Abstract
Objective
To describe darunavir pharmacokinetics with once and twice daily dosing during pregnancy and postpartum in HIV-infected women.
Design
Women were enrolled in International Maternal Pediatric Adolescent AIDS Clinical Trials Network Protocol P1026s, a prospective, non-blinded study of antiretroviral pharmacokinetics in HIV-infected pregnant women that included separate cohorts receiving darunavir/ritonavir dosed at either 800 mg/100 mg once daily or 600 mg/100 mg twice daily.
Methods
Intensive steady-state 12 or 24 hour pharmacokinetic profiles were performed during the second trimester, third trimester and postpartum. Darunavir was measured using high performance liquid chromatography (detection limit: 0.09 μg/mL).
Results
Pharmacokinetic data were available for 64 women (30 once daily and 34 twice daily dosing). Median darunavir area under the concentration-time curve (AUC) and maximum concentration (Cmax) were significantly reduced during pregnancy with both dosing regimens compared to postpartum, while the last measurable concentration (Clast) was also reduced during pregnancy with once daily darunavir. Darunavir AUC with once daily dosing was reduced by 38% during the second trimester and by 39% during the third trimester. With twice daily dosing, darunavir AUC was reduced by 26% in both trimesters. The median (range) ratio of cord blood/maternal delivery darunavir concentration in 32 paired samples was 0.18 (range: 0 – 0.82).
Conclusion
Darunavir exposure is reduced by pregnancy. In order to achieve darunavir plasma concentrations during pregnancy equivalent to those seen in non-pregnant adults, an increased twice daily dose may be necessary. This may be especially important for treatment-experienced women who may have developed antiretroviral resistance mutations.
Keywords: darunavir, pregnancy, HIV, pharmacokinetics
Introduction
HIV-1-infected pregnant women commonly receive antiretroviral drugs, both for their own health and for prevention of mother-to-child transmission of HIV-1 (HIV). Current US Public Health Service guidelines recommend that all antiretroviral naïve pregnant women receive a combination antiretroviral regimen including two nucleoside reverse transcriptase inhibitors and either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor.1
The physiological changes of pregnancy have a large impact on drug disposition.2 Previous studies have shown reduced exposure during pregnancy for many antiretroviral drugs, in particular protease inhibitors.3 Plasma concentrations of all HIV protease inhibitors studied during pregnancy, including lopinavir, nelfinavir, saquinavir, indinavir, fosamprenavir and atazanavir, are decreased during pregnancy.4-9 Subtherapeutic antiretroviral exposure during pregnancy may result in inadequate virologic control, increasing the risk of transmission of HIV to the infant and of maternal development of drug resistance mutations. Assessment of placental transfer of antiretrovirals to the fetus is of critical importance, as transplacentally-acquired antiretrovirals expose the fetus to both potential benefit, from prophylaxis against HIV infection, and harm, from drug teratogenicity and/or toxicity.3 Before any antiretroviral can be used safely and effectively in pregnancy, its pharmacology must be studied and well understood in pregnant women. Published data describing darunavir pharmacokinetics during pregnancy are limited.10-16 The primary objectives of this study were to describe darunavir pharmacokinetics in HIV-infected pregnant women receiving once and twice daily dosing and to determine if standard doses of darunavir produce equivalent drug exposure during pregnancy to that in nonpregnant adults. We also sought to evaluate transplacental passage of darunavir by comparing concentrations in cord blood and maternal blood at delivery.
Methods
Study population and design
International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) Network Protocol P1026s is an ongoing international multicenter prospective opportunistic study to evaluate the pharmacokinetics of clinically prescribed antiretroviral drugs in pregnant HIV-infected women [Clincialtrials.gov identifier NCT00042289]. This report includes women receiving darunavir with ritonavir at standard doses once and twice daily.
HIV infected pregnant women receiving darunavir 800 mg and ritonavir 100 mg orally once daily or darunavir 600 mg and ritonavir 100 mg orally twice daily as part of clinical care before the beginning of the 35th week of pregnancy were eligible. The choice of additional antiretrovirals and duration of treatment were determined by each subject's clinical care provider. Subjects received darunavir for at least 2 weeks prior to pharmacokinetic sampling and planned to continue darunavir until at least 6 weeks postpartum. Maternal exclusion criteria were: current use of medications known to interfere with darunavir disposition, multiple gestation, or clinical or laboratory toxicity that, in the opinion of the site investigator, would be likely to require a change in the antiretroviral regimen during the study. All P1026 subjects at US sites were co-enrolled in P1025, a prospective cohort study of HIV-infected pregnant women receiving care at US IMPAACT sites. Local institutional review boards approved P1026s at all participating sites and P1025 at all US sites. All subjects provided signed informed consent prior to participation.
Subjects received antiretroviral medications prescribed by their clinical care providers and all antiretrovirals were dispensed by local pharmacies. Study mothers and their infants continued on the study until 6 months after delivery. Intensive darunavir pharmacokinetic sampling was performed during the third trimester and repeated postpartum for all subjects. Women enrolling before 26 weeks gestation were also sampled during the second trimester. Samples obtained during pregnancy were assayed in real time and each subject's physician was notified of the subject's darunavir plasma concentrations and AUC within two weeks of collection. Individual physicians could elect a dosing modification if the AUC was below 70% of the median AUC in non-pregnant adults.17
Clinical and laboratory monitoring
HIV-related laboratory testing was performed at each study visit if not available as part of routine clinical care. Maternal clinical data used for this analysis were: maternal age, ethnicity, weight, concomitant medications, CD4+ lymphocyte and HIV RNA assay results. HIV RNA assays were performed locally and had lower limits of detection ranging from 20 to 400 copies/mL. Maternal clinical and laboratory toxicities were assessed through clinical evaluations and laboratory assays on each pharmacokinetic sampling day, at delivery, and at 24 weeks postpartum. Infant birth weight, gestational age at birth, and HIV infection status were collected. All infants received physical examinations after birth while infant laboratory evaluations were done only as clinically indicated. The study team reviewed toxicity reports on monthly conference calls, although the subject's physician was responsible for toxicity management. The Division of AIDS (DIADS)/NIAID Toxicity Table for Grading Severity of Adult Adverse Experience was used to grade adverse events for study subjects.18 All toxicities were followed through resolution or 24 weeks postpartum.
