Abstract
We recorded basolateral amygdala (BL) neurons in a seminaturalistic foraging task. Rats had to leave their nest to retrieve food in an elongated arena inhabited by a mechanical predator. There were marked trial-to-trial variations in behavior. After poking their head into the foraging arena and waiting there for a while, rats either retreated to their nest or initiated foraging. Before initiating foraging, rats waited longer on trials that followed failed than successful trials indicating that prior experience influenced behavior. Upon foraging initiation, most principal cells (Type-1) reduced their firing rate, while in a minority (Type-2) it increased. When rats aborted foraging, Type-1 cells increased their firing rates, whereas in Type-2 cells it did not change. Surprisingly, the opposite activity profiles of Type-1 and Type-2 units were also seen in control tasks devoid of explicit threats or rewards. The common correlate of BL activity across these tasks was movement velocity, although an influence of position was also observed. Thus depending on whether rats initiated movement or not, the activity of BL neurons decreased or increased, regardless of whether threat or rewards were present. Therefore, BL activity not only encodes threats or rewards, but is closely related to behavioral output. We propose that higher order cortical areas determine task-related changes in BL activity as a function of reward/threat expectations and internal states. Because Type-1 and Type-2 cells likely form differential connections with the central amygdala (controlling freezing), this process would determine whether movement aimed at attaining food or exploration is suppressed or facilitated.
SIGNIFICANCE STATEMENT For decades, amygdala research has been dominated by pavlovian and operant conditioning paradigms. This work has led to the view that amygdala neurons signal threats or rewards, in turn causing defensive or approach behaviors. However, the artificial circumstances of conditioning studies bear little resemblance to normal life. In natural conditions, subjects are simultaneously presented with potential threats and rewards, forcing them to engage in a form of risk assessment. We examined this process using a seminaturalistic foraging task. In constant conditions of threats and rewards, amygdala activity could be high or low, depending on the rats' decisions on a given trial. Therefore, amygdala activity does not only encode threats or rewards but is also closely related to behavioral output.
Keywords: amygdala, threat, reward, defensive behaviors, approach
Introduction
Prior conditioning studies have implicated the basolateral amygdaloid complex (BLA) in the development of conditioned responses to stimuli (CSs) that predict aversive (Maren and Quirk, 2004; Anglada-Figueroa and Quirk, 2005; Duvarci and Pare, 2014) or rewarding outcomes (Setlow et al., 2002; Everitt et al., 2003; Ambroggi et al., 2008). Consistent with this, many BLA neurons acquire robust responses to negatively or positively valenced CSs after conditioning (Rorick-Kehn and Steinmetz, 2005; Belova et al., 2008; Herry et al., 2008; Shabel and Janak, 2009; Amano et al., 2011). However, by design in conditioning studies, aversive and appetitive CSs are presented in isolation or at different times. This contrasts with natural conditions where animals are simultaneously presented with potential risks and rewards (e.g., predators, food) that are associated with opposite response tendencies (e.g., freezing vs food seeking). As a result, there is little information on how BLA activity contributes to help animals weigh the need to attain food against the associated increase in predation risk.
Choi and Kim (2010) introduced a task that reproduces natural foraging conditions. In this task, rats are confronted with a mechanical predator (“Robogator”) when they leave their nest to obtain food pellets. Intra-amygdala infusions of drugs that inhibited or excited amygdala neurons, respectively, increased or decreased risk taking (Choi and Kim, 2010). While these findings indicate that the amygdala regulates risky foraging decisions, how it does so is unclear. To address this question, we recorded neurons in the basolateral nucleus of the amygdala (BL) neurons with multishank silicon probes in rats engaged in the foraging task.
Materials and Methods
Procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University, in compliance with the Guide for the Care and Use of Laboratory Animals. We used male naive Sprague Dawley rats (310–360 g; Charles River Laboratories) maintained on a 12 h light/dark cycle. Before the experiments, they were habituated to the animal facility and handling for 1 week. After implantation, they were housed individually. All experiments were performed during the light cycle.
Surgery
Rats were anesthetized with a mixture of isoflurane and O2, and administered atropine sulfate (0.05 mg/kg, i.m.) to aid breathing. In aseptic conditions, rats were mounted in a stereotaxic apparatus with nonpuncture ear bars. A local anesthetic (bupivacaine, s.c.) was injected in the scalp. Fifteen minutes later, the scalp was incised and a craniotomy was performed above the amygdala. Then, silicon probes (NeuroNexus) were stereotaxically aimed at the BL. Silicon probes consisted of either four (three rats) or eight (three rats) shanks (intershank distance of 200 μm), each with eight recording leads (de-insulated area of 144 μm2) separated by ∼20 μm dorsoventrally. They were attached to Buzsaki-style microdrives (Vandecasteele et al., 2012), allowing us to lower them between recording sessions. In a subset of three rats, a craniotomy was also performed above NAc and mPFC. Then, pairs of tungsten stimulating electrodes (intertip spacing of 1–1.7 mm) were stereotaxically inserted in these two structures. Rats were allowed 2–3 weeks to recover from the surgery.
The following stereotaxic coordinates were used—all expressed in millimeters relative to bregma—BL: AP −2.2 to −3.6, ML 5–5.3, DV 8.8; mPFC: AP 2.7–3.7, ML 0.5, DV 3.6–5.2; NAc: AP 1.5, ML 1.35, DV 6.7.
Foraging task
After recovery from the surgery, rats were housed individually with ad libitum access to water. To ensure proper motivation in the foraging task, daily access to food was restricted in time so that the rats' bodyweight was maintained at ∼90% of age-matched subjects with continuous access to food.
Foraging apparatus.
The foraging apparatus (Fig. 1A) was a long rectangular alley (245 cm in length × 60 cm in width) with high walls (60 cm) but no ceiling. It was divided into two compartments by a door (height, 50 cm; width, 10 cm). At one end of the apparatus was a small (length 30 cm), dimly lit (10 lux) nesting area with a water bottle. The rest of the apparatus was a much longer (215 cm) and brighter (200 lux) foraging arena. An overhead digital video camera (frame rate 29.97 per second) recorded the rats' behavior in the two compartments.
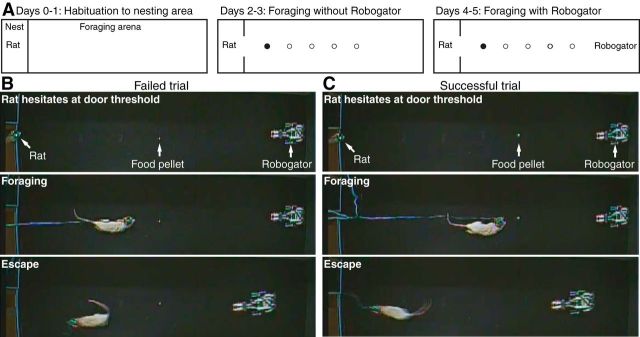
Figure 1.
Experimental paradigm and behavioral apparatus. A, The behavioral apparatus consisted of a small, dimly lit nesting area and a longer and brighter foraging arena. After 2 d of habituation to the nest (left), rats learned to retrieve food pellets in the foraging arena (middle) over a period of 2 d. One the fourth and fifth day, a mechanical predator (Robogator) was introduced (right). B, C, Examples of failed (B) and successful (C) trials.
Mechanical predator (Robogator).
On a proportion of trials (see below), a mechanical predator (length, 34 cm; width, 17 cm; height, 14 cm) on wheels was positioned at the end of the foraging arena, facing the nesting arena. This Robogator (Mindstorms; LEGO Systems) was equipped with a sensor that detected the rats' approach and triggered a sudden forward movement (80 cm at 60 cm/s) and repeated opening and closing of the jaws (nine times) followed, after 2 s, by return to its original position.
Habituation to nesting area (Days 0–1).
Rats were first habituated to the nesting area for two daily consecutive sessions of 7 h (Fig. 1A, left). During this period, they could consume up to 6 g of food (sweet cereal pellets). However, the gateway to the foraging arena remained shut at all times.
Foraging in the absence of Robogator (Days 2–3).
