Abstract
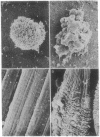
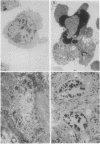
During development, large numbers of cells die by a nonpathological process referred to as programmed cell death. In many tissues, dying cells display similar changes in morphology and chromosomal DNA organization, which has been termed apoptosis. Apoptosis is such a widely documented phenomenon that many authors have assumed all programmed cell deaths occur by this process. Two well-characterized model systems for programmed cell death are (i) the death of T cells during negative selection in the mouse thymus and (ii) the loss of intersegmental muscles of the moth Manduca sexta at the end of metamorphosis. In this report we compare the patterns of cell death displayed by T cells and the intersegmental muscles and find that they differ in terms of cell-surface morphology, nuclear ultrastructure, DNA fragmentation, and polyubiquitin gene expression. Unlike the T cells, which are known to die via apoptosis, we find that the intersegmental muscles display few of the features that characterize apoptosis. These data suggest that more than one cell death mechanism is used during development.
Full text
PDF


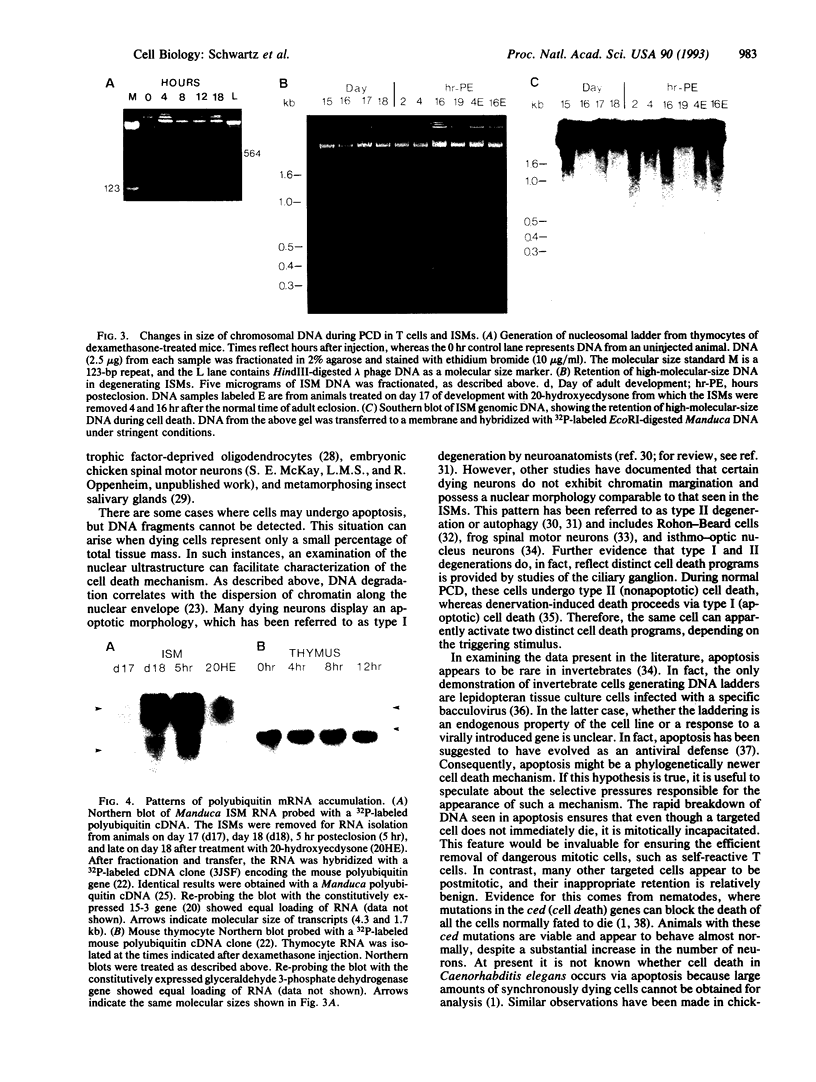

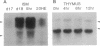
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I. W., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. I. A light and electron microscopic study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978 Jan 1;177(1):33–57. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Identical populations of phagocytes and dying neurons revealed by intravascularly injected horseradish peroxidase, and by endogenous glutaraldehyde-resistant acid phosphatase, in the brains of chick embryos. Histochem J. 1984 Sep;16(9):955–969. doi: 10.1007/BF01003851. [DOI] [PubMed] [Google Scholar]
- Clem R. J., Fechheimer M., Miller L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991 Nov 29;254(5036):1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Decker R. S. Lysosomal packaging in differentiating and degenerating anuran lateral motor column neurons. J Cell Biol. 1974 Jun;61(3):599–612. doi: 10.1083/jcb.61.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Finch J. S., Bonham K., Krieg P., Bowden G. T. Murine polyubiquitin mRNA sequence. Nucleic Acids Res. 1990 Apr 11;18(7):1907–1907. doi: 10.1093/nar/18.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Haskins K., Kubo R., White J., Pigeon M., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983 Apr 1;157(4):1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Kingston R., Smith C. A., Williams G. T., Owen J. J. Antigen-induced apoptosis in developing T cells: a mechanism for negative selection of the T cell receptor repertoire. Eur J Immunol. 1989 Nov;19(11):2175–2177. doi: 10.1002/eji.1830191132. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKSHIN R. A., WILLIAMS C. M. PROGRAMMED CELL DEATH--I. CYTOLOGY OF DEGENERATION IN THE INTERSEGMENTAL MUSCLES OF THE PERNYI SILKMOTH. J Insect Physiol. 1965 Feb;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- Lamborghini J. E. Disappearance of Rohon-Beard neurons from the spinal cord of larval Xenopus laevis. J Comp Neurol. 1987 Oct 1;264(1):47–55. doi: 10.1002/cne.902640105. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Ito A., Horigome K., Lampe P. A., Johnson E. M., Jr Biochemical characterization of programmed cell death in NGF-deprived sympathetic neurons. J Neurobiol. 1992 Nov;23(9):1205–1220. doi: 10.1002/neu.480230911. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz E., Howell D. M. CTL: virus control cells first and cytolytic cells second? DNA fragmentation, apoptosis and the prelytic halt hypothesis. Immunol Today. 1989 Mar;10(3):79–86. doi: 10.1016/0167-5699(89)90231-4. [DOI] [PubMed] [Google Scholar]
- Masters J. N., Finch C. E., Sapolsky R. M. Glucocorticoid endangerment of hippocampal neurons does not involve deoxyribonucleic acid cleavage. Endocrinology. 1989 Jun;124(6):3083–3088. doi: 10.1210/endo-124-6-3083. [DOI] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Kosz L., Kay B. K. Gene activation is required for developmentally programmed cell death. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6594–6598. doi: 10.1073/pnas.87.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Myer A., Kosz L., Engelstein M., Maier C. Activation of polyubiquitin gene expression during developmentally programmed cell death. Neuron. 1990 Oct;5(4):411–419. doi: 10.1016/0896-6273(90)90080-y. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M. The role of cell death genes during development. Bioessays. 1991 Aug;13(8):389–395. doi: 10.1002/bies.950130805. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Hormonal control of rates of metamorphic development in the tobacco hornworm Manduca sexta. Dev Biol. 1983 Sep;99(1):103–114. doi: 10.1016/0012-1606(83)90257-9. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]