Sample collection
Plasma samples for pharmacokinetic evaluation were collected during the second (20 – 28 weeks gestation) and third (30 – 38 weeks) trimesters, and between 6 to 12 weeks after delivery. Participants received a stable antiretroviral regimen for at least 2 weeks prior to pharmacokinetic sampling. Participants were instructed to take their darunavir at the same time each day for the 3 days prior to and on the day of the pharmacokinetic evaluations. Plasma samples were drawn at the antepartum and postpartum pharmacokinetic evaluation visits, starting immediately before an observed oral darunavir dose at 1, 2, 4, 6, 8 and 12 hours after the witnessed dose. A 24 hour post-dose sample was collected from once daily dosing subjects. A single maternal plasma sample and an umbilical cord blood sample after cord clamping were collected at delivery.
Darunavir concentration assays
Darunavir concentrations were measured in the Pediatric Clinical Pharmacology Laboratory of the University of California, San Diego using a validated high performance liquid chromatography assay.19 The laboratory is registered with the AIDS Clinical Trials Group (ACTG) Clinical Pharmacology Quality Assurance and Quality Control proficiency testing program and successfully passed (100%) the last 9 rounds of CPQA PT testing (Sept. 2008-Sept. 2012).20 At the lower limit of quantitation (0.09 μg/mL) over six days the within day precision (%CV) ranged from 4.17% to 7.23% and accuracy (% deviation) ranged from -5.80% to 8.8%. The within day precision for four validation samples above the lower limit of quantitation (high, middle, low and extra low concentrations) ranged from 1.33% to 5.87%. The within day accuracy for the high, middle, low and extra low validation samples ranged from 9.07% to 5.33%. The mean recovery from plasma was 99.08%.
Pharmacokinetic analyses
The concentration data collected were analyzed by direct inspection to determine the pre-dose concentration (Cpre-dose), the maximum plasma concentration (Cmax), the corresponding time (tmax), and the last measurable concentration (Clast). The area under the concentration versus time curve (AUC) from time 0 to 12 or 24 hours post dose (AUC0-12 or AUC0-24) for darunavir was estimated using the trapezoidal rule up to the last measurable concentration. Subjects whose pre-dose DRV concentrations were non-detectible were deemed to have recent non-adherence and AUC0-infinity was used to express exposure, with AUC after the last measured concentration estimated as C12/λz or C24/λz, where λz was the terminal slope of the log concentration versus time curve. The minimum exposure targets were an AUC0-24 of at least 56.5 μg*h/mL for once daily dosing and an AUC0-12 of at least 43.6 μg*h/mL for twice daily dosing, which were 70% of the average AUC in non-pregnant adults contained in the darunavir package insert at the time this study arm was developed.17 The half-life (t1/2) was calculated as 0.693/λz. Apparent clearance (CL/F) from plasma was calculated as the dose divided by AUC. The apparent volume of distribution (Vd/F) was determined as CL/F divided by λz. AUC and CL/F were also computed using a one-compartment model in WINNONLIN (Pharsight Corp., St. Louis, MO). Pharmacokinetic parameters derived from each approach were compared to assess potential limitations of each methodology.
Statistical analyses
The target sample sizes were 25 women per study arm with evaluable 3rd trimester pharmacokinetic data, of whom at least 12 had evaluable 2nd trimester data. The 3rd trimester sample size of 25 was chosen to provide at least 80% probability of concluding that the exposure of the pregnant population is lower than that of the non-pregnant population, when the value of the 10th percentile from the non-pregnant population has a true cumulative probability of 30% or higher in the pregnant population (i.e., when at least 30% of pregnant women will have exposure below the 10th percentile for the non-pregnant population). Enrollment was allowed to continue while consented subjects awaited sampling. The final sample sizes exceeded the enrollment targets due to continued enrollment during the period between the obtaining of informed consent and the availability of pharmacokinetic data.
The study design incorporated a two-stage analysis approach. Each individual woman's darunavir exposure during pregnancy was determined in real time and compared with the AUC estimated for a nonpregnant adult HIV-1-infected historical control population from the literature and was promptly reported to each subject's physician.17 Each subject's physician had the option to adjust the dose based on the pharmacokinetic results. A stopping criterion to trigger an evaluation of the adequacy of drug exposure was predefined as six of 25 women (24%; exact 80% confidence limits: 13%, 38%) falling below the target AUC. The goal was to prevent excess accrual to a cohort with known inadequate antiretroviral exposure. The statistical rationale for this early stopping criterion has been previously described.4
Descriptive statistics were calculated for pharmacokinetic parameters of interest during each study period. Darunavir pharmacokinetic parameters during the second trimester versus postpartum and during the third trimester versus postpartum were compared at the within-subject level using the Wilcoxon signed-rank test, with a p-value <0.05 considered to indicate statistical significance.
Results
Sixty six pregnant women were enrolled, of whom 34 received darunavir/ritonavir twice daily and 32 once daily. Thirty women enrolled in the second trimester and 36 in the third trimester. Two of the second trimester enrollees delivered prematurely before third trimester sampling could be completed. Twenty eight subjects (15 receiving once daily dosing and 13 receiving twice daily dosing) successfully completed second trimester pharmacokinetic sampling and 64 (30 receiving once daily dosing and 34 receiving twice daily dosing) successfully completed third trimester sampling. Fifty two subjects successfully completed postpartum sampling but two subjects (one subject in each dosing group) had no detectable darunavir concentrations and their postpartum data are not included in the summary pharmacokinetic statistics. The clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Characteristics of the study population and pregnancy outcomes, presented as median (range) or number of subjects (%).
