Abstract
In addition to the transcriptional activity of their liganded nuclear receptors, estrogens, such as estradiol (E2), modulate cell functions, and consequently physiology and behavior, within minutes through membrane-initiated events. The membrane-associated receptors (mERs) underlying the acute effects of estrogens on behavior have mostly been documented in females where active estrogens are thought to be of ovarian origin. We determined here, by acute intracerebroventricular injections of specific agonists and antagonists, the type(s) of mERs that modulate rapid effects of brain-derived estrogens on sexual motivation in male Japanese quail. Brain aromatase blockade acutely inhibited sexual motivation. Diarylpropionitrile (DPN), an estrogen receptor β (ERβ)-specific agonist, and to a lesser extent 17α-estradiol, possibly acting through ER-X, prevented this effect. In contrast, drugs targeting ERα (PPT and MPP), GPR30 (G1 and G15), and the Gq-mER (STX) did not affect sexual motivation. The mGluR1a antagonist LY367385 significantly inhibited sexual motivation but mGluR2/3 and mGluR5 antagonists were ineffective. LY367385 also blocked the behavioral restoration induced by E2 or DPN, providing functional evidence that ERβ interacts with metabotropic glutamate receptor 1a (mGluR1a) signaling to acutely regulate male sexual motivation. Together these results show that ERβ plays a key role in sexual behavior regulation and the recently uncovered cooperation between mERs and mGluRs is functional in males where it mediates the acute effects of estrogens produced centrally in response to social stimuli. The presence of an ER–mGluR interaction in birds suggests that this mechanism emerged relatively early in vertebrate history and is well conserved.
SIGNIFICANCE STATEMENT The membrane-associated receptors underlying the acute effects of estrogens on behavior have mostly been documented in females, where active estrogens are thought to be of ovarian origin. Using acute intracerebroventricular injections of specific agonists and antagonists following blockade of brain aromatase, we show here that brain-derived estrogens acutely facilitate male sexual motivation through the activation of estrogen receptor β interacting with the metabotropic glutamate receptor 1a. This behavioral effect occurring within minutes provides a mechanistic explanation of how an estrogen receptor not intrinsically coupled to intracellular effectors can signal from the membrane to govern behavior in a very rapid fashion. It suggests that different subtypes of estrogen receptors could regulate the motivation versus performance aspects of behavior.
Keywords: ERβ, estrogens, membrane-initiated effects, sexual motivation
Introduction
Liganded nuclear estrogen receptors (nERs) regulate the transcription of target genes leading to profound physiological and behavioral changes within hours to days (Tsai and O'Malley, 1994). In parallel, estrogens also trigger nongenomic effects largely through the activation of membrane estrogen receptors (mERs; Vasudevan and Pfaff, 2007). Initially described only at the cellular level, these effects have recently been shown to control within minutes several behavioral (e.g., sexual or aggressive behavior) and physiological (e.g., auditory processing, memory) processes across a range of vertebrate taxa (Cornil et al., 2012).
The receptor(s) mediating these acute actions of estrogens are only beginning to be identified. The “classical” intracellular receptors estrogen receptor α (ERα) and estrogen receptor β (ERβ) can translocate to and signal from the neuronal membrane through their interaction with other membrane receptors (Micevych and Dominguez, 2009; Levin, 2011). Although investigated in both sexes, this interaction has so far only been observed in females (Boulware et al., 2005; Dewing et al., 2007; Chaban et al., 2011). This interaction of ERα and ERβ with membrane receptors has been implicated in the control of sexual behavior (Dewing et al., 2007), learning (Boulware et al., 2013), and possibly nociception (Chaban et al., 2011; Lu et al., 2013). The “novel” G-protein ER GPER or GPR30 is known to regulate hypothalamic secretions, learning, and nociception (Hammond et al., 2009; Srivastava and Evans, 2013). Another putative G-protein-coupled receptor, Gq-mER, seems to control body temperature, energy homeostasis, hypothalamic secretions, and female sexual behavior (Kenealy et al., 2011; Roepke et al., 2011). Finally, ER-X has been described in the developing uterus and neocortex, but its molecular identity and functions remain unknown (Toran-Allerand et al., 2002).
Interestingly, most of these results concern females, where the active estrogens are thought to be of ovarian origin. Moreover, sex differences have been suggested to affect membrane-initiated cellular actions of estradiol (E2) both in vitro (Teyler et al., 1980; Boulware et al., 2005; Huang and Woolley, 2012; Meitzen et al., 2012) and in vivo (Abrahám and Herbison, 2005; Cheong et al., 2012; Small et al., 2013; Hart et al., 2014). As a consequence, it is unclear whether receptors/mechanisms underlying acute actions of estrogens on physiological and behavioral responses are the same in males where estrogens are produced locally in the brain (Roselli et al., 2009). In addition, acute cellular actions of estrogens are often transient, but the temporal features of these effects are rarely examined in vivo.
We showed previously by manipulating both estrogen synthesis and action (using different aromatase inhibitors and nonspecific ER antagonists, respectively) that brain-derived estrogens acutely control male sexual motivation through membrane-initiated events (Seredynski et al., 2013). In the present experiments, male Japanese quail were injected in the third ventricle with an aromatase inhibitor and were then tested for male sexual motivation after central injections with specific drugs targeting mERs to identify the membrane-associated receptor(s) involved in the acute regulation of this response by brain-derived estrogens and determine more precisely the temporal features of this effect. These experiments demonstrate the involvement of ERβ and its interaction with metabotropic glutamate receptor 1a (mGluR1a) in the acute and transient action of E2 on male sexual motivation.
Materials and Methods
Animals
Forty-six male Japanese quail (Coturnix japonica) belonging to three independent groups served as subjects in these experiments. Birds originated from the breeding colony established in our laboratory (Group 1) or were purchased from a local breeder at the age of 2–3 weeks (Groups 2 and 3). Throughout their life, at the breeding colony and in the laboratory, the birds were exposed to a photoperiod simulating long summer days (16 h light/8 h dark) to fully stimulate their hypothalamic–pituitary–gonadal axis. They had food and water available ad libitum. All experimental procedures were in agreement with the Belgian laws on the Protection and Welfare of Animals and on the Protection of Experimental Animals and were approved by the Ethics Committee for the Use of Animals at the University of Liège (Protocol #1442).
All birds were first housed as a group in a large cage. Birds were castrated as previously described (Balthazart et al., 1998) at the age of 3 weeks. They remained housed as a group until the age of 6 weeks, when they were moved to individual cages. At the age of 8 weeks, all birds were implanted in the third ventricle with a chronic injection cannula (see below). All birds were then implanted subcutaneously in the neck region with one 20-mm-long Silastic tube (Silclear Tubing, Degania Silicone; inner diameter, 1.57 mm; outer diameter, 2.41 mm) filled with crystalline testosterone (Sigma-Aldrich) to fully activate typical male behaviors (Seredynski et al., 2013). Two weeks after the beginning of testosterone treatment, birds were repeatedly tested for consummatory behavior until they displayed the full range of copulatory behavior. Birds were given as many tests as were necessary until their average behavioral scores had reached a plateau. Individual birds were kept in the study if at this point they had copulated in at least two different tests with a latency lower than 20 s. Typically, birds reach these criteria within 6–10 tests. They were then tested once for rhythmic cloacal sphincter movements (RCSMs; see below for description of their quantification) to habituate them to the test area. This first RCSM test constituted a reference (pretest) for the first experiment. Similar pretests were run between each individual experiment.
General approach
A total of 21 separate experiments were performed on three independent groups of castrated, testosterone-treated males to assess the temporal characteristics of the acute inhibition of brain aromatase on behavior as well as the role of the different mERs in the acute modulation of sexual motivation (Tables 1–3).
Table 1.
Details of Experiments 1–6 conducted to assess the temporal characteristics of E2 and VOR effects
| Experiment | Testing order | Group | n | Treatments | Doses | Test latency (min) | Statistics |
||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Group | Interaction | |||||||
| 1 | 17 | 3 | 13 | PG–VOR | VOR: 50 μg in 1 μl | 30, 120, 240, 480 | F(4,32) = 6.265, p < 0.001 | F(4,8) = 1.008, p = 0.457 | F(4,16) = 2.491, p = 0.014 |
| 2 | 21 | 3 | 12 | PG–VOR–VOR + E2 | VOR: 50 μg in 1 μl; E2: 50 μg in 1 μl | 30, 5 | F(2,18) = 27.946, p < 0.001 | F(2,9) = 1.842, p = 0.214 | F(4,18) = 0.703, p = 0.600 |
| 3 | 19 | 3 | 12 | PG–VOR–VOR + E2 | VOR: 50 μg in 1 μl; E2: 50 μg in 1 μl | 30, 15 | F(2,18) = 28.032, p < 0.001 | F(2,9) = 0.586, p = 0.576 | F(4,18) = 0.314, p = 0.865 |
| 4 | 18 | 3 | 12 | PG–VOR–VOR + E2 | VOR: 50 μg in 1 μl; E2: 50 μg in 1 μl | 30, 120 | F(2,18) = 45.403, p < 0.001 | F(2,9) = 0.606, p = 0.567 | F(4,18) = 1.520, p = 0.239 |
| 5 | 20 | 3 | 12 | PG–VOR–VOR + E2 | VOR: 50 μg in 1 μl; E2: 50 μg in 1 μl | 30, 240 | F(2,18) = 40.876, p < 0.001 | F(2,9) = 1.352, p = 0.306 | F(4,18) = 0.329, p = 0.855 |
| 6 | 4 | 1 | 7 | PG–E2 | E2: 50 μg in 1 μl | 15 | F(1,5) = 1.769, p = 0.241 | F(1,5) = 1.210, p = 0.321 | F(1,5) = 0.236, p = 0.647 |
The experiment numbers (Experiment) follow the order in which the results are described in the manuscript. The actual order in which the experiments were performed is provided in the second column (Testing order). The third column indicates to which group the animals involved in this experiment belonged (see Materials and Methods, General approach). The fourth column provides the number of birds tested in this experiment, while the fifth and sixth columns present the different treatments compared (separated by dashes) as well as the doses and volumes of drugs injected. The latency between injection and test is represented under Test latency. The columns under Statistics provide the results of the statistical analyses.
