Abstract
Aging-related impairments in hippocampus-dependent cognition have been attributed to maladaptive changes in the functional properties of pyramidal neurons within the hippocampal subregions. Much evidence has come from work on CA1 pyramidal neurons, with CA3 pyramidal neurons receiving comparatively less attention despite its age-related hyperactivation being postulated to interfere with spatial processing in the hippocampal circuit. Here, we use whole-cell current-clamp to demonstrate that aged rat (29–32 months) CA3 pyramidal neurons fire significantly more action potentials (APs) during theta-burst frequency stimulation and that this is associated with faster AP repolarization (i.e., narrower AP half-widths and enlarged fast afterhyperpolarization). Using a combination of patch-clamp physiology, pharmacology, Western blot analyses, immunohistochemistry, and array tomography, we demonstrate that these faster AP kinetics are mediated by enhanced function and expression of Kv4.2/Kv4.3 A-type K+ channels, particularly within the perisomatic compartment, of CA3 pyramidal neurons. Thus, our study indicates that inhibition of these A-type K+ channels can restore the intrinsic excitability properties of aged CA3 pyramidal neurons to a young-like state.
SIGNIFICANCE STATEMENT Age-related learning deficits have been attributed, in part, to altered hippocampal pyramidal neuronal function with normal aging. Much evidence has come from work on CA1 neurons, with CA3 neurons receiving comparatively less attention despite its age-related hyperactivation being postulated to interfere with spatial processing. Hence, we conducted a series of experiments to identify the cellular mechanisms that underlie the hyperexcitability reported in the CA3 region. Contrary to CA1 neurons, we demonstrate that postburst afterhyperpolarization is not altered with aging and that aged CA3 pyramidal neurons are able to fire significantly more action potentials and that this is associated with faster action potential repolarization through enhanced expression of Kv4.2/Kv4.3 A-type K+ channels, particularly within the cell bodies of CA3 pyramidal neurons.
Keywords: A-type K+ channels, action potential repolarization, aging, CA3, Kv4.2/Kv4.3, pyramidal neurons
Introduction
Nonpathological aging is associated with learning/memory impairment and cognitive decline and has been attributed to deficient encoding of information by hippocampal neurons. Thus far, most attention has been paid to the CA1 subregion of the hippocampus because it facilitates integration of multimodal sensory inputs from the entorhinal cortex and is the primary output region of the hippocampal formation (Amaral and Witter, 1989). Many studies have demonstrated that aging-related maladaptive changes in the functional properties of CA1 pyramidal neurons, including enhanced Ca2+-dependent K+ currents underlying the slow postburst afterhyperpolarization (sAHP), lead to reductions in intrinsic excitability, synaptic activation, information handling capacity, and learning impairments (Landfield and Pitler, 1984; Moyer et al., 2000; Power et al., 2002; Tombaugh et al., 2005; Burke and Barnes, 2006; Foster, 2012). Consistent with this view, fMRI studies have shown reduced hippocampal activation and excitability in pathological conditions of Alzheimer's disease (AD) and amnestic mild cognitive impairment (aMCI) as well as with normal aging (Ewers et al., 2011; Bakker et al., 2012).
Although CA1 hippocampal pyramidal neuron dysfunction clearly contributes to aging-related cognitive deficits, it is important to consider that impaired cognition is likely a multivariate product of age-related changes in the hippocampal circuit (Burke and Barnes, 2006; Wilson et al., 2006; Small et al., 2011; Morrison and Baxter, 2012). The hippocampal CA3 region, in particular, is the major source of inputs to CA1 pyramidal neurons and harbors age-related neuronal dysfunction itself. For example, higher firing rates of CA3 place cells from aged rats have been reported using in vivo single-neuron recordings (Wilson et al., 2005). Furthermore, place cells in CA3, but not in CA1, fail to remap their place fields upon introduction to a novel environment, thus demonstrating “cognitive rigidity” in aged rats. Such inability to encode and adapt to novel contexts suggests that adverse functional consequences arise from increased CA3 pyramidal neuron activity (Wilson et al., 2003, 2005). Consistent with these findings, healthy aged human subjects with impairments in pattern separation (i.e., the ability to differentiate novel but similar information from previously learned information) exhibit increased fMRI BOLD activity in the CA3/dentate gyrus region, a trend not observed in young control subjects with intact pattern separation (Yassa et al., 2011). Increased fMRI BOLD activity in CA3/DG also precedes memory impairments in patients with early AD and aMCI (Dickerson et al., 2005; Bakker et al., 2012). Furthermore, pharmacological reductions of CA3/DG subfield hyperactivity with antiepileptic levetiracetam improve cognition in aMCI subjects (Bakker et al., 2012). Thus, both decreased and increased excitability in various cell types from distinct subregions of the hippocampus likely contributes to age-associated impairment of hippocampal function.
Despite the human and animal in vivo studies emphasizing that changes in CA3 functional properties may play a critical role in aging-related cognitive deficits, the molecular substrates underlying these changes remain unclear. Our results indicate that faster action potential (AP) repolarization mediated through Kv4.2/Kv4.3 channels gives aged CA3 pyramidal neurons the capacity to fire more APs in response to synaptic stimulation, thereby promoting increased excitability.
Materials and Methods
Subjects.
Young adult (2- to 5-month-old) and aged (29- to 32-month-old) male F1 hybrid Fischer 344 × Brown Norway rats were used in this study. Rats were group housed with ad libitum access to food and water and maintained in a climate-controlled room with a 14:10 h light/dark cycle. All experimental procedures were approved by the Northwestern University Animal Care and Use Committee and conformed to National Institutes of Health standards (Publications No. 80-23). All efforts were made to minimize animals' discomfort and the number of animals used.
Brain-slice preparation.
Hippocampal slices and whole-cell current-clamp recordings were made using previously published methods and protocols (Power et al., 2002; Matthews et al., 2008). Rats were deeply anesthetized with isoflurane and decapitated. The brains were quickly removed, immersed in ice-cold modified aCSF solution containing the following (in mm): 206 sucrose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 3 MgSO4, 15 glucose, 0.1 CaCl2, pH 7.4, oxygenated with 95%:5% O2:CO2. The hippocampi were then dissected out, and 300-μm-thick slices were prepared. After a 20 min incubation period in 34°C standard aCSF bath solution (in mm as follows: 125 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2.4 CaCl2, 25 glucose, pH 7.4), slices were allowed to reach room temperature before use in patch-clamp experiments.
In vitro electrophysiology.
Whole-cell current-clamp recordings were made from visually identified CA3 pyramidal neurons using a Zeiss Axioskop upright microscope equipped with a long working distance 40× water-immersion objective (0.8 NA) and infrared differential interference contrast optics. Recording pipettes were made of glass capillaries using a horizontal Sutter P-97 puller and yielding 4–6 mΩ resistance pipettes when filled with standard intracellular (K-Meth) solution (in mm) as follows: 120 K-MeSO4, 10 KCl, 10 HEPES, 10 Na2-phosphocreatine, 4 Mg-ATP, 0.4 Na3-GTP, 0.5% neurobiotin; 285–290 mOsm; pH 7.3 adjusted with KOH. Slices were continuously perfused with oxygenated aCSF bath solution at 32°C–34°C. GABAA and GABAB receptor blockers 5 μm SR95531 hydrobromide and 10 μm SCH50911 (Tocris Bioscience) were routinely added to aCSF solution to block IPSPs unless otherwise stated. Whole-cell current-clamp data were acquired using an Axoclamp-2A amplifier (Molecular Devices) and digitized at 10 kHz (filtered at 3 kHz) with the neurons held (Vh) at −60 mV. Bipolar stimulating electrodes were placed in the stratum radiatum (SR) central to stratum pyramidale (SP) to examine aging-related differences in the characteristics of synaptically evoked APs from CA3 pyramidal neurons. The morphology corresponds to pyramidal neurons in CA3 region of the hippocampus, with cell bodies located in the SP and the visible basal dendrites in the stratum oriens (see Fig. 1A,B).
Figure 1.