| Once Daily Dosing N=32 | Twice Daily Dosing N=34 | Combined N=66 | |
|---|---|---|---|
| Race/Ethnicity: | |||
| Black Non-Hispanic | 17 (53.1%) | 12 (35.3%) | 29 (44%) |
| Hispanic/Latina (any race) | 12 (37.5%) | 14 (41.2%) | 26 (39%) |
| White Non-Hispanic | 2 (6.3%) | 4 (11.8%) | 6 (9%) |
| Asian/Pacific Islander | 0 | 3 (8.8%) | 3 (5%) |
| More than 1 race | 1 (3%) | 1 (3%) | 2 (3%) |
| Age at 3rd trimester visit (yrs) | 27.6 (19.3 - 44.9) | 27.0 (18.4- 43.7) | 27.4 (18.4 - 44.9) |
| Weight at 3rd trimester visit (kg) | 91.9 (60.5 - 155) | 76.0 (53.8- 204.1) | 81.0 (53.8 – 204.1) |
| Gestational age at 2nd trimester visit (months) | 24.6 (20.7 – 27.3) | 25.7 (20.3-26.7) | 24.9 (20.3-27.3) |
| Gestational age at 3rd trimester visit (months) | 33.7 (30.0-37.1) | 34.3 (27.4-41.0) | 34.1 (27.4-41.0) |
| Weeks after delivery at postpartum PK visit | 7.1 (3.6-12.9) | 7.0 (2.3-14.1) | 7.0 (2.3-14.1) |
| Other ARVs: | |||
| tenofovir | 17 (54%) | 18 (53%) | 35 (53%) |
| emtricitabine | 16 (53%) | 16 (47%) | 32 (48%) |
| lamivudine | 14 (47%) | 13 (37%) | 27 (41%) |
| zidovudine | 14 (47%) | 11 (32%) | 25 (38%) |
| abacavir | 5 (17%) | 3 (9%) | 8 (12%) |
| raltegravir | 1 (3%) | 10 (29%) | 2 (3%) |
| etravirine | 0 | 2 (6%) | 2 (3%) |
| didanosine | 1 (3%) | 1 (3%) | 2 (3%) |
| efavirenz | 1 (3%) | 0 | 1 (1%) |
| maraviroc | 0 | 1 (3%) | 1 (1%) |
| enfuvirtide | 0 | 1 (3%) | 1 (2%) |
| Duration of darunavir therapy at 3rd trimester visit (weeks) | 27 (6 – 121) | 17 (2 - 252) | 21 (2 - 252) |
| HIV-1 RNA at delivery (copies/mL) | <50 (<50 - 35,313) | 51 (<50 - 66,142) | <50 (<50 - 66,142) |
| HIV-1 RNA at delivery (copies/mL) | |||
| ≤ 50 copies/mL | 19/30 (63%) | 14/28 (50%) | 33/58 (57%) |
| ≤ 400 copies/mL | 28/30 (93%) | 22/28 (79%) | 50/58 (86%) |
| Infant Gestational Age at Birth (weeks) | 38.4 (31.9 - 41.1) | 38.6 (35.0 - 42.4) | 38.6 (31.9 - 42.4) |
| Infant Weight at Birth (gm) | 2792 (1800 - 4401) | 3090 (2245 - 4560) | 3023 (1800 - 4560) |
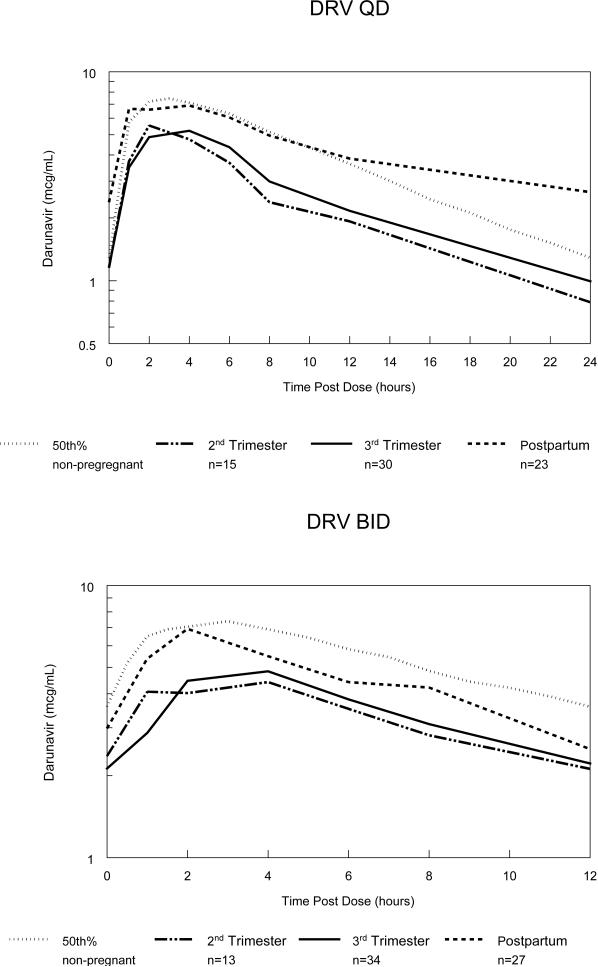
Figure 1 depicts the median antepartum and postpartum darunavir concentration–time curves. Darunavir pharmacokinetic parameters are presented in Table 2. For the subjects receiving once daily dosing, compared to postpartum, second and third trimester median AUC0-24 were reduced by 38% and 39%, median Cmax was reduced by 17% and 29% and median Clast (24 hours) was reduced by 63% and 57%, respectively. The frequency of subjects meeting the AUC0-24 target for once daily dosing was reduced during the second trimester (9/15 (60%)) and the third trimester (19/30 (63%)) compared to postpartum (22/24 (92%)). Clast (24 hours) exceeded the darunavir EC50 for wild type HIV of 0.06 μ/mL in all but 2 once daily subjects, 1 each in the second and third trimesters.
Figure 1.
Semilog plots of median darunavir (DRV) concentration–time curves during the 2nd trimester, third trimester, postpartum and the estimated 50th percentile for non-pregnant HIV-infected historical adult controls with once daily (QD) and twice daily (BID) dosing.17, 38
Table 2.