Table 2.
Details of Experiments 7–16 conducted to determine the identity of the mER involved
| Experiment | Testing order | Group | n | Treatments | Doses | Test latency (min) | Statistics |
||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Group | Interaction | |||||||
| 7 | 10 | 1 | 10 | PG–VOR–VOR + PPT–VOR + DPN | VOR: 50 μg in 1 μl; PPT/DPN: 0.4 μg in 1 μl | 30, 15 | F(3,18) = 21.411, p < 0.001 | F(3,6) = 0.2981, p = 0.826 | F(9,18) = 0.261, p = 0.978 |
| 8 | 8 | 1 | 10 | PG–VOR–VOR + PPT–VOR + DPN | VOR: 50 μg in 1 μl; PPT/DPN: 2 μg in 1 μl | 30, 15 | F(3,18) = 14.881, p < 0.001 | F(3,6) = 0.081, p = 0.968 | F(9,18) = 0.623, p = 0.763 |
| 9 | 7 | 1 | 12 | PG–VOR–VOR + PPT–VOR + DPN | VOR: 50 μg in 1 μl; PPT/DPN: 10 μg in 1 μl | 30, 15 | F(3,24) = 12.682, p < 0.001 | F(3,8) = 0.050, p = 0.984 | F(9,24) = 0.774, p = 0.641 |
| 10 | 12 | 1 | 10 | PG–VOR–VOR + PPT–VOR + DPN | VOR: 50 μg in 1 μl; PPT/DPN: 50 μg in 1 μl | 30, 15 | F(3,18) = 18.883, p < 0.001 | F(3,6) = 0.535, p = 0.675 | F(9,18) = 1.802, p = 0.138 |
| 11 | 16 | 3 | 13 | PG–VOR–VOR + PPT–VOR + DPN–VOR + PPT + DPN | VOR; 50 μg in 1 μl; PPT/DPN: 50 μg in 2 μl; PPT + DPN: 50 μg in 2 μl | 30, 15, 15 | F(4,32) = 25.154, p < 0.001 | F(4,8) = 0.707, p = 0.609 | F(16,32) = 1.300, p = 0.256 |
| 12 | 5 | 1 | 9 | PG–MPP | MPP: 50 μg in 1 μl | 30 | F(1,7) = 0.022, p = 0.886 | F(1,7) = 0.091, p = 0.772 | F(1,7) = 0.022, p = 0.886 |
| 13 | 2 | 2 | 10 | PG–VOR–VOR + G1 | VOR: 50 μg in 1 μl; G1: 50 μg in 2 μl | 30, 15 | F(2,14) = 19.762, p < 0.001 | F(2,7) = 0.251, p = 0.785 | F(4,14) = 1.933, p = 0.161 |
| 14 | 1 | 2 | 12 | PG–G15 | G15: 50 μg in 2 μl | 30 | F(1,10) = 1.104, p = 0.318 | F(1,10) = 1.001, p = 0.341 | F(1,10) = 0.161, p = 0.696 |
| 15 | 3 | 2 | 11 | PG–VOR–VOR + STX | VOR: 50 μg in 1 μl; STX: 50 μg in 2 μl | 30, 15 | F(2,16) = 22.723, p < 0.001 | F(2,8) = 1.123, p = 0.372 | F(4,16) = 0.564, p = 0.692 |
| 16 | 6 | 1 | 9 | PG–VOR– VOR + 17α-E2 | VOR: 50 μg in 1 μl; 17α-E2: 50 μg in 1 μl | 30, 15 | F(2,12) = 4.836, p = 0.029 | F(2,6) = 0.419, p = 0.675 | F(4,12) = 0.468, p = 0.758 |
The experiment numbers (Experiment) follow the order in which the results are described in the manuscript. The actual order in which the experiments were performed is provided in the second column (Testing order). The third column indicates to which group the animals involved in this experiment belonged (see Materials and Methods, General approach). The fourth column provides the number of birds tested in this experiment, while the fifth and sixth columns present the different treatments compared (separated by dashes) as well as the doses and volumes of drugs injected. The latency between injection and test is represented under Test latency. The columns under Statistics provides the results of the statistical analyses.
Table 3.
Details of Experiments 17–21 conducted to assess the involvement of mGluR1a
| Experiment | Testing order | Group | n | Treatments | Doses | Test latency (min) | Statistics |
||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Group | Interaction | |||||||
| 17 | 9 | 3 | 12 | PG–LY367385 | LY367385: 100 μg in 1 μl | 30 | F(1,10) = 48.846, p < 0.001 | F(1,10) = 3.209, p = 0.104 | F(1,10) = 7.815, p = 0.019 |
| 18 | 13 | 3 | 8 | PG–MPEP | MPEP: 100 μg in 1 μl | 30 | F(1,6) = 0.126, p = 0.735 | F(1,6) = 6.438, p = 0.044 | F(1,6) = 0.282, p = 0.614 |
| 19 | 14 | 3 | 9 | PG–LY341495 | LY341495: 100 μg in 2 μl | 30 | F(1,7) = 1.149, p = 0.319 | F(1,7) = 0.421, p = 0.537 | F(1,7) = 1.537, p = 0.255 |
| 20 | 11 | 3 | 22 | PG–VOR –VOR + E2–VOR + LY367385–VOR + LY367385 + E2 | VOR: 50 μg in 1 μl; LY367385: 100 μg in 1 μl; E2: 50 μg in 1 μl | 30, 30, 15 | F(4,68) = 36.959, p < 0.001 | F(4,17) = 0.804, p = 0.539 | F(16,68) = 1.254, p = 0.252 |
| 21 | 15 | 3 | 15 | PG–VOR–VOR + DPN–VOR + LY367385–VOR + LY367385 + DPN | VOR: 50 μg in 1 μl; LY367385: 100 μg in 1 μl; DPN: 50 μg in 1 μl | 30, 30, 15 | F(4,40) = 39.921, p < 0.001 | F(4,10) = 0.119, p = 0.973 | F(16,40) = 1.399, p = 0.191 |
The experiment numbers (Experiment) follow the order in which the results are described in the manuscript. The actual order in which the experiments were performed is provided in the second column (Testing order). The third column indicates to which group the animals involved in this experiment belonged (see Materials and Methods, General approach). The fourth column provides the number of birds tested in this experiment while the fifth and sixth columns present the different treatments compared (separated by dashes) as well as the doses and volumes of drugs injected. The latency between injection and test is represented under Test latency. The columns under Statistics provide the results of the statistical analyses.
The experimental design is similar to the design of our previous study (Seredynski et al., 2013). Briefly, each group of birds participated in several experiments testing the effects on behavioral measures of drugs targeting aromatase, ERs, or mGluRs administered at different doses and with a different timing. All drugs were injected in the third ventricle through a chronically implanted cannula allowing delivery of small volumes directly into the brain of awake subjects. All animals within an experiment were repeatedly tested in a within-subject design after injection of drugs administered alone or in combination and compared with the vehicle. Each experiment consisted of (1) a pretest to evaluate the baseline behavioral frequency, (2) 2–5 tests in which subjects were subdivided into 2–5 subgroups that received the same treatments, but in different orders, 3 d apart, and (3) a post-test to determine whether behavior returned to baseline levels. To allow direct comparisons with the experimental conditions (Phase 2), males received vehicle injections during pretests and post-tests (Phases 1 and 3).
The comparisons of results from all pretests and post-tests with paired-sample t tests failed to detect significant differences with one exception, indicating that behavioral responses studied were stable over time and that the sequence of drug injections had no long-term effects. In each experiment, data were analyzed by two-way ANOVAs with treatments as the repeated factor and the order in which the different conditions were tested (obtained by comparing subgroups) as the independent factor. This last factor was added in the analysis to assess whether the order of treatments had an impact on behavioral results. No main effect of the treatment sequence and no interaction between the treatments and their order were detected with a few exceptions that cannot be attributed to long-term effects of treatments (Tables 1–3). Together these analyses support the notion that the drugs tested do not elicit long-term changes in the behavioral responses.
Placement of intracerebroventricular cannula
All birds were implanted in the third ventricle with a chronic 22 gauge injection cannula containing a 28 gauge dummy insert (Plastics One). Coordinates of the cannula tip were 1.80 mm anterior, 2.80 mm dorsal, and 0.00 mm lateral to the zero reference point (center of the interaural axis) using an angular approach (10° away from the vertical) to avoid the blood vessel present in medial position at the surface of the brain (Cornil et al., 2005). The location of the cannula in the ventricle was confirmed at that time and before any subsequent injection by the observation of a drop of CSF flowing out of the tip of the cannula when the dummy insert was removed.