On the following day, in the absence of Robogator, rats were given the opportunity to retrieve sweetened food pellets (80–100 mg) in the foraging arena (Fig. 1A, middle). No food was available in the nesting area in this case. Sixty trials were conducted, each beginning with a period of 60 s in the nesting area with the gateway shut. This was repeated the next day. A single food pellet was placed at various distances from the door. The gateway was then opened. After a period of hesitation at the doorway, rats retrieved the food pellet and returned to the nesting area to consume it. Upon re-entry in the nesting arena, the gateway was closed. The distance between the nesting area and food pellet was gradually increased in steps of 25 cm (from 25 to150 cm), after three successful trials at each distance. Later, the distance was varied randomly from trial to trial.
Foraging in the presence of Robogator (Days 4–5).
On the following day, rats were again given the opportunity to retrieve food pellets in the foraging arena (Fig. 1A, right). However, 60% of trials were conducted with the Robogator present. Blocks of trials with (n = 10–20) or without (n = 10–15) the Robogator were conducted, for a total of 100–120 trials.
Analysis of behavior
The rats' behavior was recorded by an overhead video camera at a frame rate of 29.97 Hz. To analyze the rats' behavior, we used two approaches. First, a MATLAB script was written to determine the position of the rats based on the shifting distribution of light intensity across frames. This also allowed us to determine the rats' velocity. In addition, a trained observer performed a frame-by-frame analysis of the video file to determine when the rats started waiting at the door threshold (defined as when the rat's snout extended beyond the door into the foraging arena), when they initiated foraging (defined as the last frame of immobility before completely moving out of the nest), retrieved a food pellet, escaped, and retreated into the nest. The observer also noted whether rats failed or succeeded each trial. The start of the “escape” phase was defined as when the rat, after approaching the food pellet, abruptly turned around to run all the way to the nest. This behavior was observed whether the Robogator was present or not and whether the trial was successful or not.
Shuttle task
In this task, rats ran back and forth between two nest-like compartments (50 cm long × 20 cm wide) through a central compartment (50 cm long) to retrieve food pellets at the end of the other nest. The walls were 45 cm high. The apparatus was made of black Plexiglas and dimly illuminated (10 lux). The nests and corridor were separated by retractable doors. Rats received extensive habituation to the apparatus with the doors open. During the recordings, rats were positioned in one of the nests and a food pellet in the other. After opening the doors, rats immediately ran to the other nest to consume the food. While rats consumed the food, the door was closed. The intertrial interval was ≥ 1 min.
Open field
The open field was rectangular (60 cm wide × 180 cm long with walls 60 cm high) and made of black Plexiglas. Ambient light levels were very low (7 lux). Before the recordings, rats received extensive habituation to the apparatus.
Unit recording, clustering, and analysis
BL unit recordings were performed during all the phases of the behavioral protocols described above with the exception of habituation. In rats that had been implanted with stimulating electrodes, at the end of most behavioral sessions, electrical stimuli (300–600 μA, 0.1 ms) were delivered at 1 Hz to determine whether recorded cells could be antidromically invaded from one or more of the stimulated sites. In all rats, the silicon probes were lowered ≥ 30 μm (40 ± l0 μm) after every recording session. We waited at least 8 h until the next recording session to ensure mechanical stability of the probes. Note that it is possible that some cells were sampled on different days; however, the incidence of such recordings is impossible to assess because moving probes changes spike shapes.
The signals were sampled at 25 kHz and stored on a hard drive. The data were first high-pass filtered using a median filter (window size of 1.1 ms), then thresholded to extract spikes. We then ran principal component analysis on the spikes and the first three components were clustered using KlustaKwik (http://klustakwik.sourceforge.net/). Spike clusters were then refined manually using Klusters (Hazan et al., 2006). The reliability of cluster separation was verified by inspecting autocorrelograms and cross-correlograms. Autocorrelograms had to display a refractory period of at least 2 ms. Cross-correlograms should not show evidence of a refractory period, as this feature betrays overlap between clusters. Units with unstable spike shapes during a given recording session were excluded.
To determine spike duration, we first selected the channel where, for a given cell, action potentials had the largest peak to trough amplitude. We then measured the spike's duration as the time between spike trough and peak (Barthó et al., 2004). Antidromic action potentials were identified as such when they had a fixed latency (≤0.1 ms jitter) and collided with spontaneously occurring spikes.
Statistical analyses
All grouped data are reported as average ± SEM. When firing rates are expressed logarithmically, we used natural logarithms. All statistical tests were two sided. No subjects were excluded. When analyzing the evolution of the food retrieval interval over time, we used a mixed-effect ANOVA with subject as a random effect. When comparing behavior in the absence versus presence of the Robogator, we did not use trials (≥300 per rat) but averages of trials obtained in each rat (n = 6) for statistical comparisons. While this approach reduces statistical power, using trials as the unit of analysis would have caused an excessive risk of Type-1 error (Aarts et al., 2014).
In the foraging task, to determine whether individual neurons showed significant task-related variations in firing rates, we computed Kruskal–Wallis one-way ANOVAs during four distinct periods: (1) in the nest with the door closed (“baseline”), (2) during the waiting period at the door threshold (“waiting”), (3) during foraging, and (4) during escape (when rats turned around and ran toward the nest). Although the Kruskal–Wallis is a test for independent samples, we could not use a repeated-measure ANOVA because of variations in the number of trials available to compare the activity of each cell in the different phases of the foraging task. In particular, because on many Robogator trials, rats did not initiate foraging, we had many more trials available for the waiting phase than subsequent phases.
When instead of making cell by cell comparisons we compared the firing rate of the same group of cells in different conditions (e.g., Type-1 with Robot vs Type-1 without Robot), we could use a repeated-measure test (Wilcoxon signed rank test) because we averaged all the available trials of each cell in the two conditions separately before doing the comparison. In other words, for the ANOVA, trials were the unit of comparison, whereas for the Wilcoxon signed rank test cells were the unit of comparison.
To assess significance of correlations between firing rates and speed of movement, we computed Spearman's r and used a significance threshold of 0.05. Periods of immobility due to sleep were excluded from these analyses. Finally, when comparing proportions of cells in two or more conditions we used a Fisher exact test or χ2 test, as appropriate.
To study the relation between firing rates, movement speed, and position, for each 33 ms video frame, we determined the rats' position, velocity, and the number of spikes generated by each unit in each position-velocity bin. Data from different trials were combined. For each unit, the total number of spikes in each velocity-position bin was divided by the total amount of time spent in that velocity-position bin to obtain a raw firing rate in that velocity-position bin. This firing rate in each velocity-position bin was then divided by the baseline firing rate of the unit. Next, this process was repeated for each unit and the results obtained for each position-velocity bin averaged across units, but separately for Type-1 and Type-2 units.
To assess whether differences in firing rates as a function of movement speed and position were significant, for each cell, we shuffled the normalized spike counts in the various 33 ms bins while keeping velocity and position constant. Based on this shuffle, a map of normalized firing rate as a function of speed and position was derived for each unit. This was repeated for all units and the results averaged across units. This process was repeated a total of 1000 times allowing us to obtain, for each position-velocity bin, a null distribution against which we could compare the actual data. The normalized firing rate value in a particular position-velocity bin was considered significant when it was >97.5% or <2.5% of the shuffled values.
States of vigilance were distinguished using spectral analyses of local field potentials (LFPs) and behavioral observations. Spontaneous LFP activity was segmented in 5 s windows and frequency distributions of LFP power in different frequency bands computed. Active waking could be distinguished from all other states because it was associated with a broadband increase in the power of high frequencies (200–240 Hz), reflecting electromyographic activity. After eliminating active waking, we could easily distinguish slow-wave sleep from quiet waking because total power at frequencies <20 Hz was distributed bimodally between the two states: epochs of high power at low frequencies corresponded to periods of slow-wave sleep.
Histology
At the end of the experiments, the animals were deeply anesthetized. On each shank, one of the recording sites was marked with a small electrolytic lesion (10 μA between a channel and the animals' tail for 10 s). One day later, rats were perfused-fixed through the heart, their brains extracted and cut on a vibrating microtome, and the sections counterstained with cresyl violet. We only considered neurons that were histologically determined to have been recorded in BL.
