Abstract
Although a complete thoracic spinal cord section in various mammals induces paralysis of voluntary movements, the spinal lumbosacral circuitry below the lesion retains its ability to generate hindlimb locomotion. This important capacity may contribute to the overall locomotor recovery after partial spinal cord injury (SCI). In rats, it is usually triggered by pharmacological and/or electrical stimulation of the cord while a robot sustains the animals in an upright posture. In the present study we daily trained a group of adult spinal (T7) rats to walk with the hindlimbs for 10 wk (10 min/day for 5 days/wk), using only perineal stimulation. Kinematic analysis and terminal electromyographic recordings revealed a strong effect of training on the reexpression of hindlimb locomotion. Indeed, trained animals gradually improved their locomotion while untrained animals worsened throughout the post-SCI period. Kinematic parameters such as averaged and instant swing phase velocity, step cycle variability, foot drag duration, off period duration, and relationship between the swing features returned to normal values only in trained animals. The present results clearly demonstrate that treadmill training alone, in a normal horizontal posture, elicited by noninvasive perineal stimulation is sufficient to induce a persistent hindlimb locomotor recovery without the need for more complex strategies. This provides a baseline level that should be clearly surpassed if additional locomotor-enabling procedures are added. Moreover, it has a clinical value since intrinsic spinal reorganization induced by training should contribute to improve locomotor recovery together with afferent feedback and supraspinal modifications in patients with incomplete SCI.
Keywords: kinematics, locomotion, neuroplasticity, rat, spinal cord injury
in mammals, complete spinal cord injury (SCI) induces permanent deficits of voluntary movements below the lesion. When such SCI is located at the thoracic level, the lumbosacral neuronal network dedicated to hindlimb locomotion, termed the central pattern generator (CPG), becomes isolated from supraspinal structures, leading to permanent paralysis of the lower body parts (Rossignol et al. 2009). Despite this motor impairment, the CPG retains its intrinsic property of generating alternate rhythmic activity as shown by electrophysiological and kinematic recordings in neonatal rats and adult cats (for review, see Rossignol et al. 2014; Rossignol and Frigon 2011).
The challenge of functionally triggering the spinal circuitry to reexpress hindlimb locomotion after complete SCI in adult animals has been extensively confronted with sensorimotor rehabilitation (de Leon et al. 2002; Ichiyama et al. 2011; Martinez et al. 2012; Rossignol 1996; Tillakaratne et al. 2010; Timoszyk et al. 2002), administration of serotonin agonists such as quipazine or 8-OH-DPAT (Antri et al. 2005; Feraboli-Lohnherr et al. 1999; Fong et al. 2005; Orsal et al. 2002), graft of embryonic raphe cells or fetal brain stem 5-HT neurons (Gimenez y Ribotta et al. 2000; Slawinska et al. 2013), or combined strategies including pharmacology and spinal electrostimulation (Courtine et al. 2009; Ichiyama et al. 2008; Musienko et al. 2011; Rossignol et al. 2001). Two to three weeks after a complete thoracic spinalization, cats trained on a treadmill with the help of perineal stimulation can recover the locomotor capability of the hindlimbs and can readily adapt to the belt velocity (Rossignol et al. 2006). Some previous studies tried to replicate these results in complete spinal rats, with no success (Courtine et al. 2009; Orsal et al. 2002; Slawinska et al. 2012, 2013). All these studies show that serotonergic strategies, spinal electrostimulation, or postural changes are needed to revive the lumbosacral locomotor network in complete spinal rats.
On the other hand, we have previously shown with a clip compression model resulting in very large compressive lesions that damage the greatest part of the thoracic spinal cord, making them almost completely spinal, that rats can develop an impressive capability to walk adequately with the hindlimbs in quadrupedal posture without any additional treatment (Alluin et al. 2011). These results suggest that the reemergence of hindlimb locomotion probably results mainly from the reexpression of the lumbosacral spinal CPG (Rossignol et al. 2009). Although a positive effect of treadmill training after SCI in humans and rodents has been demonstrated in combination with other strategies (Courtine et al. 2009; Harkema et al. 2011; Van den Brand et al. 2012), the intrinsic potential of the spinal circuitry causing the recovery of locomotion in adult complete spinal rats remains poorly known.
In the present study, we induced daily treadmill training in natural horizontal posture using sustained perineal stimulation to trigger the spinal locomotor circuitry and reexpress locomotion in complete spinalized adult rats without any additional treatment. This work was deemed important since it provides a behavioral baseline that should be surpassed when claiming the need for additional enabling procedures to restore spinal locomotion.
MATERIALS AND METHODS
Animal care.
Twenty-one adult female Wistar rats (250–275 g) from Charles River Laboratory were involved in the present study. Animals were housed in standard plastic cages at 22°C before SCI and 26°C after SCI in a 12:12-h light-dark photoperiod. Food (Agribrands Purina) and drinking water were available ad libitum. Hardwood sawdust bedding (PWI brand) was used before SCI and was then replaced by soft paper bedding (Diamond Soft Bedding no. 7089; Harlan Teklad) after SCI to prevent skin lesions. Animals were examined twice a day and evaluated by a veterinarian when necessary. After SCI, the bladder was expressed twice a day until the recovery of spontaneous bladder function, generally occurring between 7 and 14 days after injury. All animal procedures were approved by the University of Montreal Research Ethics Committee and were conducted according to the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care).
Experimental framework.
After a 1-wk period of quarantine in the animal facility, rats were accustomed to walk consistently with a quadrupedal gait on the treadmill at different speeds for 30 min daily, 5 days a week, over 3 wk (see Alluin et al. 2011 for details). All rats were then video recorded for an additional 3-wk period to obtain baseline kinematic values. A complete transection of the thoracic spinal cord was next performed, and rats were assigned to two groups: 1) rats daily trained to walk on the treadmill during the whole recovery period (Trained group; n = 13) and 2) rats that received no training (Untrained group; n = 8). Treadmill locomotion elicited by perineal stimulation in trained and untrained rats was video recorded once a week during the following 10-wk period for kinematic analysis. It is thus important to remember that all rats were documented with videos exactly in the same condition (perineal pinching) so that the difference between the behavior of trained and untrained rats must be due to a training effect. At the end of the experiment, electromyography (EMG) of selected hindlimb muscles was recorded. Finally, animals were perfused with a fixative and the spinal cord was harvested for histological evaluation of the lesions. Tibialis anterior (TA) and gastrocnemius (G) muscles were removed and weighed.
It is important to note that double-blind assessments are not possible in such chronic studies because, with time and daily handling, the experimenters learn to recognize the animals according to their individual specificities (shaving for kinematic recordings, hair color tone, personality, weight, size, body marks, etc.).
Surgical procedures.
All surgeries were performed in aseptic conditions and under general gas anesthesia consisting of an O2-isoflurane (1–4%) mixture given through a mask integrated in a surgical stereotaxic frame associated with a heating pad set to 37°C. Immediately after surgery, rats were placed under a heating lamp until they recovered consciousness. Animals were given systematic postoperative analgesia (40 μg/kg Temgesic; Schering-Plough) and saline (5 ml) subcutaneously to prevent pain and dehydration. They also received antibiotic in drinking water (Clavamox drops; Pfizer Animal Health) from 3 days before to 1 week after SCI.
Prior to the SCI, the skin of the middle back was shaved and disinfected with a 1:1 mixture of 70% alcohol and povidone-iodine (Betadine; Purdue Pharma, Stamford, CT). A skin incision was made above the spinal processes between T4 and T10 (≈ 3 cm). The superficial muscles from both sides were cut along the spine and retracted from each side of the surgical area. A laminectomy was then performed at T7, and the spinal cord was completely transected with microscissors (Vannas Spring Scissors, 2.5-mm blades; Fine Science Tools). After separation of the rostral and caudal parts of the spinal cord was ensured, a piece of sterile absorbable gelatin sponge (Gelfoam; Pfizer) was placed between the two spinal cord stumps and on top of the lesion between T6 and T8. Finally, muscles were sutured (Prolene 3-0; Ethicon) and skin was closed with stainless steel wound clips (9-mm AutoClips; MikRon Precision), which were removed 1 wk later.
Treadmill training protocol after SCI.
Spinal rats were trained to walk with the hindlimbs on the motorized treadmill belt from day 1 to week 10 after SCI, while the forelimbs were actively maintained on a platform 5 mm above the belt to maintain a horizontal posture. This training procedure was performed 5 days a week and consisted of one 10-min walking session daily on the treadmill at increasing speeds ranging from 14 to 26 m/min depending on the locomotor capability of each rat. Perineal stimulation, consisting of manual pinching of the perineum area just below the base of the tail, was used to facilitate the hindlimb locomotion throughout the training sessions. During training, the belt speed was initially set at 14 m/min and the trunk of the animals was manually supported to reduce lateral body movements. Early after SCI, all rats were incapable of producing hindlimb stepping even with perineal stimulation. This evoked, in most cases, only erratic bilateral flexion/extension without paw placement on the treadmill belt. As soon as rats were capable of producing a more regular hindlimb locomotor pattern, the belt speed was incremented in steps of 2 m/min every 1.5 min until the maximum speed that could be followed by the rat was reached (i.e., in the range defined above). Then, depending on the fatigue of the animal, the treadmill speed was progressively decreased up to the end of the training session if necessary.
Kinematic recordings.