Medium and slow postburst AHPs are not altered with aging in CA3 pyramidal neurons. A, Schematic of hippocampal slice section illustrating the placement of recording and stimulating electrode in the CA3 region. B, An example of a histochemical section stained with DAB showing morphology of two representative CA3 neurons filled with neurobiotin from patch pipettes during whole-cell patch-clamp experiments. C, Illustrated are examples of postburst AHPs evoked with a 50 Hz train of suprathreshold current injections into young (blue) and aged (red) CA3 pyramidal neurons. Medium and slow postburst AHPs were measured at the peak and 1 s interval after last current pulse, respectively. D, There was no age-related difference in either the medium or slow postburst AHP from recorded CA3 pyramidal neurons. Also, changing the aCSF solution from normal aCSF to aCSF with GABAR blockers had no effect on current injection-elicited AHPs in either young or aged CA3 neurons (data not shown). E, Representative voltage traces of AHPs evoked by 5 suprathreshold synaptic stimuli at 50 Hz before and after the addition of GABAR blockers to the aCSF recorded from young and aged CA3 neurons. F, There were no age-related differences in either peak or 1 s AHP (see Table 2) before and after GABAergic inhibition. C, E, APs are truncated for clarity. Values are mean ± SEM. The number of cells (n) is represented in the middle of each bar for each group.
Resting membrane potential (Vrest) was measured immediately after breaking into the cell. Input resistance (RN) was calculated as the slope of the voltage-current curve using 500 ms current steps from −350 pA to 200 pA at 50 pA steps. Medium and slow AHPs were measured as the difference between Vh and the negative going peak and 1 s after the offset of the last current step, respectively, induced by a 50 Hz train of 15 APs evoked by 2 ms/1.2 nA current injection pulses. Single AP properties, including fast afterhyperpolarization (fAHP), were measured using direct current injection ramps (100–450 pA, 500 ms) (see Table 3). However, these single AP measures can be influenced by imperfect bridge balance when current injections and membrane potential measurements are made with the patch recording electrode (Hu et al., 2007; Oh et al., 2009). Therefore, AP amplitudes, thresholds, half-widths, and fAHP were also measured from orthodromically evoked APs using threshold voltage stimulus output from the isolated stimulator (Digitimer DS2, Digitimer). AP amplitude was calculated from Vh. AP threshold was calculated as the point where the first derivative of the upstroke phase of the trace equals 20 mV/ms. Using a 1 ms sliding average, the fAHP measurement was taken when the mean first derivative of the trace reached 0.0 ± 0.5 after each orthodromically elicited spike. AP width measurements were taken at half the AP peak amplitude relative to Vh. Theta frequency bursting was evaluated with 5 synaptically evoked bursts with a 200 ms interburst interval of 10 stimuli at 100 Hz (100 ms). The first synaptic stimulus intensity was set at threshold for generating an action potential for these theta frequency burst measurements. Threshold voltage stimulus for eliciting orthodromic AP was identified by systematically increasing voltages of stimulation on the isolated stimulator. The peak amplitudes of the IPSPs and EPSPs (with GABAR blockers added to bath aCSF) elicited from these subthreshold responses were used to generate the IPSP and the EPSP input–output slopes, which were used for statistical comparison (see Fig. 2E).
Table 3.
fAHP and AP half-width evoked synaptically or by direct somatic current injection with control (KMeth) or phrixotoxin in the internal pipette solution
| Group | 1 orthodromic APs |
Somatic current injection ramp |
||
|---|---|---|---|---|
| fAHP (mV) | AP half-widthVh (ms) | fAHP IRAMP (mV) | AP half-widththresh (ms) | |
| Young-control (n = 43) | −57.88 ± 0.32 | 1.15 ± 0.02 | −50.94 ± 0.63 (42) | 1.16 ± 0.02 |
| Young-PaTx (n = 7) | −56.79 ± 0.53 | 1.18 ± 0.03 | ||
| Aged-control (n = 28) | −61.68 ± 0.61*** | 0.98 ± 0.02** | −53.76 ± 0.74 (28)** | 1.09 ± 0.02* |
| Aged-PaTx (n = 8) | −56.25 ± 0.61 | 1.13 ± 0.02 | ||
*p < 0.05;
**p < 0.005;
***p < 0.0005.
Figure 2.
Excitability is enhanced in aged CA3 pyramidal neurons. A, Examples of representative traces from young (blue) and aged (red) CA3 pyramidal neurons are illustrated that demonstrate increased firing (or reduced accommodation) in aged CA3 pyramidal neurons to a 100 ms, 100 Hz train of synaptic stimuli. The stimulus intensity was set at threshold voltage to evoke an AP for the first stimulus in the 100 Hz train. B, Significantly more APs were observed in aged CA3 neurons during the 100 ms, 100 Hz train. C, Examples of representative traces from aged and young CA3 neurons are illustrated that show increased firing evoked during theta burst stimulation. Each 100 Hz burst (as described in A) was delivered 5 times at 5 Hz. D, Repeated-measures ANOVA revealed that aged neurons fired more APs during theta burst stimulation protocol compared with young. There was also a significant interaction of age and burst number (1–5). E, The IPSP or EPSP amplitude was measured as a function of synaptic subthreshold stimulation intensity. The slope of this relationship was determined from each cell. There was no age-related difference in IPSP (left; p = 0.98) or EPSP (right; p = 0.82) input–output slopes; thus, synaptic drive was similar. *p < 0.05 (Fisher's PLSD t test). **p < 0.005 (Fisher's PLSD t test). Values are mean ± SEM. The number of cells (n) is represented in the middle of each bar for each group.
Whole-cell current-clamp data were analyzed for those neurons meeting our cell health criteria using custom-written MATLAB scripts (The MathWorks). Neuron health criteria were series resistance (RS) < 30 mΩ, membrane RN > 45 mΩ, Vrest < −50 mV, and AP amplitude > 65 mV from holding potential. Also, none of the recorded neurons exhibited spontaneous action potentials at Vrest. Statistical analyses were conducted using Statview. All biophysical data are presented as mean ± SEM.
Drugs.
The following drugs were used at the final concentrations indicated: 2 mm 4-aminopyridine (4-AP; Sigma-Aldrich), 20 μm paxilline (Tocris Bioscience), and 1 μm phrixotoxin-1 (PaTx; Alomone Labs) were used to block A-type K+, BK, and Kv4.2/4.3 A-type K+ channels, respectively.
Drugs were prepared as a stock solution using distilled water or DMSO and diluted to the required concentration in aCSF immediately before use. Bath-applied drugs were perfused for at least 5–10 min to ensure complete equilibration within the recording chamber before the biophysical properties were remeasured. PaTx was added to the pipette solution, not to the aCSF bathing solution, and allowed to equilibrate for 10–15 min within the intracellular compartment of the cell after making a whole-cell patch.
Western blotting.
Hippocampal slices (300 μm thick) from dorsal hippocampi were prepared from young adult and aged rats as described for the brain-slice preparations. The hippocampal slices were then transferred to fresh oxygenated ice-cold aCSF containing protease and phosphatase inhibitors, frozen over dry ice, and immediately microdissected under a microscope to yield the three major hippocampal subdivisions (CA1, CA3, and dentate gyrus) (Núñez-Santana et al., 2014). The CA3 region was placed in CLB buffer (in mm) (10 HEPES, 10 NaCl, 1 KH2PO4, 5 NaHCO3, 5 EDTA, 1 CaCl2, 0.5 MgCl2) containing protease and phosphatase inhibitors and further prepared by subcellular fractionation that yielded a nuclear, a membrane, and a cytoplasmic fraction (Guillemin et al., 2005). Briefly, tissue was prehomogenized in hypotonic medium and incubated on ice for 10 min. Following further homogenization with a motorized homogenizer, the tissue sample was restored to an isotonic state with 0.1 volume 2.5 m sucrose. The sample was then centrifuged at 4°C for 10 min to remove nuclei and debris. Supernatant was carefully removed from the resulting pellet and further centrifuged for 2.5 h at 14,000 × g. The resulting supernatant containing an enriched cytoplasmic fraction was removed from the pellet containing the enriched membrane fraction. The membrane fraction was resuspended in a 1% CHAPS solution containing protease and phosphatase inhibitors. Membrane fractions were used for further analysis. Protein concentration was determined by the BCA assay using BSA as a standard (Pierce).
For quantification, samples containing equal amounts of protein (30 μg) were resolved by SDS-PAGE using 8% bis-Tris gels and subsequently transferred to nitrocellulose membranes. Upon transfer, membranes were assessed for total protein using SYPRO Ruby blot stain according to the manufacturer's instructions and imaged using a Bio-Rad chemidoc XR imager. Total protein served as the loading control. Membranes were blocked for 1 h with blocking buffer (LI-COR Biosciences) and incubated in blocking buffer with 0.2% Tween overnight at 4°C with 1:1000 of anti-Kv4.2 (NeuroMab), 1:1000 of anti-Kv4.3 (NeuroMab), and 1:500 of anti-BK (NeuroMab). The membranes were then washed in PBST and incubated with IR-dye-labeled secondary antibodies (1:15000; LI-COR Biosciences) for 1 h at room temperature, washed again in PBST, and visualized with the Odyssey infrared imaging system (LI-COR Biosciences). Blots were analyzed using ImageJ (National Institutes of Health) and normalized to loading control. All results were confirmed by repeating the experiments and analysis in duplicate. Optical density data (relative to the Sypro, total protein loading control) were calculated by obtaining the average value for young adult rat tissue in each cohort (3 cohorts, each with 3 young adult and 3 aged rat samples, for a total of 9 young adult and 9 aged rats each for BK, Kv4.2, and Kv4.3 analyses), then normalizing each young adult and aged rat optical density to the young adult average for the individual cohort. These values were then analyzed using unpaired, two-tailed Student's t test, with p < 0.05 considered significant.