Results
The foraging apparatus featured two compartments divided by a door: a dimly lit nest and a longer and brighter foraging arena (Fig. 1A). After habituation to the nest, rats were allowed to retrieve sweetened food pellets in the foraging arena in the absence of Robogator. On each of 60 trials, a food pellet was placed at various distances from the nest, beginning with 25 cm and increasing in steps of 25 cm after three successful trials, up to 150 cm. Distances were then varied randomly. Rats did not eat the food in the foraging arena, but seized the pellet and returned to the nest to consume it, at which point the doorway was shut. The next trial started ≥1 min later. On the next 2 d, another set of 100–120 trials/d was conducted with alternating trial blocks performed in the presence (n = 10–20) or absence (n = 10–15) of Robogator. The Robogator was programmed to surge forward ∼80 cm when approached by the rat. Figure 1, B and C, illustrates examples of failed and successful trials.
Foraging behavior
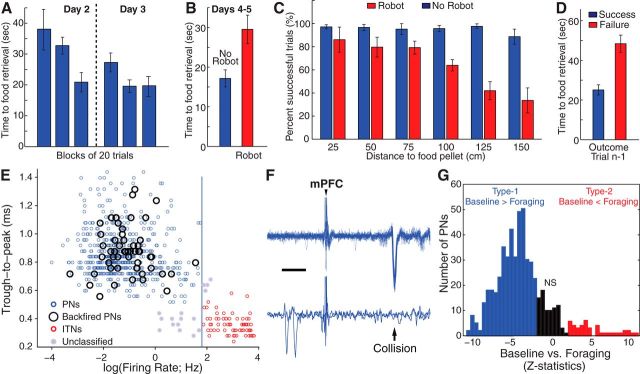
In the early stages of training, shortly after door opening, rats approached the door threshold and stayed there for a time. They then ventured into the foraging arena to retrieve the food. After obtaining the food, they abruptly turned around and ran back to the nest. In the absence of Robogator, all rats quickly learned to retrieve the food. A reliable index of progress on this task was the interval between door opening and food retrieval (Fig. 2A). Whereas it took rats nearly 40 s during the first 20 trial block (38 ± 7 s), the retrieval interval eventually dropped to ∼20 s by the third trial block and remained low the next day (Fig. 2A; last trial block, 20 ± 4 s; ANOVA, F(5,347) = 10.6, p < 0.0001; Tukey–Kramer, p ≤ 0.002).
Figure 2.
Behavior and classification of BL unit recordings. A, Time from door opening to food retrieval (y-axis) in successive blocks of 20 trials on Days 2–3 in six rats. Trials with nest to food distance ≤ 50 cm were ignored, leaving 352 trials. B, Time to food retrieval on alternating trial blocks with (red) or without (blue) Robogator. C, Proportion of successful trials (y-axis) as a function of distance to food pellet (x-axis) in trials with (red) or without (blue) Robogator. D, Time to food retrieval (y-axis) when the prior Robogator trial (trial n − 1) was a success (blue) versus a failure (red). E, Scatter plot of spike duration (y-axis), defined as trough to peak interval, as a function of firing rate (x-axis). Units were classified as PNs (blue) when they had a spike duration ≥ 0.55 ms and firing rate ≤ 6 Hz and as ITNs (red) when they generated spikes < 0.6 ms and fired at > 6 Hz. Empty black circles, units formally identified as PNs by antidromic invasion from a BL projection field. F, Example of PN backfired from mPFC (top, 25 superimposed responses; bottom, two trials where the antidromic spike collided with a spontaneously occurring action potential). Scale bar, 5 ms. G, Frequency distribution of z-statistics (from Wilcoxon rank sum test) for firing rate differences between baseline and foraging in PNs. Blue and red, Units with significantly higher or lower firing rates during baseline than foraging, respectively. Black, Cells with nonsignificant (NS) differences.
Introduction of the Robogator altered the rats' behavior in many ways (Fig. 2). First, upon door opening and approach of the door threshold, rats often retreated back into the nest instead of initiating foraging (9.4 ± 2.1% of trials), a phenomenon rarely seen in the absence of the Robogator (1.5 ± 0.5%, paired t test, p = 0.027). Second, on Robogator trials where rats initiated foraging, the food retrieval interval increased (Fig. 2B; paired t test, t(5) = 3.6, p = 0.015). Third, the proportion of successful trials was generally lower in the presence of the Robogator, particularly when the distance between the nest and food was high (Fig. 2C; two-way ANOVA, Robogator vs no Robogator, F(1,58) = 45, p < 0.0001; Tukey–Kramer, p ≤ 0.0001; Distance: F(6,58) = 3.35, p = 0.007; Interaction: F(6,57) = 4.73, p = 0.0006). Last, performance on a given Robogator trial varied depending on whether the prior trial had been successful or not (Fig. 2D). For this analysis, we only considered trials where rats succeeded in obtaining the food. Using this approach, we found that trials following successful ones had shorter times to food retrieval (Fig. 2D, blue) than following failed trials (Fig. 2D, red; paired t test, t(5) = 3.47, p = 0.017).
Activity of BL neurons during foraging
After histological determination of the silicon probes' trajectories (Fig. 3), we restricted our attention to 705 BL single-unit recordings recorded during the foraging task. It should be noted that because the silicon probes were moved down ∼40 μm at the end of each recording session, it is possible that some cells were sampled on different days. Overall, 233 unit recordings were obtained on Days 2–3 (no Robogator) and 472 on Days 4–5, when trial blocks with or without Robogator occurred. Consistent with earlier studies (Likhtik et al., 2006), recorded units were classified as presumed projection cells (PNs; 88%; Fig. 2E, blue) or interneurons (ITNs; 10%; Fig. 2E, red) on the basis of their firing rates in the nest (cutoff 6 Hz) and spike duration (trough to peak of 0.6 ms). Some unit recordings (2%) could not be classified (Fig. 2E, gray) and are not considered further. Support for this classification was found in the differential distribution of units antidromically responsive to electrical stimuli delivered in the mPFC (Fig. 2F) or NAc: 20% of tested PNs (n = 287; Fig. 2E, empty circles) compared with none of the presumed ITNs (n = 44; Fisher exact test, p < 0.0007).
Figure 3.
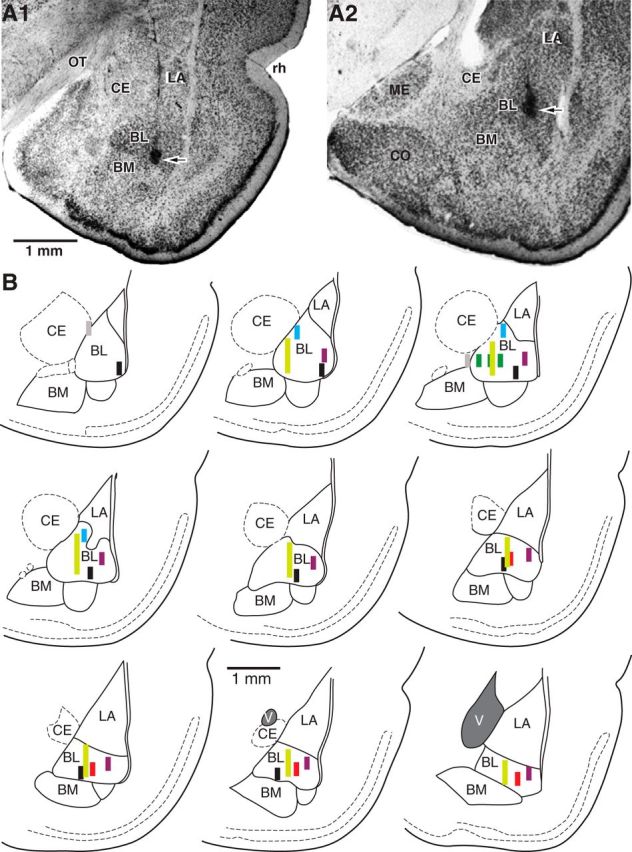
Histological verification of recording sites. A, Coronal sections of the amygdala at relatively anterior (A1) and posterior (A2) levels showing location of silicon probes in the BL nucleus and electrolytic lesions (arrows) marking the deepest recording sites. B, Schemes showing location and trajectory of silicon probes on nine coronal sections arranged from rostral (top left) to caudal (bottom right). Different colors are used for the six different rats. Gray indicates recording sites we did not consider because they were not inside the BL nucleus. CE, central nucleus of the amygdala; CO, cortical nucleus of the amygdala; BM, basomedial nucleus of the amygdala; LA, lateral nucleus of the amygdala; ME, medial nucleus of the amygdala; OT, optic tract; rh, rhinal sulcus; V, ventricle.