Kinematic baseline data were recorded at 14, 20, and 26 m/min for each rat to obtain control values before the spinal lesion. The locomotor performance on treadmill was then recorded weekly for 10 wk after SCI. The kinematic recording protocol was described previously in detail (Alluin et al. 2011). Briefly, before each recording session, the left hindquarter of the rats was shaved and three black dots were set on the skin at the bony landmarks of the ilium, great trochanter, and lateral malleolus with a felt pen while two light-reflecting markers were glued on the skin of the metatarso-phalanx (MTP) and the tip of the third toe. A left side view of the rat walking on the treadmill with the hindlimbs was captured with a high-frequency video camera (120 Hz). The kinematic data were generated from the [x, y] coordinates of each marker and from the paw contact/lift events, while the knee joint position was extrapolated by triangulation in subsequent computer analyses.
Perineal stimulation.
For training the trained group and for recording in all groups, the manual stimulation consisted in a tonic pinching of the skin on the perineum area (right under the base of the tail) between thumb and index finger while performing rubbing movements at a frequency between 1 and 2 Hz (for illustration, see finger movements of the experimenter in Supplemental Movie S1).1 Such stimulation was used in all rats from both groups for every locomotor sequence recorded for kinematic evaluation throughout the post-SCI period since, whether trained or untrained, rats performed only occasional hindlimb movements on the treadmill without stimulation. Although the stimulation intensity was not quantified in the present study, the strength applied on the skin by the experimenters was just enough to trigger locomotion depending on rat responsiveness. Specifically, the experimenters started the recording session by applying a minimal strength on the perineal skin and progressively increased the strength of pinching until it was sufficient to induce locomotion in the rat. Once hindlimb locomotor movements started, the experimenter kept the same intensity until the end of recording. For instance, at the end of the experimental series, some rats in the trained group started to walk on the treadmill with minimal intensity while some untrained rats were unresponsive even at a much higher pinch strength, which was kept, of course, at a level below which skin bruises could be produced, a counterproductive situation in daily training rats.
Individual variability assessment.
The method of individual variability assessment is detailed in Alluin et al. (2011). Briefly, the coefficient of variation (CV) of every Cartesian kinematic parameter and the circular dispersion of the circular data were used to assess the intrinsic variability of each rat during locomotion. Since the analysis of locomotion of a given rat was based on consecutive sequences of several step cycles, the kinematic data from these several steps were averages and their respective standard deviations (SDs) were calculated. Thereby, in the present study, the CV of a given parameter is calculated from the individual SD expressed in percentage of the individual mean of this parameter and then averaged by group. The group SE associated to the CV group mean was then calculated.
Homologous hindlimb coupling.
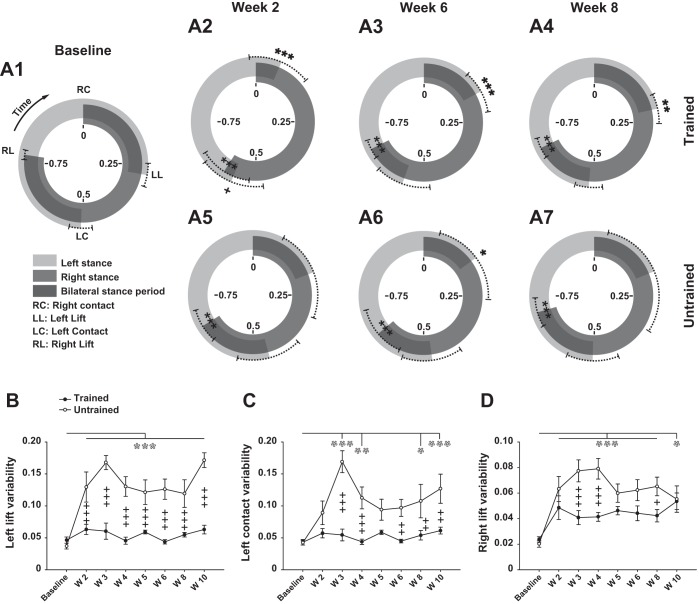
The averaged homologous hindlimb coupling is calculated (Alluin et al. 2011) and illustrated on circular charts on which the temporal relationship of the left lift, left contact, and right lift are expressed relative to the right contact (i.e., the right step cycle as reference) on the periphery of circles. The circular distance between contact and lift from the same side represents the proportion of the stance phase of this side in the reference step cycle, while the distance between lift and contact represents the proportion of the swing phases. For instance, in intact rats walking on the treadmill, the sequence of events (i.e., foot contact and lift) from one hindlimb in a given step cycle is out of phase (i.e., opposite position on the circle) with the sequence from the contralateral hindlimb. Consequently, four symmetrical locomotor periods lasting about one quarter of the whole step cycle duration each can be highlighted: 1) a first bilateral stance period from the foot contact of the reference side to the foot lift of the contralateral side, 2) the swing phase of the contralateral side from the lift to the contact of this side while the other side keeps the stance phase going, 3) the second bilateral stance period from the contact of the contralateral side to the lift of the reference side, and 4) the swing phase of the reference side from the lift to the contact of this side while the other side keeps the stance phase going (see Fig. 9A1).
Fig. 9.
Evolution of hindlimb coordination after SCI. A1–A7: circular representation of temporal relationship of hindlimb locomotor events (right contact, left lift, left contact, and right lift) before (baseline) and at weeks 2, 6, and 8 after SCI in trained (top) and untrained (bottom) groups during locomotion. Given that continuous locomotion is a repeated sequence of events (left/right foot contact/lift), the foot contact is both the beginning of a step cycle and the end of the previous one. Consequently, in this figure the relative duration of the right step cycle (reference) is represented between 0 and 1, which are at the same position on the graph (top of circles). Data are synchronized on the right contact (represented by 0 at top of each circle), and the sequence of events should be read in the clockwise direction. Dark gray circular band between RC and RL represents the averaged normalized right stance phase, and empty space between RL and RC represents the normalized average of the concomitant swing phase, both relative to the right step cycle duration. Light gray circular band between LC and LL and empty space between LL and LC represent the averaged normalized left stance and swing phases relative to the right step cycle duration, respectively. Limits of the dotted lines either side of the averaged position of each event represent the circular dispersion (equivalent to SD in the circular mathematical model). B–D: evolution of individual left lift, left contact, and right lift dispersion (step-to-step individual variability), respectively, averaged by group before and weekly throughout the recovery period. Data were recorded at 14 m/min and are expressed as circular mean ± circular dispersion in A1–A7 and as standard arithmetic mean ± SE in B–D. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, compared with group average baseline (black and white asterisks correspond to trained and untrained groups, respectively); +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after SCI.
After SCI, the interlimb coupling can change and the new temporal relationship between limbs is reflected in the new proportions and positions of the stance and swing phases around the circles.
Electromyographic recording.
Prior to death, the tips of isolated monofilament electrodes (magnet wire, 0.22 mm; Cooner Wire) were stripped and implanted (2 per muscle) in gastrocnemius lateralis (GL) and TA muscles through the skin of both hindlimbs to record EMG signals during treadmill locomotion in seven trained and six untrained rats. Signals from bipolar electrodes were differentially amplified, filtered (100 Hz–3 kHz band pass) and recorded on a computer through custom-made software for further analysis.
Spinal cord processing and analysis.
At the end of experiments, rats were deeply anesthetized with pentobarbital sodium (80 mg/kg ip) and then were perfused transcardially with 60 ml of cold phosphate-buffered saline (PBS, 0.1 M at 4°C) followed by 180 ml of 4% paraformaldehyde (PFA) solution (in 0.1 M PBS, pH 7.4). After perfusion, a 2-cm length of the spinal cord centered at the epicenter of the injury was dissected and postfixed in the perfusing solution with 10% sucrose overnight at 4°C. The cords were then cryoprotected in PBS with 20% sucrose for 48 h at 4°C. The cords were embedded in mounting medium (HistoPrep; Fisher Scientific) on dry ice. Cryostat sections (25 μm) were cut transversely and stained with cresyl violet to confirm the completeness of the spinal section.
Muscle mass assessment.
After perfusion, the left and right TA and G muscles were also dissected, weighed, and then normalized to the total body weight of the animal in order to correct for interindividual variability.
Statistical analysis.
Individual kinematic data were averaged from the number of locomotor cycles that the rats were able to execute consecutively in a range from 6 to 20. Two-way repeated-measures ANOVA followed by pairwise multiple comparison (Holm-Sidak post hoc test) when ANOVA was significant were used to compare the standard Cartesian data between the different periods and between groups (SigmaPlot; SYSTAT Software, San Jose, CA). To compare circular data (i.e., coordination measurement), the multisample Watson-Williams F-test was used (Oriana software; Kovach Computing Services). Averaged Cartesian data are reported as arithmetic means ± SE and circular data as circular mean ± circular dispersion (equivalent of SD in the circular model). The significance threshold was set to P ≤ 0.05.
RESULTS
Overview of locomotor recovery.
In the first week after the complete SCI, although strong perineal stimulation elicited a few erratic flexion and extension cycles of small amplitude, flaccid paralysis of the hindlimbs was observed in all animals in their cage. Perineal stimulation was always needed, throughout the 10-week post-SCI period in the trained group, to elicit locomotor movements during training and recording sessions for all rats. Without such stimulation the rats performed only sporadic hindlimb flexion movements. As soon as week 2 after SCI, ∼70% of rats in both groups were capable of performing alternating locomotor movements on the treadmill at 14 m/min (Fig. 1A). At the same period, ∼50% and ∼35% of trained rats could follow the treadmill belt with plantar placement of the paw at 20 and 26 m/min, respectively, while none of the untrained rats was capable of walking on the belt at these velocities, not even with dorsal foot placement (Fig. 1, B and C). With time, the proportion of trained animals capable of walking on the treadmill at the three velocities progressively increased, to reach, at week 10 after SCI, 100% at 14 m/min (Fig. 1A) and 80% at 20 m/min and 26 m/min (Fig. 1, B and C). On the other hand, the number of untrained rats capable of following the treadmill belt decreased drastically with time, to reach ∼10% at week 8 after SCI at 14 m/min and even 0% at week 10 at all speeds (Fig. 1). In fact, training exerted a strong positive effect on locomotion from week 3 and stabilized the locomotor performance as of week 4, while the locomotion of untrained rats deteriorated from week 3 until the end of the experiment. From week 7 after SCI, the great majority of untrained animals performed only bilateral alternated flexion and extension with no placement of the paw on the belt (Supplemental Movie S1).