Immunofluorescence analyses.
As previously described (Neuman et al., 2014), recorded slices were tagged with AlexaFluor-488 streptavidin (Invitrogen), 1:200 dilution and processed for immunofluorescence light microscopy and array tomography. For array tomography experiments, slices were microwave processed and embedded in LR White resin (Electron Microscopy Sciences). Arrays of 50–120 serial sections at a thickness of 70 nm were collected from CA3 and mounted on gelatin-subbed high-precision coverslips (Bioscience Tools). Arrays were blocked for 40 min for anti-Kv4.3 and anti-BK, and 50 min for anti-Kv4.2 in 5% normal goat serum (NGS), 5% BSA, 0.1% cold water fish skin gelatin, and 0.1% Tween in TBS. Blocking solution was rinsed off for 5 min with 0.1% acetylated BSA, 0.05% Tween in TBS, and incubated with primary antibody for 2 h at room temperature diluted in 2% NGS, 5% BSA, 0.1% cold water fish skin gelatin, and 0.1% Tween in TBS. Concentrations for the primary antibodies were as follows: anti-BK; mouse, NeuroMab, University of California–Davis, 1:300, anti-Kv4.2; mouse, NeuroMab, University of California–Davis, 1:600, and anti-Kv4.3; mouse, NeuroMab, University of California–Davis, 1:600. Incubation in secondary antibody was for 30 min (AlexaFluor-647 goat anti-mouse IgG1, 1:150 in 0.1% BSAc, 0.05% Tween in TBS), and subsequently rinsed with TBS and mounted onto a slide with Slowfade Gold Antifade reagent with DAPI (Invitrogen). Arrays were visualized with a Zeiss Axio Imager M2 imaging system with an Axiocam MRm digital camera and AxioVision software (Carl Zeiss, MicroImaging). The 1 × 6 mosaics of the same location in 50–100 consecutive sections were acquired with a 63×/1.4 NA Plan Apochromat oil-immersion objective. In addition to collecting from DAPI and Cy5 channels, images were also collected from Cy3 channel to visualize any autofluorescence in the sections.
Slices not used in array tomography experiments were resectioned in agar at 60 μm on a vibratome (Pelco) and rinsed in 0.01 m PBS and subsequently treated with 0.1% NaBH4 for 30 min. Slices were then rinsed with 50 mm TBS, pH 7.6, followed by a primary block (10% NGS, 0.1% Triton X-100 in TBS) for 30 min. Slices were then incubated in primary antibody (anti-Kv4.2, anti-Kv4.3, BK, mouse, NeuroMab University of California–Davis; 1:500) diluted in 2% NGS, 0.1% Triton X-100, and TBS overnight at 4°C on shaker. Slices were rinsed in TBS and incubated in secondary antibody (AlexaFluor-647, goat anti-mouse IgG1, Invitrogen; 1:500) diluted in 2% NGS in TBS, and incubated for 30 min. Slices were then washed in TBS and left to dry on subbed slides overnight and mounted with SlowFade gold antifade with DAPI. Images were acquired using Zeiss Axio Imager M2 imaging system with an Axiocam MRm digital camera and AxioVision software (Carl Zeiss, MicroImaging) with a 20×/0.8 NA objective.
For a detailed description of image processing for array tomography experiments, refer to Neuman et al. (2014). Briefly, files were opened with Fiji and processed using plugins provided by Stephen Smith's laboratory. Image stacks were aligned using the MultiStackReg plugin (Brad Busse), and a transformation matrix was saved for subsequent alignments on stacks in the Cy3 and Cy5 channels. Aligned image stacks were then imported into AxioVision for 2D deconvolution and then auto-thresholded in Fiji using the Triangle algorithm followed by the creation of sum projections in Fiji to show the channel distributions along the somatodendritic axis of area CA3. Two ROIs at 20.69 × 19.10 μm in strata lucidum (SL) and SR were chosen for analysis, whereas two ROIs at 7.26 × 7.26 μm were used in SP. Stratum oriens was not included in the analyses due to inconsistent preservation of its entire extent during processing. Autofluorescence was present in varying amounts in SP, especially in aged rats, of which a limited amount was observed in Cy5. ROIs were placed in regions lacking capillaries and signal from both DAPI and Cy3, thus eliminating the influence of autofluorescence on data analysis.
For hippocampal slice immunofluorescence analyses, average pixel intensity was determined for three ROIs each in SP, SL, and SR. For immunofluorescence array tomography, percentage area fluorescence was determined by taking the average area of the ROIs filled by nonzero pixels. Because the SP and SL lack a rigid border, perisomatic data were calculated as the average of their respective values. This average was then normalized to the values for SR to obtain the perisomatic ratio. As with the subcellular fractionation Western blot analyses, the perisomatic ratios were normalized to the young adult rat values, using the young adult average for slice immunofluorescence (n = 7 young adult and 7 aged rats each for Kv4.2 and Kv4.3 analyses) or the young adult average for immunofluorescence array tomography (n = 5 young adult and 5 aged rats each for Kv4.2 and Kv4.3 analyses). The particular parameter used to obtain the perisomatic ratio for array tomography experiments did not make a difference statistically (e.g., using puncta density also yielded the same data patterns), but percentage area fluorescence was chosen to avoid possible confound due to age-related differences in average puncta size.
Results
Postburst AHP in CA3 pyramidal neurons is unaltered with normal aging
We performed whole-cell current-clamp recordings to examine the intrinsic firing properties of visually identified CA3 pyramidal neurons from young (2–4 month) adult and aged (29–32 month) rats. Reduced excitability of CA1 pyramidal neurons via increases in both the medium and slow postburst AHP has been shown to be a biomarker of normal aging in numerous studies (Disterhoft and Oh, 2007; Matthews et al., 2009). Hence, we examined the medium and slow postburst AHPs following 50 Hz trains of APs evoked with either somatic current injections or suprathreshold synaptic stimuli in SR (Fig. 1A,B). Contrary to observations in CA1 neurons, neither the medium nor slow postburst AHP was different between the two age groups in CA3 pyramidal neurons (p > 0.656; Fig. 1C,D; Tables 1, 2). Other membrane properties (e.g., input resistance) of CA3 neurons were also constant with chronological aging (Table 1).
Table 1.
Passive intrinsic properties were not different with age
| Group | Vrest (mV) | Rn at Vrest (mΩ) | Rs at Vrest (mΩ) | Saga (mV) | AP amplitude from Vh (mV) | Vthresh (mV) |
|---|---|---|---|---|---|---|
| Young (n = 52) | −68.97 ± 0.75 | 85.01 ± 4.41 | 21.56 ± 0.77 | −2.28 ± 0.13 | 83.74 ± 1.1 (31) | −48.26 ± 0.86 (31) |
| Aged (n = 44) | −67.56 ± 1.02 | 79.99 ± 4.73 | 21.33 ± 1.08 | −2.32 ± 0.17 | 86.11 ± 1.19 (29) | −47.89 ± 0.87 (29) |
aAfter −350 pA current injection. Vrest, Resting potential; RN, input resistance; RS, series resistance; Vh, holding potential; Vthresh, AP threshold potential.
Table 2.