Darunavir pharmacokinetic parameters, presented as median (interquartile range) or number/total (%)
| DRV/r800/100mg Once Daily | DRV/r 600/100 mg Twice Daily | |||||
|---|---|---|---|---|---|---|
| 2nd trim (n=15) | 3rd trim (n=30) | PP (n=23) | 2nd trim (n=13) | 3rd trim (n=34) | PP (n=27) | |
| AUC (μg*hr/mL) | 64.6* (35.9, 72.3) | 63.5* (46.0, 75.2) | 103.9 (85.9, 135.7) | 45.8* (36.1, 53.4) | 45.9* (29.3, 52.5) | 61.7 (49.7, 80.9) |
| Met AUC target#/total | 9/15* (60%) | 19/30* (63%) | 22/24 (92%) | 7/13 (54%) | 19/34* (56%) | 22/27 (81%) |
| Cmax (μ/mL) | 6.77* (4.35, 7.84) | 5.78* (4.31, 7.29) | 8.11 (6.93, 10.30) | 5.64* (3.78, 6.07) | 5.53* (4.44-7.10) | 7.78 (6.11-9.54) |
| Clast (hours) | 0.99* (0.43,1.81) | 1.17* (0.73 -1.72) | 2.78 (2.05, 2.98) | 2.12 (1.76, 2.85) | 2.22 (1.68-3.26) | 2.51 (2.04, 3.27) |
| Cl/F (L/hr) | 12.4* (11.1, 22.9) | 12.6* (10.6, 17.4) | 7.7 (5.9, 9.3) | 13.1* (11.2, 16.6) | 13.1* (11.4, 20.5) | 9.5 (7.4-11.7) |
p<0.05, compared to postpartum, Wilcoxon signed-rank test
AUC minimum targets were AUC0-24 of at least 56.5 μg*h/mL for once daily dosing and AUC0-12 of at least 43.6 μg*h/mL for twice daily dosing
For the subjects receiving twice daily dosing, compared to postpartum, second and third trimester median AUC0-12 was reduced by 26% and median Cmax was reduced by 28% and 29%, respectively, while there were no significant differences in Clast. The frequency of subjects meeting the AUC0-12 target for twice daily dosing was 7/13 (54%) during the 2nd trimester and 19/34 (56%) during the 3rd trimester, compared to 22/27 (81%) postpartum; these differences reached statistical significance only for the third trimester to postpartum comparison. Clast exceeded the darunavir EC50 for wild type HIV of 0.06 μg/mL in all twice daily subjects except 1 postpartum subject.
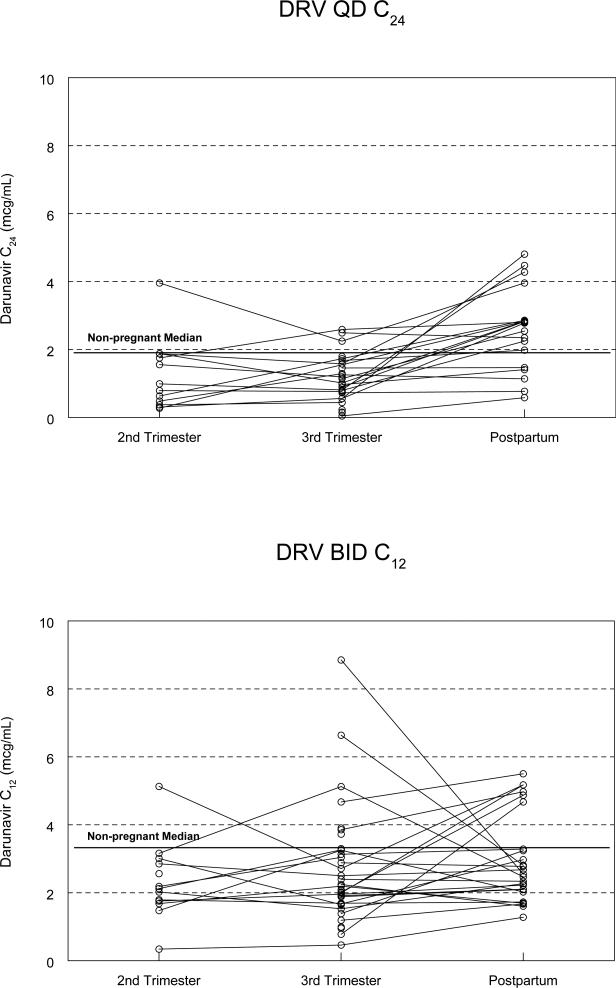
No care provider elected to increase the darunavir dose for any subject in either arm. Graphs presenting darunavir AUC and Clast concentrations for individual subjects across sampling times are depicted in Figure 2. The one-compartment analysis yielded similar darunavir exposure parameters to the non-compartmental analysis (data not presented).
Figure 2.
Darunavir (DRV) Clast (C12 or C24) across sampling times.
Median (interquartile range) ritonavir pharmacokinetic parameters with once daily dosing during the second trimester, third trimester and postpartum periods were: AUC0-24: 3.7 μg*h/mL(2.5-4.7), 3.7 μg*h/mL (2.3-5.0), 8.2 μg*h/mL (5.8-10.8); Cmax: 0.29 μg/mL (0.22-0.41), 0.27 μg/mL (0.22-0.42), 0.64 μg/mL (0.35-0.90); Clast <0.09 μg/mL (<0.09-0.09), <0.09 μg/mL (<0.09-0.08), 0.09 μg/mL (<0.09-0.14). With twice daily dosing, median (interquartile range) ritonavir pharmacokinetic parameters during the second trimester, third trimester and postpartum periods were: AUC0-12: 3.9 μg*h/mL (3.1-5.2), 3.8 μg*h/mL (3.1-4.8), 5.6 μg*h/mL (3.7-11.3); Cmax: 0.47 μg/mL (0.32-0.64), 0.46 μg/mL (0.36-0.67), 0.63 μg/mL (0.438-1.08); Clast: 0.16 μg/mL (0.14-0.24), 0.18 μg/mL (0.11-0.25), 0.20 μg/mL (0.12-0.35).
