Abstract
Norepinephrine (NE) is a key modulator of synaptic plasticity in the hippocampus, a brain structure crucially involved in memory formation. NE boosts synaptic plasticity mostly through initiation of signaling cascades downstream from beta (β)-adrenergic receptors (β-ARs). Previous studies demonstrated that a β-adrenergic receptor agonist, isoproterenol, can modify the threshold for long-term potentiation (LTP), a putative cellular mechanism for learning and memory, in a process known as “metaplasticity.” Metaplasticity is the ability of synaptic plasticity to be modified by prior experience. We asked whether NE itself could engage metaplastic mechanisms in area CA1 of mouse hippocampal slices. Using extracellular field potential recording and stimulation, we show that application of NE (10 µM), which did not alter basal synaptic strength, enhances the future maintenance of LTP elicited by subthreshold, high-frequency stimulation (HFS: 1 × 100 Hz, 1 sec). HFS applied 30 min after NE washout induced long-lasting (>4 h) LTP, which was significantly extended in duration relative to HFS alone. This NE-induced metaplasticity required β1-AR activation, as coapplication of the β1-receptor antagonist CGP-20712A (1 µM) attenuated maintenance of LTP. We also found that NE-mediated metaplasticity was translation- and transcription-dependent. Polysomal profiles of CA1 revealed increased translation rates for specific mRNAs during NE-induced metaplasticity. Thus, activation of β-ARs by NE primes synapses for future long-lasting plasticity on time scales extending beyond fast synaptic transmission; this may facilitate neural information processing and the subsequent formation of lasting memories.
Long-term potentiation (LTP) of synaptic strength is a putative cellular mechanism for learning and memory (Bliss and Lomo 1973; Bliss and Collingridge 1993; Bear and Malenka 1994; Larkman and Jack 1995; Martin et al. 2000). The mammalian hippocampus is critical for making enduring memories, and it is densely innervated by the noradrenergic system, which can modulate memory formation and consolidation (Sara 2009). Indeed, noradrenergic receptors are found on hippocampal principal neurons (reviewed by Gelinas and Nguyen 2007), and the locus coeruleus, the primary source of neural norepinephrine (NE), critically regulates behavioral memory in rodents (Berridge and Waterhouse 2003; Lemon et al. 2009; Sara 2009). Modulation of LTP and of learning and memory can occur through NE acting on β-adrenergic receptors (β-ARs) to enhance LTP and boost memory endurance (Stanton and Sarvey 1984; Harley et al. 1996; Katsuki et al. 1997; reviewed by Gelinas and Nguyen 2007). Additionally, β-ARs can enhance learning by boosting trafficking of the α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor subunit, GluA1 (Hu et al. 2007).
Translation regulation is a key component of enduring forms of synaptic plasticity (reviewed by Sossin and Lacaille 2010). Neuromodulators can modify the threshold of future synaptic plasticity in a process called “metaplasticity” (Abraham and Bear 1996; Abraham 2008; Abraham and Williams 2008). For example, ryanodine receptors enhanced the maintenance of homosynaptic and heterosynaptic LTP when these receptors were activated 30 min prior to a subthreshold electrical stimulus (Mellentin et al. 2007; Sajikumar et al. 2009). Similarly, previous studies have found that, when paired with a subthreshold high-frequency stimulus (HFS) that alone did not elicit persistent LTP, direct β-AR activation by a β-agonist, isoproterenol (ISO), induced protein synthesis-dependent LTP that persisted for several hours in hippocampal slices (Gelinas and Nguyen 2005; see also Thomas et al. 1996, for 5-Hz stimulation data). In contrast, β-AR antagonism prevented novelty-induced LTP enhancement (Straube et al. 2003). Importantly, ISO engaged metaplastic mechanisms to recruit protein synthesis-dependent LTP when a subthreshold HFS was applied 1 h after ISO washout (Tenorio et al. 2010). β-AR activation by ISO recruited cAMP-dependent protein kinase (PKA) to phosphorylate GluA1 subunits of AMPA receptors, which increased cell surface GluA1 expression in a protein synthesis-dependent manner (Tenorio et al. 2010).
It is not known whether NE can elicit metaplastic enhancement of LTP maintenance in a manner that requires β-adrenergic receptor activation, translation, and transcription. It is also unclear whether the translation-dependence of β-AR-mediated metaplasticity reflects increased translation of specific mRNAs, particularly those encoding AMPAR subunits involved in regulating trafficking. To this end, we sought to determine if NE could elicit metaplasticity and if so, whether translation and transcription were required. We also used polysomal profiling to begin to identify which specific mRNAs display increased rates of translation in response to NE application 30 min prior to HFS. Our findings establish that NE can prime synapses for subsequent enhancement of LTP maintenance, and that this requires transcription and translation. Importantly, we show, for the first time in the metaplasticity literature, that NE, alone or paired with subsequent HFS, boosts translation rates of mRNAs encoding AMPAR subunits. Thus, de novo translation of mRNAs for transmitter receptor subunits may represent a critical mechanism for synaptic metaplasticity in the mammalian brain. In a broader perspective, our biochemical data provide direct support for the notion that protein synthesis, measured as increased translation rates of mRNAs, is critical for specific forms of long-lasting synaptic potentiation.
Results
Norepinephrine (NE) induces metaplasticity of LTP
In the dentate gyrus of rat hippocampal slices, acute application of NE elicits population spike potentiation (Stanton and Sarvey 1985). In area CA1 of mouse hippocampal slices, activation of β-ARs by acute application of a β-AR agonist, isoproterenol (ISO), enhances LTP induction when paired with a subthreshold stimulus (Thomas et al. 1996). Also, NE paired with high-frequency stimulation (HFS) enhanced field EPSP (fEPSP) potentiation in CA1 of rat hippocampal slices (Katsuki et al. 1997). Additionally, Tenorio et al. (2010) found that ISO enhanced future LTP through a process known as “metaplasticity” (see Abraham and Bear 1996).