Intracerebroventricular infusions
Injections in the third ventricle were performed with a 25 μl Hamilton syringe connected to a microinfusion pump (model KDS-220, KD Scientific). The dummy cannula was replaced by a 28 gauge internal cannula (C313C, Plastics One) attached to the syringe by a cannula connector. The liquid (1 or 2 μl, depending on the dose and drug) was infused at the rate of 0.5 μl/min, resulting in injection durations of 2 and 4 min for the injection of 1 or 2 μl, respectively. The internal cannula was left in place for 1 min before being removed to allow diffusion of the solution into the CSF and avoid its leakage outside of the brain. The internal cannula was then slowly lifted and replaced by the dummy cannula. If during the infusion process it was noticed that a cannula had become loose, the animal was considered as an animal with a lost cannula (see below) and removed from this and following experiments.
Behavioral tests
To assess appetitive sexual behavior, which reflects the propensity of the male to approach a female and engage in copulation (i.e., a measure of his underlying sexual motivation), we recorded the frequency of the RCSMs, a response considered as a good index of sexual motivation (Ball and Balthazart, 2010). The frequency of RCSMs was quantified by placing the experimental male in one side of a glass aquarium (40 × 20 × 25 cm) located on a raised platform with a mirror placed underneath at a 45° angle, which provided an unobstructed view of the cloacal area (Balthazart et al., 1998). During the first phase of the test, a stimulus female was placed on the other side of the aquarium separated from the male by a glass partition and a vertically sliding opaque panel that prevented the animals from seeing each other. In the second phase of this test, the opaque panel was raised, allowing visual access to the female although the male could still not physically interact with her. The number of RCSMs was recorded by direct observation for 2.5 min during each of these two phases (female visible or not). The experimenter was not strictly blind to the treatment of the subjects but multiple birds were injected each day with, in most cases, two injections and the list of injections was not in the testing room. As a consequence, remembering who had received what treatment was almost impossible. In addition, the contraction of the cloacal sphincter muscles is a fairly discrete phenomenon—almost all or none—and its identification is subject to very little subjectivity. Therefore we believe that no bias was introduced in the results even if the experimenter was not completely blind to the subjects' treatment regimen.
Specific experimental procedures
Time course of vorozole and estradiol effects.
Birds were assigned to three, four, or five subgroups, depending on the number of treatments compared in a specific experiment (Table 1). This assignment was made semirandomly based on the frequencies of RCSMs displayed during the pretest to insure that subgroups were not different before the experimental procedures and to prevent any putative interaction between a pre-existing behavioral difference and an effect of the tested drug(s). The absence of difference between subgroups was confirmed by a one-way ANOVA. A total of five experiments were performed on one independent group of birds (Group 3, n = 13) each consisting of three, four, or five repeated experimental tests (vehicle vs drugs) surrounded by two “baseline” tests (one pretest and one post-test) to assess the time course of action of an aromatase inhibitor, vorozole (VOR), and E2 compared with the vehicle, propylene glycol (PG).
To test the effect of a bolus of E2 alone (without prior blockade of aromatase), birds received a central injection of E2 (50 μg) 15 min before testing (Experiment 1). To test the duration of the effect of VOR, birds received a central injection of VOR (50 μg) 30, 120, 240, and 480 min (Experiment 2) before testing. To test the latency and duration of the effect of E2, E2 was administered 15 min after VOR and behavior was tested 5, 15, 120, and 240 min (Experiments 2–6) after this E2 injection. One bird lost its cannula between Experiments 2 and 3.
Acute effects of agonists and antagonists of the different mERs.
Based on the frequencies of RCSMs displayed during the pretest, birds were assigned to one of two, three, four, or five subgroups, depending on the number of conditions compared in the specific experiment (Table 2). A total of 10 experiments were performed on the three independent groups of birds (n = 37; Experiments 7–10, 12, and 16 on Group 1; Experiments 13–15 on Group 2; Experiment 11 on Group 3) each consisting of two, three, or four repeated experimental tests (vehicle vs drugs) surrounded by two baseline tests (one pretest and one post-test) to assess the effects of blockade or activation of mERs on the frequency of RCSMs elicited by the presence of a female.
The impact of the activation of given mERs was tested following the acute blockade of local estrogen synthesis by the aromatase inhibitor VOR, with VOR and the mER agonist(s) tested injected 30 and 15 min respectively before the behavioral test (Table 2). The effects of the following drugs were compared with those of the vehicle, PG (positive control), and of VOR alone (negative control): the ERα-specific agonist, 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT; 0.4–50 μg); the ERβ-specific agonist, diarylpropionitrile (DPN; 0.4–50 μg); the agonist of GPR30, G1 (50 μg); the agonist of Gq-mER, STX (50 μg); and 17α-E2 (50 μg), a potential agonist of ER-X (50 μg).
Experiments testing the impact of receptor blockade compared in subjects chronically treated with testosterone (and thus exposed to the activation of both androgen receptors and ERs) the effect of the following antagonists to the effect of vehicle PG: the ERα antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP; 50 μg) and GPR30 antagonist G15 (50 μg). Three birds lost their cannula in Group 1 and two birds in Group 2 at some point of the experiment.
Acute effects of metabotropic glutamate receptors antagonists.
Based on the frequencies of RCSMs displayed during the pretest, birds were assigned to one of two or five subgroups (Table 3). A total of five experiments were performed on one independent group of birds (n = 22). Each experiment consisted of two or five repeated experimental tests (vehicle vs drugs) surrounded by two baseline tests (one pretest and one post-test) to assess the effects of blocking mGluRs on the frequency of RCSMs. The details of these experiments and the order in which they were actually conducted are represented in Table 3.
Experiments testing the impact of receptor blockade compared the effect of the following antagonists to the effect of vehicle: the antagonist of mGluR1a, LY367385 [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid, 100 μg]; the antagonist of group II mGluRs (mGluR2/3), LY341495 [(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid; 100 μg], and the antagonist of mGluR5, 2-methyl-6-(phenylethynyl)-pyridine (MPEP; 100 μg). The effect of these drugs was tested after a latency of 30 min. The choice of the dose was based on a literature search that indicated that intracerebroventricular injections of LY367385, LY341495, and MPEP produced detectable effects at doses of 68 μg (Johnson et al., 2001), 5–18 μg (Johnson et al., 2001; Folbergrová et al., 2005), and 2–200 μg (Aoki et al., 2004; Miyatake et al., 2005; Zhu et al., 2005; Popkirov and Manahan-Vaughan, 2011), and the knowledge that birds have a higher metabolic rate compared with mammals so that the minimal efficient doses are usually higher in birds compared with mammals.
To test the interaction between E2 and mGluR1a, birds received an injection of the aromatase inhibitor VOR, immediately followed by the mGluR1a antagonist 30 min before behavioral testing and/or E2 was injected 15 min later (that is, 15 min before testing). All tests were conducted 3 d apart. All birds were thus used as their own control. Four birds lost their cannula (three during Experiment 17 and one during Experiment 18).
Drugs
VOR was graciously provided by Dr. R. DeCoster (Janssen Research Foundation, Beerse, Belgium). PG, 17β-E2, and 17α-E2 were obtained from Sigma-Aldrich. PPT, DPN, MPP, G1, G15, LY367385, LY341495, and MPEP hydrochloride were obtained from Tocris Bioscience. STX was synthetized and kindly provided by Martin J. Kelly (Oregon Health & Sciences University, Portland, OR).
Drugs selected to specifically target mGluR1a, mGluR2/3, and mGluR5 were successfully used in previous studies conducted in avian species. LY367385, the specific mGluR1a antagonist, was shown to alter consolidation and reconsolidation of passive avoidance memory in chicks in a manner similar to what is observed in rodents (Salinska, 2006; Gieros et al., 2012). Likewise, MPEP, the specific mGluR5 antagonist, was found to reduce memory formation but also memory recall (Gieros et al., 2012). Finally, the specific antagonist of mGluR2/3, LY341495, modulates the endogenous activity measured in slice preparations of the chicken cochlear nucleus magnocellularis (Lu, 2007; Tang and Lu, 2012). In particular, it was shown to prevent the induction by (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), a specific agonist of type 2 mGluRs, of LTD in GABAergic synapses of the chicken cochlear nucleus magnocellularis, where intense mGluR2/3 immunolabeling was detected (Tang et al., 2013). Similarly, DCG-IV induced a suppression of EPSCs in the chicken nucleus laminaris that was prevented by LY341495 (Okuda et al., 2013). Moreover, the genes coding for mGluR1, mGluR/3, and mGluR5 in zebra finches (Taeniopygia guttata), the only avian species in which these genes have been cloned to date, exhibit ∼80% sequence identity to their human counterpart (Wada et al., 2004), which provides further confidence that the drugs that were used here are working in birds in a selective manner, as they do in mammalian species. Finally, the analysis of peptide sequences published online by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) provides further information regarding the sequence homology for these receptors in chickens and mammals. The comparison of the amino acid sequences of the full-length chicken (Gallus gallus) mGluR1 (XP_419652), mGluR2 (XP_004944551), mGluR3 (XP_004937557), and mGluR5 (NP_989469) revealed a 84, 79–80, 91, and 83–95% sequence identity with their rat (NP_058707.1, NP_001099181.1, NP_001099182.1, and XP_006229721.1), mouse (NP_058672.1, NP_001153825.1, NP_862898.1, and NP 001074883.1/NP_001137306.1), and human (AAB05337.1, NP_000830.2, NP_000831.2, and NP_001137303.1/NP_000833.1) counterparts, respectively, with a percentage coverage between 95 and 100%. The sequence identity tends to become even higher when blasting a sequence identified as the ligand-binding domain. Indeed, for mGluR1, blasting a sequence sharing 100% amino acid sequence identity (amino acids 28–502) between cloned chicken isoforms of this protein yielded 93% amino acid sequence identity with human, rat, and mouse with 100% coverage. The ligand-binding domain of the chicken mGluR2 (amino acids 48–498) share 81–82% sequence identity with its human, mouse, and rat counterparts with 100% coverage. Similarly, the ligand-binding domain of the chicken mGluR3 (amino acids 47–504) is 89–90% identical to the human, rat, and mouse sequences with 100% coverage. Finally, the ligand-binding domain of the chicken mGluR5 (amino acids 32–503) shares 96% amino acid sequence identity with, mouse, rat. and its human counterparts with 100% coverage.
