotional or daily disturbance), and each item had a stronger correlation with its own score than with the other score. These results are similar to those reported for the ENT questionnaire [18] that formed the basis of our questionnaire. We also found no redundancy of questions, as all item–item correlation coefficients were <0.8.
The internal consistency reliability of our questionnaire was good to excellent, similar to results from Berdeaux et al. [18] for the ENT questionnaire (Cronbach’s α = 0.83–0.92) and from Boruk et al. [4] for an otitis media questionnaire (Cronbach’s α = 0.88); and higher than that reported by Dubé et al. [6] for a telephone AOM questionnaire (Cronbach’s α = 0.81 for caregivers). Our questionnaire also had good discriminant validity, as shown by the significant correlations between impact scores and parental perception of AOM severity (Faces Scale).
Overall, our questionnaire was found to be a good tool for measuring parental QoL during an episode of childhood AOM. This questionnaire, which has been used in another study of the incidence of AOM and its effect on child and parental QoL in five East European countries (ClinicalTrials.gov Identifier: NCT01365390) [36], can be downloaded for use from Online Resource 2.
QoL Results
When applying our questionnaire, childhood AOM was found to have a detrimental effect on parental QoL, which worsened with increasing parental perception of AOM severity, younger child age and increasing numbers of AOM episodes. Parents particularly reported interrupted sleep, worry, altered daily schedule and less time for leisure activities.
Berdeaux et al. [18], who developed PAR-ENT-QoL questionnaire that formed the basis of our AOM questionnaire, found that recurrent ENT infections also had a detrimental effect on parental QoL. Although comparisons between studies should be undertaken with caution, our results showed a somewhat smaller detrimental effect of AOM (mean 1.2 episodes/child in children aged <6 years) on parental emotional scores compared to recurrent ENT infections (mean 1.4 episodes/child of rhinopharyngitis, 0.1 of AOM and 0.7 of AOM and rhinopharyngitis in children aged <5 years [18]), but similar detrimental effects on daily disturbance. Another study has recently found that childhood acute gastroenteritis also has a detrimental effect on parental QoL (worry, distress and impact on daily activities) [37].
In our study, the aspects of parental QoL most affected by childhood AOM were: being woken during the night, worry and having to alter their daily schedule. This is similar to results from Dubé et al. [6], who found sleep to be most affected, followed by concern, changes to daily activities, emotional distress and cancelling family activities. Boruk et al. [4] found three aspects of caregiver QoL to be most affected, namely lack of sleep, feeling nervous/agitated/irritable and feeling helpless/frustrated.
To the best of our knowledge, only two studies have specifically compared caregiver QoL for caregivers of children with otitis media and those of healthy children [8, 15]. Lee et al. [8] reported that caregiver emotional and personal time and family activities were all significantly more affected for caregivers with a child with otitis media than for those with a healthy child (p ≤ 0.05). Barber et al. [15] reported that parents of a child who had experienced AOM during the previous 6 months reported a higher burden than those whose child had never had AOM in terms of cancelled holidays and rearranged family plans and childcare. Furthermore, various studies have found that parental QoL improves after tympanostomy tube insertion [14, 24, 27, 29, 35], indicating that recurrent AOM and otitis media with effusion (prior to tube insertion) have a negative impact on parental QoL, similar to the observation in our study of AOM.
Although our study was not specifically designed to examine QoL differences between countries, we observed that parents in Italy reported less impact on their QoL than parents in the other four countries. Whether this is a true or biased representation of parents in Italy is unclear. However, in the ENT study by Berdeaux et al. [18], Italian parents also reported less effect on their QoL, particularly emotionally. Interestingly, we also found very little variation in impact scores with increasing AOM severity as measured by the Faces Scale in Italy. There were also some differences between countries depending on sibling status: impact scores were higher for only children in Italy and the UK, but for the oldest children in Germany. However, it should be noted that this study was not designed to test differences between countries, and many of the groups were small, so these results should be interpreted with caution. That said, differences in scores between countries could potentially be due to differences in cultural beliefs, standards of living and healthcare, perceptions, etc.