To assess whether each unit showed task-related variations in activity, we computed a Kruskal–Wallis one-way ANOVA on its firing rate (threshold p of 0.05) during four periods: (1) in the nest with the door closed (baseline), (2) during the waiting period at the door threshold (waiting), (3) during foraging, and (4) escape. Whether they were recorded in the absence or presence of Robogator, most BL unit recordings showed significant task-related activity. Moreover, as described below, their activity in these two conditions was similar. Therefore, we first describe the activity of BL units in the foraging task regardless of trial type and then perform within-cell comparisons of activity in Robogator versus no-Robogator trials.
PNs
Overall, 88% of PNs showed significant activity variations in the foraging task. The proportion of unit recordings with significant firing rate comparisons (Dunn test, threshold p = 0.05) was highest for differences between baseline and foraging (86%) or escape (83%); the two are highly correlated (r = 0.57, p < 0.0001). Other comparisons yielded a lower incidence of significant differences (24–55%). Most PNs exhibited a significant decrease in firing rate from baseline to foraging (Fig. 2G, blue, 69%) and a minority showed the opposite (red, 7%). Hereafter, we will refer to these two groups of unit recordings as Type-1 and Type-2 cells, respectively. See Figure 4, A and B, for individual examples and Figure 4C for their average behavior. It should be noted that the two types of unit recordings were recorded in all subjects and that they were homogenously distributed throughout the BL nucleus. Because they were less frequent, Type-2 unit recordings were observed in 23% of shanks and sessions. In all these cases, however, Type-1 unit recordings were present on the same shank and in the same session.
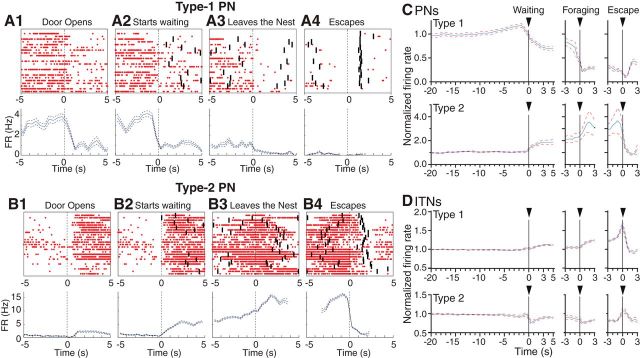
Figure 4.
Examples and average activity of PNs. A1–A4, Top, Type-1 PN. Rasters showing spikes (red dots) generated within ±5 s of salient task events in 25 trials: door opening (A1), waiting onset (A2), foraging onset (A3), and escape (A4). The “start waiting” time point was defined as when the rat approached the door threshold and had at least its snout extending past the door, into the foraging arena. The start of the “escape” phase was defined as when the rat, after approaching the food pellet, abruptly turned around to run all the way to the nest. This behavior was observed whether the Robogator was present or not and whether the trial was successful or not. However, only successful trials are shown in this figure. Black bars indicate, for each trial, when the preceding and following task events occurred. A1–A4, Bottom, Average firing rate (±SEM) for a larger sample of trials than depicted in rasters (0.5 s bins). Type-2 PN. B1–B4, Top, Rasters showing spikes (red dots) generated within ±5 s of salient task events in 25 trials: door opening (B1), waiting onset (B2), foraging onset (B3), and escape (B4). Only successful trials are shown. Black bars indicate, for each trial, when the preceding and following task events occurred. B1–B4, Bottom, Average firing rate (±SEM) for a larger sample of trials than depicted in rasters (0.5 s bins). C, D, Population analyses. Normalized average firing rates (thick lines) ± SEM (thin dashed lines) of different types of units in relation to the main task events (marked above). Data were normalized to baseline firing rates (C, Type-1 0.22 ± 0.03, Type-2 1.64 ± 0.31; D, Type-1 24.2 ± 2.5, Type-2 23.8 ± 4.3). C, PNs of Type-1 (top; n = 425) and Type-2 (bottom, n = 41). D, ITNs of Type-1 (top, n = 44) and Type-2 (bottom, n = 14).
The predominant PN type (Type-1) showed a firing rate reduction from baseline to waiting and a further decrease upon foraging initiation and food retrieval (Fig. 4A,C, top). There was heterogeneity among Type-1 unit recordings, particularly with respect to their baseline firing rates (range: 0.03–3.36 Hz). Nevertheless, as a group, they exhibited little spontaneous activity (average: 0.22 ± 0.03 Hz) and their firing rates were lowest during escape, when they were virtually silent (0.05 ± 0.01 Hz; Fig. 4C, top). In contrast, Type-2 PNs (Fig. 4B,C, bottom) exhibited a slight and gradual increase in firing rate from baseline to waiting, continuing during foraging, and abruptly reversing to a firing rate reduction after food retrieval. Last, we observed a heterogeneous group of unit recordings (Type-3, 13%) that lacked sustained firing rate changes during foraging, but instead displayed short-lived alterations in activity, usually upon food retrieval. They are not considered further.
To shed light on the behavioral significance of the activity patterns of Type-1 and Type-2 PNs, we examined how their firing rates varied depending on whether the prior trial was a failure or success. The reader will recall that following a failed Robogator trial, rats took longer to retrieve food pellets. Type-1 PNs fired at higher rates during waiting (Fig. 5A1) and foraging (Fig. 5A2) following failed than successful trials (Wilcoxon signed-ranks tests, p's < 0.0001). In contrast, no differences were seen in Type-2 cells depending on prior trial outcome (Waiting, p = 0.11; Foraging, p = 0.23).
Figure 5.
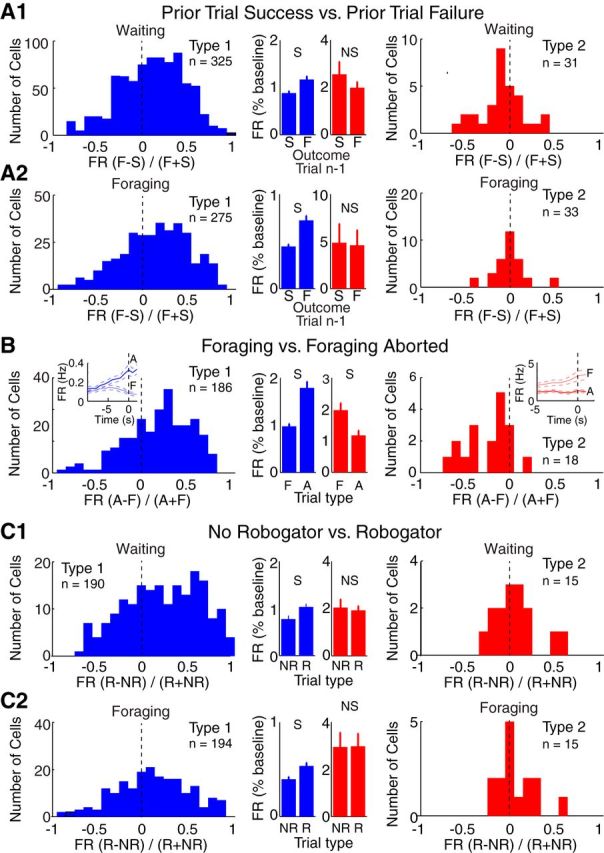
Firing rates of Type-1 and Type-2 PNs as a function of trial type. A–C, Comparison of firing rates as a function of trial type in Type-1 (blue, left) and Type-2 (red, right). Comparisons are as follows: (A) depending on whether the prior Robogator trial was a failure (F) or success (S) during waiting (A1) or foraging (A2); (B) depending on whether waiting at door threshold is followed by retreat back to the nest (A) or foraging (F); (C) depending on whether Robogator was present (R) or not (NR) during waiting (C1) and foraging (C2). Units were only included if ≥5 trials of each type were available and if they fired one spike in at least one trial. These exclusion criteria explain variations in the number of unit recordings considered in the various analyses (Type-1: A1, n = 325; A2, n = 275; B, n = 186; C1, n = 190; C2, n = 194; Type-2: A1, n = 31; A2, n = 33; B, n = 18; C1, n = 15; C2, n = 15). B, Insets, Firing rate as a function of time during waiting period followed by foraging (thin line) versus retreat in the nest (thick line). S, significant at 0.05 level. Central histograms and insets, averages ± SEM.