Data analysis
In each of the 21 separate experiments, results obtained in pretests and post-tests were compared with each other with paired-samples t tests. All these analyses identified no significant difference (t ≤ 1.800, p ≥ 0.069) with the exception of Experiment 6 (t = 2.27, df = 11; p = 0.044).
When the two-way ANOVAs (see General approach) identified a significant treatment effect, they were followed by a post hoc Fisher's least significant difference test (for main effect of treatment in Experiment 17), Newman–Keuls test (main effect of treatment in all other experiments), or Tukey's honestly significant difference test (all significant interactions) as appropriate, depending on the number of comparisons comparing all conditions to each other.
No effect of the order of treatments (F ≤ 3.209, p ≥ 0.104) was detected with the exception of an order effect detected for the mGluR5 antagonist MPEP (Experiment 12: F(1,6) = 6.438, p = 0.044; the baseline between subgroups was slightly different).
No interaction between the treatments and their order (F ≤ 4.470, p ≥ 0.137) was observed with the exception of an interaction detected for the mGluR1a antagonist LY367385 tested alone (Experiment 17: F(1,10) = 7.815, p = 0.019) and for the time–response curve of VOR (Experiment 2: F(16,32) = 2.491, p = 0.014). The interaction detected in Experiment 17 is associated with a higher baseline in subjects that received the mGluR1a antagonist first, suggesting that the preceding blockade of mGluR1a might have induced an increased sexual motivation, which seems unlikely. The interaction relative to the time–response curve (Experiment 2) is explained by much lower RCSM frequencies detected 30 min after injection compared with the baseline, the 2 h and 8 h conditions in subjects translating a particularly pronounced effect of VOR in these few birds.
All statistical analyses were performed with Statistica version 9 (StatSoft). Differences were considered significant for p < 0.05. All data are expressed as mean ± SEM.
Results
Time course of changes in male sexual behavior after aromatase inhibition and E2 supplementation
Our initial investigation focused on the temporal characteristics of the effects of an acute depletion of brain-derived estrogens on male sexual motivation. The effect of the aromatase inhibitor VOR was tested in different subgroups 30, 120, 240, or 480 min after injection (Table 1; Fig. 1A). As predicted, VOR significantly inhibited RCSM frequency (p < 0.001; Exp. 1). Post hoc tests showed that aromatase blockade induced a significant behavioral reduction after 30–240 min as compared with the positive control (vehicle vs 30, 120, and 240 min; p < 0.05). This inhibition was no longer detected after 480 min, as indicated by the absence of a difference with vehicle (p = 0.219) and the significant difference with 30 min (p = 0.004). These results confirm that blocking estrogen synthesis impairs behavior within a few minutes (Cornil et al., 2006; Seredynski et al., 2013; 30 min here and even 15 min in Seredynski et al., 2013). This behavioral reduction not only results from the termination of the effects induced by the estrogens present before aromatase blockade intervened, but also from the rapid elimination of these estrogens (since providing E2 15 min after aromatase blockade restores normal behavioral frequencies). This observation thus suggests that locally produced estrogens are rapidly cleared, thus resulting in the termination of their effects.
Figure 1.
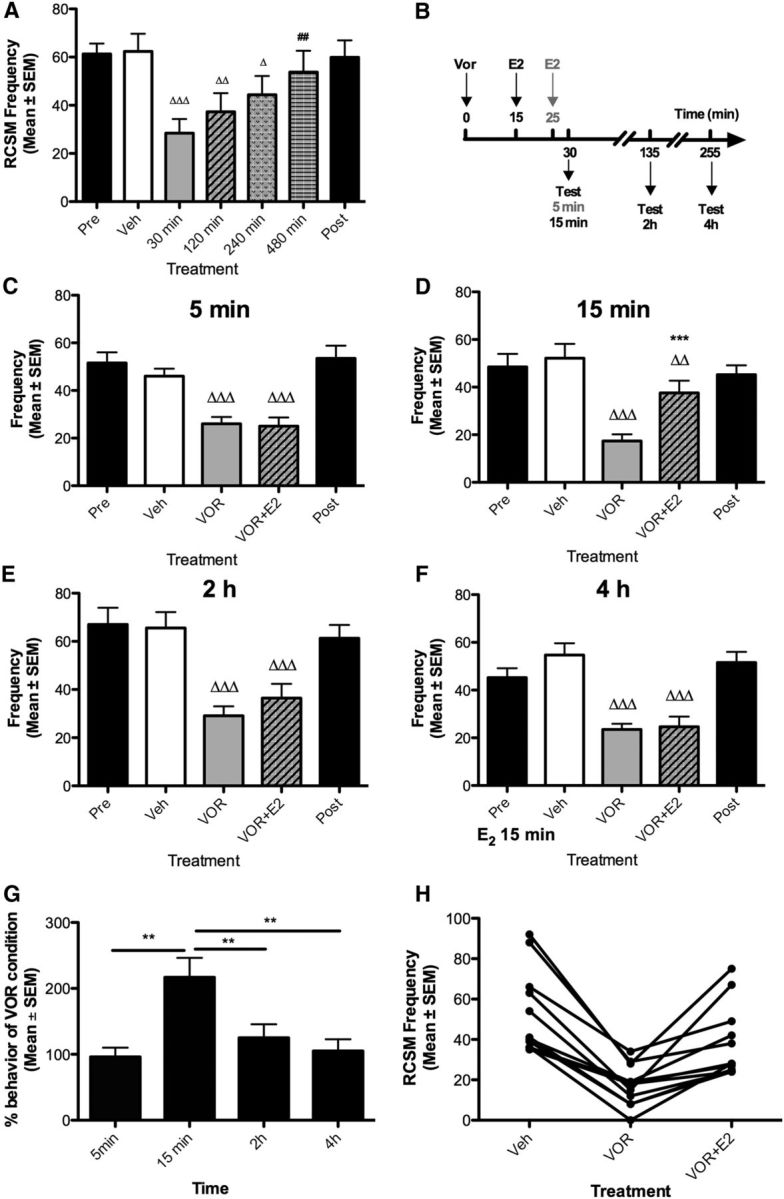
Time course of aromatase inhibition and acute E2 action on male sexual motivation. A, Blockade of local estrogen synthesis by the aromatase inhibitor VOR (50 μg) inhibits RCSM frequency within 30–240 min. B, Experimental design used to test the time course of acute E2 action (50 μg) on behavior acutely inhibited by aromatase blockade. Black arrows indicate how long E2 was injected before 15 min, 2 h, or 4 h testing conditions; the gray arrow indicates how long E2 was injected before the 5 min test condition. C–F, The behavioral inhibition resulting from acute estrogen deprivation is prevented by E2 (50 μg) injected 15 min (D) before the test, but not 5 (C), 120 (E), and 240 min (F) before the test. The Pre and Post black bars provide reference behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments, but these data are not included in the statistical analyses. Δp < 0.05, ΔΔp < 0.01, and ΔΔΔp < 0.001 versus vehicle (Veh) and ##p < 0.01 versus VOR 30 min by Newman–Keuls post hoc tests after identification of a significant overall treatment effect by two-way ANOVA (repeated measure; n = 13). H, The behavioral inhibition resulting from acute neuroestrogen depletion is prevented by E2 injected 15 min but not after 2 or 4 h before. **p < 0.01 by Newman–Keuls post hoc tests following a significant treatment effect by one-way ANOVA (repeated measure; n = 12) on data expressed as percentage of VOR condition. G, Representation of individual values comparing the effect of E2 after 15 min to the positive (Veh) and negative (VOR) controls .
The time window provided by the inhibition of local estrogen production resulting from a single injection of VOR then enabled us to investigate the temporal properties of E2 action in four independent experiments performed in random order. E2 was administered 15 or 25 min after the aromatase inhibitor and behavior was tested 5 min, 15 min, 2 h, or 4 h later (Fig. 1B; Table 1; Exps. 2–5). An overall significant effect of treatments was detected in each experiment (p < 0.001; Fig. 1C–F). Post hoc tests confirmed that VOR significantly reduced RCSM frequency compared with the positive control at the four time points tested (vehicle vs VOR, p < 0.001). This behavioral decrease was counteracted by E2 after 15 min (VOR vs VOR + E2; p < 0.001). When tested at shorter (5 min) or longer (120 and 240 min) time points, E2 did not significantly restore behavior (VOR vs VOR + E2, p ≥ 0.087).