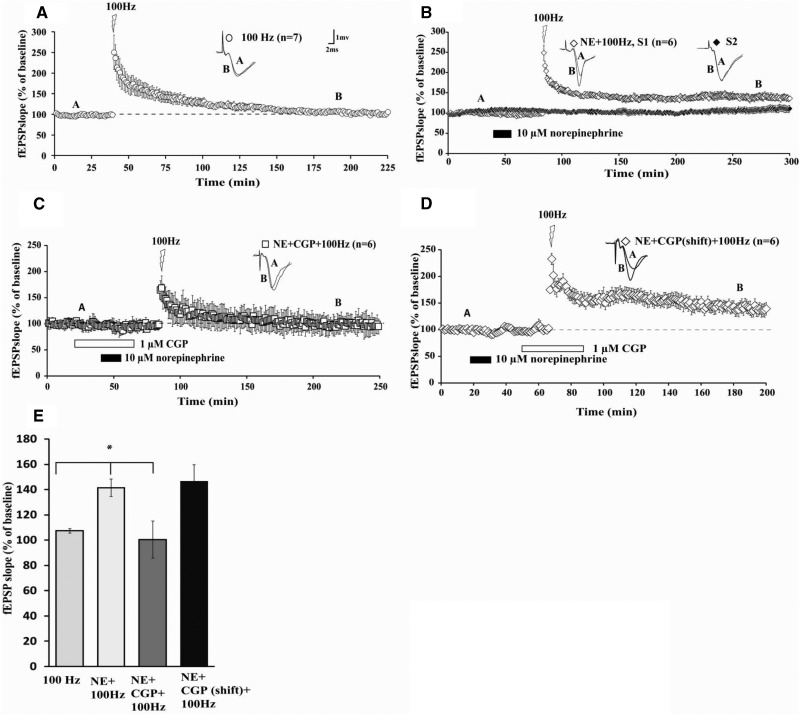
Although β-AR activation by a specific β-AR agonist elicited metaplasticity of subsequent LTP, it is unclear whether the natural transmitter, NE, could do the same. In order to address this issue, we sought to determine whether acute application of NE could “prime” synapses to boost subsequent LTP elicited by high-frequency tetanization that was by itself subthreshold for eliciting long-lasting LTP. Application of HFS (1 × 100 Hz, 1 sec) alone to area CA1 of mouse hippocampal slices generated fEPSP potentiation that decayed to baseline within 2 h (Fig. 1A; mean fEPSP slope 120 min after HFS was 107% ± 2% of baseline fEPSP slopes). However, prior application of 10 µM NE for 15 min facilitated LTP when HFS was applied 30 min after NE washout (Fig. 1B; mean fEPSP slope was 142% ± 7% 120 min after HFS). In a previous study (Tenorio et al. 2010), a 1-h time lag between washout of ISO and HFS was used successfully to elicit metaplastic enhancement of LTP maintenance similar to our present study. Thus, acute bath application of NE can prime synapses to boost subsequent expression of long-lasting LTP.
Figure 1.
NE-induced metaplasticity is maintained for several hours and is mediated through β1-adrenergic receptors. (A) HFS alone (open circles) induces transient (<2 h) LTP. (B) Applying HFS 30 min after NE induces NE-LTP (S1; open diamonds) that lasts for several hours (>3 h) after stimulation. (C) Application of a β1-AR antagonist, CGP-20712A (“CGP”) inhibits LTP generated by HFS given 30 min after NE application (open squares). (D) Application of CGP during 100-Hz HFS did not reduce the NE-primed LTP, compared with NE + 100 Hz (P > 0.05 n = 6). (E) Summary histograms of fEPSP slopes obtained 120 min after HFS. Sample traces were taken at points A and B on graphs. NE did not alter basal synaptic transmission in a second independent pathway (1B: S2, filled diamonds). Results in E represent means ± SEM. (*) P < 0.05, see text for group comparison data. Calibration: 2 mV, 1 msec.
β-Adrenergic receptors can boost LTP duration (Connor et al. 2011). To determine whether β1 receptors are contributing to NE-induced metaplasticity, we applied a β1-AR specific antagonist, CGP-20712A (1 µM). Co-application of CGP overlapping with NE substantially reduced the maintenance of subsequently induced LTP (Fig. 1C; mean fEPSP slope was 100% ± 15% of baseline at 120 min after HFS). Also, shifting application of CGP to overlap only with HFS, after washout of NE, did not affect expression of subsequent primed LTP (Fig. 1D; mean fEPSP slope was 147% ± 13% of baseline at 120 min after HFS in slices treated with CGP only during HFS, P > 0.05 compared with NE + 100 Hz in Fig. 1B). Thus, metaplasticity of LTP is not the result of incomplete washout of NE. An ANOVA comparing all four groups (100 Hz, NE + 100 Hz, NE + CGP + 100 Hz, NE + CGP shift + 100 Hz) at 120 min post-HFS revealed a significant difference between groups [F(3,21) = 5.46, P < 0.05]. Subsequent Tukey–Kramer post hoc tests showed that NE + 100 Hz enhanced the maintenance of LTP relative to HFS alone, and that this effect was blocked by CGP (Fig. 1C; P < 0.05). Collectively, these data indicate that NE acts through β1-ARs to prime future induction of long-lasting potentiation.
NE-induced metaplasticity requires translation
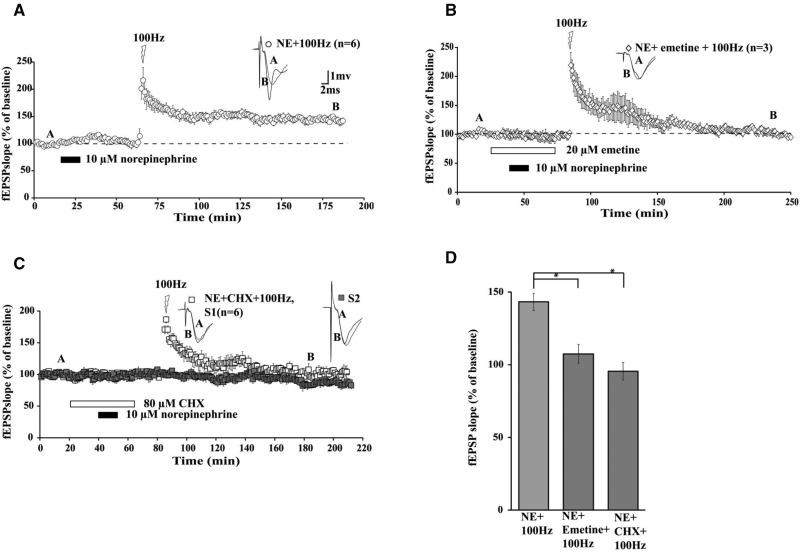
Activation of β-ARs by pairing ISO with HFS elicits protein synthesis-dependent LTP in area CA1 of mouse hippocampal slices (Gelinas and Nguyen 2005). ISO applied prior to HFS can also promote translation-dependent LTP when one train of 100-Hz was applied well after ISO washout (Tenorio et al. 2010). To determine whether the natural transmitter, NE, could prime synapses in CA1 to express translation-dependent LTP, we used two translation inhibitors, emetine (EME, 20 µM) and cycloheximide (CHX, 80 µM). Coapplication of EME and NE attenuated the maintenance of LTP induced by subsequent HFS (Fig. 2B; mean fEPSP slope was 108% ± 7% of baseline, 120 min after HFS). CHX treatment overlapping with NE also reduced the maintenance of LTP (Fig. 2C; mean fEPSP slope was 96% ± 6% of baseline, 120 min after HFS). An ANOVA comparing all three groups (Fig. 2A, NE control; Fig. 2B, NE + EME; Fig. 2C, NE + CHX) 120 min after HFS demonstrated a significance difference between groups (F(2,12) = 17.84), P < 0.001). Subsequent Tukey–Kramer post hoc tests showed that EME and CHX impaired LTP maintenance relative to NE-treated controls (Fig. 2D; P < 0.05). Emetine and CHX did not differ significantly in their impairment of LTP (P > 0.05). These data indicate that the facilitation of LTP maintenance by NE requires translation. A second set of inputs converging on the same postsynaptic cells (pathway “S2” in Fig. 2C) was monitored to confirm that CHX did not adversely affect basal fEPSPs elicited at test stimulation frequency of once per minute.