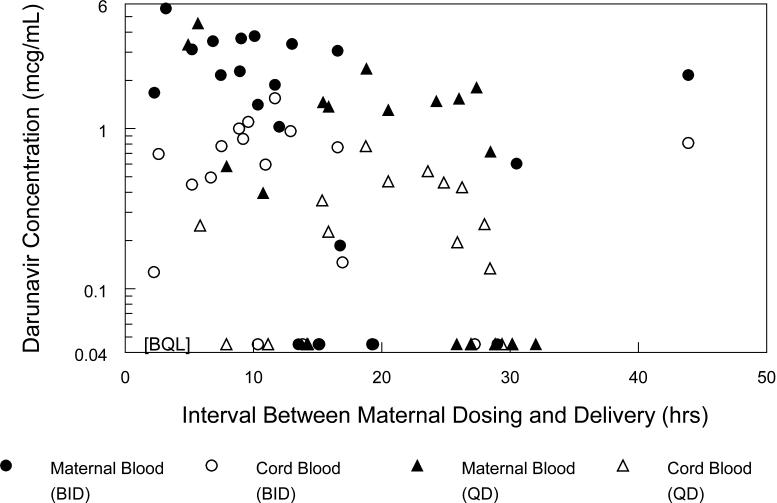
Maternal plasma samples at delivery and umbilical cord blood samples were collected from 43 subjects. Darunavir concentration was below the assay limit of quantitation in 11 maternal and 15 cord blood samples. When maternal plasma darunavir concentration at delivery was above the limit of quantitation, the median (range) darunavir cord blood concentration was 0.41 μg/mL (range: less than 0.09 – 1.55 μg/mL), maternal plasma delivery concentration was 1.98 μg/mL (range: 0.19 – 5.62 μg/mL), ratio of cord blood to maternal delivery concentration was 0.18 (range: 0 – 0.82) and the median time between administration of the last antenatal darunavir dose and delivery was 15.1 hours (range 2.2 - 43.9 hours). Figure 3 presents the cord blood and maternal delivery darunavir concentrations plotted against the time between maternal dosing and delivery.
Figure 3.
Maternal darunavir concentrations at delivery and cord blood darunavir concentrations plotted against the time interval between maternal dosing and delivery. Circles present data for twice daily subjects and triangles present data for once daily subjects. Maternal plasma darunavir concentrations at delivery are represented with filled symbols, cord blood darunavir concentrations with open symbols. BQL = below quantitation limit of assay.
Overall, darunavir was well tolerated during pregnancy and postpartum. No subjects indicated side effects of darunavir as a reason for discontinuation. There was one toxicity thought by the care providers to be related to study treatment, life threatening grade 4 anemia in one subject, although the study team thought it most likely due to concomitant nucleoside reverse transcriptase inhibitor use. Six subjects had toxicities thought to be possibly related to darunavir treatment, including 3 with grade 2 liver function test elevations, 1 with intrauterine growth restriction, 1 with grade 2 hypercholesterolemia and 1 with gestational diabetes.
Study infants were delivered at a median of 38.6 weeks of gestation (range: 31.6 – 42.4 weeks) with a median birth weight of 3022 g (range: 1800 – 4560 g) and a median length of 49 cm (range: 41- 54.6 cm). Congenital anomalies were reported in 9 infants: two cases of postaxial polydactyly (supernumerary digit), and one case each of congenital cystic adenomatoid malformation (congenital pulmonary airway malformation), cardiac murmur, patent foramen ovale, patent ductus arteriosus with right ventricular hypertrophy, genitourinary chordee, ASD, VSD, and a hearing impairment. With the exception of the cardiac murmur and the patent foramen ovale, which were thought to be possibly related, these were deemed not related to darunavir exposure by the clinical care providers and by the study team.
No infant HIV infection data are available for three infants whose mothers withdrew from the study shortly after delivery. Of the remaining 63 infants, one was HIV infected. This infant had an initial positive HIV RNA on day 3 of life, subsequently confirmed with a second test, and also had congenital toxoplasmosis. The infant was born with weight appropriate for gestational age at 38 weeks gestation to a mother on the twice daily arm who enrolled in the third trimester and had an HIV RNA of 66,142 copies/mL at delivery. Her predose darunavir concentration on the day of her third trimester pharmacokinetic assessment was below the assay limit of detection, suggesting poor adherence. Fifty eight infants were confirmed uninfected and 4 had negative tests through age 2-9 weeks with no further testing available.
Discussion
Darunavir is an HIV protease inhibitor recommended for use as a first-line agent in combination with ritonavir dosed once daily for treatment of antiretroviral-naïve HIV infected adults and dosed twice daily for treatment of antiretroviral-experienced adults with at least one darunavir resistance mutation.21 The physiologic changes associated with pregnancy have been shown to decrease drug exposure of other HIV protease inhibitors by 28% to 41% compared to postpartum.4-9 Our study is the first to describe darunavir pharmacokinetics in a large group of pregnant women receiving both once and twice daily administration. Among our subjects, median darunavir AUC in the second and third trimesters decreased 38-39% with once daily dosing and 26% with twice daily dosing compared to postpartum. These results are consistent with previously published case reports and smaller series of patients.10-16 Zorrilla, et al, reported a decrease of 17-24% in darunavir AUC in 14 women dosed twice daily in the second and third trimesters.15 Colbers, et al reported a mean decrease in darunavir AUC of 34% in 6 women with twice daily dosing and 22% in18 women with once daily dosing in the third trimester.16 Our data are also consistent with a recent presentation describing darunavir exposure in pregnant women. Courbon, et al described DRV trough concentrations in 33 mostly African women in France receiving once and twice daily dosing. Trough DRV concentrations were decreased 20-25% in the second and third trimesters compared to the first trimester and were lower with once daily dosing than with twice daily dosing.22
Although trough concentration is considered the pharmacokinetic parameter that correlates best with protease inhibitor efficacy, trough concentration targets have not been established for darunavir.23 The darunavir EC50 of wild type HIV is 0.06 μg/mL, below the lower limit of sensitivity of 0.09 μg/mL of the assay used in this study.23 Resistance of HIV to protease inhibitors develops with accumulation of multiple mutations in the HIV genome, so that trough concentrations effective in treatment-naïve subjects who lack prior exposure to protease inhibitors may not be effective in treatment-experienced subjects with HIV strains that include protease resistance mutations. Darunavir trough concentrations are lower with once daily than twice daily dosing, and once daily dosing is recommended only for antiretroviral-naïve patients and for treatment-experienced patients with no darunavir resistance mutations.21 Our once daily dosing subjects had median trough concentrations during the second and third trimester of 0.99 and 1.17 μg/mL, compared to median troughs of 2.71 μg/mL in the same women postpartum and 2.16 - 2.28 μg/mL in clinical trials of once daily darunavir in HIV-infected adults.17 Median trough concentrations were less affected by pregnancy in our twice daily dosing subjects, who had second and third trimester median troughs of 2.12 μg/mL and 2.22 μg/mL, compared to 2.51 μg/mL postpartum and 3.39 - 3.58 μg/mL in clinical trials of HIV-infected adults receiving twice daily darunavir.17
It is difficult to determine the clinical significance of a reduction in darunavir exposure of the magnitude we observed and whether HIV-infected pregnant women would benefit from use of an increased dose during pregnancy. In our study, HIV RNA at delivery was below 400 copies/mL in 90% of once daily dosing subjects and 81% of twice daily dosing subjects. In the study by Zorrilla, et al, 3 of 14 subjects had detectable HIV RNA (>50 copies/mL) during pregnancy and 2 of these subjects became undetectable during postpartum follow up while continuing on darunavir.15 In the study by Colbers, et al of pregnant women receiving once daily dosing, 73% had an HIV RNA below 50 copies/mL and 93% below 1000 copies/mL approaching delivery.24 There were no infected infants born to the 12 mothers in the Zorrilla study who remained on therapy at delivery or the 15 mothers in the Colbers study.15,24 In our study, one infant was infected out of 64 with at least some virologic testing results available.