Impact scores were slightly worse for physician-confirmed AOM than for physician-diagnosed AOM. However, laboratory-confirmed AOM seemed to have less impact despite such cases generally being more severe. This is probably due to a decrease in pain following either spontaneous rupture of the tympanic membrane or after tympanocentesis performed by a physician. This would agree with various reports that tympanostomy tube insertion improves the QoL of children and their parents [14, 24–27, 29, 35]. However, our results should be interpreted with caution due to the low number of children with laboratory-confirmed AOM (n = 18).
Interestingly, the impact on parent emotional status was lower if the affected child had siblings. This could indicate a higher emotional stress for parents who have limited experience with a sick child affected by AOM. However, parental QoL impact scores worsened with increasing numbers of AOM episodes. This was also found for increasing numbers of ENT infections in the study by Berdeaux et al. [18]. Brouwer et al. [12] reported that children with ≥4 versus 2–3 episodes of AOM in the past year had significantly worse QoL and their family was significantly more affected (both p < 0.001). Boruk et al. [4] reported significantly worse child QoL with increasing numbers of otitis media episodes and time spent with otitis media problems during the prior 3 months. Lee et al. [8] reported that caregiver concerns correlated moderately, but significantly, with the frequency of otitis media episodes in the previous month (p < 0.001).
Strengths and Limitations
In our study, questionnaires were completed for 1063/1419 episodes of AOM (67.8–82.5 % across countries). This good response rate, coupled with high rates of complete questionnaires (88.4–96.7 %) and those with computable scores (99.5–100 %) mean that results are likely to be representative of the recruited population within each country. In addition, the baseline characteristics of the children whose parents completed the questionnaire were similar to those of all the children enrolled into the larger cohort study [20]. However, as acknowledged in our previous publication [20], the study population may not have been representative of the general population because most children were enrolled during a medical visit (except in Sweden, where children were recruited via mailing based on population registers). Although selection bias may therefore be present, it is unlikely that any such bias would have an important effect on the performance of the questionnaire other than potentially contributing to a slight overestimation of the QoL scores if concerned parents of more frequently sick children were more likely to respond. It should also be noted that it may not be possible to extrapolate the results to other countries, due to differences in cultural beliefs, standards of living and healthcare, perceptions, etc.
Other limitations of our study include the lack of a comparison population (i.e. parents of age- and practice-matched children without AOM) and the fact that QoL data were not collected at baseline (when children did not have AOM). However, this would not have been possible with our AOM-specific questionnaire. Rather, a generic questionnaire would allow the comparison of parental QoL in the absence and presence of AOM in their child. Also, we did not assess the reproducibility of the questionnaire. However, this would be difficult, given that results varied by child age and episode number, which would inevitably change between one episode of AOM and the next.
In addition, no conventional guidelines were applied for the diagnosis of AOM in this study, since this was a non-interventional study that relied on the diagnosis made by the physician as documented in the medical file. However, as discussed in our previous publication [20], the prospective follow-up (based on both physician and parental reports) allowed us to collect data on all cases and episodes of possible AOM (physician-diagnosed, physician- or laboratory-confirmed, or suspected by the parents) with a holistic view to capture the entire burden of disease, considering under-reporting of undiagnosed episodes. Studies of AOM that use conventional guidelines for diagnosis only focus on clinically-confirmed AOM, and are likely to miss mild cases and episodes for which medical care is not sought.
QoL reporting is very subjective, as indicated by the wide range of responses. However, as QoL is also subjective, with similar adversities affecting some people more than others, this is to be expected in any such assessment. Another potential weakness is that parents were asked to complete the questionnaire up to 14 days after their physician visit, by which time their child is likely to be better. While this approach was taken to try to capture QoL information for the whole AOM episode, parents may have forgotten how they felt during their child’s AOM episode, or at least underestimated the impact.