Figure 2.
Protein synthesis is required for NE-induced metaplasticity. (A) NE-induced metaplasticity by timed HFS (open circles). Emetine (B; open diamonds) and CHX (C; open squares) inhibited the maintenance of NE-LTP. (D) Summary histogram of mean fEPSP slopes at 120 min after HFS, comparing effects of emetine and CHX on NE-LTP. Sample traces were taken at points A and B on graphs. CHX did not alter basal synaptic transmission in a second independent pathway (2C: S2, filled squares) that did not receive HFS. Results in D represent means ± SEM. (*) P < 0.05, see text for group comparison data. Calibration: 2 mV, 1 msec. Calibration: 2 mV, 1 msec.
Overall, these data demonstrate that NE elicits metaplasticity of subsequent LTP in CA1 by recruiting mechanisms that depend on translation. These mechanisms appear to be engaged during and/or shortly after NE application. Does this NE-induced priming of subsequent long-lasting potentiation also require transcription?
Role of transcription in NE-induced metaplasticity
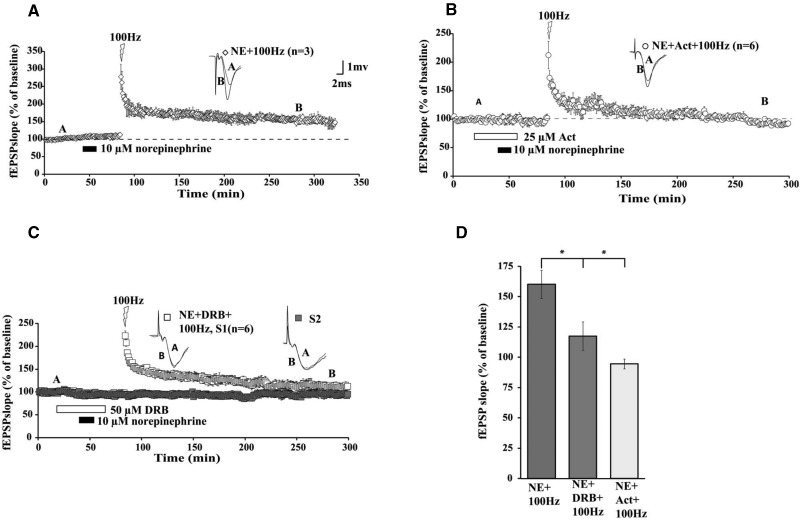
Altered gene expression is a crucial element of long-term memory and also of late phases of LTP that persist for several hours (Abraham et al. 1993; Nguyen et al. 1994; Frey et al. 1996; Sossin 1996). To date, it is unclear whether NE can recruit transcription to prime extended maintenance of subsequent LTP. Since our present data show that NE can boost maintenance of LTP so that potentiation can last for several hours after HFS, we hypothesized that a transcriptional component for metaplasticity may be initiated by NE treatment prior to LTP induction. To address this idea, we used two transcription blockers, actinomycin D (Act-D, 25 µM) and dichlorobenzimidazole 1-β-D-ribofuranoside (DRB, 50 μM), to determine whether transcription is required for NE-induced metaplasticity. Act-D at 25 µM has been shown to inhibit transcription by >70% in hippocampal slices (Nguyen et al. 1994). When coapplied with NE, Act-D reduced the maintenance of LTP (Fig. 3B; mean fEPSP slope was 95% ± 4% of baseline at 3 h after HFS). To mitigate against the possibility that the LTP decay in response to Act-D could result from nonspecific effects of the drug, we repeated this experiment using another transcription inhibitor, DRB, while monitoring a second control pathway (S2). DRB likewise reduced the maintenance of LTP (Fig. 3C; mean fEPSP slope was 118% ± 12% of baseline at 3 h after HFS). An ANOVA followed by a Tukey–Kramer post hoc test revealed a significant difference (Fig. 3D; F(2,12) = 9.60, P < 0.05) between the groups when compared at 3 h after HFS. Act-D and DRB-treated groups did not differ significantly (P > 0.05) from each other.
Figure 3.
NE-induced LTP is transcription-dependent. (A) HFS (open diamonds) 30 min after 15 min of NE application elicited NE-LTP lasting several (>3) hours. Application of Act-D (B; open circles) or DRB (C; open squares) prevented the NE-LTP maintenance. (D) Summary histogram of fEPSP slopes obtained 180 min after HFS comparing effects of application of Act-D and DRB on NE-LTP. Sample traces were taken at points A and B on graphs. Addition of DRB did not alter basal synaptic transmission in a second independent pathway (3C: S2, filled squares) that did not receive HFS. Results in D represent means ± SEM. (*) P < 0.05, see text for group comparison data. Calibration: 2 mV, 1 msec.
Overall, these data complement our findings with translation inhibitors to underscore critical requirements for both translation and transcription in mediating the priming effects of NE on subsequent maintenance of LTP. Which specific transcripts display increased translation rates following NE treatment that promotes extended duration of subsequent LTP?
Polysome profiling reveals that NE elicits increased translation rates of mRNAs encoding AMPAR subunits
To date, it is unclear which specific mRNAs are translated in response to β-AR activation by NE. Activation of β-ARs by ISO persistently increases phosphorylation of GluA1 subunits of AMPA receptors (AMPARs), and it also elicits a translation-dependent increase in cell surface levels of GluA1-containing AMPARs during metaplasticity (Tenorio et al. 2010; see also Hu et al. 2007). This finding, along with our present data obtained using NE as the trigger for translation-dependent metaplasticity of LTP, suggests that a possible mechanism for mediating this metaplasticity may involve increased translation rates of mRNAs encoding GluA1. Many transcripts may be modulated by NE treatment, and it is a formidable task to pursue a thorough, complete screen of the “transcriptome” in the mouse hippocampus following NE treatment. However, it is feasible to examine specific transcripts and their rates of translation following NE treatment in hippocampal slices. As such, we sought to assess whether the translation rates of mRNAs encoding GluA1 and GluA2 are increased following acute treatments of mouse hippocampal slices with NE. GluA2 is a subunit of AMPARs that is known to also be important, along with GluA1, for regulating trafficking of AMPARs in hippocampal neurons during LTP (reviewed by Shepherd and Huganir 2007; Henley et al. 2011). Thus, increased translation rates for mRNAs encoding GluA1 and GluA2 may underlie NE-induced metaplasticity of LTP.