Consistent with this, Type-1 PNs fired at higher rates when, after approaching the door, rats retreated back in the nest versus initiated foraging (Fig. 5B; Wilcoxon signed ranks test, p < 0.0001). However, in this case, Type-2 cells showed the opposite (p = 0.001). These results suggest that successful foraging is associated with decreased firing in Type-1 unit recordings and increased firing in Type-2 unit recordings.
Last, we compared PN activity in trials with versus without Robogator during waiting (Fig. 5C1) and foraging (Fig. 5C2). Type-1 PNs fired at significantly higher rates during Robogator trials (Wilcoxon signed ranks tests: Waiting, p < 0.0001; Foraging, p < 0.0001). In contrast, Type-2 PNs fired at similar rates in the two trial types (Waiting, p = 0.9; Foraging, p = 0.21; Table 1). The effects described above were not due to interdependence between the variables examined (Table 2).
Table 1.
Comparison between firing rates of Type-1 and Type-2 PNs as a function of trial type (same analyses as in Figure 5)
| PNs Type 1 | PNs Type 2 | |
|---|---|---|
| Prior trial success versus failure | ||
| Waiting | N = 325, p < 0.0001 | N = 31, p = 0.11 |
| ΔFR = 33.1 ± 6.5% | ΔFR = −22.3 ± 17.9% | |
| Foraging | N = 275, p < 0.0001 | N = 33, p = 0.23 |
| ΔFR = 62.4 ± 9.0% | ΔFR = −5.7 ± 10.0% | |
| Foraging versus aborted foraging | N = 186, p < 0.0001 | N = 18, p = 0.001 |
| ΔFR = 83.2 ± 11.6% | ΔFR = −40.9 ± 13.2% | |
| No Robogator versus Robogator | ||
| Waiting | N = 190, p < 0.0001 | N = 15, p = 0.9 |
| ΔFR = 33.2 ± 9.1% | ΔFR = −6.5 ± 12.6% | |
| Foraging | N = 194, p < 0.0001 | N = 15, p = 0.21 |
| ΔFR = 35.4 ± 8.5% | ΔFR = 3.6 ± 9.6% |
FR, firing rate.
Table 2.
Comparison between firing rates of Type-1 and Type-2 PNs as a function of trial type
| PNs Type 1 | PNs Type 2 | |
|---|---|---|
| Prior trial success versus failure (control for period duration) | ||
| Waiting | N = 306, p < 0.0001 | N = 31, p = 0.34 |
| ΔFR = 31.6 ± 7.2% | ΔFR = −7.0 ± 17.3% | |
| Foraging | N = 263, p < 0.0001 | N = 33, p = 0.03 |
| ΔFR = 60.0 ± 9.0% | ΔFR = −12.2 ± 10.2% | |
| Foraging versus aborted foraging (restricted to successful prior trial) | N = 117, p < 0.0001 | N = 12, p = 0.015 |
| ΔFR = 136.1 ± 24.8% | ΔFR = −27.2 ± 16.0% | |
| Foraging versus aborted foraging (restricted to failed prior trial) | N = 144, p < 0.0001 | N = 12, p = 0.03 |
| ΔFR = 70.3 ± 18.3% | ΔFR = −19.3 ± 10.9% | |
| No Robogator versus Robogator (control for period duration and restricted to successful prior trial) | ||
| Waiting | N = 174, p = 0.03 | N = 15, p = 0.72 |
| ΔFR = 20.9 ± 11.7% | ΔFR = 3.1 ± 10.6% | |
| Foraging | N = 189, p = 0.04 | n = 15, p = 0.25 |
| ΔFR = 32.9 ± 8.7% | ΔFR = 1.7 ± 10.1% |
Analyses shown in Figure 5 were repeated after controlling for duration of the examined period and nature of the prior trial (success or failure). Because not all types of trial restrictions occurred for all cells, the number of unit recordings included in the various analyses varies. Cells were included if at least five trials were available. To control for period duration, the beginning of the longer period was ignored. FR, firing rates.
Interneurons
Of the 71 presumed ITNs, 96% showed statistically significant variations in activity during the task. However, these variations were minor, relative to their high baseline firing rates (<10%), except for when rats approached the food pellet and escaped. Figure 4D (grouped data) contrasts the activity of the two main ITN types. Most ITNs (Type-1; n = 44; Fig. 4D) displayed a slight increase in firing rate upon approach of the door threshold, a further progressive increase during foraging, followed by a steep increase as they approached the food. Upon escape, their firing rates rapidly returned toward baseline. In contrast, most of the remaining ITNs (Type-2; n = 14; Fig. 4D, bottom) displayed a minor firing rate reduction during waiting and foraging. Their activity in relation to approach of the food and escape was similar to the first ITN class. In addition, 27 and 36% of Type-1 and Type-2 ITNs showed a phasic increase in firing rate when the door opened. In contrast to PNs (Fig. 5), ITNs displayed little or no differences in activity as a function of trial type (Table 3).
Table 3.
Comparison between firing rates of Type-1 and Type-2 ITNs as a function of trial type
| ITN Type 1 | ITN Type 2 | |
|---|---|---|
| Prior trial success versus failure | ||
| Waiting | N = 31, p = 0.98 | N = 10, p = 0.005 |
| ΔFR = 1.2 ± 1.9% | ΔFR = 9.2 ± 3.1% | |
| Foraging | N = 33, p = 0.03 | N = 10, p = 0.16 |
| ΔFR = −1.3 ± 2.1% | ΔFR = 7.11 ± 3.3% | |
| Foraging versus aborted foraging | N = 16, p = 0.17 | N = 6, p = 0.22 |
| ΔFR = −1.8 ± 3.3% | ΔFR = 8.5 ± 5.4% | |
| No Robogator versus Robogator | ||
| Waiting | N = 23, p = 0.69 | N = 9, p = 0.42 |
| ΔFR = 1.3 ± 3% | ΔFR = 4.5 ± 2.9% | |
| Foraging | N = 23, p = 0.04 | N = 9, p = 0.91 |
| ΔFR = −4.1 ± 1.5% | ΔFR = 5.4 ± 4.9% |
Same analyses as in Figure 5 but for ITNs. FR, firing rates.
Projection site and state-dependent variations in the firing rates of Type-1 and Type-2 cells
The incidence of antidromically responsive units was similar among the different PN types (χ2 test, p = 0.27; Table 4). Differences in the incidence of neurons responsive to mPFC or NAc stimuli did not reach significance (χ2 test, p = 0.38; Table 4). In contrast, Type-1 and Type-2 units exhibited significant differences in firing rates and a dissimilar modulation by behavioral states (Fig. 6A,B). In particular, Type-1 units fired at lower rates than Type-2 cells in quiet waking (QW; Type-1, 0.34 ± 0.02 Hz; Type-2, 1.58 ± 0.27 Hz; Wilcoxon rank sum test, z value = 6.82, p < 0.0001) and slow-wave sleep (SWS; Type-1, 0.51 ± 0.02 Hz; Type-2, 1.49 ± 0.16 Hz; Wilcoxon rank sum test, z-statistics = 7.28, p < 0.0001). Also, whereas the firing rate of Type-1 units was higher in SWS than QW (221 ± 12% higher, Wilcoxon signed rank test, z value = 12.7, p < 0.0001), Type-2 unit recordings showed inconsistent activity modulation by behavioral states, resulting in an insignificant average modulation (z-value = 0.65, p = 0.48). Importantly, the probability that a particular PN would belong to the Type-1 or Type-2 class varied inversely with firing rate in QW and SWS (Fig. 6C).