Medium and slow AHP measures with and without GABA blockers
| Group | 15 AP current injection |
5 orthodromic APs |
||
|---|---|---|---|---|
| Peak AHP | 1 s | Peak AHP | 1 s | |
| Young aCSF | −8.01 ± 0.43 (52) | −5.15 ± 0.35 | −14.39 ± 1.08 (13) | −7.22 ± 1.03 |
| Aged aCSF | −7.84 ± 0.42 (44) | −4.87 ± 0.36 | −15.49 ± 1.03 (18) | −6.63 ± 0.53 |
| Young aCSF-GABA | −7.00 ± 0.83 (13) | −4.56 ± 0.72 | −7.35 ± 0.91 (13) | −4.30 ± 0.78 |
| Aged aCSF-GABA | −7.78 ± 0.85 (13) | −5.11 ± 0.67 | −8.47 ± 0.64 (18) | −4.91 ± 0.44 |
The hyperpolarization resulting from five synaptically evoked APs is comprised of both the postburst AHP and IPSPs. To isolate the postburst AHP, 5 μm SR95531 hydrobromide and 10 μm SCH50911 (GABAA and GABAB receptor blockers, respectively) were added to the aCSF after the initial intrinsic properties were measured. Addition of GABA receptor (GABAR) blockers reduced the postburst hyperpolarization as it eliminated the synaptically evoked IPSP from the response. However, there was no age-related difference in either medium or slow AHP measures after elimination of the inhibitory component of the response (repeated-measures ANOVA for peak AHP: F(1,29) = 0.98, p = 0.33; 1 s: F(1,29) = 0.0002, p = 0.99; Figure 1E,F; Table 2). Further, there was no interaction of age with GABAR blockers, suggesting that inhibition onto CA3 pyramidal neurons is not altered with normal aging. Because GABAR blockers had no effect on the postburst AHP elicited with direct current injection or other passive intrinsic cellular properties (Table 2), GABAR blockers were routinely added to aCSF solution in the subsequent experiments.
Age-related increase in excitability in CA3 hippocampal neurons
The enlarged postburst AHP in CA1 pyramidal neurons from aged rats augments spike frequency adaptation (accommodation) compared with those from young adult rats (Disterhoft and Oh, 2007). Based on our observation that the postburst AHP in CA3 pyramidal neurons is similar in young adult and aged rats, we hypothesized that accommodation in these neurons is also similar, again in contrast to what has been observed in CA1 pyramidal neurons.
To test this, we measured the number of action potentials evoked from CA3 pyramidal neurons to varying steps of 1 s depolarizing current injections (550–700 pA, 50 pA steps). As expected based on our postburst AHP observations, CA3 pyramidal neurons from both young and aged rats accommodated very strongly to 1 s depolarizing current injections, firing on an average 4.5–6.5 APs. Thus, there were no significant differences due to age (repeated ANOVA: F(1,42) = 0.04, p = 0.84, young: n = 24; aged: n = 20) or age by current injection interaction (repeated ANOVA: F(3,126) = 0.84, p = 0.48; data not shown) in the accommodation measures with the 1 s depolarizing current injections. While using a 1 s direct depolarizing current injection is a way to measure accommodation, neurons are not naturally depolarized for such a prolonged duration. Rather, trains of orthodromic synaptic stimulation more closely mimic the input patterns experienced by neurons in vivo. Therefore, we examined aging-related changes in the ability of CA3 pyramidal neurons to accommodate to 10 synaptic stimuli delivered as a single 100 Hz train (Fig. 2A) or as five 100 Hz trains delivered at theta frequency (Fig. 2B). The stimulus intensity used throughout the protocol was adjusted for each neuron to the threshold level needed to evoke a single orthodromic AP.
Despite their similar membrane properties (Tables 1, 2), aged CA3 pyramidal neurons fired significantly more APs than young neurons during a single 100 Hz train (young: 4.82 ± 0.41 APs, n = 11; aged: 6.93 ± 0.36 APs, n = 7; t test p < 0.005; Fig. 2A,B). Aged CA3 neurons also fired significantly more APs than young during theta burst stimulation (repeated-measures ANOVA: F(1,48) = 10.46, p < 0.01; Fig. 2C,D). Moreover, analyses revealed a significant interaction between age and burst number (repeated-measures ANOVA: F(4,48) = 5.54, p = 0.001), attributable to the higher number of APs fired by aged neurons during the first and second theta bursts compared with young adult CA3 neurons (Fisher's PLSD post hoc test for first burst: p = 0.001, second burst: p < 0.05; age-related differences in spike number within bursts 3–5 were not significant with p values ranging from 0.074 to 0.064). Thus, there is an age-related increase in the ability of CA3 pyramidal neurons to fire APs in high-frequency bursts, consistent with the enhanced firing rate found in vivo in the CA3 region of aged subjects (Wilson et al., 2003).
Importantly, these theta burst stimulation data further illustrate the spike frequency adaptation by CA3 pyramidal neurons to trains of synaptic stimuli. Furthermore, although more APs were observed in the initial bursts with a greater decline in AP firing with successive bursts in aged CA3 neurons (Fig. 2C,D), this latter result may be due in part to an increased Ca2+-dependent slow AHP in response to the higher number of APs fired during the first burst of the theta burst protocol. The medium and slow AHPs are generated between each burst with larger postburst AHPs following bursts with more APs (Wu et al., 2004). Therefore, we compared the sAHPs generated with 5 suprathreshold synaptic stimuli, which generated 5 APs (Fig. 1E,F), with those that fired 6 times with those same stimuli to determine whether increasing numbers of APs also increased the sAHP. Repeated-measures ANOVA revealed that additional APs significantly increase the sAHP in CA3 pyramidal neurons (F(1,28) = 20.3; p = 0.0001; n = 16 young, 14 aged; the mean AHP values (mV) were −7.9 ± 0.6, −8.7 ± 0.7, −7.9 ± 0.9, and −8.2 ± 0.8 for young 5 and 6 APs and aged 5 and 6 APs, respectively) in both young and aged CA3 pyramidal neurons (no significant age-related difference: p = 0.794). Hence, it is possible that the greater number of APs fired by aged CA3 pyramidal neurons during the first and second burst generated larger Ca2+-dependent postburst AHPs leading to a sharper decline in their ability to fire APs in the last 3 bursts.
Moreover, the fact that the IPSP and EPSP amplitude to orthodromic stimuli was unaltered with aging (Fig. 2E) suggests that there was no alteration in the synaptic drive to CA3 pyramidal neurons contributing to the observed changes in excitability.
AP repolarization is faster in aged CA3 pyramidal neurons
Given that the observed age-related increase in CA3 neuron excitability is not due to changes in the medium or slow AHP, or other membrane properties (Table 1), we hypothesized that enhanced AP repolarization may contribute to this aging-related increased excitability. Indeed, among the numerous channels contributing to AP kinetics, A-type K+ channels contribute to the fAHP and AP duration (Storm, 1987; Zhang and McBain, 1995; Kim et al., 2005), and expression of the Kv4.2 channel subunit in the CA3 region correlates with learning impairment in aged rats (Haberman et al., 2011). Intuitively, neurons with increased A-type channel expression would have larger fAHPs and narrower APs, allowing them to return to Vrest more quickly, and thus shortening their refractory phase (Eccles et al., 1957; Kandel and Spencer, 1961; Storm, 1987; Sah and McLachlan, 1992). Either scenario would confer on neurons the potential for higher AP firing frequency, as we observed in aged CA3 pyramidal neurons (Fig. 2). Thus, we assessed the fAHP and half-width of APs in CA3 pyramidal neurons from young adult and aged rats.
In support of our hypothesis, aged CA3 neurons exhibited a substantial increase in the fAHP after a single orthodromically evoked AP (young: −57.87 ± 0.32 mV, n = 43; aged: −61.68 ± 0.31 mV, n = 28; t test p < 0.0001; Fig. 3A,B). Moreover, with repeated suprathreshold orthodromic stimuli (5 synaptically evoked APs at 50 Hz), the age-related differences in fAHP amplitudes became even larger (repeated-measures ANOVA for age: F(1,276) = 29.58, p < 0.0001; young: n = 43, aged: n = 28; Fig. 3D,E) with no significant interaction of age and spike number.
Figure 3.