The impact of antiretroviral use during pregnancy on the durability of maternal antiretroviral treatment continuing after delivery should also be considered in determining antiretroviral doses during pregnancy. It is unknown whether a sustained period of decreased darunavir exposure during pregnancy could result in a more rapid development of resistant virus and treatment failure during long term postnatal treatment.
Protease inhibitors are highly protein bound drugs, and plasma protein binding of drugs generally decreases during pregnancy due to decreases in the quantity of albumin and alpha-1-acid glycoprotein.25 As a result, concentration of free (unbound) drug tends to be higher in pregnant women compared to nonpregnant women with the same total drug concentration. Since free drug is the pharmacologically active fraction of drug in plasma, reductions in protein binding during pregnancy have the potential to at least partially compensate for the reduction in total plasma protease inhibitor concentrations, as has been shown for lopinavir.26-28
Interpretation of the lopinavir protein binding data has been controversial, with some authors concluding that the protein binding changes negate the impact of differences in total lopinavir concentration during pregnancy, while others disagree.26-29 Concentrations of free darunavir during pregnancy have been reported in 2 studies. Zorrilla, et al reported free darunavir concentrations in 6 second trimester, 7 third trimester and 11 postpartum subjects. While total AUC was significantly decreased during pregnancy, there were no significant differences in free darunavir AUC or Cmin, or in darunavir free fraction.15 In contrast, Colbers, et al reported no difference in mean (95%CI) darunavir free fraction in 19 women during pregnancy compared to 14 women postpartum (12% (11-14%) in the third trimester and 10% (7-13%) postpartum).16
These studies are too small and lack sufficient clinical correlations to allow definitive conclusions as to whether changes in protein binding during pregnancy may mitigate the decreases in total plasma darunavir concentrations. However, the free fraction of darunavir is much greater than that of lopinavir, which has a free fraction in nonpregnant adults of 1-2%, suggesting that changes in protein binding associated with pregnancy are more likely to have a significant impact on free drug concentration of lopinavir than of darunavir.30
The magnitude of the reduction in darunavir exposure associated with pregnancy that we observed is consistent with the decreases that have been observed for other protease inhibitors.5-9 For some of these drugs, including lopinavir, atazanavir and nelfinavir, use of increased doses in pregnancy has been shown to result in plasma concentrations equivalent to those seen in nonpregnant adults receiving standard dosing.31-33 Two randomized studies of standard versus increased dose lopinavir in pregnancy suggest that increased lopinavir doses may be beneficial for pregnant women with a detectable viral load at initiation of treatment in pregnancy or with detectable lopinavir resistance mutations.34,35 The package insert for atazanavir, the only protease inhibitor approved in the US for use in pregnancy, includes a recommendation for an increased dose for treatment-experienced pregnant women in the second or third trimester who are also receiving tenofovir or an H2-receptor antagonist, which have been shown to reduce atazanavir exposure.36 There are no data on darunavir pharmacokinetics or clinical outcomes with use of an increased dose in pregnancy, and an increased twice daily darunavir dose (800 mg or 900 mg darunavir with 100 mg ritonavir) is currently under study in another arm of P1026s. Ritonavir exposure was reduced during pregnancy in our subjects, consistent with the reduction seen in other pregnancy studies of ritonavir boosted protease inhibitors in pregnant women.15,31,32 While it is not known if use of an increased ritonavir dose would increase darunavir exposure during pregnancy, the poor tolerability of ritonavir makes increasing the ritonavir dose unattractive to pregnant women and their care providers.
Darunavir was well tolerated in our subjects, who demonstrated little toxicity attributed to darunavir use. Placental transfer of darunavir was poor, as has been demonstrated for other protease inhibitors.37 The median ratio of maternal to cord blood darunavir was 0.18, consistent with that seen in previous studies, and this ratio reached a steady state at around 8 hours after administration of the last maternal dose prior to delivery.15,24
There are several limitations to our study. Our study used an opportunistic design, enrolling pregnant women receiving darunavir as part of clinical care, and enrolled a very heterogeneous population. Women enrolled at various stages of pregnancy and with varying past histories of antiretroviral use, ranging from none to years of treatment with multiple regimens. Their duration of darunavir treatment at the time of third trimester sampling ranged from 2 weeks to nearly 5 years. Our study population was biased towards those who tolerated and responded well to darunavir therapy, as pregnant women with early darunavir toxicity or lack of efficacy may have been switched to other antiretrovirals and would not have been eligible for enrollment. Clinical care providers determined whether subjects received once or twice daily dosing and were responsible for making adjustments to the antiretroviral regimens due to drug toxicity and therapeutic response. Our study included no rigorous measure of adherence, although inspection of the intensive pharmacokinetic profiles did reveal whether subjects were at steady state at the time of sampling. In addition, we measured concentrations of total but not free darunavir, so we can provide no new data to address the question of the impact of protein binding changes on free darunavir concentrations during pregnancy.