Table 4.
Incidence of antidromically invaded neurons among different types of BL unit recordings
| Tested cells (336) | Backfired cells | Backfired from NAc | Backfired from mPFC | Backfired from NAc and mPFC | |
|---|---|---|---|---|---|
| PNs Type 1 | 217 | 48 (22%) | 21 (10%) | 22 (10%) | 5 (2%) |
| PNs Type 2 | 14 | 2 (14%) | 0 (0%) | 2 (14%) | 0 (0%) |
| PNs Type 3 | 36 | 4 (11%) | 2 (6%) | 1 (3%) | 1 (3%) |
| PN Not Significant | 20 | 2 (10%) | 0 (0%) | 2 (10%) | 0 (0%) |
| ITNs | 44 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unclassified | 5 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
All units were tested for antidromic responsiveness to stimulation of NAc and mPFC.
Figure 6.
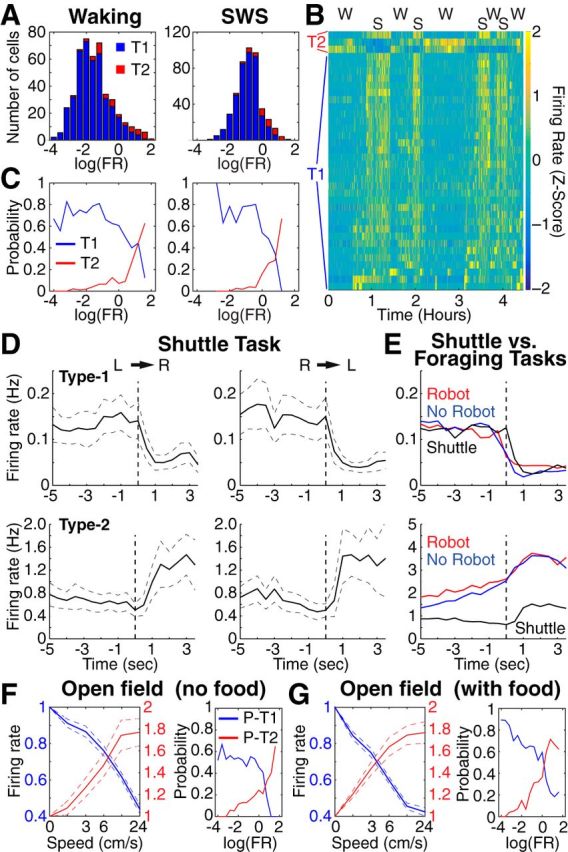
Activity variations of Type-1 and Type-2 PNs during QW, SWS, and in control tasks. A, Frequency distribution of firing rates among Type-1 (blue, n = 425) and Type-2 (red, n = 41) PNs in QW (left) and SWS (right). B, Z-scored firing rate of 36 simultaneously recorded PNs during spontaneous alternations between QW and SWS. C, Probability that a PN belongs to the Type-1 (blue, n = 425) or Type-2 (red, n = 41) classes plotted as a function of firing rate. D, Firing rate of Type-1 (top) or Type-2 (bottom) during shuttle task (left, left to right trials; right, right to left trials). D, E, Population averages (Type-1, n = 186; Type-2, n = 14). E, Comparison between PN activity during Robogator and shuttle tasks. Vertical dashed lines in D and E indicate when rats left the nest. F, Left, Firing rate during open field exploration (no food) plotted as a function of movement speed. Blue (n = 189) and red (n = 51), Units with significant negative or positive correlation to speed, respectively. Right, Probability that the firing rate of a PN is negatively (blue) or positively (red) correlated with speed plotted as a function of overall firing rate in QW. G, Left, Firing rate during open field exploration (with food pellets) plotted as a function of movement speed. Blue (n = 141) and red (n = 49), Units with significant negative or positive correlation to speed, respectively. Right, Probability that the firing rate of a PN is negatively (blue) or positively (red) correlated with speed plotted as a function of overall firing rate in QW.
Control tasks
The above suggests that BL contains two main PN types that exhibit a differential regulation by behavioral states and opposite activity profiles in the foraging task. However, the seminaturalistic character of this task complicates analysis of the factors that drive neuronal activity. Are predator risk, reward availability, both, or neither required? Also, this task features uncontrolled behavioral variables such as the rats' movements and speed. Therefore, to shed light on the impact of these various factors, we compared the activity of BL units in two additional tasks that did not include explicit threats or rewards.
Food-seeking shuttle task
In this task, rats ran back and forth across a central arena to retrieve food pellets placed in two enclosures (hereafter termed “nests”) located at opposite ends of the central arena. Light levels were uniformly low to minimize perceived threat. Moreover, recordings began after two daily 3 h sessions of habituation to the apparatus. Three of the rats used for the foraging task also performed the shuttle task, allowing us to compare the activity of the same Type-1 (n = 186) and Type-2 (n = 14) cells in the two tasks.
Behaviorally, there was no sign of apprehension in the shuttle task. Upon door opening, rats immediately entered the central compartment and ran to the other nest to retrieve the food. This contrasts with the foraging task where rats waited at the door threshold for ∼16 s before leaving the nest. Also contrasting with the foraging task, rats never skipped a trial in the shuttle task. Yet, the activity of Type-1 units in the foraging and shuttle tasks was similar. As shown in Figure 6, D and E (top), their firing rate decreased to a similar degree when rats initiated foraging (red and blue curves) or a shuttle trial (black curve). Type-2 units started shuttle trials at a lower average firing rate than during Robogator trials, presumably because of the lack of a waiting period. However, they also showed an increase in discharge rate upon door opening (Fig. 6D,E, bottom).
Open field exploration
Since the above suggests that the opposite activity of Type-1 and Type-2 units are not strictly dependent on threat, we next obtained BL unit recordings while rats explored an open field, devoid of explicit rewards. To minimize threat, this test was performed under low light levels and rats were habituated to the apparatus for two daily 3 h sessions before the recordings began. To ensure that rats would not expect food in the open field, we used naive rats.
Spontaneous exploration occurs regardless of external events under investigator control. Thus we could only relate neuronal activity to the rats' movements and position in the open field. We first examined whether there was a relationship between firing rates and movement speed. We reasoned that if the inverse activity modulation of Type-1 and Type-2 units in Robogator and shuttle tasks are related to movement, we should observe two subsets of units that display decreasing versus increasing activity with movement speed and a different distribution of spontaneous firing rates, as in the foraging task (Fig. 6C). We found that 67% of PNs (n = 355) showed significant variations in firing rates as a function of speed (Fig. 6F, left; 53 and 14% decreasing and increasing with speed, respectively). Critically, the probability that the firing rate of a particular PN would show a negative (blue, presumed Type-1) versus positive (red, presumed Type-2) correlation with speed varied inversely with firing rate in QW (Fig. 6F, right), reminiscent of the results obtained the foraging task (Fig. 6C).
After completion of the above tests, when food pellets were dropped at random spots in the open field to motivate movement, an even higher proportion of PNs showed a significant relation to speed (Fig. 6G; n = 209; 90% with significant modulation; 67 and 23% decreasing and increasing with speed, respectively). However, the higher proportion of significant units is not due to rewards but to the fact that rats ran more, increasing statistical power. Indeed, when we reduced the number of movements analyzed to mimic the spontaneous exploration, the proportion of significant unit recordings was comparable to that seen in the absence of food (56 and 18% decreasing and increasing with speed, respectively; χ2 test, p = 0.41). Last, to ensure that the relation with movement was not dependent on differences in firing rates as a function of the rats' position in the center versus periphery of the open field (Likhtik et al., 2014), we repeated the above analyses for movements starting and ending in these two sectors. Qualitatively identical results were obtained.