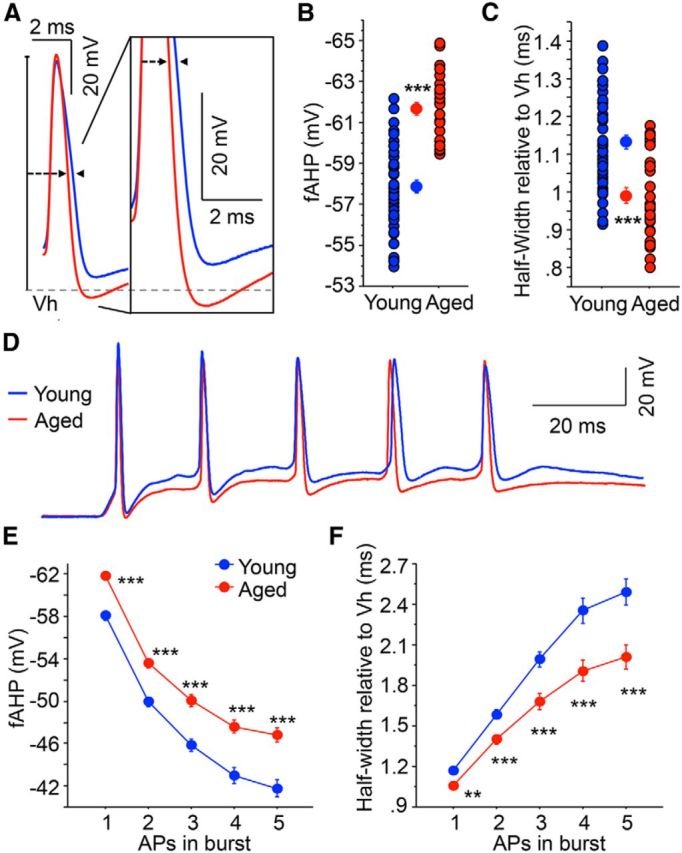
Aged CA3 pyramidal neurons exhibit increased fAHP and reduced spike broadening. A, Illustrated are overlays of representative traces from young (blue; n = 43) and aged (red; n = 28) CA3 neurons that show fAHP evoked with single orthodromic AP. fAHP is measured as absolute membrane voltage. AP half-width is measured as the duration of the AP at half of the AP amplitude (relative to holding potential −60 mV). B, The fAHP evoked with a single orthodromic stimulus was significantly enhanced (more hyperpolarized) in aged CA3 neurons than young neurons. C, AP half-width was significantly narrower in aged CA3 neurons than young neurons. D, Overlay of representative traces of young (blue) and aged (red) CA3 neurons after repeated orthodromic stimulations at suprathreshold intensities (50 Hz). E, Significantly larger fAHPs were observed in aged CA3 neurons after 5 synaptically evoked APs at 50 Hz (repeated-measures ANOVA, F(1,276) = 29.58, p < 0.0001). Planned post hoc analysis further revealed that the fAHP was significantly different after each of the 5 synaptically evoked APs at 50 Hz. F, Significantly less spike broadening was observed in aged CA3 neurons after a 50 Hz train of 5 suprathreshold orthodromic APs. Repeated-measures ANOVA revealed not only a decrease in the spike half-width with age but also an age by spike number interaction, suggesting that the rate of change with each subsequent spike is higher in young CA3 neurons. **p < 0.005 (Fisher's PLSD t test). ***p < 0.0005 (Fisher's PLSD t test). Values are mean ± SEM.
Similarly, the half-width of a single AP in aged CA3 pyramidal neurons was significantly narrower than in young neurons (young: 1.15 ± 0.02 ms, n = 43; aged: 0.98 ± 0.02 ms, n = 28; t test p < 0.0001; Fig. 3A,C). Furthermore, the AP half-widths during 5 sequential synaptically elicited APs at 50 Hz were significantly smaller in aged pyramidal neurons compared with young (repeated-measures ANOVA for age: F(1,276) = 19.37, p < 0.0001; Fig. 3D,F). Moreover, spike broadening during burst firing was reduced in aged CA3 neurons, as revealed by a significant interaction between age and spike number (repeated-measures ANOVA for age/spike number: F(1,276) = 9.36, p < 0.0001). Importantly, the fAHP and AP half-width were similarly altered with somatic current injection ramp protocols with no changes in AP amplitude or threshold (fAHP t test: p < 0.001; HW from threshold potential t test: p < 0.05; young n = 42 aged n = 28; Table 3). The decreased spike broadening together with increased fAHP in aged CA3 neurons reflects an increase in the spike repolarization rate, suggesting a progressive increase in the repolarizing outward currents during the spike train. Furthermore, we examined whether spike broadening (using the ratio of the fifth/first spike HW) or fAHP of the first AP of a 5 AP burst correlates with medium and/or slow AHP. The R2 values for all of these comparisons were <0.06 for young, aged, or combined CA3 neurons. Hence, there is no correlation between fast AHP or spike broadening and the postburst AHP in our dataset.
Faster AP repolarization in aged CA3 neurons is mediated by Kv4.2/Kv4.3 channels
Accumulating evidence has shown that the repolarizing phase of the neuronal action potential relies on voltage-gated K+ conductances, such as IA (A-type), ID (delayed rectifiers), and IC (Ca2+-activated BK) (Connor and Stevens, 1971; Storm, 1987; Mitterdorfer and Bean, 2002). Spike broadening in hippocampal pyramidal neurons is attributed to the inactivation of A-type and/or BK channels, as delayed rectifiers have much slower inactivation kinetics and cannot account for changes in fAHP (Giese et al., 1998; Shao et al., 1999; Hu et al., 2001; Kim et al., 2005). We hypothesized that the faster AP repolarization in aged CA3 neurons is due to an enhanced functional expression of A-type K+ channels.
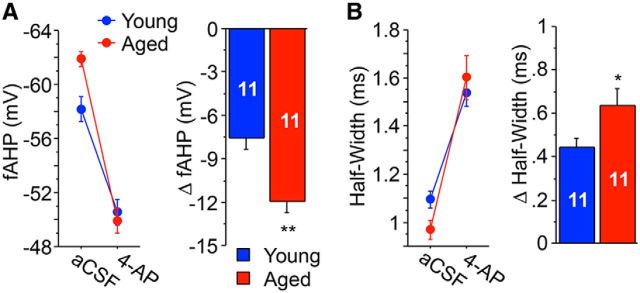
Measurements of fAHP and AP half-width were made before and after at least 5 min of 2 mm 4-AP (a nonspecific A-type K+ channel blocker) (Yao and Tseng, 1994) bath application with suprathreshold orthodromically elicited APs. Addition of 4-AP reduced the fAHP after a single AP in young and aged CA3 neurons to a similar membrane potential. Thus, the magnitude of change of the fAHP (ΔfAHP) was significantly larger for aged CA3 pyramidal neurons compared with young (young, ΔfAHP = −7.61 ± 0.73 mV, n = 11; aged, ΔfAHP = −11.97 ± 0.76 mV, n = 11; repeated-measures ANOVA revealed interaction of age/4-AP treatment: F(1,20) = 17.21, p = 0.0005; Fig. 4A) because the fAHP of aged neurons was larger. Similarly, 4-AP also caused a larger magnitude increase in AP half-width in aged CA3 neurons compared with young neurons (young, Δhalf-width = 0.44 ± 0.04 ms, n = 11; aged, Δhalf-width = 0.63 ± 0.08 ms, n = 11; repeated-measures ANOVA revealed interaction of age/4-AP treatment: F(1,20) = 4.37, p = 0.05; Fig. 4B). Importantly, we did not see a time-dependent effect on any of the parameters (e.g., spike amplitude and half-width) after perfusing slices with aCSF with GABAR blockers for 10–20 min after initial baseline measures were made (data not shown).
Figure 4.
A-type K+ channel blocker 4-AP produces a larger magnitude reduction in fAHP and increase in half-width in aged CA3 neurons than young. A, The fAHP was recorded after a single orthodromically induced AP before and after the addition of 2 mm 4-AP to the aCSF bathing solution. 4-AP incubation caused a larger magnitude reduction in fAHP in aged CA3 neurons compared with young CA3 neurons. B, 4-AP application also caused a larger magnitude increase in AP half-width in aged neurons compared with young neurons. *p < 0.05 (Fisher's PLSD t test), **p < 0.005 (Fisher's PLSD t test). Values are represented as mean ± SEM. The number of cells (n) is represented in the middle of each bar for each group.
These results suggest that increased A-type K+ channel function underlies the age-related changes in fAHP and AP half-width of CA3 neurons. However, 4-AP can also impact other voltage-gated K+ channels (Kv1.4, Kv3.3/3.4), the delayed rectifier K+ channels, and the β subunit of voltage-gated calcium channels (Wu et al., 2009). Thus, we further examined the source of altered fAHP and half-width using a specific Kv4.2/Kv4.3 channel blocker, PaTx (1 μm), added to the internal pipette solution. PaTx has been shown to preferentially bind the closed or inactivated form of the Kv4.2/Kv4.3 channels with high specificity, preventing further activation (Diochot et al., 1999; Chagot et al., 2004; Whyment et al., 2011). To ensure complete equilibration within the perisomatic region of CA3 pyramidal neurons, recordings were made 10–15 min after breaking into whole-cell configuration.