We note that >15 % of respondents answered ‘not at all’/‘never’/‘unchanged’ for 13 of the questions, indicating a floor effect. This can occur if a questionnaire is used in a different population than its intended one, but this floor effect was also apparent in Berdeaux et al.’s results [18]. However, we note that the floor effect may have had an impact on the performance of the questionnaire.
We recognise that our questionnaire assessment process was not a full ‘validation’. Heidemann et al. [13] used confirmatory factor analysis to test their questionnaire validity, but various other authors [6, 12, 18, 38] have used an approach similar to ours, using Cronbach α internal consistency reliability. Although all Spearman item–item correlation coefficients were <0.8, indicating no redundancy, the correlations between Q4 (frustration/annoyance) and Q5 (mood affected) and between Q9 (daily schedule affected) and Q10 (quality of activities affected) were ≥0.70, showing that these questions, not surprisingly, had some similarities. Although all Spearman item–scale correlations were higher between items and the relevant scale, some questions had reasonably strong correlations with the opposite scale, e.g. Q7 (less time for family) and Q10 (quality of activities affected) were strongly correlated to the daily disturbance scale, but also had a moderate correlation with the emotional scale. However, this is not surprising, as having less time for other family members disturbs daily life, but can also have an emotional effect, especially if the parent feels that their other children are being affected. Lastly, we did not asses the external construct validity of our questionnaire, apart from assessing the correlation to the overall QoL score (Q15).
Conclusions
A new AOM-specific parental QoL questionnaire was successfully adapted and implemented, demonstrating good performance in measuring the impact of AOM on parental QoL across five European countries. Future research could include further psychometric testing of the questionnaire, in terms of structure, reproducibility and external validity. The majority of parents of children with AOM reported a worsening of their QoL, including interrupted sleep, worry and reduced time for activities. Parental QoL was affected more when they perceived their child’s AOM to be more severe, if their child was aged <3 years and with an increasing number of episodes of AOM. Although AOM is generally a benign disease, it affects a very high number of children and has a negative impact on QoL for them and their parents. Prevention of, or improved treatments for, AOM could help to reduce the negative impact on the QoL of children with AOM and their parents.
Electronic supplementary material
Online Resource 1: The procedure used to adapt the ENT questionnaire to AOM (PDF 355 kb)
Online Resource 2: The parental QoL questionnaire used in the study. (PDF 181 kb)
Acknowledgments
GlaxoSmithKline Biologicals SA funded this study and was involved in all stages of the study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript. We thank Jenny Lloyd (Compass Medical Communications Ltd.) for medical writing services on behalf of GSK Vaccines, and Abdelilah Ibrahimi (XPE Pharma and Science on behalf of GSK Vaccines), Cédric Laloyaux and Fabien Debailleul (Business and Decision Life Sciences on behalf of GSK Vaccines) for publication coordination, and TransPerfect for the translation of the Quality of Life Questionnaire on behalf of GSK Vaccines.
Compliance with Ethical Standards
Conflict of interest
KH is an employee of the GSK group of companies and reports ownership of stock options/restricted shares from the GSK group of companies. MR was an employee of the GSK group of companies and reports ownership of stock options/restricted shares from the GSK group of companies at the time of the study. CG received grants from the GSK group of companies, Bristol-Myers Squibb, Sanofi Pasteur MSD and Gilead for board membership, travels, lecture and research. S-AS received institutional payments from the GSK group of companies, Pfizer, Sanofi and Merck for costs associated with recruitment of subjects and collection of data in different studies; received grants from the GSK group of companies for advisory board meetings on pneumococcal vaccines, from Astra-Zeneca for advisory board meeting on Fluenz™ (Fluenz™ is a trademark of MedImmune, LLC.) and travel support from Pfizer and the GSK group of companies for poster presentation at international meetings. AC received institutional payments from the GSK group of companies to fund the costs associated with recruitment of subjects and collection of data. JL received institutional research grants from the GSK group of companies for data collection. JG-S received institutional grants from the GSK group of companies. AF received institutional research grants from the GSK group of companies. MEM received investigator grant from the GSK group of companies. MLA received research and travel grant from the GSK group of companies. BS and JV are employees of the GSK group of companies and reports ownership of stock options/restricted shares from the GSK group of companies. J-YP is an employee of the GSK group of companies. JGL received a travel grant and a research grant from the GSK group of companies, a research grant from Pfizer, and honorary payments or travel grants for board memberships and scientific lectures from the GSK group of companies, Sanofi Pasteur MSD, AstraZeneca, Abbott and Pfizer.