Relation between firing rates and movement in the foraging and shuttle tasks
The correlation between PN activity and movement velocity in the open field led us to examine whether this relationship was also present in the foraging and shuttle tasks (Fig. 7). Complicating this analysis, however, were the different amounts of movement periods available for analysis in the open field (∼20 or 90 min) versus Robogator (∼15 min) and shuttle (∼3 min) tasks, reducing statistical power for the latter task. Nevertheless, there was a strong bias for the firing rate of Type-1 and Type-2 units to respectively, show a negative and positive relation to movement velocity in the foraging and shuttle tasks (Fig. 7A).
Figure 7.
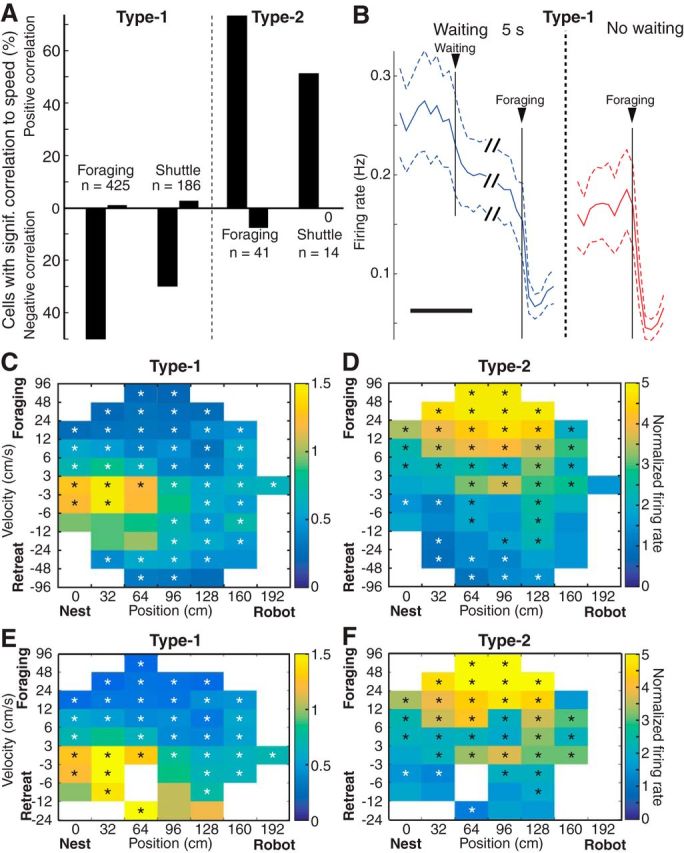
Relation between the firing rate of PNs and movement speed in the foraging and shuttle tasks. A, Proportion of Type-1 (left) and Type-2 (right) PNs whose firing rate shows a significant negative (bottom) or positive (top) correlation to movement speed. B, Firing rates of Type-1 PNs on Robogator trials with long waiting periods (≥5 s, blue) or no waiting (red; n = 244; averages ± SEM). Scale bar, 10 s. Units were only included if ≥5 trials of each type were available. C, D, Color-coded firing rates of Type-1 (n = 339) and Type-2 (n = 33) PNs plotted as a function of movement speed (y-axis) and position (x-axis). Firing rates are normalized to baseline values. E, F, Same analysis as in C and D, with the exception that the final escape phase was excluded. C–F, Rectangles are only shown if (1) rats spent ≥4 s at the given position and velocity and (2) data from ≥50% of unit recordings had to be available for that position and speed. To assess significance, we shuffled the actual spike trains 1000 times while keeping velocity and position constant and then compared the actual data to the distribution of shuffled values. Black and white asterisks indicate bins that were higher or lower than 97.5% of shuffled values, respectively.
Another important consideration is whether Type-1 and Type-2 units exhibit the same relation to movement speed regardless of motion direction and Robogator proximity. To address this, we computed the firing rate of Type-1 and Type-2 units (Fig. 7C,D) as a function of position (x-axis) and speed (y-axis), with the latter separating forward (toward the food, top) and backward (toward the nest, bottom) movements. In these conditions, the firing rate of Type-1 units exhibited a similar negative relation to movement speed regardless of direction (Fig. 7C). However, this analysis also disclosed an influence of position as the firing rates of the cells decreased with increasing distances from the nest at low movement velocities. To test whether Robogator proximity was responsible for this effect, we compared the normalized firing rates of the cells at a long foraging distance (150 cm) in trials with versus without the Robogator as well as in naive animals with the Robogator absent. However, we found no difference (p = 0.23). Thus the positional effect does not depend on Robogator proximity. Because many variables covary with position in this task (distance to food, distance to Robogator, and distance from nest), it is not possible to tease out the factors contributing to this apparent influence of position.
Importantly, when we excluded the escape phase to separately consider trials where rats hesitated as they approached food (first moving forward, then backward, then forward again), an interesting phenomenon was observed: the firing rate of Type-1 units showed decreased activity when the rats approached the food, but increased activity, when rats moved backward (Fig. 7E). In contrast, Type-2 units showed a positive relation to movement in the forward direction and the opposite upon retreat, regardless of whether we considered the entire foraging trials (Fig. 7D, foraging and retreat), or only the foraging phase (Fig. 7F).
Other dissociations between PN activity and movement occurred during the waiting period, when rats are immobile. A first dissociation is the differential activity of PNs during the waiting period on trials where rats, after approaching the door, retreated back in the nest versus initiated foraging. Anticipating movement by several seconds, the firing rate of Type-1 PNs increased when rats retreated back in the nest rather than initiating foraging; Type-2 units showed the opposite (Fig. 5B, insets). Second, on trials where rats initiated foraging, both types of unit recordings showed firing rate changes in the absence of movement (Fig. 4C). This was seen even when we compared trials where rats initiated foraging after a ≥5 s waiting period versus those rare trials (≤4%) where rats initiated foraging immediately (Fig. 7B).
Discussion
We studied BL activity in a seminaturalistic foraging task and control tasks devoid of explicit threats or rewards. Threat proximity and reward availability did not consistently predict BL activity. Instead, BL activity covaried with behavior, even though threat and reward levels were clamped at low or high levels. Our results suggest that BL activity not only encodes threats or rewards, but is closely related to behavioral output. Below, we consider the origin of the behavioral variations we observed and discuss the significance of the associated changes in BL activity.
Relation to prior work on coding by amygdala neurons
Early lesion studies (Klüver and Bucy, 1939; Weiskrantz, 1956; Blanchard and Blanchard, 1972) led to the view that the amygdala plays a key role in defensive behaviors. This notion was reinforced by subsequent studies implicating the amygdala in classically conditioned fear, which showed that fear conditioning leads to the emergence of amygdala cells with potentiated responses to CSs predicting aversive outcomes (Maren and Quirk, 2004). Overall, this work suggested that amygdala neurons signal threat and trigger defensive behaviors. Extrapolated to the foraging task, this suggests that the rats' cautious behavior depends on threat signaling by amygdala neurons. In apparent agreement with this inference, local infusions of drugs that enhanced or reduced threat signaling by amygdala neurons diminished or increased risk taking, respectively (Choi and Kim, 2010). Yet, our recordings revealed a different situation.
We distinguished two types of principal neurons based on their activity in the foraging task. Increased firing rates in the prevalent type (Type-1) anticipated aborted foraging and retreat into the nest. In contrast, augmented activity in Type-2 cells anticipated foraging. Thus Type-1 cells seem to correspond to the threat-signaling neurons expected from prior work. Surprisingly, however, upon foraging initiation, these cells showed a marked firing suppression, despite increasing predator proximity. In other words, Type-1 cells do not signal threat during foraging. Thus these results suggest that amygdala inactivation might not increase risk taking because it suppresses threat signaling, but because it reproduces the state of Type-1 cell suppression that occurs during foraging.