PaTx significantly reduced the fAHP of aged CA3 neurons after a single orthodromically elicited AP compared with aged controls but had no effect on young neurons (F(3,82) = 32.26, p < 0.0005; Fig. 5A,B; Table 3). Moreover, PaTx treatment reduced the fAHP of aged CA3 neurons to young-like values (Fig. 5A,B; Table 3). Analyses of fAHPs evoked with a 50 Hz train of synaptically evoked APs revealed significant effects for age/internal (F(3,324) = 11.751, p < 0.0001), for AP number (F(4,324) = 616.867, p < 0.0001), and a significant interaction of age/internal by AP number (F(12,324) = 2.332, p < 0.01). Further analysis for each individual AP within the train revealed that PaTx significantly reduced the fAHP in aged CA3 neurons, but not in young CA3 neurons (Fisher's PLSD p < 0.01; Fig. 5C).
Figure 5.

Inhibition of Kv4.2/Kv4.3 K+ channels with PaTx reverses aging-related alterations in fAHP and AP half-width. A, Representative traces of single orthodromically elicited APs from aged (red) and young (blue) CA3 neurons with (dashed line) and without (solid line) 1 μm PaTx in recording pipette (from AP threshold). PaTx diffusion into the cell significantly reduced the fAHP of aged CA3 neurons with no significant effect in young after a single orthodromically induced AP (B) and after 5 synaptically evoked AP at 50 Hz (C). PaTx also enhanced AP half-widths in aged CA3 neurons after single (D) or 5 sequential (E) synaptically evoked APs to durations similar to young CA3 neurons while having no effect on young half-widths (see Table 3). *p < 0.05 (Fisher's PLSD t test). **p < 0.005 (Fisher's PLSD t test). ***p < 0.0005 (Fisher's PLSD t test). Values are mean ± SEM. The number of cells (n) is represented in the figure legend.
Finally, PaTx significantly increased the AP half-width of aged neurons compared with aged controls while having no effect on young neurons (F(3,82) = 15.51, p < 0.001; Fig. 5A,D; Table 3). PaTx treatment increased the AP half-width of aged CA3 neurons to young-like values (Fig. 5A,D; Table 3). With repeated synaptic stimulation at 50 Hz, PaTx increased spike broadening with each subsequent AP in aged CA3 neurons, again to values similar to those in young neurons. Analyses of AP half-widths revealed significant effects for age/internal (F(3,324) = 10.82, p < 0.0001), for AP number (F(4,324) = 249.72, p < 0.0001), and a significant interaction of age/internal by AP number (F(12,324) = 5.72 p < 0.0001; Fig. 5E). Further analysis for each individual AP within the train revealed that PaTX significantly increased the AP half-widths in aged CA3 neurons, but not in young CA3 neurons (Fisher's PLSD p < 0.01; Fig. 5C).
Together, the 4-AP and PaTx results strongly suggest that the aging-related increase in fast repolarizing outward currents is mediated by enhanced functional expression of A-type Kv4.2/Kv4.3 K+ channels in CA3 pyramidal neurons.
Faster AP repolarization in aged CA3 neurons is not mediated by BK channels
As both A-type K+ and BK channels play a role in AP repolarization, we also investigated the contribution of BK channels to the age-related alterations in the fAHP and AP half-width in CA3 pyramidal neurons. After gathering baseline data, BK channels were blocked with 20 μm paxilline added to the aCSF solution, which was allowed to perfuse into the slices for 10–15 min before biophysical properties were measured again. Paxilline reduced the fAHP equally in both young and aged CA3 neurons, with no interaction of age with paxilline after one orthodromic AP, resulting in still larger fAHPs in aged CA3 neurons after BK channel block (ΔfAHP: young, −4.08 ± 0.6 mV, n = 8; aged, −4.54 ± 0.66 mV, n = 7; repeated-measures ANOVA for age: F(1,13) = 23.21, p < 0.0005; age/paxilline interaction: F(1,13) = 0.27, p = 0.61; Figure 6A). Further, fAHP measures with 5 synaptically evoked APs at 50 Hz were taken before and after the addition of paxilline. The ΔfAHP was calculated for each spike, and repeated-measures ANOVA revealed no significant difference in ΔfAHP between age groups and no interaction of age and spike number (young, n = 7; aged, n = 7; age effect: F(1,48) = 0.88, p = 0.37; age/spike interaction: F(1,48) = 0.44, p = 0.78; Figure 6B,C).
Figure 6.
Faster AP kinetics in aged CA3 neurons are not mediated by BK channels. A, Addition of BK channel antagonist, paxilline, to the aCSF bathing solution significantly reduced the fAHP of a single orthodromic-elicited AP of both young and aged neurons. B, Paxilline also increased the half-width of both young and aged CA3 neurons by similar magnitude after a single orthodromically evoked AP. Five synaptically evoked APs before and after the addition of paxilline induced a reduction in fAHP (C) and an increase in half-width (D) for both young and aged CA3 neurons. The magnitude of fAHP (E) and half-width (F) change was calculated for each AP spike. Repeated-measures ANOVA did not reveal an age effect for ΔfAHP or Δhalf-width measures (ΔfAHP: p = 0.37, Δhalf-width: p = 0.74, n = 8 young and n = 7 aged). Values are mean ± SEM. A, C, The number of cells (n) is represented in the middle of each bar for each group.
Paxilline also had an equivalent impact on the half-width of a single orthodromically evoked spike in young and aged CA3 neurons, thus preserving the narrowed AP in aged CA3 neurons after BK channel block (ΔHW: young: 0.47 ± 0.05 ms, n = 8; aged: 0.36 ± 0.05 ms, n = 7; repeated-measures ANOVA for age: F(1,13) = 5.27, p < 0.05, age/paxilline interaction: F(1,13) = 2.35, p = 0.15; Figure 6D). Moreover, paxilline induced a large increase in AP half-width with each subsequent AP in a 50 Hz train of synaptic stimulation in both young and aged CA3 neurons, but the change in magnitude (ΔHW) was similar in both age groups (age effect: F(1,48) = 0.11, p = 0.74; age/spike interaction: F(1,48) = 0.39, p = 0.82; Figure 6E,F).
Enhanced membrane expression of A-type K+ channels from aged CA3 hippocampus
Our biophysical results strongly suggest that the increased excitability in aged CA3 neurons is due to faster AP repolarization mediated by Kv4.2/Kv4.3, but not BK, channels. To determine whether there are more Kv4.2/Kv4.3, but not BK, channels in the neuronal membranes of aged CA3 tissue, we performed subcellular fractionation and Western blot analyses on microdissected CA3 tissue, probing the membrane fraction for Kv4.2, Kv4.3, and BK channel proteins.
Consistent with the patch-clamp experiments, the membrane fraction of aged CA3 tissue was enriched in both Kv4.2 and Kv4.3 channel protein (normalized to total membrane protein as a loading control) compared with tissue from young rats (Kv4.2: t test: p < 0.02; n = 9 animals per age group; Kv4.3: t test: p = 0.08; n = 9 animals per age group; Fig. 7). Also consistent with our biophysical results, BK channel expression level was not altered by aging (t test: p = 0.5; n = 9 animals per age group; Fig. 7).
Figure 7.
A-type Kv4 channel expression is enhanced in the membrane fraction of aged CA3. A, Western blot analysis of CA3 hippocampal tissue from young (blue) and aged (red) rats using BK, Kv4.2, and Kv4.3 antibodies. Loading control total protein stained with Sypro (large blot on the right). B, The optical density values for BK, Kv4.2, and Kv4.3 (relative to total membrane protein as a loading control) were normalized to the young rat CA3 tissue optical densities for each Western blot. Whereas BK channel expression was similar between young adult and aged rats, expression of both Kv4.2 and Kv4.3 was increased. Numbers report p values for the independent-samples t tests. *p = 0.02 (Fisher's PLSD t test).
Increased perisomatic ratio of A-type K+ channels observed in aged CA3 hippocampus
The combined biophysical and Western blot results strongly indicate that alterations in Kv4.2/Kv4.3 channels are a source of the faster AP repolarization in aged CA3 pyramidal neurons. Although the Western blot analyses revealed increased expression of these channels in the aged CA3 region, they do not provide definitive evidence regarding the neuronal compartment in which these channels are increased. To determine the regional distribution of the upregulated Kv4.2/Kv4.3 channel expression, we immunolocalized these two channel subunits and estimated their expression using light microscopy and immunofluorescence array tomography on slices used in patch-clamp experiments (Fig. 8).
Figure 8.