Fig. 1.
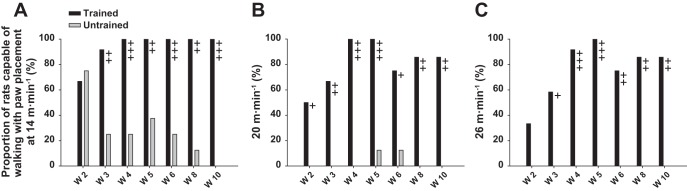
General walking capacity throughout the recovery period [weeks (W) 2–10]. A: proportion of rats in trained (n = 13) and untrained (n = 8) groups capable of walking with paw placements (plantar or dorsal) on treadmill at 14 m/min, expressed as % of total number of animals in each group. B: same as A at 20 m/min. C: same as A and B at 26 m/min. +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after spinal cord injury (SCI).
Although the intensity of perineal stimulation was not measured in the present study, it was very clear to the experimenters that the strength of pinching needed to elicit locomotion was drastically reduced with time in trained rats, from maximal intensity immediately after SCI to minimal in some trained rats at the end of experiment. However, the trend was reversed in untrained animals. The strength of pinching increased with time, so that in several animals of this group only, the maximum strength applied did not elicit any organized locomotor movement at the end of the experimental series (see materials and methods). In addition, in trained rats no limitation of the consecutive number of step cycles was noted during training session from week 3 to the end of the experiment (i.e., they walked throughout the training session). In contrast, the average number of consecutive steps that untrained rats were capable of producing at the end of the experiment during recording sessions was clearly decreased (14.1 ± 2.2 step cycles at week 10) compared with trained animals (P < 0.05), even with strong perineal stimulation (see the progressive exhaustion of locomotor movements despite the perineal stimulation in typical untrained rat at 14 m/min in week 7 in Supplemental Movie S1). Finally, no improvement of spontaneous treadmill locomotion (i.e., without perineal stimulation) was observed in animals from either group throughout the experiment.
Treadmill training improves spatial characteristics of step cycle.
Spatial characteristics of locomotion such as step cycle length, foot contact and lift position relative to the hip, and maximum foot height during the swing phase were assessed and are illustrated in Fig. 2.
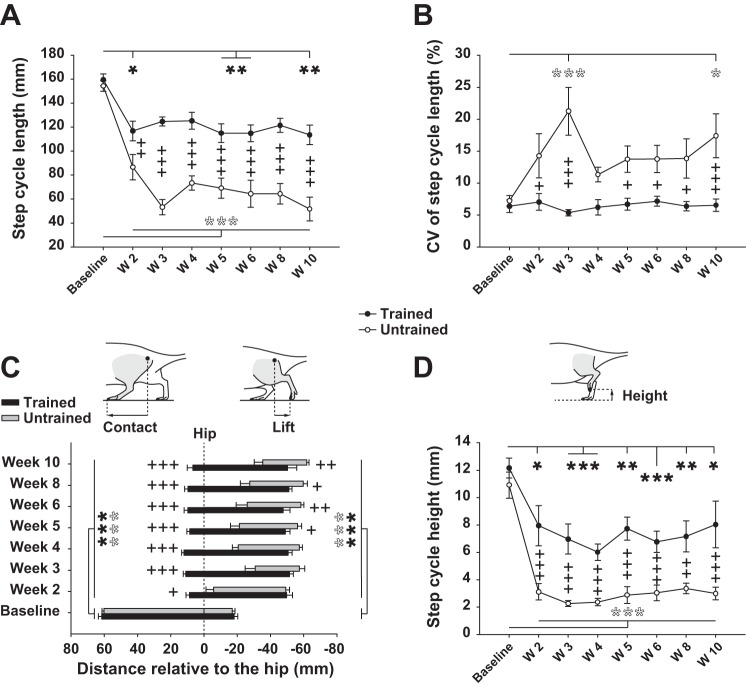
Fig. 2.
Recovery of step cycle length, position, and height after SCI. A: length of step cycle (from foot contact on treadmill belt to the next one) before (baseline) and weekly after SCI in trained and untrained rats. B: individual coefficient of variation (CV; individual step-to-step variability averaged by groups and expressed in %) of data depicted in A. C: position of the foot relative to the vertical projection of the great trochanter (vertical dashed line corresponding to 0) for the time points specified above. Left end of each bar represents the contact position of the left foot with the treadmill belt (onset of stance phase); right end represents the foot lift position (end of the stance phase) in trained and untrained rats. Sketches representing the foot contact and lift position measurement are given above the graph. D: maximum height of the foot during the swing phase of locomotion in trained and untrained rats for the time points specified above. Sketch depicting the foot height measurement is given above the graph. Data were recorded at 14 m/min and are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, compared with group average baseline (black and white asterisks correspond to trained and untrained groups, respectively); +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after SCI.
After SCI the step cycle length decreased drastically throughout the recovery period in untrained rats, while treadmill training clearly restricted this decrease (Fig. 2A). In addition, the recovery of the step cycle length remained greatly improved in trained compared with untrained rats until the end of the experiment (Fig. 2A). The step-to-step variability of the step length (represented by the CV) is an indicator of the rats' capability to maintain a constant step cycle length throughout the locomotor session. In normal rats, the CV of the step cycle length is ∼7% and can be attributed to intrinsic biological and experimental noises. After complete SCI, trained rats could keep the same degree of step length variability as in the normal state all along the recovery period while the CV of untrained rats increased drastically early after SCI (Fig. 2B, week 3) and then, after some fluctuations, remained high until the end of the experiment compared with trained animals (Fig. 2B, week 10).
The foot contact and lift position relative to the hip during walking were assessed weekly in all rats throughout the experiment. The position of the foot relative to the rest of the body during the stance phase gives information about the efficiency of the forward propulsion. As indicated above, in spinalized rats the hindlimbs' locomotor movements shifted backward of the body as demonstrated by the displacement of the relative contact and lift position compared with the baseline in both groups throughout the recovery period (Fig. 2C). In trained animals this shift remained constant until the end of the experiment and its impact on locomotion was limited by the preservation of the foot contact in front of the hip vertical projection (Fig. 2C). In untrained rats, however, the position of the foot contact was highly degraded from about −15 mm compared with trained rats at week 2 after SCI to about −50 mm at week 10 (Fig. 2C). Although the foot lift position was similar between trained and untrained animals at week 2 after SCI, it worsened in untrained rats from week 3 to be significantly shifted backward at week 5 and finally reached −15 mm compared with trained animals at week 10 after SCI (Fig. 2C). Consequently, the position of the ground contact period of untrained rats was shifted backward and shorter and the degradation increased with time after SCI compared with trained rats.
We also measured the vertical amplitude of the foot trajectory during locomotion (Fig. 2D). This parameter decreased in both groups after SCI. Although the decrease was much greater and stable compared with the baseline in untrained animals throughout the postinjury period, it remained present in trained animals, with a trend for improvement by the end of the recovery period (Fig. 2D). Finally, the recovery of the vertical amplitude performance was clearly greater in trained animals all along the experiment (average of 58.5% of the baseline value) compared with untrained animals (average of 22.5% of the baseline value; Fig. 2D).
Positive effect of treadmill training on recovery of angular excursion and amplitude of hindlimb joints.
To assess the dynamic angular properties of rats' locomotion we measured the angular excursion, minimum angle, maximum angle, and amplitude of hip, knee, ankle, and MTP throughout the post-SCI period as depicted in Fig. 3 and Fig. 4.
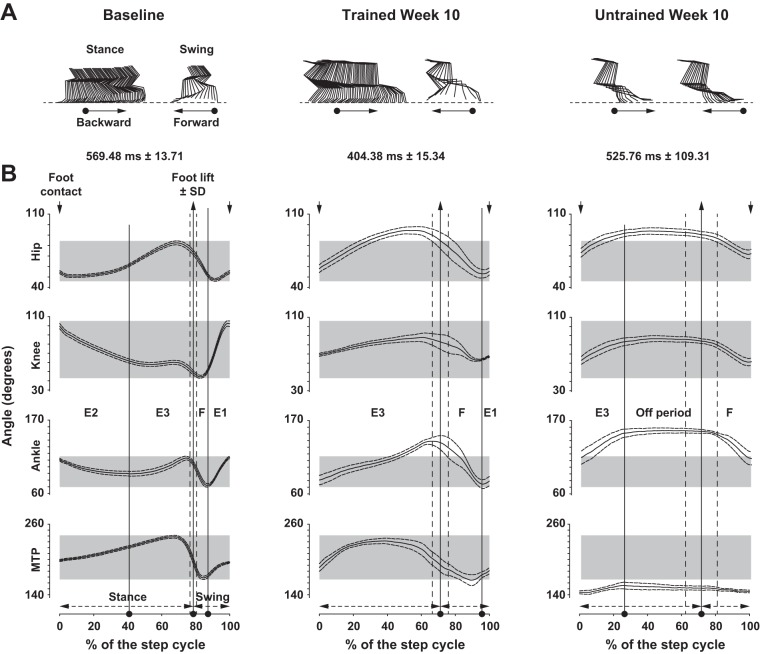
Fig. 3.