To enable direct comparisons between data collected after different latencies, RCSM frequencies measured after E2 treatment were expressed as percentages of the frequencies measured in each subject after VOR alone. A one-way ANOVA confirmed that E2 differentially increased RCSM frequency after different latencies. Post hoc tests indicated a significant difference between the 15 min and shorter or longer latencies (Fig. 1G; general ANOVA: p < 0.001). Moreover, the representation of individual values at the 15 min time point clearly shows that, regardless of testing order, all subjects responded to aromatase inhibition by a reduced RCSM frequency and that E2 supplementation restored behavioral frequencies close to basal values (Fig. 1H). These results confirm that brain-derived estrogens robustly modulate male sexual motivation (decrease and recovery within 15 min) and further indicate that these effects are transient as they are no longer detected 2 h after E2 injection.
E2 does not increase sexual motivation above control levels in males with active aromatase
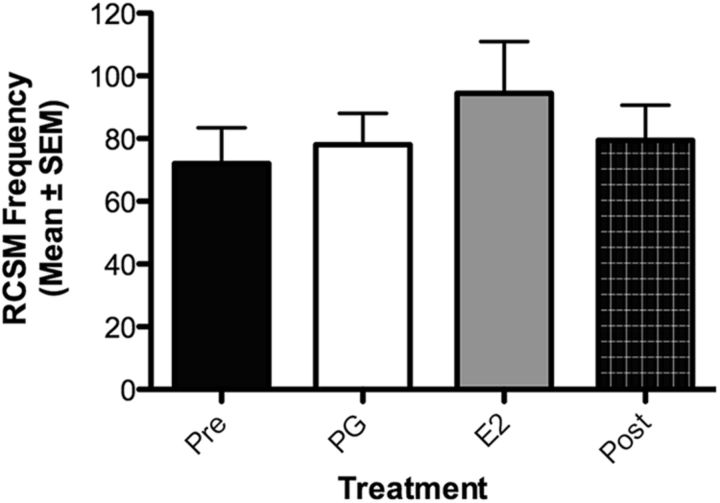
We next wondered whether supplementation with exogenous E2 would enhance sexual motivation above normal levels in the absence of a previous inhibition of aromatase (Table 1, Exp. 6). Castrated males chronically treated with testosterone to restore the full spectrum of sexual behavior received an intracerebroventricular injection of E2 and were tested for female-induced RCSM frequency 15 min later (Fig. 2). Although two males with an intermediate RCSM frequency increased their frequency of contraction in response to E2, no treatment effect was observed in all other birds (p = 0.24), independent of whether they had a low or high baseline activity, suggesting that males with a functional brain aromatase produce estrogens in optimal amounts to maximally activate sexual motivation, contrary to what has been observed for other behavioral variables that were enhanced by additional exogenous E2 [e.g., aggressive behavior in quail (Ubuka et al., 2014) and in songbirds (Heimovics et al., 2015)]. However, it cannot be ruled out that this absence of effect results from a ceiling effect due to a physical limitation to produce more cloacal contractions.
Figure 2.
The acute intracerebroventricular injection of a bolus of E2 (50 μg, n = 7) without concurrent depletion in neuroestrogens does not affect male sexual motivation. The Pre and Post black bars provide reference behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments, but these data are not included in the statistical analyses.
Acute effects of brain-derived estrogens on male sexual motivation are mediated through ERβ
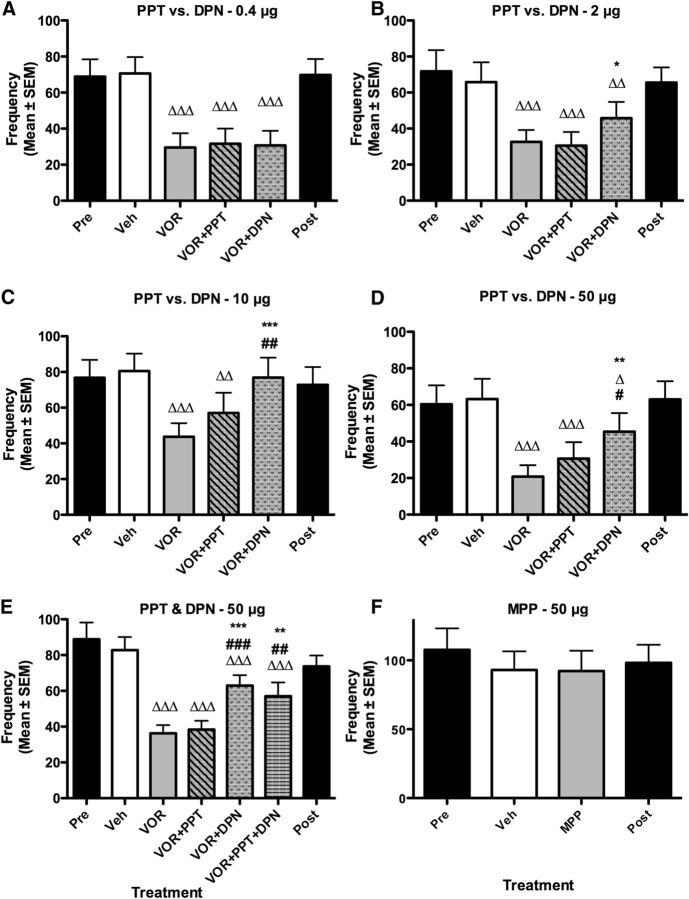
We tested the effects of PPT, the specific agonist of ERα, and of DPN, the specific agonist of ERβ. DPN has an ∼70-fold higher selectivity for ERβ over ERα, while PPT shows a 410-fold higher selectivity for ERα over ERβ (Stauffer et al., 2000; Meyers et al., 2001). To discriminate more clearly between effects mediated by each of these receptors, the effects of increasing doses (0.4–50 μg) of both agonists were compared in four independent experiments performed in random order such that effects could not be attributed to cumulative effects of increasing doses (Table 2). These experiments all showed a significant effect of treatments (Exps. 7–10: 0.4 μg, p < 0.001; 2 μg, p < 0.001; 10 μg, p < 0.001; 50 μg, p < 0.001). Post hoc analyses confirmed that VOR significantly reduced the frequency of RCSMs compared with the positive control (vehicle, all four comparisons, p < 0.001; Fig. 3A–D). This behavioral decrease was prevented by the ERβ agonist, DPN, at doses as low as 2 μg (DPN vs VOR: p < 0.044), while no significant effect was induced by the ERα agonist throughout the range of doses tested (PPT vs VOR: p > 0.066). The two higher doses of DPN also activated significantly more RCSMs than PPT did (10 μg, p = 0.029; 50 μg, p = 0.008). These results thus suggest that brain-derived estrogens acutely modulate male sexual behavior through ERβ activation.
Figure 3.
Effects of acute activation of ERα and ERβ on the frequency of RCSMs. A–E, Blockade of local estrogen synthesis by the aromatase inhibitor VOR (50 μg) inhibits RCSM frequency within 30 min. The behavioral inhibition induced by acute estrogen deprivation is prevented by the highest doses of the specific agonist of ERβ, DPN (2, 10, and 50 μg; B–D), but not its lowest dose (0.4 μg; A). Similarly, the administration of both PPT and DPN prevents the behavioral inhibition induced by VOR (E). However, the ERα agonist PPT alone has no effect at any of the doses tested (A–E). F, The ERα antagonist has no effect on behavioral frequencies. The Pre and Post black bars provide reference behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments, but these data are not included in the statistical analyses. Δp < 0.05, ΔΔp < 0.01, and ΔΔΔp < 0.001 versus vehicle (Veh); *p < 0.05, **p < 0.01, and ***p < 0.001 versus VOR; #p < 0.05, ##p < 0.01, and ###p 0.001 versus VOR + PPT by Newman–Keuls post hoc tests after identification of a significant treatment effect (repeated measure; A, B, D, n = 10; C, n = 12; E, n = 9; F, n = 9) by two-way ANOVA.
The qualitative analysis of these results indicated, however, that PPT induces a slight yet nonsignificant increase in RCSM frequency when administered at the highest doses (Fig. 3C,D). To test whether this minimal effect of PPT results from a cross-reaction with the ERβ receptor, we tested whether this effect synergizes with DPN by coinjecting the highest dose (50 μg) of both agonists along with VOR. These acute treatments significantly affected RCSM frequency (Fig. 3E; p < 0.001; Exp. 11). Post hoc analyses confirmed that RCSM frequency was acutely inhibited by aromatase inhibition (p < 0.001) and that the ERβ agonist DPN significantly increased RCSM frequency compared with VOR alone (p < 0.001) and with VOR plus PPT (p < 0.001). However, when injected together, PPT and DPN did not produce a larger effect than DPN alone (p = 0.267). Thus activation of ERβ alone is sufficient to rapidly increase male sexual motivation.
There is no ERβ antagonist available with a relative affinity sufficient to discriminate between ERα and ERβ but blocking ERα with MPP did not alter RCSM frequency (Fig. 3F; p = 0.886; Table 2, Exp. 12). Together, these results indicate that the acute modulation of sexual motivation by locally produced estrogens depends to a large extent on ERβ activation and is independent of ERα stimulation.