In summary, our study of a large number of women demonstrates a significant reduction in darunavir plasma concentrations during pregnancy with once and twice daily dosing, consistent with reductions seen with other proteases inhibitors during pregnancy. Given the absence of established darunavir trough concentration targets, it is reasonable to use typical plasma concentrations seen in non-pregnant adults as a therapeutic target when darunavir is used during pregnancy. In order to achieve darunavir plasma concentrations during pregnancy equivalent to those seen in non-pregnant adults, an increased twice daily dose may be necessary. This may be especially important for treatment experienced women who may have developed antiretroviral resistance mutations.
Acknowledgements
We thank the study participants and their families. In addition to the authors, members of the IMPAACT P1026s protocol team include Francesca Aweeka, Michael Basar, Emily Barr, Mark Byroads, Nantasak Chotivanich, Lisa M. Frenkel, Kathy George, Elizabeth Hawkins, Kathleen Adriane Hernandez, Amy Jennings, Rita Patel and Pra-ornsuda Sukrakanchana. We also thank the follow investigators and staff at the enrolling sites:
New Jersey Medical School CRS (Linda Bettica, RN; Charmane Calilap-Bernardo, MA, PNPC; Arlene Bardeguez, MD, MPH); Texas Children's Hospital CRS (Shelley Buschur, RN, CNM; Chivon Jackson, RN, BSN, ADN; Mary Paul, MD); Columbia CRS (Philip La Russa, MD); University of Miami Pediatric Perinatal HIV/AIDS CRS ( Claudia Florez, MD; Patricia Bryan, BSN, MPH; Monica Stone, MD); University of California San Diego Mother-Child-Adolescent Program CRS (Andrew D. Hull, MD (I think to be consistent, we should not include society memberships for some and not for others. The other OBs are FACOG also.); Mary Caffery, RN, MSN; Stephen A. Spector, MD); Duke University Medical Center CRS (Joan Wilson, RN, BSN, MPH; Julieta Giner, RN, ACRN; Margaret A. Donnelly, PA-C); New York University, New York NICHD CRS (Nagamah Deygoo, MD; Aditya Kaul, MD; William Borkowsky, MD); Jacobi Medical Center Bronx NICHD CRS (Mindy Katz, MD; Raphaelle Auguste, RN; Andrew Wiznia, MD); University of South Florida - Tampa NICHD CRS (Karen L. Bruder, MD; Gail Lewis, RN; Denise Casey, RN); University of Southern California School of Medicine– Los Angeles County NICHD CRS (Françoise Kamer, MD; LaShonda Spencer, MD;James Homans, MD); University of Colorado Denver NICHD CRS (Torri Metz, MD; Jenna Wallace, MSW; Alisa Katai, MHA); Hospital dos Servidores Rio de Janeiro NICHD CRS (Esau C. Joao MD, PhD; Plinio Tostes Berardo Carneiro da Cunha, MD, PhD; Maria Isabel Fragoso da Silveira Gouvêa, MD); Hospital General de Agudos Buenos Aires NICHD CRS (Marcelo H. Losso, MD; Silvina A. Ivalo, MD; Alejandro Hakim, MD); Miller Children's Hospital NICHD CRS (Audra Deveikis MD; Jagmohan Batra MD; Janielle Jackson Alvarez RN); Hopsital Santa Casa Porto Alegre Brazil NICHD CRS (Regis Kreitchmann, PhD, MD; Debora Fernandes Coelho, MN, PhD; Marcelo Comerlato Scotta, MSc, MD); St Jude CRS (Katherine M. Knapp, MD; Nina Sublette, FNP, PhD; Thomas Wride, MS); University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Irma L. Febo MD; Ruth Santos RN, MPH; Vivian Tamayo MD); The Children's Hospital of Philadelphia (Steven D. Douglas, MD; Carol A. Vincent, PhD, CRNP; Samuel Parry, MD); Bronx-Lebanon Hospital CRS (Jenny Gutierrez, MD; Mary Elizabeth Vachon, MPH; Murli Purswani, MD); Siriraj Hospital Mahidol University, Bangkok, Thailand CRS (Thanomsak Anekthananon, MD; Amphan Chalermchokcharoenkit, MD; Kulkanya Chokephaibulkit, MD).
Funding source: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
These data were presented in part at the 3rd International Workshop on HIV Pediatrics; Rome, Italy; July 15-16, 2011.