Recovery of angular excursions. A: stick figures of stance and swing phases from representative rats before (left) and at week 10 after SCI in trained (center) and untrained (right) groups. The direction of movement is given (arrows). B: angular excursions averaged by groups of hip, knee, ankle, and metatarso-phalanx (MTP) before (left) and at week 10 in trained (center) and untrained (right) groups. Step cycle duration (x-axis) is normalized in % of the step cycle beginning and finishing with a foot contact (down arrows at top). Average ± SE duration of the step cycle is depicted above each panel. The position of foot lift ± SD (up arrows at top), the proportion of stance and swing phases (bottom), and the Philippson's subphases are also given. The “off period” (right) corresponds to the absence of active hindlimb movement. Data were recorded at 14 m/min and are expressed as means ± SE.
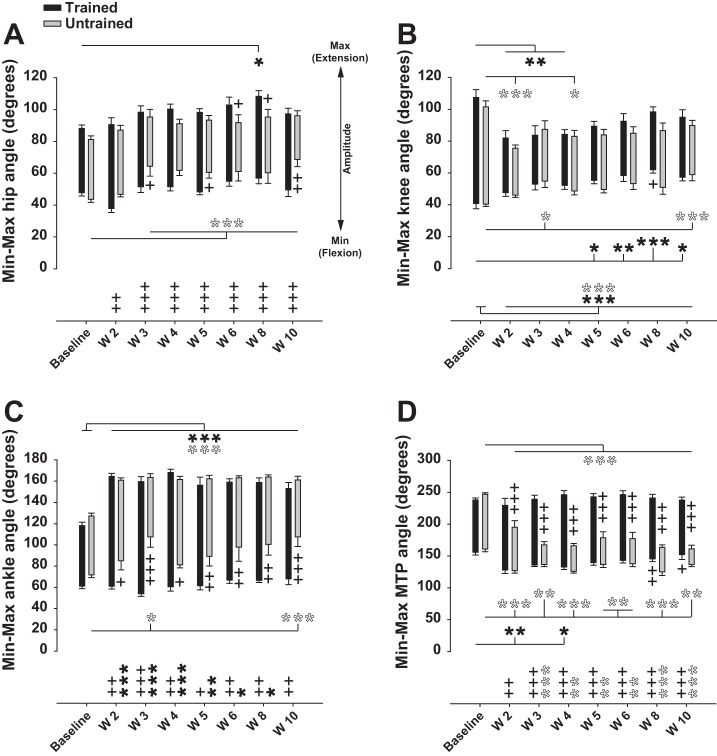
Fig. 4.
Recovery of hindlimb angle joint amplitude after SCI. A: averaged minimum, maximum, and amplitude of hip joint angle during locomotion on treadmill before and weekly after SCI in trained and untrained rats. B: same as A for knee joint angle. C: same as A and B for ankle joint angle. D: same as A–C for MTP joint angle. Black and white asterisks below and above the sticks represent the statistical comparison of minimum and maximum angle values with baseline in trained and untrained groups, respectively. Plus signs below and above the sticks represent the statistical comparison between both groups for minimum and maximum angle values, respectively, at each time point. Symbols at bottom graphs relate to the angle amplitude (max − min illustrated by length of sticks) and follow the same rules as described above. Data were recorded at 14 m/min and are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, compared with group average baseline; +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after SCI.
At the end of the experiment (week 10) the global posture of the hindquarters during locomotion had shifted in all animals. The locomotor movements shifted backward relative to the hip because of pelvic anteversion (i.e., posterior rotation; compare the first stick at the top of the stick illustrations representing the orientation of the hip in Fig. 3A). These changes in the biomechanical characteristics of the locomotor apparatus changed the hindlimb angular properties during the step cycle in trained animals toward foot contact occurring more caudally in front of the hip and foot lift occurring further back the hip (compare Fig. 3A, left and center stick illustrations). Consequently, the limb extension part of the swing phase (defined as E1 in Philippson 1905) was clearly reduced (compare Fig. 3B, left and center) and its extension counterpart at the beginning of the stance phase (defined as E2, corresponding to weight acceptance) was so reduced that it was not possible to identify. Aside from these changes, the angular coordination (i.e., coordination of the flexion and extension phases between joints) was recovered in trained animals (compare the synchronicity of flexions and extensions between joints in E3, F, and E1 subphases in Fig. 3B, left and center) as well as the plantar placement of the foot during the stance phase. The whole step cycle amplitude also returned close to normal values in trained animals (compare Fig. 3A, left and center).
In untrained animals, the locomotor movements occurred far behind the hip at the end of the recovery period and their amplitude was drastically reduced compared with trained rats (Fig. 3A, right vs. center). In addition, the plantar placement was lost (Fig. 3A, right), an “off period” with no active movement emerged at the stance-swing transition (Fig. 3B, right), and the E1 and E2 subphases were completely gone (Fig. 3B, right). This difference of the whole step cycle amplitude between trained and untrained rats is clearly demonstrated by the angle amplitudes of hip, ankle, and MTP joints. The amplitudes of these joints were much higher in the trained group at week 10 after SCI (Fig. 4, A, C, and D). These greater amplitudes are explained by the obvious higher flexion capacity of hip and ankle joints (Fig. 4, A and C) together with the higher extension capacity of the MTP joint (Fig. 4D). Interestingly, the amplitude of the knee joint remained reduced in similar proportions in trained and untrained rats week 10 after SCI (Fig. 4B), but the increased ankle flexion capacity in the trained group at the same time point and consequently the increased ankle amplitude (Fig. 4C) demonstrate that trained animals compensated the knee deficit by increasing ankle amplitude.
Taken together, these results show that our training protocol greatly improved the overall kinematics of the angular joint excursions toward a return of coordination and amplitude of the angles.
Treadmill training improves temporal characteristics of locomotion.
Although the swing phase length remained longer in the trained group throughout the recovery period (swing length is the exact half of the step cycle length depicted in Fig. 2A), the swing phase duration was similar in both groups except weeks 5 and 6 (Fig. 5A) and reached normal values (∼120 ms) at the end of the experiment. The proportion of the two constitutive subphases, F (∼37% of the normal swing phase duration) and E1 (∼63%), were increased and decreased, respectively, compared with baseline throughout the recovery period in both groups (Fig. 5, B and C). However, the treadmill training improved the recovery of F and E1 subphases' proportion all along the post-SCI period to reach ∼70% and ∼30%, respectively, at week 10 after SCI in the trained group while the untrained rats, stagnating ∼95% and ∼5%, respectively, failed to show any change (Fig. 5, B and C).
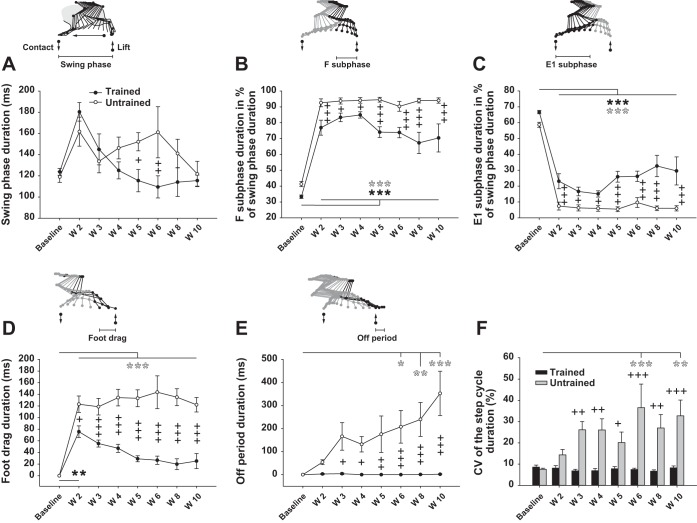
Fig. 5.
Effect of treadmill training on temporal characteristics of locomotion. A: duration of the swing phase of locomotion averaged by group in trained and untrained rats before (baseline) and weekly throughout the recovery period. Schematic representation of the swing phase of locomotion demarcated by the foot lift and contact (up and down arrows, respectively) and the movement direction are given above the graph. B: duration of the F subphase of the swing (flexion part) expressed in % of the whole swing duration. C: duration of the E1 subphase of the swing (extension part) expressed in % of the whole swing duration. D: duration of the foot drag period on the treadmill belt during the swing phase of locomotion. E: duration of the period with no movement of the hindlimb, named the “off period,” before the onset of the swing phase. F: individual CV (step-to-step variability expressed in %) of the whole step cycle duration and averaged in trained and untrained rats. Data were recorded at 14 m/min and are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, compared with group average baseline; +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after SCI.
A foot drag period, consisting of dragging forward the dorsal surface of the foot on the treadmill belt, appeared in the first part of the swing phase after SCI in all animals. In untrained rats, the duration of this deficit remained at ∼120 ms throughout the post-SCI period (Fig. 5D), which was 100% of the swing phase duration at week 10 after SCI (Fig. 5A). In contrast, the duration of the foot drag period decreased week after week with training so that it was not significantly higher than baseline in trained rats as soon as week 3 after SCI, to reach ∼17% of the swing phase duration at week 10 (Fig. 5D). In addition, the treadmill training decreased the foot drag duration as early as week 2 until it was greatly reduced at week 10 after SCI so that it completely disappeared in 33% of the trained group, while at the same time point all animals in the untrained group were highly affected by this deficit (Fig. 5D). This is a clear case of a locomotor parameter (foot drag) improving with training week after week.