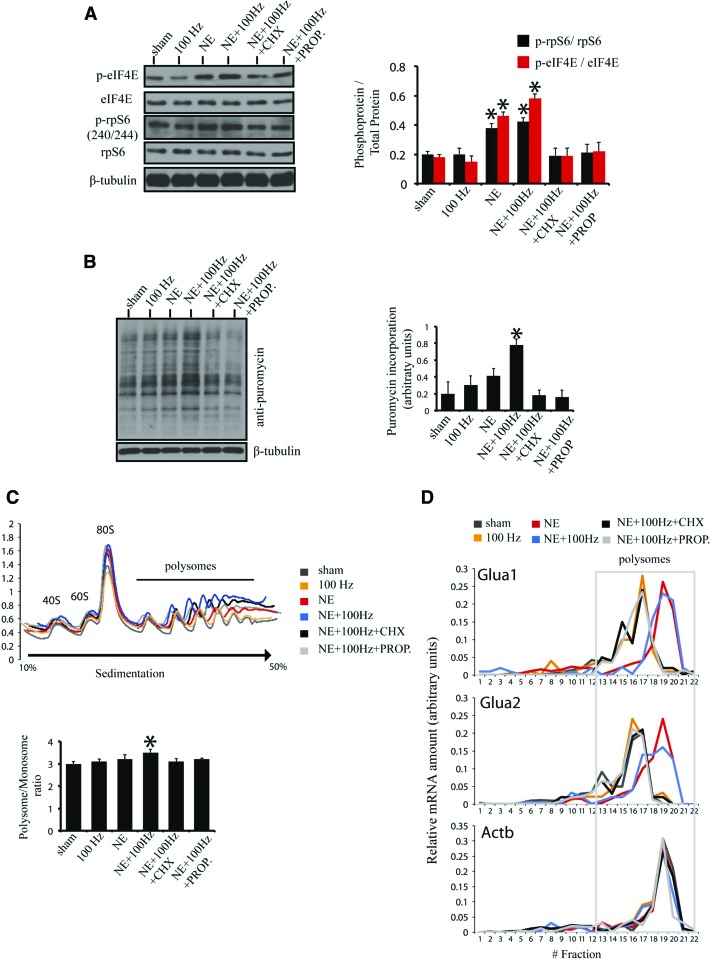
To investigate which signaling pathways upstream of translation are affected by our metaplasticity treatment groups, we used immunoblotting of major hubs in those signaling pathways. Postsynaptically, there are two major signaling pathways downstream from glutamatergic receptors, which affect translation by phosphorylating key translation initiation factors: the phosphoinositide-3-kinase/mammalian target of rapamycin (PI3K/mTOR) and Ras/mitogen-activated protein kinase/MAPK-interacting kinase (Ras/MAPK/Mnk) pathways. Phosphorylation of ribosomal protein S6 (rpS6) is an indicator for the activity of the PI3K/mTOR branch, whereas phospho-eIF4E (eukaryotic initiation factor 4E) is a marker for the activation levels of the Ras/MAPK/Mnk pathway.
Using immunoblotting in lysates from acute hippocampal slices for our treatment groups, we noted a marked increase in both signaling pathways impinging on translation in the NE and NE + 100 Hz groups, as evidenced by combined increased phosphorylation of rpS6 and eIF4E (Fig. 4A). Subsequently, to assess any changes in global protein synthesis, we used puromycin labeling of nascent polypeptides (Schmidt et al. 2009) in acute slices for all of our treatment groups (Fig. 4B). Using an antibody against puromycin, we detected puromycin incorporation in our slices using immunoblotting (Fig. 4B). Out of all the treatment groups, only NE + 100 Hz was able to sustain a ∼50% increase in total protein synthesis as measured by puromycin incorporation (Fig. 4B). This notable increase in protein synthesis was also reflected in the association of mRNAs with different populations of ribosomes. Using polysome profiling (Gkogkas et al. 2013) in acute slices from our treatment groups, we showed that NE + 100 Hz induced an increase in mRNAs associated with polysomes, as evidenced by the increased polysome/monosome ratio (Fig. 4C). To examine whether translation of AMPAR mRNAs (Glua1, Glua2) is altered following our various treatments, we carried out qPCR on polysomal fractions, using specific primers for Glua1, Glua2, and Actb (actin-b mRNA) as a control. Notably, NE and NE + 100 Hz treatments elicited a shift of Glua1 and Glua2 mRNAs toward heavier polysomal fractions, suggesting that they are translated more efficiently following those treatments (Fig. 4D). Taken together, these data suggest that NE and NE + 100 Hz activate postsynaptic signaling pathways that increase translation. However, only NE + 100 Hz induced a change in global translation under these conditions, whereas both NE and NE + 100 Hz increased translation of Glua1 and Glua2 mRNAs.
Figure 4.
NE-induced metaplasticity activates translational signaling and stimulates translation of Glua1 and Glua2 mRNAs. (A, Left) Immunoblotting of hippocampal slices probed with antibodies against the indicated proteins. (Right) Quantification of immunoblotting (phosphoprotein/total protein for rps6 and eIF4E). n = 4, one-way ANOVA; (*) P < 0.05: for marked groups as compared with the sham, 100 Hz, NE + 100 Hz + CHX and NE + 100 Hz + PROP groups; for all other group comparisons P > 0.05 (not significant); β-tubulin is a loading control. (B) De novo protein synthesis assay with puromycin labeling for all treatment groups. (Left) Immunoblotting of hippocampal slices treated with puromycin and probed with anti-puromycin antisera; β-tubulin is a loading control. (Right) Quantification of puromycin incorporation. n = 4, one-way ANOVA; (*) P < 0.05: for NE + 100 Hz as compared with all other groups; for all other group comparisons P > 0.05 (not significant). (C, Top) Polysome profiling in hippocampal slices for the depicted treatment groups. Positions of the 40S, 60S, and 80S ribosome peaks, and polysomes are indicated. (Bottom) Polysome to monosome ratio is shown in the histogram, for all the treatment groups analyzed with polysome profiling. n = 4, one-way ANOVA; (*) P < 0.05: for NE + 100 Hz as compared with all other groups; for all other group comparisons P > 0.05 (not significant). (D) RT-qPCR for the indicated groups of polysome-extracted RNAs, with specific primers for Glua1, Glua2, and Actb mRNAs. Relative mRNA amount per fraction (sedimentation) is depicted for all treatment groups.
These data reveal that NE alone, or NE followed later by 100 Hz (which elicits metaplastic enhancement of LTP maintenance), significantly increases the rates of translation of mRNAs for GluA1 and GluA2 in CA1 extracts of mouse hippocampal slices. Total translation was increased, as evidenced from the puromycin assays. These data shed novel light on the molecular mechanisms by which NE elicits metaplasticity of LTP by revealing de novo translation of mRNAs for GluA1 and GluA2 as a possible mechanism for boosting maintenance of LTP.