GPR30 and Gq-mER do not contribute to the rapid effects of neuroestrogens on sexual motivation
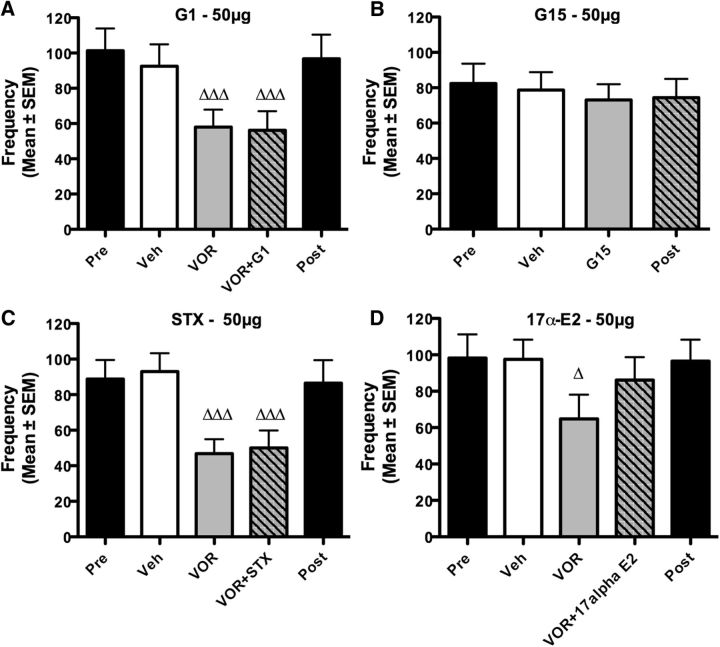
We then tested whether the mER GPR30 could also contribute to these rapid effects of E2 on sexual motivation. The acute treatments with VOR followed 15 min later with GPR30-specific agonist G1 significantly affected RCSM frequency (Fig. 4A; p < 0.001; Table 2, Exp. 13). Post hoc comparisons confirmed that VOR significantly decreased sexual motivation compared with the positive control (vehicle; p < 0.001) but this behavioral inhibition was not prevented by G1 (VOR + G1 vs VOR: p = 0.790). Accordingly, the GPR30 antagonist G15 did not affect RCSM frequency (Fig. 4B; vehicle vs G15: p = 0.318; Exp. 14). Thus, GPR30 does not appear to be involved in the acute control of sexual motivation.
Figure 4.
The acute activation of GPR30, Gq-mER, and ER-X has no effect on the frequency of RCSMs. A, C, D, Blockade of local estrogen synthesis by the aromatase inhibitor VOR (50 μg) inhibits RCSM frequency within 30 min. G1, the specific agonist of GPR30 (50 μg, n = 12, A), injected 15 min after VOR, did not counteract the effect of VOR on RCSMs, nor did STX, the specific agonist of Gq-mER (50 μg, n = 11, C), and 17α-E2, a potential agonist of ER-X (50 μg, n = 9, D). B, No change in RCSM frequency was observed 30 min after the injection of G15, the specific antagonist of GPR30 (50 μg, n = 10). The Pre and Post black bars provide reference behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments, but these data are not included in the statistical analyses. Δp < 0.05, ΔΔΔp < 0.001 versus vehicle (Veh) by Newman–Keuls post hoc tests after identification of a significant treatment effect (repeated measure) by two-way ANOVA.
Similarly the acute treatment with VOR followed 15 min later with the Gq-mER-specific agonist STX significantly affected RCSM frequency (Fig. 4C; p < 0.001; Table 2, Exp. 15). Post hoc tests showed that this effect results solely from the significant behavioral inhibition induced by VOR compared with the control condition (vehicle vs VOR: p < 0.001; vehicle vs VOR + STX, p < 0.001) and STX did not rescue behavior after VOR injection (VOR + STX vs VOR, p = 0.679). Collectively, these data suggest that GPR30 and Gq-mER are not involved in the acute control of sexual motivation.
Injection of 17α-E2 slightly stimulates male sexual behavior
ER-X potential involvement in the rapid modulation of male sexual motivation was investigated by injection of 17α-E2, which has been described as a potential ER-X agonist (Toran-Allerand et al., 2002). Acute treatments significantly affected RCSMs (Fig. 4D; p = 0.029; Table 2, Exp. 16). Post hoc analyses showed that the behavioral inhibition induced by VOR (vehicle vs VOR; p = 0.025) was partially counteracted by 17α-E2, as is apparent by the absence of difference between the 17α-E2 condition and the positive controls (vehicle vs VOR + 17α-E2, p = 0.305), although the increased behavioral frequency was not significant in and of itself (VOR vs VOR + 17α-E2, p = 0.069). ER-X could thus contribute to the rapid effects of brain-derived estrogens on male sexual motivation.
The acute effect of E2 is mediated through an interaction between ERβ and mGluR1a
nERs can translocate to and signal from the neuronal membrane through their association with mGluRs (Boulware et al., 2005; Meitzen et al., 2013). The next set of experiments investigated the possible involvement of mGluRs in the acute behavioral response triggered by E2.
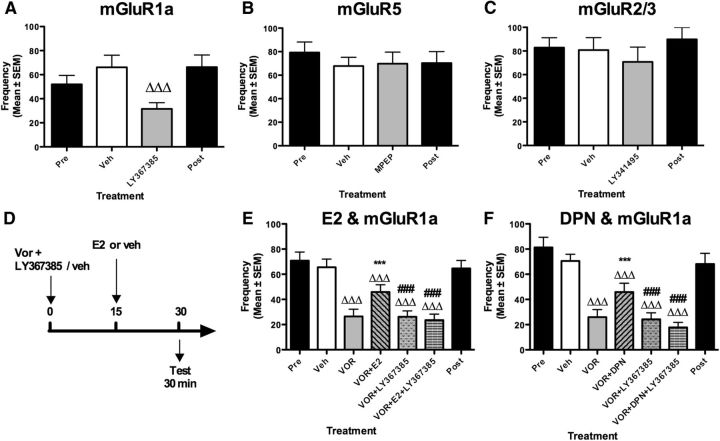
mGluRs have been subdivided into three groups based on sequence homology and coupling to second messenger systems (Niswender and Conn, 2010). ERs have been shown to signal through Group I or II mGluRs in a region-specific manner (Meitzen and Mermelstein, 2011). As a first approach to examine a role of mGluRs in the control of male sexual behavior, three independent experiments tested the effect of three antagonists specific for the Group I mGluR1a (LY367385, Exp. 17) and mGluR5 (MPEP, Exp. 18) and the Group II mGluR2/3 (LY341495; Table 3, Exp. 19) injected 30 min before the test (Fig. 5A–C). No effect was detected after injection of MPEP (p = 0.735) and LY341495 (p = 0.319) while LY367385 significantly decreased RCSM frequency (p < 0.001). Collectively, these results thus suggest that the activation of mGluR1a is involved in the control of male sexual motivation but they do not indicate whether this action depends on E2. Indeed, such an activation of mGluR1a could result from a release of glutamate occurring when the male is looking at the female, as shown in the medial preoptic area during copulation in male rats (Dominguez et al., 2006). Glutamate release could occur independently from brain estrogen synthesis/action or could also be part of the chain of events activated by neuroestrogens. It could also be postulated that activation of ERs could in turn activate mGluR1a through a process called transactivation (Micevych and Mermelstein, 2008).
Figure 5.
Effects of acute blockade of mGluRs on the frequency of RCSMs. A–C, The specific antagonist of mGluR1a (LY367385) inhibits RCSM frequency (100 μg, n = 12, A) within 30 min, while specific antagonists of mGluR2/3 (LY341495) and mGluR5 (MPEP) have no effect on RCSM within 30 min (100 μg, n = 9 and 8 respectively, B, C). D, The combined effect of mGluR1a blockade and E2 was tested following acute blockade of brain aromatase using the described experimental design. E, F, Blockade of local estrogen synthesis by the aromatase inhibitor VOR (50 μg) inhibits RCSM frequency within 30 min. E2 or DPN (50 μg; E, F) injected 15 min after VOR prevents the effect of VOR on RCSMs. LY367385 (100 μg) prevents the restoration of behavior by E2 (E, n = 22) or DPN (F, n = 15). The Pre and Post black bars provide reference behavior frequencies after vehicle intracerebroventricular injections performed before and after the acute treatments, but these data are not included in the statistical analyses. ΔΔΔp < 0.001 versus vehicle (Veh) by Fisher's LSD post hoc test (A) and Newman–Keuls post hoc test; ***p < 0.001 versus VOR; ###p < 0.001 versus VOR + E2 or VOR + DPN by Newman–Keuls post hoc tests after identification of a significant treatment effect (repeated measure) by two-way ANOVA.
To determine whether mGluR1a activation potentially results from the action of brain-derived estrogens, five treatments were administered in a counterbalanced order: the positive and negative controls (vehicle and VOR), VOR plus E2, VOR plus LY367385, and VOR plus LY367385 plus E2. If mGluR1a activation controls male sexual motivation in parallel to E2 action, one could predict that the combined blockade of aromatase and mGluR1a should produce a more pronounced behavioral inhibition than the inhibition resulting from the effect of each of these blockers given separately (additive effects). By contrast, if mGluR1a activation and E2 action work in concert, blocking one or the other or both at the same time should result in effects of similar amplitude.