Our results also show, in untrained animals, the appearance of a period of inactivity at the transition between the end of the stance phase and the onset of the swing phase that we have named the “off period,” during which no active movement of the hindlimbs were present despite continuous perineal stimulation. The duration of the off period increased in untrained rats throughout the recovery to reach 352.6 ± 96.1 ms (294% of the swing phase duration) at the end of the experiment (Fig. 5E). The consequence of this increase in the period of inactivity in untrained rats was a growing incapacity to follow the treadmill speed. In contrast, treadmill training prevented such a period of inactivity at the end of stance (Fig. 5E).
Variability of step cycle duration was also a function of treadmill training after SCI. The step-to-step variability of the cycle duration greatly increased throughout the recovery period in untrained rats to reach 32.7 ± 7.4% at week 10 (Fig. 5F), while the trained animals remained at the baseline level during the whole post-SCI period with a CV of 8.2 ± 0.9% at the end of the experiment (Fig. 5F). These data suggest that treadmill training prevented the increase of step duration instability and kept the spinal trained animal at a level of variability similar to the intact (baseline) condition.
Foot trajectory during swing phase improved in trained rats.
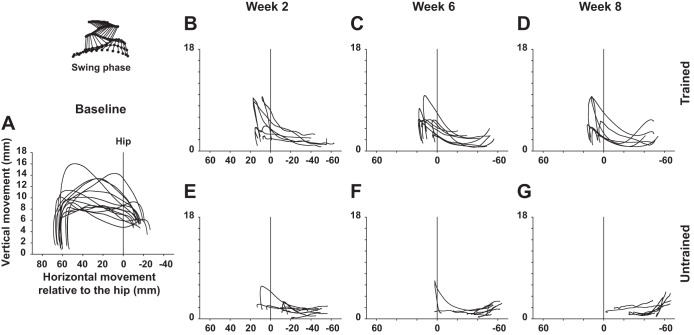
We also assessed the averaged trajectory in the sagittal plan of the limb end point in trained and untrained rats during the swing phase of treadmill locomotion in the normal state and at weeks 2, 6, and 8 after SCI as depicted in Fig. 6. In normal rats, the foot leaves the ground at the onset of the swing phase, reaches the greatest height in the second part of the forward movement (E1 subphase), and finishes the swing phase by a vertical trajectory corresponding to the final extension of the ankle until the foot hits the ground (Fig. 6A). As soon as week 2 after spinal cord transection the trajectory of the foot in trained animals showed a greatest vertical amplitude in the second part of the movement and more regularity in the group than in untrained animals (Fig. 6, B and E). Then the trajectory of the foot continued to improve throughout the recovery period in trained animals including greatest vertical amplitude and regularity in the trajectories themselves (Fig. 6, C and D), while the foot trajectories of untrained rats worsened and became short, disorganized, and almost flat near the end of the experiment (Fig. 6, F and G).
Fig. 6.
Evolution of hindlimb foot trajectory during the swing phase after SCI. A: superimposed averaged trajectory of the third toe in normal rats during the swing phase of locomotion on treadmill. Scale of the y-axis (vertical foot displacements) is increased (zoomed in) compared with the x-axis for better depiction and comparison of the vertical amplitude. Vertical line labeled as Hip represents the vertical projection of the great trochanter and corresponds to the reference (0 value) on the x-axis. B–D: representations similar to A in trained rats weeks 2, 6, and 8 after SCI, respectively. E–G: representations similar to B–D in untrained rats at the same time points. Data are expressed as averaged instant position relative to the hip (x-axis) and the treadmill belt (y-axis).
Treadmill training improves velocity of swing phase of locomotion.
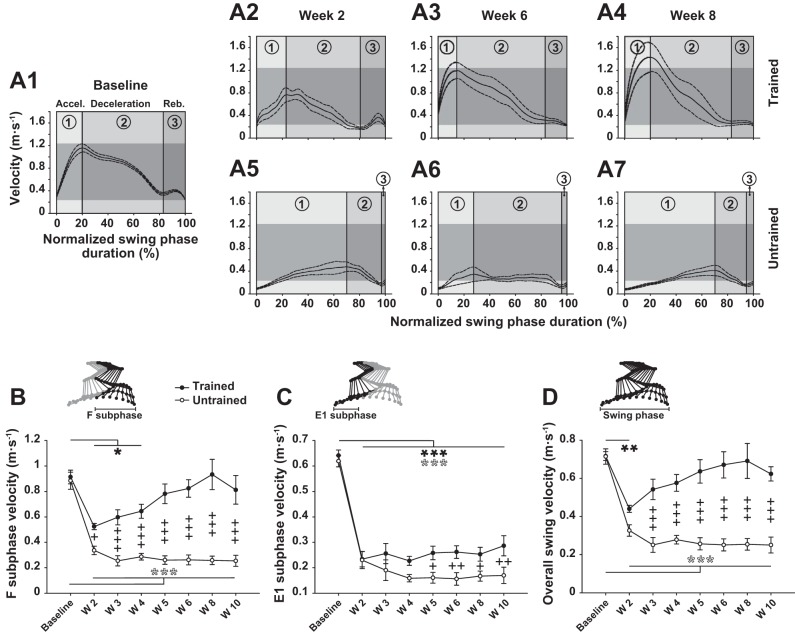
The instant and averaged velocity of the limb end point were assessed during the swing phase and are illustrated in Fig. 7. Briefly, in intact rats, the instant velocity of the swing phase can be divided into three main parts, acceleration, deceleration, and rebound (labeled 1, 2, and 3, respectively, in Fig. 7A1). As early as week 2 after SCI, trained rats showed a return of the three-part organization of the instant velocity of the swing in similar proportions to the baseline while the untrained rats remained deficient, with a low and long acceleration followed by a short deceleration and the disappearance of the third part (compare subdivisions 1, 2, and 3 in Fig. 7, A2 and A5). This disorganization remained present without improvement all along the recovery period (Fig. 7, A6 and A7). On the other hand, in trained rats, the instant velocity improved throughout the post-SCI period so that at week 8 after the lesion not only was the normalized duration of each part recovered compared with the baseline but also the magnitude of the acceleration peak increased (compare Fig. 7, A4 and A1).
Fig. 7.
Effect of treadmill training on recovery of swing phase velocity. A1–A7: averaged instant velocity of the left foot against normalized swing phase duration before (A1) and at weeks 2, 6, and 8 after SCI in trained (A2–A4) and untrained (A5–A7) groups. Height of horizontal grey strips depicted on each panel reflects range of normal instant velocity (baseline) for direct comparison with the different epochs after SCI. The subdivisions of the instant velocity [i.e., acceleration (1), deceleration (2), and rebound (3)] are specified in each chart and separated by vertical lines. B–D: averaged velocity of the left foot during F and E1 subphases of swing and the overall swing phase, respectively, in trained and untrained groups. The corresponding phase and subphases of the step cycle are indicated above each panel on the stick diagram. Data were recorded at 14 m/min and are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, compared with group average baseline; +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001, for comparison between experimental groups at the same time point after SCI.
Similarly to the instant velocity, whether for the subparts or the whole swing phase, the averaged velocity of the limb end point in untrained rats was strongly deficient and no improvement was present throughout the 10-week recovery period compared with the baseline (Fig. 7, B–D). In trained rats, however, the averaged velocity of the F subphase was improved as early as week 2 compared with the untrained group and became similar to the baseline from week 5 to the end of the experiment (Fig. 7B). Although E1 subphase velocity remained deficient in both groups at the end of the experiment, this parameter was partially recovered with training from week 5 to the end of the experiment (Fig. 7C). Finally, the averaged velocity of the overall swing phase in the trained group became similar to the baseline from week 3 after SCI and was fully recovered at the end of the experiment (Fig. 7D).
These results show that treadmill training strongly improves the instant and averaged velocity of the foot during the swing phase early after SCI and throughout the 10-wk recovery period.
Treadmill training improves recovery of swing phase component relationship.
Swing phase duration depends on the length of the foot trajectory and the hindlimb's velocity in the air, which are driven by the spinal cord. Consequently, the swing phase features are very relevant indicators of the modulation exerted by the spinal locomotor network because they are essentially dependent of the sensorimotor control capacity of the animals.
Although swing phase duration remained similar between groups at the end of experiment (Fig. 5A), we have shown that its principal constituents, namely, swing phase length, height, and velocity, had been strongly improved by our training protocol (Fig. 4, A and D, and Fig. 7D). This apparent inconsistency between a parameter (dependent variable) and its constituents (independent variables) can be only explained by compensatory changes of the constituents' relationship keeping constant the parameter value. To explore the change of this relationship, we computed polynomial multiple regressions in normal, trained, and untrained rats week 10 after SCI as illustrated by the regression hyperplanes in Fig. 8.
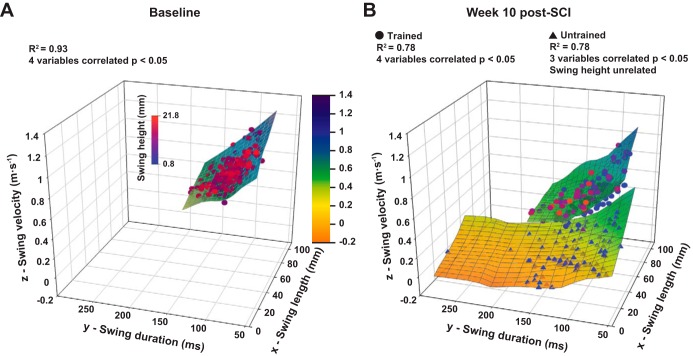
Fig. 8.