Perisomatic redistribution of A-type K+ channels in aged hippocampal CA3 region. A, Monochrome images of free-floating brain sections immunostained for Kv4.2 channels from a young adult (top) and an aged (bottom) rat. Pink arrows indicate approximate borders of hippocampal region CA3. Unfilled arrows indicate approximate borders of CA3b, which was targeted in the patch-clamp experiments. Inset, Triple-immunofluorescence image of aged pyramidal neuron from which patch-clamp data were obtained (green), with Kv4.2 immunofluorescence (red) and DAPI (blue). Scale bar, 250 μm. B, Monochrome images at lower magnification (left) and higher magnification (right) of Kv4.2 immunoreactivity in hippocampal slices used in patch-clamp experiments from a young adult (top) and an aged (bottom) rat. Purple arrows in this and all other panels indicate the approximate borders between the perisomatic region (SP and SL) and stratum oriens (so) and SR (sr). Scale bars: left, 150 μm; right, 50 μm. C, D, Monochrome array tomography immunofluorescence images at low (C) and higher (D) magnification from brain slices used in patch-clamp experiments obtained from young (C, D, left panel) and aged (C, D, right panel) rats. Scale bars: C, 75 μm; D, 25 μm. E–H, Same as A–D, but for Kv4.3 immunoreactivity. I, Ratio of perisomatic-to-dendritic immunosignal in CA3 from brain slice immunofluorescence experiments (circles) and array tomography immunofluorescence experiments (triangles) in young adult (blue) and aged (red) rats normalized to young adult rat values. *p < 0.05, significant difference (unpaired t test). J, Same as I, but for Kv4.3 immunoreactivity. I, *p < 0.05, significant difference (unpaired t test). I–J, Lines connect data from the paired cohorts. Group means are presented as ± SEM.
The hippocampal CA3 region of young adult rats has a nonuniform expression pattern of both Kv4.2 and Kv4.3 channels in that perisomatic regions express noticeably fewer channels than more distal regions in the basal and apical dendrites (Rhodes et al., 2004) (Fig. 8). Although this pattern was largely maintained in aged hippocampal slices (Fig. 8A–H), the level of immunoexpression for both Kv4.2 (Fig. 8A–D) and Kv4.3 (Fig. 8E–H) was elevated in the perisomatic region of CA3 from aged rats. Indeed, the ratio of perisomatic immunoexpression over dendritic expression for both Kv4.2 and Kv4.3 was significantly elevated in aged CA3 hippocampal region. Notably, unpaired t tests on either the perisomatic ratios themselves or the ratios normalized to the young adult rat values yielded the same pattern of results. Specifically, unpaired t tests on the perisomatic ratios yield t(22) = 2.497, p = 0.02 for Kv4.2; and t(22) = 2.194, p = 0.039 for Kv4.3 (Kv4.2 young adult mean, 0.90 ± 0.014; range, 0.82–0.99; aged mean, 1.02 ± 0.04; range, 0.82–1.31; Kv4.3 young adult mean, 0.62 ± 0.04; range, 0.50–0.98; aged mean, 0.77 ± 0.05; range, 0.54–1.07). Similarly, unpaired t tests on the ratios normalized to the young adult group averages yield t(22) = 2.45, p = 0.02 for Kv4.2; and t(22) = 3.428, p = 0.002 for Kv4.3 (perisomatic ratio: p < 0.05; Figure 8I,J; Kv4.2 young adult mean, 1.0 ± 0.02; range, 0.92–1.08; aged mean, 1.12 ± 0.04; range, 0.90–1.46; Kv4.3 young adult mean, 1.0 ± 0.05; range, 0.78–1.20; aged mean, 1.20 ± 0.04; range, 0.94–1.42). Thus, these data indicate that Kv4.2 and Kv4.3 channels are redistributed to the perisomatic compartment in aged CA3 pyramidal neurons, placing them in close proximity to the final integration zone in the soma/axon.
Discussion
We provide a cellular mechanism (i.e., increased fAHP and narrower APs due to increased perisomatic expression of A-type K+ channels) that can explain the reported increase in CA3 activity with aging observed in vivo (Wilson et al., 2005, 2006; Haberman et al., 2008, 2011; Yassa et al., 2011; Bakker et al., 2012). Because age-related differences in CA3 pyramidal neurons were found exclusively in the AP half-width and fAHP, we focused on conductances involved in the repolarization phase of the action potential. fAHP and AP half-width rely on fast voltage-gated K+ conductances (Storm, 1987; Shao et al., 1999; Hu et al., 2001; Kim et al., 2005); thus, we examined the magnitude of change after incubation with A-type channel blockers (4-AP and PaTx) to determine whether functional expression of these channels was altered with age in CA3 neurons. PaTx, a specific Kv4.2/Kv4.3 channel blocker (Diochot et al., 1999; Chagot et al., 2004), reversed the aging effect in both the fAHP and half-width in CA3 neurons, converting these values to a young-like state, while having no effect in young CA3 neurons (Fig. 5). This suggests that the contribution of Kv4.2/Kv4.3 channels to AP repolarization is very small in young CA3 neurons, but that, with aging Kv4, function and membrane expression (specifically Kv4.2 and Kv4.3) are enhanced substantially (Figs. 5, 7). Moreover, the magnitude of change in fAHP and AP half-width after blocking BK channels with paxilline was similar between young and aged CA3 neurons (Fig. 6), indicating that the BK conductance is not the source of the functional alteration in aging. Subcellular fractionation Western blot analyses and immunolocalization experiments using immunofluorescence light microscopy and array tomography support our electrophysiological findings by showing that A-type K+ channel expression (specifically Kv4.2 and Kv4.3) is indeed increased in the CA3 region of aged rats (Fig. 7), particularly within the perisomatic compartment (Fig. 8).
Our study does not exclude that other conductances (e.g., IM, Kv7-mediated) or modulation by accessory proteins involved in regulation of cellular electrophysiological properties may also be altered with aging in CA3 pyramidal neurons. Further experimentation would be necessary to examine their contributions to age-related increases in CA3 neuron excitability. Hyperpolarization-activated cyclic nucleotide-gated (HCN) conductances were ruled out as a possible source for age-related alterations of fAHP and AP half-width as there was no age-related difference in the depolarizing sag (Table 1) nor age-related changes in dendritic expression of hyperpolarization-activated cyclic nucleotide-gated 1 using array tomography and immunogold electron microscopy (data not shown).
Importantly, our findings with regard to CA3 pyramidal neuron excitability differ substantially from those described in studies of CA1 pyramidal neurons during normal aging: excitability is increased in CA3 but decreased in CA1. In CA3 neurons, aging is associated with hyperexcitability that is attributable to increased fAHP and reduced AP half-width. In contrast, aged CA1 neurons are hypoexcitable, with enlarged sAHP (Landfield and Pitler, 1984; Power et al., 2002). Moreover, neither fAHPs nor AP half-widths in CA1 pyramidal neurons changed with chronological aging (Matthews et al., 2009), and the sAHP did not show age-related changes in CA3 pyramidal neurons (Fig. 1). The dichotomy of aging-related effects on the sAHP, fAHP, and AP half-width of CA1 and CA3 hippocampal pyramidal neurons underscores the complex nature of age-related degradation within the hippocampal circuit. Such regional specificity indicates that pharmacotherapies designed to help reverse cognitive aging may benefit from molecular targeting to particular brain regions.
What are possible translational consequences of the differing age-associated changes in CA3 and CA1 excitability? The subcellular expression pattern of Kv4 channels in young CA3 and CA1 regions has been shown to be primarily at the distal dendrites (Johnston et al., 2000; Aronica et al., 2009; Menegola et al., 2012), and we propose that the age-related increase in perisomatic CA3 A-type channel expression we found could enhance repolarization and excitability. Thus, it is conceivable that mildly blocking these distal dendritic A-type conductances with a specific Kv4.2/.3 blocker in vivo would increase CA1 excitability (Chen et al., 2006; Andrásfalvy et al., 2008) while simultaneously reversing the aging-related effect of enhanced somatic A-type conductance to reduce CA3 excitability.
In CA3 neurons, the aging-related increase in fAHP and decrease in AP half-width have the potential to increase the number of APs fired during each burst by speeding up interspike repolarization (Fig. 2). Such a view is consistent with in vivo studies reporting an increase in CA3 neuron firing rate with age (Wilson et al., 2005; Moradi-Chameh et al., 2014) and could provide a cellular basis for strengthened synaptic transmission through the auto-associative, recurrent collateral fibers in the proximal dendrites (Gilbert and Brushfield, 2009). Several studies have suggested that CA3 recurrent collaterals contribute to pattern completion mechanisms. Specifically, hyperactivity of CA3 neurons has been postulated to drive overgeneralization in both aged human and animal models, wherein some aged subjects exhibit difficulty distinguishing between similar memory experiences with overlapping features (Wilson et al., 2005, 2006; Haberman et al., 2008, 2011; Yassa et al., 2011; Bakker et al., 2012). The results of the experiments described here provide a cellular mechanism (increased fAHP and reduced AP half-width via A-type K+ channels) to help explain the aging-related CA3 hyperactivity reported in vivo. Indeed, our data suggest that pharmacological inactivation of A-type K+ conductances may alleviate pattern separation impairments in aging, as PaTx returned biophysical properties of CA3 pyramidal neurons from aged rats to values closely resembling those found in CA3 pyramidal neurons from young adult rats.