Ethical standards
This study was approved by the appropriate ethics committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All parents gave their informed consent prior to their inclusion in the study.
Footnotes
Katsiaryna Holl and Mats Rosenlund contributed equally to this work and are both first authors.
References
- 1.Garces-Sanchez M, Diez-Domingo J, Alvarez de Labiada T, Planelles V, Graullera M, Baldo JM, et al. Epidemiology and burden of acute otitis media in Valencia (Spain) An Pediatr (Barc). 2004;60:125–132. doi: 10.1016/S1695-4033(04)78232-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan K, Sparks RA, Berryhill WE. Diagnosis and treatment of otitis media. Am Fam Physician. 2007;76:1650–1658. [PubMed] [Google Scholar]
- 3.Grevers G, First International Roundtable ENT Meeting Group Challenges in reducing the burden of otitis media disease: an ENT perspective on improving management and prospects for prevention. Int J Pediatr Otorhinolaryngol. 2010;74:572–577. doi: 10.1016/j.ijporl.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Boruk M, Lee P, Faynzilbert Y, Rosenfeld RM. Caregiver well-being and child quality of life. Otolaryngol Head Neck Surg. 2007;136:159–168. doi: 10.1016/j.otohns.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld RM, Goldsmith AJ, Tetlus L, Balzano A. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg. 1997;123:1049–1054. doi: 10.1001/archotol.1997.01900100019002. [DOI] [PubMed] [Google Scholar]
- 6.Dube E, De Wals P, Ouakki M. Quality of life of children and their caregivers during an AOM episode: development and use of a telephone questionnaire. Health Qual Life Outcomes. 2010;8:75. doi: 10.1186/1477-7525-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grindler DJ, Blank SJ, Schulz KA, Witsell DL, Lieu JE. Impact of otitis media severity on children’s quality of life. Otolaryngol Head Neck Surg. 2014;151:333–340. doi: 10.1177/0194599814525576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Witsell DL, Dolor RJ, Stinnett S, Hannley M. Quality of life of patients with otitis media and caregivers: a multicenter study. Laryngoscope. 2006;116:1798–1804. doi: 10.1097/01.mlg.0000231305.43180.f6. [DOI] [PubMed] [Google Scholar]
- 9.Kubba H, Swan IR, Gatehouse S. How appropriate is the OM6 as a discriminative instrument in children with otitis media? Arch Otolaryngol Head Neck Surg. 2004;130:705–709. doi: 10.1001/archotol.130.6.705. [DOI] [PubMed] [Google Scholar]
- 10.Brouwer CN, Rovers MM, Maille AR, Veenhoven RH, Grobbee DE, Sanders EA, et al. The impact of recurrent acute otitis media on the quality of life of children and their caregivers. Clin Otolaryngol. 2005;30:258–265. doi: 10.1111/j.1365-2273.2005.00995.x. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer CN, Maille AR, Rovers MM, Veenhoven RH, Grobbee DE, Sanders EA, et al. Effect of pneumococcal vaccination on quality of life in children with recurrent acute otitis media: a randomized, controlled trial. Pediatrics. 2005;115:273–279. doi: 10.1542/peds.2004-0778. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer CN, Schilder AG, van Stel HF, Rovers MM, Veenhoven RH, Grobbee DE, et al. Reliability and validity of functional health status and health-related quality of life questionnaires in children with recurrent acute otitis media. Qual Life Res. 2007;16:1357–1373. doi: 10.1007/s11136-007-9242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidemann CH, Godballe C, Kjeldsen AD, Johansen EC, Faber CE, Lauridsen HH. Otitis media and caregiver quality of life: psychometric properties of the modified danish version of the caregiver impact questionnaire. Otolaryngol Head Neck Surg. 2014;151:142–149. doi: 10.1177/0194599814528245. [DOI] [PubMed] [Google Scholar]