Recovery of hindlimb duration, length, velocity, and height relationship at the end of the experiment. A: fitted hyperplane of 3 independent variables {swing length (x), swing velocity (z), and swing height [dot color gradient] predicting the swing duration [dependent variable (y)]} in normal animals. The hyperplane color gradient (from orange to dark blue) represents the z-axis values to help the spatial representation of the plane. Each dot represents 1 step cycle, and the swing height values are represented by the blue to red color gradient of the dots. R2 of the multiple correlation, number of variables correlated, and P value are given at top. B: same representation as A at week 10 after SCI in trained (top plane) and untrained (bottom plane) animals. Data are raw data.
In normal rats, the four variables (i.e., swing duration, length, velocity, and height) are strongly interrelated (R2 = 0.93), meaning that the swing duration can be reliably predicted by the values of the three other variables. Ten weeks after SCI, in trained animals the four variables remained correlated (R2 = 0.78) and the fitting hyperplane remained in the same orientation and position as in normal animals (compare Fig. 8A and hyperplane corresponding to trained rats in Fig. 8B). Even if the coefficient of determination was decreased compared with the normal animals (which can be explained by the increase of the measurement variability after SCI), it remained high enough and significant to attest to a strong relationship between the different variables similarly to the baseline. In untrained rats, only three variables were interrelated at week 10 after SCI (R2 = 0.78); the swing height remained unrelated and the orientation and position in the three-dimensional graph is clearly different from normal animals (compare Fig. 8A and hyperplane corresponding to untrained rats in Fig. 8B). These data show that our training model allowed maintenance, in complete spinalized rats, of interrelations similar to those in normal rats between different swing phase components, while these interrelations were changed without training.
Effect of training on recovery of hindlimb coupling.
Interlimb coordination and variability were measured at the time points of interest and are illustrated in Fig. 9 (see materials and methods for details about coordination calculation and illustration).
Two weeks after SCI the out-of-phase coordination of the hindlimb locomotor movements was recovered in both groups (Fig. 9, A2 and A5). However, the foot lifts occurred earlier after the contralateral contact compared with the baseline (Fig. 9, A2 and A5). Consequently, the proportion of bilateral stance periods were decreased in both groups, although this effect was more pronounced in trained animals. The proportion of bilateral stance periods then increased progressively in the trained group to reach values similar to the untrained group at week 6 after SCI (Fig. 9, A3 and A6). By the end of the recovery period, the hindlimb coordination remained similar between groups while the left and right lifts still occurred earlier in both groups compared with the baseline, keeping the bilateral stance periods shortened (Fig. 9, A4 and A7). In trained animals, each locomotor period and event tended to return progressively to the baseline value after SCI, while the dispersion around the mean of each event decreased (see dotted lines in Fig. 9A4), demonstrating the improvement of the rats' homogeneity in this group, which is not the case in untrained animals (see dotted lines in Fig. 9A7).
The step-to-step variability of the left lift, left contact, and right lift coordination in each rat during locomotion was averaged by group and plotted against each time point in Fig. 9, B–D, respectively. In trained rats, the regularity of left lift and left contact coordination remained similar to the baseline all along the post-SCI period, while a nonsignificant upward trend in right lift variability was present (Fig. 9, B–D). However, these parameters were greatly increased in untrained rats throughout the recovery period (Fig. 9, B–D). In addition, the inconstancy of coordination was clearly higher in the untrained group compared with the trained group all along the recovery period for the left lift (Fig. 9B) and the left contact (weeks 3–4 and 6–10 in Fig. 9C). Although right lift variability was higher in the untrained group during almost the whole post-SCI period (weeks 3–4 and 8) it became similar at week 10 after SCI (Fig. 9D).
These results show that the averaged coordination of both hindlimbs was similar between trained and untrained rats after SCI, independently from the other kinematic parameters highly degraded in untrained compared with trained rats. In addition, the step-to-step coordination in each rat and the coordination regularity between rats in the group were more consistent in trained than untrained animals.
Recovery of EMG pattern.
At week 11 after SCI we recorded, through transcutaneous electrodes, the EMG activity of TA and GL muscles on both hindlimbs during locomotion on the treadmill to assess the recovery of central pattern output. Comparison of raw EMG activity in normal, trained, and untrained rats at the end of the recovery period is depicted in Fig. 10.
Fig. 10.
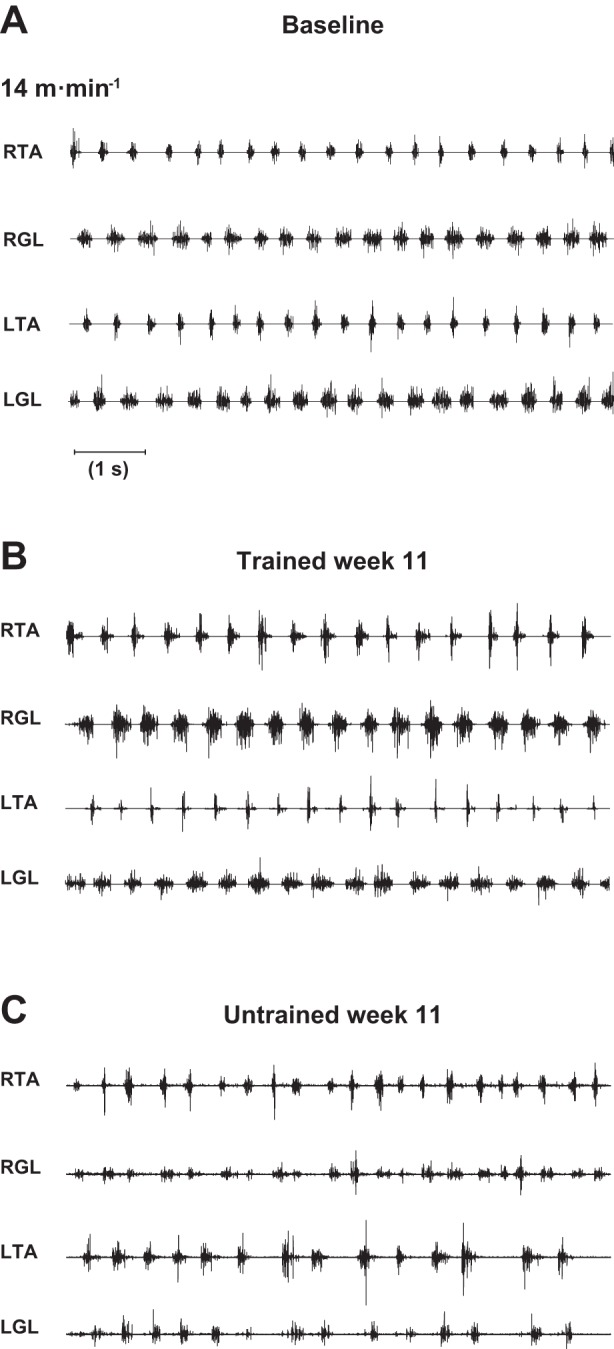
Recovery of hindlimb EMG pattern during locomotion on treadmill. A: raw EMG activity of right (RTA) and left (LTA) tibialis anterior and right (RGL) and left (LGL) gastrocnemius lateralis in typical normal rat walking on the treadmill at 14 m/min. B: representation similar to A in a typical trained rat at the same velocity at week 11 after SCI. C: representation similar to B in a typical untrained rat at the same time point and same velocity. Data from each muscle were recorded transcutaneously. Graphs depict ∼8 s of locomotion.
At the end of the 10-wk recovery period, concomitantly with the kinematic parameters, electromyographic recording showed differential muscle activity patterns between groups during locomotion. In trained rats, the hindlimb locomotor EMGs were well organized and coordinated and the regularity of bursting was so similar to those recorded in normal rats that it was impossible to visually differentiate (compare Fig. 10, A and B). In addition, the recovered EMG pattern in trained animals was not restricted in short bouts of locomotion but was very stable over the whole locomotor session, similarly to their kinematic locomotor pattern. The extensor activity (i.e., related to the weight bearing and forward propulsion during locomotion) was also increased with training (compare right GL and left GL in Fig. 10, B and C). Comparatively, although EMG rhythmic activity was still present in untrained rats at the same time point, the electromyogram indicates that the normal pattern and organization were lost, including transient coactivations of antagonist muscles and consequently the failure of muscle coordination (see right TA vs. right GL and left TA vs. left GL in Fig. 10C). This uncoordinated activity of the EMGs in untrained animals is coherent with our results on kinematics (Fig. 9, B–D). This result reinforces the statement that our treadmill training protocol positively interacts with the rats' locomotor spinal network plasticity below the complete lesion and allows the reexpression of a near-normal EMG pattern.
Assessment of hindlimb muscle mass.
Since muscular properties could also interact with the locomotor performance, especially muscle mass, which is related to muscle strength, contraction velocity, and endurance, we compared the weight of selected hindlimb muscles in both groups in order to perhaps correlate the differences measured in locomotor performances with the potential differences in muscle properties that would be changed by training. At the end of the experiment rats were weighed and then perfused; afterward the left ankle flexor TA and extensor G muscles were harvested and weighed. The rats' weight and the muscle mass of TA and G expressed in percentage of total body weight were then averaged by group and are depicted in Fig. 11. No significant difference was measured in total body weight (Fig. 11A) or TA and G (both gastrocnemius lateralis and gastrocnemius medialis) muscle mass (Fig. 11B) between trained and untrained rats at the end of the experiment. These results confirm that the mass of muscles directly involved in hindlimb locomotor reexpression was not changed by training in our protocol and consequently highlight the key role played by changes in the spinal circuitry rather than changes in muscle mass. However, no histochemical analyses were performed.