Increased intrinsic excitability of CA3 neurons may also affect other hippocampal subregions, in particular the CA1 subregion. CA1 neurons receive synaptic inputs from CA3 neurons via Schaffer collaterals, and CA3 burst activity sets into motion a short-lived, dynamic interaction between CA1 pyramidal neurons and interneurons, which enable sharp-wave ripples and synchronized discharge of CA1 ensembles to occur (Buzsáki et al., 1992). This intrahippocampal circuit interaction and the resultant oscillatory activity have been suggested to facilitate synchronized communication within the hippocampus and across distributed neural networks between the hippocampus and other brain regions, such as the neocortex, to enable memory processes (Fell et al., 2002). Thus, the opposing effects of aging on CA1 and CA3 neurons through differential changes in biophysical properties (e.g., decreased CA1 excitability due to increased sAHP vs increased CA3 excitability due to increased fAHP and reduced AP half-width) may lead to the disruption of optimal CA3-CA1 interactions and a subsequent attenuation of oscillatory activity, ultimately contributing to or producing cognitive deficits associated with aging. At the cellular level, enhanced fAHP and reduced half-width in aged CA3 neurons enhance interspike AP repolarization, allowing more spikes to occur within a single burst epoch. On the other hand, enhanced sAHP in aged CA1 neurons may lead to increased interburst intervals, slowing down the frequency at which a burst occurs. It is unlikely that alteration in the fast repolarization impacts the postburst afterhyperpolarization. Several studies have demonstrated that antagonists of BK (Lancaster and Nicoll, 1987; Lin et al., 2014) and A-type (Kim et al., 2005) channels reduce the fast AHP and increase spike width without altering the postburst AHP, and we did not observe any correlation between the fast repolarization and medium or slow AHP in the present report. Thus, the disparate firing patterns between the two structures may interfere with dynamic CA3-CA1 interactions that normally give rise to hippocampal oscillatory patterns necessary for learning. An interesting possibility is that aging-associated increases in CA3 excitability may lead to an adaptive, neuroprotective response that promotes reduced excitability in CA1 pyramidal neurons. This adaptation, however, may actually be maladaptive in that the CA3 hyperactivity inadvertently creates deficits in memory functions, such as pattern separation, and impairs the ability of the CA1 output region of the hippocampus to perform its normal functional contribution to learning and cognition.
Although the opposing changes in intrinsic excitability with normal aging in the CA1 and CA3 hippocampal subregions are surprising, they are not totally unexpected. Several studies have demonstrated that hippocampal subregions contribute differentially to information encoding and retrieval of memory (Leutgeb et al., 2004; Burke and Barnes, 2006; Small et al., 2011). Studies have also shown that enhanced CA3 hippocampal excitability may serve as a biomarker for aging-related cognitive impairments and a predictor of more severe, incipient impairments, such as AD and aMCI (Dickerson et al., 2005; Bakker et al., 2012). Importantly, our study is the first to describe a molecular mechanism for the widely reported aging-associated CA3 pyramidal neuron hyperactivity. When viewed in light of previous findings in CA1 pyramidal neurons of aged animals, our findings highlight the complexity of the age-related modifications throughout the hippocampal circuit, which interfere with optimal signal integration and obstruct information throughput, ultimately impeding circuit computation and producing age-related cognitive deficits.
Footnotes
This work was supported by National Institutes of Health Grants AG08796 and AG017139 to J.F.D., and the Charles and M.R. Shapiro Foundation, the Schild Fund, and National Institutes of Health Grant AG047073 to D.A.N. We thank Zoltan Nusser for advice regarding immunolocalization of the Kv4 channels, and Felix Núñez-Santana for assistance developing a brain slicing protocol for patching aged CA3 neurons.
The authors declare no competing financial interests.
References
- Amaral DG, Witter MP. The 3-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andrásfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J Physiol. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Boer K, Doorn KJ, Zurolo E, Spliet WG, van Rijen PC, Baayen JC, Gorter JA, Jeromin A. Expression and localization of voltage dependent potassium channel Kv4.2 in epilepsy associated focal lesions. Neurobiol Dis. 2009;36:81–95. doi: 10.1016/j.nbd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Chagot B, Escoubas P, Villegas E, Bernard C, Ferrat G, Corzo G, Lazdunski M, Darbon H. Solution structure of Phrixotoxin 1, a specific peptide inhibitor of Kv4 potassium channels from the venom of the theraphosid spider Phrixotrichus auratus. Protein Sci. 2004;13:1197–1208. doi: 10.1110/ps.03584304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6:327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Durations of after-hyperpolarization of motoneurones supplying fast and slow muscles. Nature. 1957;179:866–868. doi: 10.1038/179866a0. [DOI] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elger CE, Fernández G. The interaction of rhinal cortex and hippocampus in human declarative memory formation. Rev Neurosci. 2002;13:299–312. doi: 10.1515/REVNEURO.2002.13.4.299. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-d-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. doi: 10.1101/lm.5.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin I, Becker M, Ociepka K, Friauf E, Nothwang HG. A subcellular prefractionation protocol for minute amounts of mammalian cell cultures and tissue. Proteomics. 2005;5:35–45. doi: 10.1002/pmic.200400892. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Lee HJ, Colantuoni C, Koh MT, Gallagher M. Rapid encoding of new information alters the profile of plasticity-related mRNA transcripts in the hippocampal CA3 region. Proc Natl Acad Sci U S A. 2008;105:10601–10606. doi: 10.1073/pnas.0804292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, Gallagher M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging. 2011;32:1678–1692. doi: 10.1016/j.neurobiolaging.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27:1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol. 2000;525:75–81. doi: 10.1111/j.1469-7793.2000.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA. Electrophysiology of hippocampal neurons: II. After-potentials and repetitive firing. J Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lin M, Hatcher JT, Wurster RD, Chen QH, Cheng ZJ. Characteristics of single large-conductance Ca2+-activated K+ channels and their regulation of action potentials and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. Am J Physiol. 2014;306:C152–C166. doi: 10.1152/ajpcell.00423.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009;29:4750–4755. doi: 10.1523/JNEUROSCI.0384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola M, Clark E, Trimmer JS. The importance of immunohistochemical analyses in evaluating the phenotype of Kv channel knockout mice. Epilepsia. 2012;53(Suppl 1):142–149. doi: 10.1111/j.1528-1167.2012.03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci. 2002;22:10106–10115. doi: 10.1523/JNEUROSCI.22-23-10106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi-Chameh H, Peng J, Wu C, Zhang L. Intracellular activities related to in vitro hippocampal sharp waves are altered in CA3 pyramidal neurons of aged mice. Neuroscience. 2014;277:474–485. doi: 10.1016/j.neuroscience.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KM, Molina-Campos E, Musial TF, Price AL, Oh KJ, Wolke ML, Buss EW, Scheff SW, Mufson EJ, Nicholson DA. Evidence for Alzheimer's disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0848-z. doi: 10.1007/s00429-014-0848-z. Advance online publication. Retrieved Jul 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Santana FL, Oh MM, Antion MD, Lee A, Hell JW, Disterhoft JF. Surface l-type Ca2+ channel expression levels are increased in aged hippocampus. Aging Cell. 2014;13:111–120. doi: 10.1111/acel.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci U S A. 2009;106:1620–1625. doi: 10.1073/pnas.0807708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22:7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol. 1992;68:1834–1841. doi: 10.1152/jn.1992.68.5.1834. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyment AD, Coderre E, Wilson JM, Renaud LP, O'Hare E, Spanswick D. Electrophysiological, pharmacological and molecular profile of the transient outward rectifying conductance in rat sympathetic preganglionic neurons in vitro. Neuroscience. 2011;178:68–81. doi: 10.1016/j.neuroscience.2010.12.061. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/S0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol. 2004;92:2346–2356. doi: 10.1152/jn.00977.2003. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Li DP, Chen SR, Pan HL. Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit. J Biol Chem. 2009;284:36453–36461. doi: 10.1074/jbc.M109.075523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JA, Tseng GN. Modulation of 4-AP block of a mammalian A-type K channel clone by channel gating and membrane voltage. Biophys J. 1994;67:130–142. doi: 10.1016/S0006-3495(94)80462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995;488:661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]