Discussion
Activity-dependent synaptic plasticity is a critical mechanism for learning and memory, and neuromodulatory transmitters play a vital role in regulating synaptic strength (Moody et al. 1999; Mann and Greenfield 2003; van Dam et al. 2004). Previous studies have found that when β-ARs are activated by ISO, maintenance of LTP is prolonged following a single train of HFS that, alone, is insufficient to elicit long-lasting potentiation (Gelinas and Nguyen 2005; see also Dahl and Li 1994 for dentate gyrus data). Our present results show that the endogenous modulatory transmitter, NE, can transform decremental synaptic potentiation to a persistent form of LTP when NE is applied well before HFS. Within a 30-min time window, NE primes β-adrenergic receptor signaling to initiate molecular events that can enhance the duration of LTP induced by one train of HFS applied well after NE application. Our findings extend previous studies of neuromodulator-induced metaplasticity (Christie and Abraham 1992; Cohen and Abraham 1996), and they demonstrate for the first time that NE primes subsequent expression of LTP by increasing translation of specific mRNAs in area CA1.
The locus coeruleus (LC) is a primary source of NE in the mammalian brain. Noradrenergic fibers from the LC project widely throughout the cortex, cerebellum, midbrain, and spinal cord (reviewed by Sara 2009; Gelinas and Nguyen 2007). A central function of noradrenergic neuromodulation is to facilitate rapid reorganization of neural networks in response to environmental challenges that require cognitive and behavioral adaptations (Sara 2009). As the hippocampus is involved in memory processing, NE release in hippocampal networks may modulate acquisition, storage, and retrieval of memories. Activation of β-ARs accounts for much of the NE-dependent effects on hippocampal memory. β-AR receptor antagonists injected into the hippocampus of rats block contextual fear conditioning (Ji et al. 2003a) and spatial memory (Ji et al. 2003b). Norepinephrine injected into area CA1 enhances long-term memory without altering short-term memory (Izquierdo et al. 1998). Interestingly, LC activation in rats enhances conditions for synaptic plasticity by promoting θ-frequencies and facilitating noradrenergic synaptic potentiation (Brown et al. 2005). LC stimulation in awake rats can elicit potentiation of field EPSPs in the dentate gyrus that is blocked by anisomycin, an inhibitor of translation (Walling and Harley 2004), and it can promote retrieval of memory for food motivated maze and inhibitory avoidance tasks (Devauges and Sara 1991; Barros et al. 2001). We do not know whether our in vitro protocol of applying NE to hippocampal slices has a clear behavioral correlate in awake rodents. However, it is reasonable to predict that behavioral expectations may elicit release of NE in brain circuits relevant for updating and modifying ongoing behaviors. For example, exposure to a novel environment may elicit arousal and NE release to prepare an organism for future actions requiring attention and adaptation to current contexts. At the synaptic level, there is evidence in awake rats that exploration of a novel environment can boost induction of long-lasting hippocampal LTP that requires activation of β-ARs (Kemp and Manahan-Vaughan 2008). Thus, it is likely that endogenous norepinephrine from the LC, released through exposure to novel contexts or to other stimuli, can play key roles in initiating and modulating long-lasting synaptic potentiation and memory processing.
How activation of β-adrenergic receptors is linked to LTP maintenance is still incompletely understood. ISO activates β-ARs and triggers molecular mechanisms including the activation of PKA (Madison and Nicoll 1986; Dunwiddie et al. 1992; Thomas et al. 1996; Brown et al. 2000) and ERK cascades (Winder et al. 1999; Giovannini et al. 2001; Gelinas et al. 2007). Activation of β-ARs also recruits translation initiation factor eIF4E (Gelinas et al. 2007), and β-ARs can trigger PKA-mediated phosphorylation of AMPARs (Man et al. 2007; Tenorio et al. 2010). Insertion of GluA1-containing AMPA receptors at synaptic sites occurs following LTP induction (Barry and Ziff 2002; Malinow and Malenka 2002; Song and Huganir 2002; Bredt and Nicoll 2003; Sheng and Hyoung Lee 2003; Malenka and Bear 2004). Interestingly, a translation-dependent increase in cell surface levels of GluA1-containing AMPA receptors was elicited by β-AR activation prior to HFS (Tenorio et al. 2010). This trafficking of GluA1-containing AMPA receptors to the cell surface through PKA-dependent serine-845 phosphorylation (Man et al. 2007) represents one mechanism for β-AR-induced metaplasticity of LTP. Phosphorylation of serine-845 on GluA1 also boosts subsequent insertion of extrasynaptic GluA1 (Oh et al. 2006). A similar effect has been reported following LTP in the dentate gyrus in vivo, for subunits other than GluA1 (Williams et al. 2007).
Our present results with NE show that maintenance of NE-primed LTP was blocked by inhibitors of translation. Importantly, our data revealed increased translation rates for mRNAs encoding GluA1 and GluA2. This finding consolidates our previous study (Tenorio et al. 2010) by showing that activation of β-ARs by NE may recruit newly synthesized GluA1/2 to the cell surface. Indeed, we showed in the present study that blockade of β-ARs by propranolol prevented the increase in translation rates of mRNAs encoding GluA1/2, whereas emetine treatment blocked β-AR-induced increases in cell surface GluA1 expression (Tenorio et al. 2010). Other studies demonstrated that NE-LTP is associated with transient phosphorylation of GluA1 subunits at serine-845 (Hu et al. 2007). The importance of GluA1 in LTP and fear memory was shown by experiments on mice expressing mutations at serine-845, the PKA phosphorylation site of GluA1. The ability of NE to enhance contextual fear conditioning was disrupted in mutant mice where PKA phosphorylation of AMPAR GluA1 was impaired (Hu et al. 2007). Also, in mouse cerebellar stellate cells, fear-inducing stimuli can trigger new transcription of GluA2, accompanied by a switch to expression of AMPA receptors containing GluA2 subunits (Liu et al. 2010). The latter was dependent on β-AR activation by norepinephrine (Liu et al. 2010). Thus, GluA1 and GluA2 subunits are importantly regulated at transcriptional, translational, and post-translational stages of intracellular metabolism, within multiple neural circuits relevant for behavioral learning and memory.
The late-phase of LTP (L-LTP), often induced by multiple trains of HFS, requires transcription (Abraham et al. 1993; Nguyen et al. 1994; Frey et al. 1996; reviewed by Steward and Schuman 2001). It is noteworthy that β-AR activation by acute application of ISO overlapping with one 100-Hz train of HFS elicited persistent LTP that was unaffected by acute application of a transcription inhibitor, actinomycin-D (Gelinas and Nguyen 2005). In contrast, in the present study, we showed that metaplastic enhancement of LTP persistence by application and washout of NE before one train of HFS was attenuated by inhibitors of transcription. This finding underscores a potentially important mechanistic difference between these two protocols. With NE priming metaplasticity of future LTP in the present study, transcription may be optimally engaged by β-AR activation occurring well before HFS. Synapse-to-nucleus signaling by β-ARs, followed by increased translation rates of mRNAs for GluA1 subunits, alongside trafficking and insertion of newly synthesized subunits to the cell surface (Tenorio et al. 2010), may require a spaced protocol that temporally separates the priming stimulus (β-AR activation by NE) and the electrical LTP-inducing stimulus (100-Hz HFS). Indeed, Tenorio et al. (2010) showed that a critical time window of 1–2 h, between washout of ISO and subsequent HFS, existed for successful priming of LTP by ISO. A pairing protocol that conjointly applies HFS overlapping with β-AR activation (Gelinas and Nguyen 2005) may be less sensitive to transcriptional inhibition because the mechanisms for nuclear modulation by β-ARs are less optimally engaged. The molecular basis for this time-dependent difference in sensitivity to transcriptional inhibition is unclear. One potential contributing factor is the spatial location of β-ARs on hippocampal neurons in CA1. β-ARs are found in all principal cell layers in the hippocampus, including area CA1 (Hillman et al. 2005; Guo and Li 2007; Cox et al. 2008). A concentration of distally situated dendritic β-ARs could, hypothetically, require some lag time to relay synaptic signals to the nucleus of pyramidal neurons. Further research is needed to more definitively map β-AR distribution on single neurons in hippocampal slices, and to probe the roles of activation of these receptors in trans-synaptic nuclear signaling.