Fig. 11.
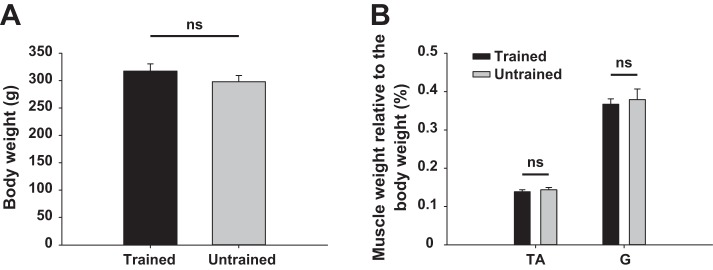
Whole body weight and ankle flexor and extensor muscle weight at end of experiment. A: averaged body weight in trained and untrained groups. B: average weight of left TA and gastrocnemius (G) muscles relative to whole body weight at week 11 after SCI is depicted for trained and untrained rats. Data are expressed as means ± SE. ns, Not significant.
DISCUSSION
Since the first work on cats completely spinalized as adults, demonstrating that 2–3 wk of treadmill training could induce reexpression of involuntary locomotion including weight support and plantar foot placement (Barbeau and Rossignol 1987), several studies have concluded that adult complete spinal rats could not walk with the hindlimbs without invasive and/or complex therapeutic strategies (Antri et al. 2002; Courtine et al. 2009; Slawinska et al. 2000). We hypothesized that sustained perineal stimulation in rats could trigger the lumbar locomotor network to induce active treadmill training in order to reexpress locomotion in conditions similar to those in adult spinal cats. To address these points, we investigated the effects of daily treadmill locomotor training elicited by perineal stimulation provided while the animal was held in a quadrupedal posture on the functional plasticity of the lumbar spinal locomotor network in adult rats completely spinalized at T7. In accordance with the previous studies in cats, our detailed kinematic analysis shows strong evidence of intrinsic and sustained remodeling of the spinal locomotor circuitry induced by our treadmill training protocol bringing back near-normal hindlimb locomotion, as demonstrated by the recovery of well-organized and adaptive locomotion in trained rats while untrained rats showed poor locomotor performance decreasing with time despite the fact that the untrained rats were recorded in the same conditions as trained rats (i.e., perineal stimulation).
Noninvasive triggering of spinal locomotor network.
When the tail of an adult spinal rat is manually stimulated, it generally produces short bouts of uncoordinated hindlimb flexions followed by prolonged extensions, defective foot placements, and abnormal swing phases (Slawinska et al. 2012). Our results reflect the fact that tonic stimulation of the perineal area is more efficient to elicit robust hindlimb stepping, allowing locomotor training in adult spinal rats. Although this strategy is commonly used in adult spinal cats to induce locomotion, the underlying mechanisms are not yet understood. Fictive locomotion recordings in rats clearly suggest that the CPG below the lesion site can be spontaneously active after the intraspinal graft of embryonic brain stem cells (Yakovleff et al. 1995) or activated by the stimulation of the mesencephalic locomotor region (Canu et al. 2001; Iles and Nicolopoulos-Stournaras 1996). The spinal locomotor network is not created by these stimulations, it is revealed (Rossignol et al. 2014), suggesting that such locomotor activity emerges in response to the change of excitatory/inhibitory balance within the spinal cord.
Perineal stimulation seems to adequately modulate the excitability level of the spinal locomotor circuitry and compensates for the decrease of supraspinal input in the incomplete SCI rat model (Alluin et al. 2011, 2014) or for the complete absence of brain input as in the present study. This triggering capability is probably innate since it was effective as early as a few days after SCI as demonstrated by the reexpression of alternated bilateral flexion-extension in untrained animals. However, unlike cats (Barbeau and Rossignol 1987), none of the rats walked without perineal stimulation even at the end of the post-SCI period. This suggests common mechanisms underlying spinal cord modulation between species but different tuning giving less weight to hindlimb cutaneous and proprioceptive information during spinal locomotion in rats compared with cats. This predominant influence of the perineal afferents in our protocol could be a double-edged sword, because if their stimulation was sufficient to trigger the CPG it could also be responsible for the differences observed in the locomotor pattern of trained compared with normal rats (for instance, the overextension at the swing/stance transition) given that a variety of sensory inputs can influence the spinal locomotor network output (Rossignol and Frigon 2011). Another hypothesis concerning this overextension could be that complete thoracic SCI also affects the tone of abdominal muscles, favoring an anterior pelvic tilt and consequently a backward shift of the hindlimb step cycles through an exaggerated extension at the end of the stance phase (Supplemental Movie S1). We have previously observed this also in incomplete spinal rats (Alluin et al. 2011, 2014).
From a clinical perspective, it is of primary interest to have a clearer idea of the spinal cord functional potential devoid of supraspinal influence since optimization of the spinal locomotor circuitry will have a major impact on the feedback provided to the remnant structures after partial spinal cord lesions.
Treadmill training as a key factor to positively drive spinal plasticity.
Different strategies have been investigated to induce hindlimb locomotion in complete spinal rats, such as cell graft (Gimenez y Ribotta et al. 2000; Majczynski et al. 2005), excitatory drugs (Antri et al. 2002; Feraboli-Lohnherr et al. 1999), epidural electrostimulation and/or robotic assistance (Cha et al. 2007; de Leon et al. 2002; Timoszyk et al. 2002), upright posture (Slawinska et al. 2012), or a combination of some of these (Courtine et al. 2009; de Leon and Acosta 2006; Dominici et al. 2012; Gerasimenko et al. 2007; Hsieh and Giszter 2011; Ichiyama et al. 2005). In some of these studies, treadmill training had also been provided in combination with other strategies in order to produce synergistic effects. When treadmill training was administered alone, its beneficial effect on locomotor reexpression was very moderate (Ilha et al. 2011; Zhang et al. 2007) or no beneficial effect was observed even in facilitating upright posture (Ichiyama et al. 2008).
Our data clearly demonstrate that if treadmill training is not passive but triggered by perineal stimulation, it positively interacts with functional spinal plasticity insomuch that the locomotor parameters of trained rats returned close to normal values, while untrained rats progressively lost the capability to walk on the treadmill. For instance, the amplitude of hip and MTP (Fig. 4), the swing velocity (Fig. 7), the EMG activity of ankle flexors and extensors (Fig. 10), and the variability of several kinematic parameters (Figs. 2, 5, and 9) returned to normal values only in trained rats. We also have analyzed the interrelations of the different swing phase components (i.e., duration, length, velocity, and height; Fig. 8), which are very representative of the sensorimotor and adaptive control capacity of the spinal cord because the movement of the limb in air is devoid of direct environmental influence and only driven by the locomotor network and proprioceptive inputs from the joints, in contrast to the stance phase, which is directly driven by proprioceptors and cutaneous receptors activated by foot contact with the belt. These fine interrelations are also greatly restored by training, while the absence of activity induces a deterioration. Such impressive recovery was previously shown in spinal rats trained on the treadmill in upright posture with a combination of 5-HT agonists and epidural electrostimulation (Courtine et al. 2009). The locomotor effect of these agonists is attributed to the activation of 5-HT2 and 5-HT7 receptors (Dunbar et al. 2010; Garraway and Hochman 2001; Liu et al. 2009; Madriaga et al. 2004; Pearlstein et al. 2005; Schmidt and Jordan 2000). However, the mechanisms induced by epidural electrostimulation in rats remain unknown, although it has been suggested that such stimulation activates the spinal locomotor network through afferent fibers (Courtine et al. 2009). Although no spasticity was measured in the present study, it has also been shown that spinal 5-HT receptors below the lesion site are upregulated after complete SCI, leading to serotonin denervation supersensitivity and the subsequent spasticity (Barbeau and Bédard 1981; Kong et al. 2010, 2011). Taken together, these considerations suggest that the rehabilitative strategy used in the present study could act on the spinal cord through similar mechanisms implicating afferent recruitment via the perineum and concomitantly the neurochemical normalization of the spinal cord induced by step training. Specific upregulation of brain-derived neurotrophic factor (BDNF) in the lumbar spinal cord of trained rats could also be implicated in the strong training effect observed in our results (Joseph et al. 2012).
Although Smith et al. (2009) have reported deleterious effect of exercise training starting too early after SCI, the precocious locomotor improvements at week 2 after SCI in our trained rats shows that starting it immediately after complete SCI has positive effects on spinal plasticity and highlights the benefit of stimulating the spinal cord even when the spinal circuits do not produce locomotor output in response to the stimulation as we observed in the present study. The adaptive processes of the spinal cord were also effective by early training, as trained rats could adapt the locomotor pattern to the belt velocity from week 2 (Fig. 1) similar to the observations made in spinal cats (Barbeau and Rossignol 1987). In addition, the duration of the daily treadmill training session previously used in complete spinal cats and rats was at least 20 min (Barbeau and Rossignol 1987; Courtine et al. 2009; de Leon et al. 1998; Lovely et al. 1990;). The robust results obtained in the present study with a daily exercise period as short as 10 min raise the issue of the optimal duration and frequency of treadmill training to induce long-lasting plastic changes and concomitant locomotor reexpression. Studies involving a variety of treadmill training periods could be performed but would be very demanding on human resources and would probably add little to the locomotor performance seen here since the locomotor parameters return to values obtained in the animals during the control period before spinalization.