These treatments produced an overall significant effect on RCSM frequency (Fig. 5D, p < 0.001; Table 3, Exp. 20). Post hoc tests confirmed that acute aromatase inhibition significantly decreased behavioral frequencies (vehicle vs VOR: p < 0.001), an effect that was prevented by E2 (VOR vs VOR + E2: p < 0.001). The mGluR1a antagonist did not amplify the reduction of RCSM frequency induced by VOR (vehicle vs VOR + LY367385: p < 0.001; VOR + LY367385 vs VOR: p = 0.957) but most importantly the mGluR1a antagonist prevented the behavioral restoration induced by E2 (VOR + E2 vs VOR + E2 + LY367385: p < 0.001). Collectively, these results indicate that mGluR1a blockade does not inhibit behavior further in the absence of local aromatase activity (when behavior is obviously already low but could still be further decreased) and that blocking mGluR1a is sufficient to abrogate behavioral restoration by E2. Therefore, these results demonstrate that E2 and mGluR1 control sexual motivation by acting in sequence and not in parallel. Furthermore they show that mGluR1 acts downstream of E2 since E2 effects are blocked by the mGluR1 antagonist.
Similar results were obtained when E2 was replaced by DPN, the specific ERβ agonist (Fig. 5E; general ANOVA: p < 0.001; Table 3, Exp. 21). Acute aromatase inhibition induced a significant decrease in RCSM frequency (vehicle vs VOR: p < 0.001), which was prevented by DPN when administered alone (VOR vs VOR + DPN: p < 0.001), but not in the presence of the mGluR1a antagonist (VOR + DPN vs VOR + DPN + LY367385: p < 0.001). As previously, the mGluR1a antagonist alone did not further reduce behavior compared with VOR (p = 0.721). Together, these results show that when the activation of mGluR1a is blocked, E2 or the ERβ agonist DPN no longer restore sexual motivation, thus indicating that the acute action of brain-derived estrogens on behavior is mediated through ERβ and likely relies on a downstream transactivation of mGluR1a.
Discussion
Sex differences in brain structure and function are widespread (Jazin and Cahill, 2010) and analysis of their functional significance has become a priority in the neurosciences (Clayton and Collins, 2014). Interestingly, these sex differences also affect rapid brain estrogen effects (Krentzel and Remage-Healey, 2015). Blocking brain estrogen action/synthesis decreases male sexual motivation within minutes without affecting sexual performance in quail (Seredynski et al., 2013). Here we confirm that blockade of brain aromatase acutely and robustly alters male sexual motivation. This effect is prevented within 15 min by a central injection of E2 or a specific agonist of ERβ, but not of ERα. Furthermore, E2 cannot restore this behavior when mGluR1a is blocked. Together with the previous demonstration that these effects are membrane initiated (Seredynski et al., 2013), the acute modulation of sexual motivation by E2 thus seems to depend on its binding to membrane ERβ, leading to the transactivation of the mGluR1a. Additionally, the acute effects of aromatase inhibition confirm that locally produced estrogens are cleared rapidly and their effects acutely terminated (Cornil et al., 2006; Seredynski et al., 2013). Along with the rapid behavior restoration following E2 injection, these results indicate that these effects are not only rapid but also transient. Because this behavioral response is induced within 15 min, it must depend on a chain of rapid events. In comparison, recent demonstrations of ER/mGluRs in behavioral processes whose functional consequences were observed after much longer latencies (Dewing et al., 2007; Boulware et al., 2013; Martinez et al., 2014).
Our data also suggest an intriguing effect of 17α-E2, the supposedly inactive enantionmer of 17β-E2. Although 17α-E2 was proposed to act as an ER-X agonist (Toran-Allerand et al., 2002), this notion or the existence of this receptor have never been confirmed. Yet, 17α-E2 effects have been reported and discussed before (Barha et al., 2010; Inagaki et al., 2010).
Early work on ER knock-out (ERKO) mice suggested that ERα is essential for the development of reproductive organs as well as for sexual behaviors, while ERβ regulates nonreproductive organs and social and cognitive aspects of behaviors (Gustafsson, 1999; Rissman et al., 1999). However, because ERKO mice lack a functional ERs both during ontogeny and adulthood, these models cannot discriminate organizational from activational impacts of estrogens. Moreover, these models are potentially biased by compensatory developmental mechanisms. Numerous isoforms have also been described for both receptors (Sellers et al., 2015), some of which are still present in some mutant mice (Lubahn et al., 1993; Krege et al., 1998). Incomplete ERβ ablations may have led to the persistence of ERβ-dependent functions, as suggested by discrepancies observed between different ERβ knock-out mouse lines (Krege et al., 1998; Antal et al., 2008), resulting in an overall underappreciation of ERβ's role in reproductive behavior.
The recent development of specific receptor agonists has enabled the pharmacological assessment of the respective roles of ERα and ERβ at specific life stages. PPT and DPN were developed based on the low sequence identity of the C-terminal ligand-binding domain of the two receptors (Malamas et al., 2004; Manas et al., 2004) and have been used to investigate their role in many behavioral processes (Mazzucco et al., 2008; Clipperton-Allen et al., 2011; Russell et al., 2012; Boulware et al., 2013). Although both agonists have a relatively good selectivity for their receptor (Stauffer et al., 2000; Meyers et al., 2001), cross-activation can occur at high doses. However, the present dose–response studies combined with the absence of synergistic effects of PPT and DPN clearly show that only DPN activates RCSMs, while PPT is inactive even at high doses. As PPT displays a higher selectivity than DPN for its specific receptor, these observations indicate that E2 acutely modulates sexual motivation through ERβ activation. Along with the recent report of mild impairments in male and female sexual behavior in a new mouse line deleted for ERβ (Antal et al., 2012), these data demonstrate that ERβ is involved in the activation of male sexual behavior and that initial conclusions based on knock-out studies should be revised, at least for the rapid actions presumably mediated at the membrane level.
ERα and ERβ can translocate from the intracellular compartments to the cell membrane (Marino and Ascenzi, 2008; Micevych and Dominguez, 2009; Levin, 2011), where they signal through the transactivation of mGluRs (Micevych and Mermelstein, 2008). Different combinations of an ER and an mGluR subtype activate different intracellular signaling cascades, which exhibit region-specific profiles (Meitzen and Mermelstein, 2011). The behavioral consequences of such ER–mGluR interactions have only been documented in female rats (Dewing et al., 2007; Boulware et al., 2013; Martinez et al., 2014). The existence of a sex difference in the brain signaling pathways rapidly modulated by E2 (Abrahám and Herbison, 2005; Meitzen et al., 2012) and in the ability of ER and mGluR to associate (Boulware et al., 2005; Huang and Woolley, 2012) has been suggested, thus raising questions about the existence of such an interaction in males. Here, we show that blocking mGluR1a, but not mGluR2/3 or mGluR5, impairs RCSM frequencies. Interestingly, mGluR1a blockade does not further reduce RCSM frequencies when aromatase activity is inhibited, providing a first indication that mGluR1a might work in concert with ERβ to regulate this response. Importantly, blocking mGluR1a prevents behavioral restoration to normal frequencies by E2 or DPN, following acute aromatase inhibition. These data thus provide functional evidence that ERβ acts in sequence and potentially associates with mGluR1a to acutely regulate male sexual motivation.
Where this interaction occurs is unknown at present. As estrogens involved in this response arise from local aromatization, cell populations expressing aromatase in the social behavior network (Newman, 1999; Goodson, 2005) constitute the primary candidate regions. The medial preoptic nucleus, the bed nucleus of the stria terminalis, and the tuberal hypothalamus appear as the most likely action sites because they additionally exhibit acute variations in local estrogen synthesis following sexual interactions or stress (de Bournonville et al., 2013; Dickens et al., 2014). Changes in brain estrogen production and bioavailability thus take place in a time frame that matches the rapid behavioral actions of E2 described here. These rapid changes in endogenous estrogen production might modulate sexual motivation in a moment-to-moment fashion similar to the action of traditional neuromodulators (Balthazart and Ball, 2006; Saldanha et al., 2011).
One important aspect of our findings indeed relates to the timing of behavioral changes. Although ER–mGluR associations have been implicated in the regulation of behaviors, the behavioral consequences of such membrane-initiated effects were only observed several hours after E2 administration. For example, the μ-opioid receptor internalization induced by an ERα–mGluR1a interaction is observed in the preoptic area 30 min after E2 treatment in the female arcuate nucleus of the hypothalamus, but the facilitation of female receptivity resulting from these early events is only detected 30 h later (Dewing et al., 2007). Similarly, membrane-initiated E2 effects on spatial memory and cocaine-induced locomotor sensitization are detected from 4 h to several days after treatment (Boulware et al., 2013; Martinez et al., 2014). Such latencies are likely explained by an indirect induction of transcription by estrogens. Indeed, various E2-activated intracellular cascades can lead to transcription regulation (Abrahám et al., 2004; Boulware et al., 2005; Heimovics et al., 2012). These indirect genomic effects potentially concern the synthesis of proteins required for behavioral activation, such as those involved in synaptic plasticity (Christensen et al., 2011; Peterson et al., 2015). Compared with these relatively long latencies, the present effects occurring within 15 min are quite rapid, demonstrating that neuroestrogens can acutely activate the circuitry underlying male sexual behavior by interacting with glutamate signaling.