References
- 1. [April 3, 2014];Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 2.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997 Nov;33(5):328–343. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mirochnick M, Best B, Clarke D. Antiretroviral Pharmacology: Special Issues Regarding Pregnant Women and Neonates. Clinics in Perinatology. 2010:37. doi: 10.1016/j.clp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006 Oct 3;20(15):1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 5.Read JS, Best BM, Stek AM, et al. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and postpartum. HIV medicine. 2008 Nov;9(10):875–882. doi: 10.1111/j.1468-1293.2008.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta EP, Bardeguez A, Zorrilla CD, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004 Feb;48(2):430–436. doi: 10.1128/AAC.48.2.430-436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cressey TR, Best BM, Achalapong J, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013 Sep;76(3):475–483. doi: 10.1111/bcp.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capparelli EV, Stek A, Best B, Rossi S, Burchett SK, Li H, Read JS, Smith E, Hawkins E, Basar M, Mirochnick M. Boosted FosAmprenavir Pharmacokinetics During Pregnancy.. The 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. February, 2010. [Google Scholar]
- 9.Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruela Correa JC, D'Arcy DM, dos Reis Serra CH, Nunes Salgado HR. Darunavir: a critical review of its properties, use and drug interactions. Pharmacology. 2012;90(1-2):102–109. doi: 10.1159/000339862. [DOI] [PubMed] [Google Scholar]
- 11.Pinnetti C, Tamburrini E, Ragazzoni E, De Luca A, Navarra P. Decreased plasma levels of darunavir/ritonavir in a vertically infected pregnant woman carrying multiclass-resistant HIV type-1. Antivir Ther. 2010;15(1):127–129. doi: 10.3851/IMP1473. [DOI] [PubMed] [Google Scholar]
- 12.Ivanovic J, Bellagamba R, Nicastri E, et al. Use of darunavir/ritonavir once daily in treatment-naive pregnant woman: pharmacokinetics, compartmental exposure, efficacy and safety. AIDS. 2010 Apr 24;24(7):1083–1084. doi: 10.1097/QAD.0b013e32833653b2. [DOI] [PubMed] [Google Scholar]
- 13.Pacanowski J, Bollens D, Poirier JM, et al. Efficacy of darunavir despite low plasma trough levels during late pregnancy in an HIV-hepatitis C virus-infected patient. AIDS. 2009 Sep 10;23(14):1923–1924. doi: 10.1097/QAD.0b013e32832e534b. [DOI] [PubMed] [Google Scholar]
- 14.Furco A, Gosrani B, Nicholas S, et al. Successful use of darunavir, etravirine, enfuvirtide and tenofovir/emtricitabine in pregnant woman with multiclass HIV resistance. AIDS. 2009 Jan 28;23(3):434–435. doi: 10.1097/QAD.0b013e32832027d6. [DOI] [PubMed] [Google Scholar]
- 15.Zorrilla CD, Wright R, Osiyemi OO, et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100 mg administered twice daily. HIV medicine. 2014 Jan;15(1):50–56. doi: 10.1111/hiv.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colbers A, Molto J, Ivanovic J, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015 Feb;70(2):534–542. doi: 10.1093/jac/dku400. [DOI] [PubMed] [Google Scholar]
- 17.PrezistaTM package insert. Janssen Therapeutics; Titusville, NJ: Available at http://www.prezista.com/sites/default/files/pdf/us_package_insert.pdf. [Google Scholar]
- 18. [May 5, 2010];The Division of AIDS (DAIDS) Standardized Toxicity Table for Grading Severity of Adult Adverse Experiences. 1992 Aug; Available from http://rsc.techres.com/safetyandpharmacovigilance.
- 19.Croteau D, Rossi SS, Best BM, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90% inhibitory concentration. J Antimicrob Chemother. 2013 Mar;68(3):684–689. doi: 10.1093/jac/dks441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiFrancesco R, Rosenkranz SL, Taylor CR, et al. Clinical pharmacology quality assurance program: models for longitudinal analysis of antiretroviral proficiency testing for international laboratories. Ther Drug Monit. 2013 Oct;35(5):631–642. doi: 10.1097/FTD.0b013e31828f5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [April 9, 2014]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 22.Courbon E, Matheron S, et al. Safety, efficacy, and pharmacokinetics of darunavir/ritonavir-containing regimen in pregnant HIV+ women.. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA. March 5-8, 2012; Abstract #1011. [Google Scholar]
- 23.Mirochnick M. Antiretroviral pharmacology in pregnant women and their newborns. Ann N Y Acad Sci. 2000 Nov;918:287–297. doi: 10.1111/j.1749-6632.2000.tb05498.x. [DOI] [PubMed] [Google Scholar]
- 24.Colbers A, Moltó J, Ivanovic J, et al. Low Darunavir Exposure during Pregnancy with 800/100 mg Darunavir/r QD Dosing.. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. March, 2014; Abstract #887. [Google Scholar]
- 25.Mattison D, Zajicek A. Gaps in knowledge in treating pregnant women. Gender medicine. 2006 Sep;3(3):169–182. doi: 10.1016/s1550-8579(06)80205-6. [DOI] [PubMed] [Google Scholar]
- 26.Aweeka FT, Stek A, Best BM, et al. Lopinavir protein binding in HIV-1-infected pregnant women. HIV medicine. 2010 Apr;11(4):232–238. doi: 10.1111/j.1468-1293.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson KB, Dumond JB, Prince HA, et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013 May 1;63(1):51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fayet-Mello A, Buclin T, Guignard N, et al. Free and total plasma levels of lopinavir during pregnancy, at delivery and postpartum: implications for dosage adjustments in pregnant women. Antivir Ther. 2013;18(2):171–182. doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 29.Ripamonti D, Cattaneo D. Do lopinavir and ritonavir require a dose adjustment during pregnancy? J Acquir Immune Defic Syndr. 2014 Apr 1; doi: 10.1097/QAI.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 30.Kaletra package insert. Abbott Laboratories; North Chicago, IL.: Jun, 2008. [November 25, 2008]. at http://www.rxabbott.com/pdf/kaletratabpi.pdf. [Google Scholar]
- 31.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010 Aug 1;54(4):381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreitchmann R, Best BM, Wang J, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr. 2013 May 1;63(1):59–66. doi: 10.1097/QAI.0b013e318289b4d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormack SA, Best BM, Stek A, Wang J, Kreitchmann R, Shapiro D, Smith E, Mofenson LM, Capparelli EV, Mirochnick M, the IMPAACT P1026s Protocol Team Pharmacokinetics of an Increased Nelfinavir Dose During the 3rd Trimester of Pregnancy.. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. March 2014. [Google Scholar]
- 34.Bonafe SM, Costa DA, Vaz MJ, et al. A randomized controlled trial to assess safety, tolerability, and antepartum viral load with increased lopinavir/ritonavir dosage in pregnancy. AIDS Patient Care STDS. 2013 Nov;27(11):589–595. doi: 10.1089/apc.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini-Oliveira M, Estrela Rde C, Veloso VG, et al. Randomized clinical trial comparing the pharmacokinetics of standard- and increased-dosage lopinavir-ritonavir coformulation tablets in HIV-positive pregnant women. Antimicrob Agents Chemother. 2014 May;58(5):2884–2893. doi: 10.1128/AAC.02599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capparelli E, Rakhmanina N, Mirochnick M. Pharmacotherapy of perinatal HIV. Semin Fetal Neonatal Med. 2005 Apr;10(2):161–175. doi: 10.1016/j.siny.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid). Antivir Ther. 2011;16(8):1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 38.Boffito M, Winston A, Jackson A, et al. Pharmacokinetics and antiretroviral response to darunavir/ritonavir and etravirine combination in patients with high-level viral resistance. AIDS. 2007 Jul 11;21(11):1449–1455. doi: 10.1097/QAD.0b013e3282170ab1. [DOI] [PubMed] [Google Scholar]