Since rats continue to forage during amygdala inactivation, the drive to seek food does not require the amygdala. Yet, many studies have implicated the amygdala in reward seeking (see above, Introduction). A critical question emerging from these studies is whether the same or different pools of amygdala neurons process threats and rewards. In monkeys, different populations of BLA neurons signal positively or negatively valenced CSs (Belova et al., 2008). In contrast, a study using a mixed appetitive aversive learning paradigm emphasized the similar behavior of rat BLA neurons in response to CSs predicting rewarding or aversive outcomes (Shabel and Janak, 2009). Related to this, Roesch et al. (2010) reported that rat BLA neurons signal unexpected deviations in reward outcomes, whether positive or negative. Further complicating this picture, Genud-Gabai et al. (2013) reported a mixture of excitation and inhibition with aversive, neutral, and positively valenced CSs in monkeys. These findings suggest that the amygdala contains multiple cell populations related to processing of negative, neutral, or positively valenced CSs. This raises the question of what factors drive the inverse activity fluctuations of Type-1 and Type-2 cells in the foraging task.
Behavioral correlates of activity fluctuations in BL cells
We observed that in constant conditions of rewards and threats, BL activity varied with behavior. In the foraging task, Type-2 cells fired at higher rates when rats approached food pellets and at lower rates during the escape phase. In contrast, Type-1 cells fired at low rates when the rats' behavior was directed toward attaining the food or seeking refuge from the predator. Overall, Type-1 cells were most active when the rats' behavior betrayed apprehension such as if they failed to initiate foraging and retreated into the nest or when, after initiating foraging, they showed signs of hesitation, moving backward and forward again. Type-1 cells also fired at higher rates when rats hesitated at the door threshold, particularly if the prior trial was a failure. The longer waiting period and higher firing rates of Type-1 cells after failed than successful trials suggest that behavior on the foraging task and the associated activity of Type-1 cells are not only correlated with movement speed or reward/threat contingencies, but are also function of prior experience and related internal states.
To shed light on the significance of these observations, we examined BL activity in two control tasks devoid of explicit threats or rewards. Whether rats moved to secure food pellets (shuttle task) or spontaneously for no apparent reason (open field), the firing rate of Type-1 cells diminished as the rats' velocity increased. Similarly, the firing rate of Type-2 cells in the control tasks showed a strong relation to movement speed, but it was in the opposite direction. Although a relation between BL firing rates and movement velocity was also observed in the foraging task, it depended on the direction and context of movements. For instance, when rats moved forward to attain food pellets, the firing rates of Type-1 and Type-2 cells, respectively, decreased and increased as a function of velocity, as in the control tasks. In contrast, when rats hesitated during the foraging phase, moving forward, backward and forward again, the firing rate of Type-1 cells decreased when the rats approached the food, but increased when rats moved backward. Furthermore, the firing rate of Type-1 cells was also affected by position in the foraging arena.
Impact, origin, and significance of the inverse activity fluctuations of Type-1 and Type-2 cells
How could these task-related changes in BL activity affect behavior? A possible answer to this question comes from prior fear conditioning experiments. In particular, researchers (Herry et al., 2008; Amano et al., 2011; Sangha et al., 2013) distinguished two main types of BL cells among neurons with conditioning-induced changes in CS responsiveness. Most plastic neurons, likely corresponding to Type-1 cells, developed increased responses to the CS that were either abolished by or persisted despite extinction training. A second class of neurons, likely corresponding to Type-2 cells, displayed inhibitory CS responses after conditioning, which reversed to excitatory responses following extinction training. It was later found that the two cell types project to different sectors of the medial prefrontal cortex (Senn et al., 2014) and it is likely that they also form contrasting connections with the medial part of the central amygdala (CeM; for review, see Duvarci and Pare, 2014), with many Type-1, but not Type-2 cells, projecting to CeM (Popescu and Paré, 2011). The significance of this inference stems from the fact that most amygdala projections to the periaqueductal gray (PAG), controlling behavioral freezing, originate from CeM (Hopkins and Holstege, 1978). Therefore, suppressed firing in Type-1 PNs would cause a disfacilitation of PAG-projecting CeM neurons, reducing freezing, a prerequisite for the initiation of movement in the three tasks examined here. This view parallels the conclusions of a prior study (Jacobs and McGinty, 1972).
In addition to CeM, BL contributes projections to other structures involved in fear and anxiety (Walker and Davis, 1997; Felix-Ortiz et al., 2013) or reward-seeking behaviors (Stuber et al., 2011). It will be important to determine whether Type-1 and Type-2 principal cells also contributed to the behavioral variations observed here via segregated or overlapping projections to different BL targets.
So far, concepts about amygdala function have centered on the processing of threat and rewards. Yet, given that the activity of BL cells can be high or low in constant conditions of threats and rewards, an inescapable conclusion is that BL activity is closely related to behavioral output, not only driven by threats and rewards. We propose that BL activity is determined by at least two opposing (and possibly competing) processes: one driven by threats and one driven by actual (foraging and shuttle tasks) or potential (open field) rewards (including threat reduction as when escaping). These two processes likely involve distinct subsets of higher order afferent neurons that recruit different populations of BL cells (Type-1 and Type-2 cells, respectively), either directly or through dedicated subsets of local interneurons. Crucially, which of these two processes dominates is not only a function of stimulus contingencies, but also of prior experience and internal states. This statement is supported by the longer wait times and associated increases in the firing rate of Type-1 cells in trials that follow failed relative to successful trials.
These considerations lead us to speculate that the task-related changes in BL activity we observed result from a continuous evaluative process where internal states as well as reward and threat expectations determine how rats will behave on a moment-to-moment basis. The outcome of this evaluative process would differentially recruit two distinct groups of BL afferents, ultimately determining whether Type-1 or Type-2 cells will be more active. Because of the contrasting connections they form with CeM, when Type-1 cells are more active, movement whether aimed at attaining food or spontaneous exploration would be suppressed, whereas when Type-2 cells are more active, it would be facilitated. An important challenge for future studies will be to determine whether cells acquiring increased responses to CSs predicting aversive or rewarding outcomes are differentially distributed among Type-1 and Type-2 cells.
Footnotes
The authors declare no competing financial interests.
This material is based upon work supported by National Institute of Mental Health grants RO1 MH-083710 and MH-107239 to D.P.
References
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci U S A. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. doi: 10.1111/j.1749-6632-2003.tb07085.x. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genud-Gabai R, Klavir O, Paz R. Safety signals in the primate amygdala. J Neurosci. 2013;33:17986–17994. doi: 10.1523/JNEUROSCI.1539-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods. 2006;155:207–216. doi: 10.1016/j.jneumeth.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, McGinty DJ. Participation of the amygdala in complex stimulus recognition and behavioral inhibition: evidence from unit studies. Brain Res. 1972;36:431–436. doi: 10.1016/0006-8993(72)90750-0. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobe in monkeys. Arch NeurPsych. 1939;42:979–1000. doi: 10.1001/archneurpsyc.1939.02270240017001. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Popescu AT, Paré D. Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J Neurophysiol. 2006;96:3257–3265. doi: 10.1152/jn.00577.2006. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Popescu AT, Paré D. Synaptic interactions underlying synchronized inhibition in the basal amygdala: evidence for existence of two types of projection cells. J Neurophysiol. 2011;105:687–896. doi: 10.1152/jn.00732.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci. 2010;30:2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Steinmetz JE. Amygdalar unit activity during three learning tasks: eyeblink classical conditioning, Pavlovian fear conditioning, and signaled avoidance conditioning. Behav Neurosci. 2005;119:1254–1276. doi: 10.1037/0735-7044.119.5.1254. [DOI] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH. Safety encoding in the basal amygdala. J Neurosci. 2013;33:3744–3751. doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecasteele M, M S, Royer S, Belluscio M, Berényi A, Diba K, Fujisawa S, Grosmark A, Mao D, Mizuseki K, Patel J, Stark E, Sullivan D, Watson B, Buzsáki G. Large-scale recording of neurons by movable silicon probes in behaving rodents. J Vis Exp. 2012;61:e3568. doi: 10.3791/3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in light enhances versus fear-potentiated startle. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]