Numerous proteins have been implicated in translation-dependent LTP (Abraham and Williams 2008). Much of this evidence derives from experiments aimed at blocking or enhancing the functions of specific proteins. A more objective, and just as effective, strategy for identifying proteins recruited for expression of LTP is polysome profiling (PP) of mRNAs extracted from hippocampal slices. The basic principle behind PP is that mRNAs that are intensively translated will be bound to more ribosomes than those that are weakly translated. These strongly translated mRNAs are heavier, and will separate out in the polysomal fraction during sucrose gradient fractionation (Melamed and Arava 2007).
Which proteins are synthesized during NE-induced metaplasticity? In awake rodents, endogenous NE can modulate the transcription of many genes to promote synaptic potentiation (Cirelli and Tononi 2004). For metaplasticity experiments using in vitro hippocampal slices and more tightly controlled conditions of drug applications, it is important to identify which transcripts display increased translation in response to priming stimuli that are known to boost subsequent synaptic potentiation. Here, we have done a focused screen for mRNAs that encode specific AMPA receptor subunits known to be critical for LTP expression. The critical issue here is not whether these specific subunits are required for LTP; it is whether their synthesis (i.e., mRNA translation) is increased following LTP induction. This is an important general question because it sheds light on the molecular bases of long-term synaptic plasticity. Longevity of LTP can be regulated by preexisting proteins and/or by synthesizing proteins de novo. Using translational profiling (for global translation and for specific candidate mRNAs), we showed that the PI3K/mTORC1 and ERK/Mnk pathways are activated by NE + 100 Hz and NE alone, evident from the increased phosphorylation of their downstream targets: rpS6 and eIF4E. NE treatment alone activates signaling; however, it did not increase global protein synthesis or GluA1/2 translation initiation under the present conditions (Fig. 4B,D). The NE + 100 Hz treatment did enhance both global translation (Fig. 4B) and translation of GluA1/2 subunit mRNAs (Fig. 4D). Collectively, these data suggest that NE + 100 Hz, and NE alone, can engage translation pathways that are capable of recruiting translation of GluA1/2 subunit mRNAs. Thus, NE perhaps replenishes the pool of GluA1/2 subunits by promoting, when followed by HFS, de novo translation of their mRNAs to enable persistence of LTP. These findings lend credence to the idea that NE can modulate synapse-to-nucleus signaling, to gate the production of mRNAs that can be translated to maintain LTP.
Our results provide a novel mechanism for NE-induced metaplastic enhancement of LTP. Biochemical signaling during priming by NE engages transcription and translation to generate new mRNAs and plasticity-related proteins. These proteins likely include GluA1/2 subunits of AMPA receptors and they could constitute a critical “front line” of molecules for maintaining LTP. Metaplastic enhancement of LTP could facilitate encoding of information presented on time scales much longer than fast synaptic transmission. In a broader perspective, the noradrenergic neuromodulatory system plays critical roles in the regulation of memory, especially during periods of heightened emotional arousal (McGaugh 1989; Cahill et al. 1994; Sara 2009). Such roles may entail fine-tuning of synaptic strength through priming of neural circuits that are critically positioned to affect behavioral adaptations and memory processing. Future studies should reveal which other mRNAs display altered translation rates during NE-induced metaplasticity. Also, it is unclear whether translation- and transcription-dependent structural changes in synaptic networks can occur during priming by NE.
Materials and Methods
Ethical approval
The experiments and methods of this paper were approved by the University Animal Policy and Welfare Committee (UAPWC) at the University of Alberta using guidelines approved by the Canadian Council on Animal Care (CCAC). Male C57BL/6 mice (aged 7–12 wk) were used for these experiments. Hippocampal brain slices were harvested following cervical dislocation and decapitation of mice, in accordance with UAPWC and CCAC guidelines.
Animals
Male mice (Charles River Canada, C57BL/6, aged 7–12 wk) were used for all experiments. Animals were housed in the University of Alberta's animal facility center under the guidelines of the Canadian Council on Animal Care (CCAC). Animals were kept on a 12-h light–dark cycle with no environmental enrichment in cages, with all experiments conducted during the light portion of the cycle.
Electrophysiology
Following cervical dislocation and decapitation, the intact brain was removed quickly and placed in a beaker of ice-cold artificial cerebrospinal fluid (aCSF) composed of (in mM): 124 NaCl, 4.4 KCl, 1.3 MgSO4, 1.0 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, and 10 glucose, aerated with 95% O2 and 5% CO2. The brain was divided into two hemispheres and each hippocampus was removed from its surrounding tissue and placed on a manual tissue chopper (Stoelting Co.). Transverse hippocampal slices (400-μm thick) were collected and transferred to an interface recording chamber as described previously (Nguyen and Kandel 1997; Nguyen 2006) and maintained at 30°C. The slices were continuously perfused with aCSF (1 mL/min). Electrophysiological recordings of extracellular field excitatory postsynaptic potentials (fEPSPs) began following a 90-min recovery period. A glass microelectrode (resistance: 2–3 MΩ) filled with aCSF was positioned in stratum radiatum of area CA1 for recording fEPSPs. For some experiments, the Schaeffer collateral fibers were stimulated at two separate sets of inputs (S1 and S2) converging onto the same postsynaptic population of neurons using two bipolar nickel–chromium electrodes (diameter 130 µm; AM Systems). Test stimulus intensity was set to elicit baseline fEPSP sizes that were 40% of maximal amplitude (Woo and Nguyen 2003; Gelinas and Nguyen 2007). Subsequent fEPSPs were obtained at a stimulation rate of once per minute at this test intensity, with S2 stimulation following S1 stimulation by 200 msec. To confirm independence of pathways, we used interpathway paired-pulse facilitation (PPF) elicited by successive stimulation through the two electrodes (S1 and S2) at 50, 100, 150, and 200-msec inter-pulse intervals. Pathways were considered independent when no PPF was observed. After establishing a 20-min baseline, LTP was induced on S1 alone through application of one train of high-frequency stimulation (HFS; 100 Hz, 1-sec duration) 30 min after application of NE (10 µM for 15 min). All fEPSPs were measured by an amplifier and were low-pass filtered at 2 kHz. Responses were digitized at a rate of 20 kHz by a Digidata 1200 system and recordings were analyzed offline with pClamp 10 software (Axon Instrument Inc.).