Altogether, these results demonstrate that the acute regulation of male sexual motivation by brain-derived estrogens is under the control of ERβ through mGluR1a transactivation. This finding is significant because (1) it reveals that ERβ is important for reproduction; (2) it demonstrates a sequential action and potentially functional ER–mGluR cooperation in males, where estrogens are produced centrally in response to social stimuli (Cornil et al., 2013); (3) it suggests a mechanistic explanation of how an ER that is not intrinsically coupled to intracellular effectors, such as G-proteins, can signal from the membrane to govern complex behavioral processes in very rapid fashion; and (4) it demonstrates the presence of an ER–mGluR interaction in birds, which suggests that this mechanism is relatively ancient and conserved in the brain of amniotes. Finally, although evidence obtained from ERKO mice, site-specific ER silencing, and pharmacological studies indicate that the long-term control of male sexual behavior depends on ERα (Rissman et al., 1999; Paisley et al., 2012; Russell et al., 2012), these findings open the exciting possibility that the motivation versus performance aspects of behavior are regulated by different subtypes of ERs, thus supporting the dual hypothesis of estrogen action (Cornil et al., 2015) stating that these two aspects of behavior are also regulated by estrogens acting at different sites in different time scales. The specific implications of ER–mGluR interaction thus provide an additional tool to identify and characterize the neural networks that specifically control motivational and performance aspects of typical male behavior by estrogens.
Footnotes
This work was supported by grants from the National Institutes of Health/National Institute of Mental Health (R01MH50388) to C.A.C., G.F.B., and J.B., the Belgian Collective Fundamental Research Fund (No. 2.4537.9) to J.B., and the University of Liège (Crédits spéciaux) to J.B. and C.A.C. C.A.C. is a Funds for Scientific Research–Funds National de la Recherche Scientifique research associate. We thank Martin J. Kelly (Oregon Health & Sciences University, Portland, OR) for generously providing the STX compound.
The authors declare no competing financial interests.
References
- Abrahám IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–951. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Abrahám IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal MC, Petit-Demoulière B, Meziane H, Chambon P, Krust A. Estrogen dependent activation function of ERbeta is essential for the sexual behavior of mouse females. Proc Natl Acad Sci U S A. 2012;109:19822–19827. doi: 10.1073/pnas.1217668109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Narita M, Shibasaki M, Suzuki T. Metabotropic glutamate receptor 5 localized in the limbic forebrain is critical for the development of morphine-induced rewarding effect in mice. Eur J Neurosci. 2004;20:1633–1638. doi: 10.1111/j.1460-9568.2004.03609.x. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentialy regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong RY, Kwakowsky A, Barad Z, Porteous R, Herbison AE, Ábrahám IM. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology. 2012;153:3792–3803. doi: 10.1210/en.2012-1232. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Almey A, Melichercik A, Allen CP, Choleris E. Effects of an estrogen receptor alpha agonist on agonistic behaviour in intact and gonadectomized male and female mice. Psychoneuroendocrinology. 2011;36:981–995. doi: 10.1016/j.psyneuen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Ball GF. Dopamine binds to alpha2-noradrenergic receptors in quail brain: implications for the activation of male sexual behavior. Horm Behav. 2005;48:95. [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Seredynski AL, de Bournonville C, Dickens MJ, Charlier TD, Ball GF, Balthazart J. Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J Neuroendocrinol. 2013;25:1070–1078. doi: 10.1111/jne.12062. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. The dual action of estrogens hypothesis. Trends Neurosci. 2015;38:408–416. doi: 10.1016/j.tins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA. Dynamic changes in brain aromatase activity following sexual interactions in males: where, when and why? Psychoneuroendocrinology. 2013;38:789–799. doi: 10.1016/j.psyneuen.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, de Bournonville C, Balthazart J, Cornil CA. Relationships between rapid changes in local aromatase activity and estradiol concentrations in male and female quail brain. Horm Behav. 2014;65:154–164. doi: 10.1016/j.yhbeh.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrová J, Druga R, Otáhal J, Haugvicová R, Mares P, Kubová H. Seizures induced in immature rats by homocysteic acid and the associated brain damage are prevented by group II metabotropic glutamate receptor agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate. Exp Neurol. 2005;192:420–436. doi: 10.1016/j.expneurol.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Gieros K, Sobczuk A, Salinska E. Differential involvement of mGluR1 and mGluR5 in memory reconsolidation and retrieval in a passive avoidance task in 1-day old chicks. Neurobiol Learn Mem. 2012;97:165–172. doi: 10.1016/j.nlm.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor beta—a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, Clark SM, Vasudevan N. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids. 2014;81:49–56. doi: 10.1016/j.steroids.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153:1364–1376. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Ferris JK, Soma KK. Non-invasive administration of 17β-estradiol rapidly increases aggressive behavior in non-breeding, but not breeding, male song sparrows. Horm Behav. 2015;69:31–38. doi: 10.1016/j.yhbeh.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Kelly G, Chamberlain M. Changes in rat serum corticosterone after treatment with metabotropic glutamate receptor agonists or antagonists. J neuroendocrinol. 2001;13:670–677. doi: 10.1046/j.1365-2826.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:3182–3191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor b. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Remage-Healey L. Sex differences and rapid estrogen signaling: a look at songbird audition. Front Neuroendocrinol. 2015;38:37–49. doi: 10.1016/j.yfrne.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Endogenous mGluR activity suppresses GABAergic transmission in avian cochlear nucleus magnocellularis neurons. J Neurophysiol. 2007;97:1018–1029. doi: 10.1152/jn.00883.2006. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17beta-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERalpha and GPR30. Endocrinology. 2013;154:2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith JC, Jr, Harris HA. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem. 2004;47:5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- Manas ES, Unwalla RJ, Xu ZB, Malamas MS, Miller CP, Harris HA, Hsiao C, Akopian T, Hum WT, Malakian K, Wolfrom S, Bapat A, Bhat RA, Stahl ML, Somers WS, Alvarez JC. Structure-based design of estrogen receptor-beta selective ligands. J Am Chem Soc. 2004;126:15106–15119. doi: 10.1021/ja047633o. [DOI] [PubMed] [Google Scholar]
- Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LA. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191:111–117. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153:4616–4621. doi: 10.1210/en.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake M, Narita M, Shibasaki M, Nakamura A, Suzuki T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur J Neurosci. 2005;22:1476–1488. doi: 10.1111/j.1460-9568.2005.04325.x. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda H, Yamada R, Kuba H, Ohmori H. Activation of metabotropic glutamate receptors improves the accuracy of coincidence detection by presynaptic mechanisms in the nucleus laminaris of the chick. J Physiol. 2013;591:365–378. doi: 10.1113/jphysiol.2012.244350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley JC, Huddleston GG, Carruth LL, Petrulis A, Grober MS, Clancy AN. Sexual responses of the male rat medial preoptic area and medial amygdala to estrogen I: site specific suppression of estrogen receptor alpha. Horm Behav. 2012;62:50–57. doi: 10.1016/j.yhbeh.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015;220:2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/S0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci (Landmark Ed) 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NV, Ogaga-Mgbonyebi EV, Habteab B, Dunigan AI, Tesfay MA, Clancy AN. Sexual responses of the male rat medial preoptic area and medial amygdala to estrogen II: site specific effects of selective estrogenic drugs. Horm Behav. 2012;62:58–66. doi: 10.1016/j.yhbeh.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinska E. The role of group I metabotropic glutamate receptors in memory consolidation and reconsolidation in the passive avoidance task in 1-day-old chicks. Neurochem Int. 2006;48:447–452. doi: 10.1016/j.neuint.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 2015;36:72–89. doi: 10.1016/j.yfrne.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Christophe VJ, Ball GF, Cornil CA. Neuroestrogens rapidly regulate sexual motivation but not performance. J Neurosci. 2013;33:164–174. doi: 10.1523/JNEUROSCI.2557-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Nag S, Mokha SS. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an ERK-dependent non-genomic mechanism. Neuroscience. 2013;255:177–190. doi: 10.1016/j.neuroscience.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25:1219–1230. doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Tang ZQ, Lu Y. Development of GPCR modulation of GABAergic transmission in chicken nucleus laminaris neurons. PloS One. 2012;7:e35831. doi: 10.1371/journal.pone.0035831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZQ, Liu YW, Shi W, Dinh EH, Hamlet WR, Curry RJ, Lu Y. Activation of synaptic group II metabotropic glutamate receptors induces long-term depression at GABAergic synapses in CNS neurons. J Neurosci. 2013;33:15964–15977. doi: 10.1523/JNEUROSCI.0202-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Sander Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Haraguchi S, Tobari Y, Narihiro M, Ishikawa K, Hayashi T, Harada N, Tsutsui K. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nat Commun. 2014;5:3061. doi: 10.1038/ncomms4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Hsieh G, Ei-Kouhen O, Wilson SG, Mikusa JP, Hollingsworth PR, Chang R, Moreland RB, Brioni J, Decker MW, Honore P. Role of central and peripheral mGluR5 receptors in post-operative pain in rats. Pain. 2005;114:195–202. doi: 10.1016/j.pain.2004.12.016. [DOI] [PubMed] [Google Scholar]