Ultimately, the absence of difference between trained and untrained rats in terms of hindlimb muscle mass (Fig. 11) strengthens the idea that the very positive effect of training on the detailed locomotor characteristics reported in the present study is largely attributed to the activity-dependent plasticity occurring within the spinal circuitry (Edgerton et al. 2004). On the other hand, the absence of activity after such drastic spinal lesion induces a dramatic impairment of the spinal locomotor capability that degrades with time as demonstrated previously (Courtine et al. 2009).
Conclusion and perspectives.
The afferent inputs from the perineum and sacral area can access the rat's spinal locomotor circuitry (Etlin et al. 2010) to elicit step training and induce the activity-dependent spinal plasticity underlying the recovery of locomotion in our study. Treadmill locomotor training in combination with perineal stimulation could appear as key components to easily induce locomotion in the complete spinal rat and should question the need to use more invasive central stimulations (i.e., pharmacology, epidural electrostimulation, cell graft . . .) that should, at least, be superior to the intrinsic capacity highlighted in the present report. Further neuroanatomical studies in adult spinal rats submitted to training should improve our understanding of intrinsic spinal plasticity. In the clinical context mainly involving incomplete spinal injuries, it is of great interest to exploit the intrinsic locomotor capacity of the spinal cord since potentiation of the spinal locomotor network improves the functional interactions between afferent feedback and remnant structures that are essential for the overall recovery of locomotion. This latest point is crucial because safely strengthening the spared nervous components after SCI should be the first priority before considering more invasive therapeutic approaches.
GRANTS
This work was supported by individual operating grants from the Canadian Institutes of Health Research (CIHR) to S. Rossignol as well as the Sensorimotor Rehabilitation Research Team (SMRRT) team grant and a Canada Research Chair. O. Alluin was supported by a postdoctoral fellowship from the Fonds de Recherche du Québec-Santé (FRQS) and by SMRRT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.A. and S.R. conception and design of research; O.A. and H.D.-M. performed experiments; O.A. analyzed data; O.A. and S.R. interpreted results of experiments; O.A. prepared figures; O.A. drafted manuscript; O.A. and S.R. edited and revised manuscript; O.A. and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Philippe Drapeau and Claude Gagner for their technical assistance in software design and electronics.
Present address of O. Alluin: Institut des Sciences du Mouvement (UMR 7287), Aix-Marseille Université, CNRS, Marseille, France.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Alluin O, Delivet-Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S. Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One 9: e111072, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma 28: 1963–1981, 2011. [DOI] [PubMed] [Google Scholar]
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett 384: 162–167, 2005. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. In: Spinal Cord Trauma: Regeneration, Neural Repair and Functional Recovery, edited by McKerracher L, Doucet G, Rossignol S. Amsterdam: Elsevier Science, 2002, p. 467–476. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Bédard P. Denervation supersensitivity to 5-HT in rats following spinal transection and 5,7 dihydroxytryptamine injection. Neuropharmacology 20: 611–616, 1981. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- Canu MH, Falempin M, Orsal D. Fictive motor activity in rat after 14 days of hindlimb unloading. Exp Brain Res 139: 30–38, 2001. [DOI] [PubMed] [Google Scholar]
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, de Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma 24: 1000–1012, 2007. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J Neurotrauma 23: 1147–1163, 2006. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 80: 83–91, 1998. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev 40: 267–273, 2002. [DOI] [PubMed] [Google Scholar]
- Dominici N, Keller U, Vallery H, Friedli L, Van den Brand R, Starkey ML, Musienko P, Riener R, Courtine G. Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nat Med 18: 1142–1147, 2012. [DOI] [PubMed] [Google Scholar]
- Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol 588: 139–156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 27: 145–167, 2004. [DOI] [PubMed] [Google Scholar]
- Etlin A, Blivis D, Ben-Zwi M, Lev-Tov A. Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J Neurosci 30: 10324–10336, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res 55: 87–98, 1999. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci 25: 11738–11747, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway SM, Hochman S. Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br J Pharmacol 132: 1789–1798, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98: 2525–2536, 2007. [DOI] [PubMed] [Google Scholar]
- Gimenez y Ribotta M, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci 20: 5144–5152, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FH, Giszter SF. Robot-driven spinal epidural stimulation compared with conventional stimulation in adult spinalized rats. Conf Proc IEEE Eng Med Biol Soc 2011: 5807–5810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Broman J, Roy RR, Zhong H, Edgerton VR, Havton LA. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci 31: 26–33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, Van den Brand R, Lazrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci 28: 7370–7375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 383: 339–344, 2005. [DOI] [PubMed] [Google Scholar]
- Iles JF, Nicolopoulos-Stournaras S. Fictive locomotion in the adult decerebrate rat. Exp Brain Res 109: 393–398, 1996. [DOI] [PubMed] [Google Scholar]
- Ilha J, Centenaro LA, Broetto CN, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M. The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res 36: 1046–1055, 2011. [DOI] [PubMed] [Google Scholar]
- Joseph MS, Tillakaratne NJ, de Leon RD. Treadmill training stimulates brain-derived neurotrophic factor mRNA expression in motor neurons of the lumbar spinal cord in spinally transected rats. Neuroscience 224: 135–144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XY, Wienecke J, Chen M, Hultborn H, Zhang M. The time course of serotonin 2A receptor expression after spinal transection of rats: an immunohistochemical study. Neuroscience 177: 114–126, 2011. [DOI] [PubMed] [Google Scholar]
- Kong XY, Wienecke J, Hultborn H, Zhang M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: an immunohistochemical study. Brain Res 1320: 60–68, 2010. [DOI] [PubMed] [Google Scholar]
- Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol 102: 337–348, 2009. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cat. Brain Res 514: 206–218, 1990. [DOI] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92: 1566–1576, 2004. [DOI] [PubMed] [Google Scholar]
- Majczynski H, Maleszak K, Cabaj A, Slawinska U. Serotonin-related enhancement of recovery of hind limb motor functions in spinal rats after grafting of embryonic raphe nuclei. J Neurotrauma 22: 590–604, 2005. [DOI] [PubMed] [Google Scholar]
- Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. J Neurosci 32: 10961–10970, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko P, Van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci 31: 9264–9278, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsal D, Barthe JY, Antri M, Feraboli-Lohnherr D, Yakovleff A, Ribotta M, Privat A, Provencher J, Rossignol S. Locomotor recovery in chronic spinal rat: long-term pharmacological treatment or transplantation of embryonic neurons? Prog Brain Res 137: 213–230, 2002. [DOI] [PubMed] [Google Scholar]
- Pearlstein E, Ben MF, Pflieger JF, Vinay L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci 21: 1338–1346, 2005. [DOI] [PubMed] [Google Scholar]
- Philippson M. L'autonomie et la centralisation dans le système nerveux des animaux. Trav Lab Physiol Inst Solvay (Bruxelles) 7: 1–208, 1905. [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems Bethesda, MD: Am. Physiol Soc, 1996, sect. 12, p. 173–216. [Google Scholar]
- Rossignol S, Barriere G, Alluin O, Frigon A. Re-expression of locomotor function after partial spinal cord injury. Physiology (Bethesda) 24: 127–139, 2009. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34: 413–440, 2011. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharmacological aids to locomotor training after spinal injury in the cat. J Physiol 533: 65–74, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Schmidt BJ, Jordan LM. Spinal plasticity underlying the recovery of locomotion after injury. In: Textbook of Neural Repair and Rehabilitation, edited by Selzer ME, Clarke S, Cohen LG, Kwakkel G, Miller RH. Cambridge, UK: Cambridge Univ. Press, 2014, p. 166–195. [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. [DOI] [PubMed] [Google Scholar]
- Slawinska U, Majczynski H, Dai Y, Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol 590: 1721–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Majczynski H, Djavadian R. Recovery of hindlimb motor functions after spinal cord transection is enhanced by grafts of the embryonic raphe nuclei. Exp Brain Res 132: 27–38, 2000. [DOI] [PubMed] [Google Scholar]
- Slawinska U, Miazga K, Cabaj AM, Leszczynska A, Majczynski H, Nagy JI, Jordan LM. Grafting of fetal brainstem 5-HT neurons into the sublesional spinal cord of paraplegic rats restores coordinated hindlimb locomotion. Exp Neurol 247: 572–581, 2013. [DOI] [PubMed] [Google Scholar]
- Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma 26: 1017–1027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillakaratne NJ, Guu JJ, de Leon RD, Bigbee AJ, London NJ, Zhong H, Ziegler MD, Joynes RL, Roy RR, Edgerton VR. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience 166: 23–33, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoszyk WK, de Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol 88: 3108–3117, 2002. [DOI] [PubMed] [Google Scholar]
- Van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336: 1182–1185, 2012. [DOI] [PubMed] [Google Scholar]
- Yakovleff A, Cabelguen JM, Orsal D, Ribotta M, Rajaofetra N, Drian MJ, Bussel B, Privat A. Fictive motor activities in adult chronic spinal rats transplanted with embryonic brainstem neurons. Exp Brain Res 106: 69–78, 1995. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ji SR, Wu CY, Fan XH, Zhou HJ, Liu GL. Observation of locomotor functional recovery in adult complete spinal rats with BWSTT using semiquantitative and qualitative methods. Spinal Cord 45: 496–501, 2007. [DOI] [PubMed] [Google Scholar]