Drugs
Fresh stock solutions of NE were made daily to minimize oxidation. NE (L-(−)-norepinephrine (+)-bitartrate salt monohydrate; Sigma) was prepared in aCSF as 1 mM stock solution and diluted to a final concentration of 10 µM for bath application. A specific competitive β1-AR antagonist, (+)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide methanesulfonate salt (CGP-20712A; CGP; Sigma) was prepared in distilled water as 1 mM stock solution and was bath-applied at 1 µM. A protein synthesis inhibitor, emetine (EME; Sigma-Aldrich), was prepared in distilled water as 20 mM stock solution and diluted to 20 µM final concentration before bath application. EME has been previously shown to block protein synthesis by >80% at similar concentrations as that used in our experiments (Stanton and Sarvey 1984). A second translation inhibitor, cycloheximide (CHX, Sigma), was dissolved in aCSF as 2.5 mM stock solution and diluted to 80 µM prior to bath application. A transcription inhibitor, actinomycin-D (Act-D, Sigma), was made at a stock solution of 25 mM in dimethyl sulfoxide (DMSO) and diluted to a final concentration of 25 µM for bath application. A second transcription inhibitor, 5,6-dichlorobenzimidazole 1-β-D ribofuranoside (DRB, Sigma), was dissolved in DMSO as 50 mM stock solution and diluted to 50 µM. NE was applied for a duration of 15 min. Drugs were applied for durations as shown by bars in the data graphs. Experiments were done under dimmed light conditions to mitigate against photolysis of bath-applied drugs.
Polysome profiling
For all biochemical experiments, area CA1 of isolated mouse hippocampal slices was excised and flash-frozen in liquid nitrogen at 20 min after HFS. For tissue collections, 8–12 CA1 microslices were pooled in each vial. NE and drug treatments prior to HFS were done exactly as described in our electrophysiology experiments. Propranolol was applied (50 µM) with a time course identical to CGP as in Figure 1C.
Polysome profile analysis was carried out as described (Gkogkas et al. 2013). Intact hippocampi were washed with ice-cold phosphate-buffered saline (PBS) containing 100 μg/mL cycloheximide and lysed in a hypotonic lysis buffer (5 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 1.5 mM KCl, 100 μg/mL cycloheximide, 2 mM DTT, 0.5% Triton X-100, and 0.5% sodium deoxycholate). Lysates were loaded onto 10%–50% sucrose density gradients (20 mM HEPES–KOH (pH 7.6), 100 mM KCl, 5 mM MgCl2) and centrifuged at 35,000 rpm for 2 h at 4°C. Gradients were fractionated and the optical density (OD) at 254 nm was continuously recorded using an ISCO fractionator (Teledyne ISCO). Total RNA from each fraction was isolated using TRIzol (Invitrogen) and reverse transcribed using the Superscript III kit (Invitrogen) (Gkogkas et al. 2013). Primers for GluA1, GluA2, and Actb were previously described (Ran et al. 2013). Polysome-to-monosome ratio was calculated as the area under the A254 absorbance curve, using the function describing the absorbance values, processed with the definite integral command in MATLAB.
Western blotting
All tissues were dissociated in RIPA buffer (unless otherwise specified). Western blotting was previously described (Gkogkas et al. 2013). Antibodies against indicated proteins were: eIF4E, phospho-eIF4E (Ser209) (BD Biotechnologies), rpS6, phospho-rpS6(240–244) (Cell Signaling), anti-puromycin (Kerafast) and β-tubulin (SIGMA). Secondaries were anti-mouse and anti-rabbit (GE Healthcare), and anti-goat (Santa-Cruz) antibodies. Quantification of immunoblots was performed using ImageJ (NIH). Values were normalized to β-tubulin or another control where specified, and presented as a ratio of phosphoprotein/total protein.
Measurement of de novo protein synthesis
Transverse hippocampal slices (400 μm) were prepared from mice (ages 5–6 wk) and allowed to recover for at least 3 h Puromycin labeling was performed as described previously (Hoeffer et al. 2011; Bhattacharya et al. 2012) with some modifications. Briefly, the slices were incubated with puromycin (Sigma, 5 μg/mL in ACSF), and then processed for Western blotting, as described above, using an anti-puromycin antibody. For groups treated with CHX or propranolol, these drugs were applied with the same time courses as for CHX in Figure 2C and for CGP in Figure 1C. Slices processed in parallel but not incubated with puromycin served as unlabeled controls. Protein synthesis was determined by measuring total lane signal from 250 to 15 kDa (top to bottom) and subtracting unlabeled protein control. Signals were quantified using ImageJ, normalized to β-tubulin and presented as arbitrary units.
Data analysis
The initial slope of the fEPSP was measured as an index of synaptic strength. fEPSP slopes were compared with baseline slope values (20 min average at test stimulation intensity) and were plotted as a percentage of baseline. To compare LTP between groups, we used data points at 120 or 180 min after LTP induction. One-way ANOVA and Tukey–Kramer post hoc tests (Graphpad Instat Software) were done for comparison of more than two groups to determine which groups were significantly different from the others. Data are reported as means ± SEM, with n = number of slices, 3–6 mice per group.
Acknowledgments
This research was funded by grants from the Canadian Institutes of Health Research (MOP-114994 to N.S.; MOP-74453 to P.V.N.) and from Azrielli/Brain Canada (to N.S.). P.V.N. is a Scientist of the Alberta Heritage Foundation for Medical Research. We thank Dr. S. Connor for constructive comments and Ms. H. Edison for assisting with slice collection for biochemical assays.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.039222.115.