- 14.Heidemann CH, Lauridsen HH, Kjeldsen AD, Faber CE, Johansen EC, Godballe C. Caregiver quality of life and daily functioning in relation to ventilating tube treatment. Otolaryngol Head Neck Surg. 2014;151:341–347. doi: 10.1177/0194599814529911. [DOI] [PubMed] [Google Scholar]
- 15.Barber C, Ille S, Vergison A, Coates H. Acute otitis media in young children—what do parents say? Int J Pediatr Otorhinolaryngol. 2014;78:300–306. doi: 10.1016/j.ijporl.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Blank SJ, Grindler DJ, Schulz KA, Witsell DL, Lieu JE. Caregiver Quality of Life Is Related to Severity of Otitis Media in Children. Otolaryngol Head Neck Surg. 2014;151:348–353. doi: 10.1177/0194599814531912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman AA, Meesters CM, Anteunis LJ, Chenault MN, Haggard MP. Psychometric evaluation of the OM8-30 questionnaire in Dutch children with otitis media. Eur Arch Otorhinolaryngol. 2008;265:1047–1056. doi: 10.1007/s00405-008-0591-2. [DOI] [PubMed] [Google Scholar]
- 18.Berdeaux G, Hervie C, Smajda C, Marquis P. Rhinitis Survey Group. Parental quality of life and recurrent ENT infections in their children: development of a questionnaire. Qual Life Res. 1998;7:501–512. doi: 10.1023/A:1008874324258. [DOI] [PubMed] [Google Scholar]
- 19.Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 20.Liese JG, Silfverdal SA, Giaquinto C, Carmona A, Larcombe JH, Garcia-Sicilia J, et al. Incidence and clinical presentation of acute otitis media in children aged <6 years in European medical practices. Epidemiol Infect. 2014;142:1778–1788. doi: 10.1017/S0950268813002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman NR, McCormick DP, Pittman C, Chonmaitree T, Teichgraeber DC, Uchida T, et al. Development of a practical tool for assessing the severity of acute otitis media. Pediatr Infect Dis J. 2006;25:101–107. doi: 10.1097/01.inf.0000199290.73333.89. [DOI] [PubMed] [Google Scholar]
- 22.Bellussi L, Mandala M, Passali FM, Passali GC, Lauriello M, Passali D. Quality of life and psycho-social development in children with otitis media with effusion. Acta Otorhinolaryngol Ital. 2005;25:359–364. [PMC free article] [PubMed] [Google Scholar]
- 23.Kubba H, Swan IR, Gatehouse S. Measuring quality of life in preschool children with sore throats and otitis media using the TAPQOL questionnaire. Otolaryngol Head Neck Surg. 2005;132:647–652. doi: 10.1016/j.otohns.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Chow Y, Wabnitz DA, Ling J. Quality of life outcomes after ventilating tube insertion for otitis media in an Australian population. Int J Pediatr Otorhinolaryngol. 2007;71:1543–1547. doi: 10.1016/j.ijporl.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Witsell DL, Stewart MG, Monsell EM, Hadley JA, Terrell JE, Yueh B, et al. The Cooperative Outcomes Group for ENT: a multicenter prospective cohort study on the outcomes of tympanostomy tubes for children with otitis media. Otolaryngol Head Neck Surg. 2005;132:180–188. doi: 10.1016/j.otohns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Timmerman AA, Anteunis LJ, Meesters CM. Response-shift bias and parent-reported quality of life in children with otitis media. Arch Otolaryngol Head Neck Surg. 2003;129:987–991. doi: 10.1001/archotol.129.9.987. [DOI] [PubMed] [Google Scholar]