References
- Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9: 387. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. 1996. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Williams JM. 2008. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem 89: 260–268. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason SE, Demmer J, Williams JM, Richardson CL, Tate WP, Lawlor PA, Dragunow M. 1993. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience 56: 717–727. [DOI] [PubMed] [Google Scholar]
- Barros DM, Mello e Souza T, De David T, Choi H, Aguzzoli A, Madche C, Ardenghi P, Medina JH, Izquierdo I. 2001. Simultaneous modulation of retrieval by dopaminergic D(1), β-noradrenergic, serotonergic-1A and cholinergic muscarinic receptors in cortical structures of the rat. Behav Brain Res 124: 1–7. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. 2002. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. 1994. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. 2003. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. 2012. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232: 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. 2003. AMPA receptor trafficking at excitatory synapses. Neuron 40: 361–379. [DOI] [PubMed] [Google Scholar]
- Brown GP, Blitzer RD, Connor JH, Wong T, Shenolikar S, Iyengar R, Landau EM. 2000. Long-term potentiation induced by θ frequency stimulation is regulated by a protein phosphatase-1-operated gate. J Neurosci 20: 7880–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Walling SG, Milway JS, Harley CW. 2005. Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances θ frequencies in rat dentate gyrus. J Neurosci 25: 1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. 1994. β-Adrenergic activation and memory for emotional events. Nature 371: 702–704. [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. 1992. Priming of associative long-term depression in the dentate gyrus by θ frequency synaptic activity. Neuron 9: 79–84. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. 2004. Locus ceruleus control of state-dependent gene expression. J Neurosci 24: 5410–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. 1996. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol 76: 953–962. [DOI] [PubMed] [Google Scholar]
- Connor SA, Wang YT, Nguyen PV. 2011. Activation of {β}-adrenergic receptors facilitates heterosynaptic translation-dependent long-term potentiation. J Physiol 589: 4321–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DJ, Racca C, LeBeau FE. 2008. β-Adrenergic receptors are differentially expressed in distinct interneuron subtypes in the rat hippocampus. J Comp Neurol 509: 551–565. [DOI] [PubMed] [Google Scholar]
- Dahl D, Li J. 1994. Induction of long-lasting potentiation by sequenced applications of isoproterenol. Neuroreport 5: 657–660. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. 1991. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by β-receptors. Behav Brain Res 43: 93–97. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. 1992. Long-term increases in excitability in the CA1 region of rat hippocampus induced by β-adrenergic stimulation: possible mediation by cAMP. J Neurosci 12: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Frey S, Schollmeier F, Krug M. 1996. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol 490 (Pt 3): 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. 2005. β-Adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci 25: 3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. 2007. Neuromodulation of hippocampal synaptic plasticity, learning, and memory by noradrenaline. CNS Agents Med Chem 7: 17–33. [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. 2007. ERK and mTOR signaling couple β-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem 282: 27527–27535. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM. 2001. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J Neurosci 21: 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, Vasuta C, Yee S, Truitt M, Dallaire P, et al. 2013. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature 493: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo NN, Li BM. 2007. Cellular and subcellular distributions of β1- and β2-adrenoceptors in the CA1 and CA3 regions of the rat hippocampus. Neuroscience 146: 298–305. [DOI] [PubMed] [Google Scholar]
- Harley CW, Lalies MD, Nutt DJ. 1996. Estimating the synaptic concentration of norepinephrine in dentate gyrus which produces β-receptor mediated long-lasting potentiation in vivo using microdialysis and intracerebroventricular norepinephrine. Brain Res 710: 293–298. [DOI] [PubMed] [Google Scholar]
- Henley JM, Barker EA, Glebov OO. 2011. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman KL, Knudson CA, Carr PA, Doze VA, Porter JE. 2005. Adrenergic receptor characterization of CA1 hippocampal neurons using real time single cell RT-PCR. Brain Res Mol Brain Res 139: 267–276. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, et al. 2011. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci 108: 3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. 2007. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131: 160–173. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. 1998. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem 69: 219–224. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Wang XM, Li BM. 2003a. Deficit in long-term contextual fear memory induced by blockade of β-adrenoceptors in hippocampal CA1 region. Eur J Neurosci 17: 1947–1952. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Zhang XH, Li BM. 2003b. Deficient spatial memory induced by blockade of β-adrenoceptors in the hippocampal CA1 region. Behav Neurosci 117: 1378–1384. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. 1997. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol 77: 3013–3020. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2008. β-Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex 18: 1326–1334. [DOI] [PubMed] [Google Scholar]
- Larkman AU, Jack JJ. 1995. Synaptic plasticity: hippocampal LTP. Curr Opin Neurobiol 5: 324–334. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. 2009. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb Cortex 19: 2827–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Formisano L, Savtchouk I, Takayasu Y, Szabó G, Zukin RS, Liu SJ. 2010. A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat Neurosci 13: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. 1986. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol 372: 221–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. 2004. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. 2002. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. 2007. Regulation of {α}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci 104: 3579–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. 2003. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol 551: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: 649–711. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. 1989. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci 12: 255–287. [DOI] [PubMed] [Google Scholar]
- Melamed D, Arava Y. 2007. Genome-wide analysis of mRNA polysomal profiles with spotted DNA microarrays. Methods Enzymol 431: 177–201. [DOI] [PubMed] [Google Scholar]
- Mellentin C, Jahnsen H, Abraham WC. 2007. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. Neuropharmacology 52: 118–125. [DOI] [PubMed] [Google Scholar]
- Moody TD, Carlisle HJ, O'Dell TJ. 1999. A nitric oxide-independent and β-adrenergic receptor-sensitive form of metaplasticity limits θ-frequency stimulation-induced LTP in the hippocampal CA1 region. Learn Mem 6: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV. 2006. Comparative plasticity of brain synapses in inbred mouse strains. J Exp Biol 209: 2293–2303. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. 1997. Brief θ-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem 4: 230–243. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. 1994. Requirement of a critical period of transcription for induction of a late phase of LTP. Science 265: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281: 752–758. [DOI] [PubMed] [Google Scholar]
- Ran I, Gkogkas CG, Vasuta C, Tartas M, Khoutorsky A, Laplante I, Parsyan A, Nevarko T, Sonenberg N, Lacaille JC. 2013. Selective regulation of GluA subunit synthesis and AMPA receptor-mediated synaptic function and plasticity by the translation repressor 4E-BP2 in hippocampal pyramidal cells. J Neurosci 33: 1872–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Li Q, Abraham WC, Xiao ZC. 2009. Priming of short-term potentiation and synaptic tagging/capture mechanisms by ryanodine receptor activation in rat hippocampal CA1. Learn Mem 16: 178–186. [DOI] [PubMed] [Google Scholar]
- Sara SJ. 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10: 211–223. [DOI] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P. 2009. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6: 275–277. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. 2003. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res 46: 127–134. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. 2007. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23: 613–643. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. 2002. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588. [DOI] [PubMed] [Google Scholar]
- Sossin WS. 1996. Mechanisms for the generation of synapse specificity in long-term memory: the implications of a requirement for transcription. Trends Neurosci 19: 215–218. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Lacaille JC. 2010. Mechanisms of translational regulation in synaptic plasticity. Curr Opin Neurobiol 20: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1984. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci 4: 3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1985. Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res 361: 276–283. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. 2001. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24: 299–325. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. 2003. Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol 552: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio G, Connor SA, Guévremont D, Abraham WC, Williams J, O'Dell TJ, Nguyen PV. 2010. ‘Silent’ priming of translation-dependent LTP by β-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn Mem 17: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. 1996. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17: 475–482. [DOI] [PubMed] [Google Scholar]
- van Dam EJ, Kamal A, Artola A, de Graan PN, Gispen WH, Ramakers GM. 2004. Group I metabotropic glutamate receptors regulate the frequency-response function of hippocampal CA1 synapses for the induction of LTP and LTD. Eur J Neurosci 19: 112–118. [DOI] [PubMed] [Google Scholar]
- Walling SG, Harley CW. 2004. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel β-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci 24: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Guévremont D, Mason-Parker SE, Luxmanan C, Tate WP, Abraham WC. 2007. Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals. J Neurosci 27: 14171–14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. 1999. ERK plays a regulatory role in induction of LTP by θ frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24: 715–726. [DOI] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. 2003. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci 23: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]