- 27.Richards M, Giannoni C. Quality-of-life outcomes after surgical intervention for otitis media. Arch Otolaryngol Head Neck Surg. 2002;128:776–782. doi: 10.1001/archotol.128.7.776. [DOI] [PubMed] [Google Scholar]
- 28.Rovers MM, Krabbe PF, Straatman H, Ingels K, van der Wilt GJ, Zielhuis GA. Randomised controlled trial of the effect of ventilation tubes (grommets) on quality of life at age 1–2 years. Arch Dis Child. 2001;84:45–49. doi: 10.1136/adc.84.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld RM, Bhaya MH, Bower CM, Brookhouser PE, Casselbrant ML, Chan KH, et al. Impact of tympanostomy tubes on child quality of life. Arch Otolaryngol Head Neck Surg. 2000;126:585–592. doi: 10.1001/archotol.126.5.585. [DOI] [PubMed] [Google Scholar]
- 30.Baumann I, Gerendas B, Plinkert PK, Praetorius M. General and disease-specific quality of life in patients with chronic suppurative otitis media—a prospective study. Health Qual Life Outcomes. 2011;9:48. doi: 10.1186/1477-7525-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung KH, Cho YS, Hong SH, Chung WH, Lee GJ, Hong SD. Quality-of-life assessment after primary and revision ear surgery using the chronic ear survey. Arch Otolaryngol Head Neck Surg. 2010;136:358–365. doi: 10.1001/archoto.2010.24. [DOI] [PubMed] [Google Scholar]
- 32.Nadol JB, Jr, Staecker H, Gliklich RE. Outcomes assessment for chronic otitis media: the Chronic Ear Survey. Laryngoscope. 2000;110:32–35. doi: 10.1097/00005537-200003002-00009. [DOI] [PubMed] [Google Scholar]
- 33.Wang PC, Nadol JB, Jr, Merchant S, Austin E, Gliklich RE. Validation of outcomes survey for adults with chronic suppurative otitis media. Ann Otol Rhinol Laryngol. 2000;109:249–254. doi: 10.1177/000348940010900302. [DOI] [PubMed] [Google Scholar]
- 34.Choi SY, Cho YS, Lee NJ, Lee J, Chung WH, Hong SH. Factors associated with quality of life after ear surgery in patients with chronic otitis media. Arch Otolaryngol Head Neck Surg. 2012;138:840–845. doi: 10.1001/archoto.2012.1800. [DOI] [PubMed] [Google Scholar]
- 35.Mui S, Rasgon BM, Hilsinger RL, Jr, Lewis B, Lactao G. Tympanostomy tubes for otitis media: quality-of-life improvement for children and parents. Ear Nose Throat J. 2005;84(418):20–22. [PubMed] [Google Scholar]
- 36.Usonis V, Jackowska T, Petraitiene S, Sapala A, Neculau A, Devadiga R, et al. Incidence of acute otitis media in children below 6 years of age in medical practices in five East European countries [abstract]. In: European Society for Paediatric Infectious Diseases. 31st Annual Meeting, Milan, Italy, May 28 to June 1, 2013. http://w3.kenes-group.com/apps/espid2013/abstracts/pdf/594.pdf. Accessed 26 Aug 2013.
- 37.Diez-Domingo J, Patrzalek M, Cantarutti L, Arnould B, Meunier J, Soriano-Gabarro M, et al. The impact of childhood acute rotavirus gastroenteritis on the parents’ quality of life: prospective observational study in European primary care medical practices. BMC Pediatr. 2012;12:58. doi: 10.1186/1471-2431-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips JS, Haggard M, Yung M. A new health-related quality of life measure for active chronic otitis media (COMQ-12): development and initial validation. Otol Neurotol. 2014;35:454–458. doi: 10.1097/MAO.0000000000000205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1: The procedure used to adapt the ENT questionnaire to AOM (PDF 355 kb)
Online Resource 2: The parental QoL questionnaire used in the study. (PDF 181 kb)