Abstract
We compared the effect of vortioxetine, paroxetine and placebo after three days of dosing on sleep architecture. This was a randomised, double-blind, four-way crossover, placebo-controlled, multiple-dose study in 24 healthy young men. Subjects received 20mg vortioxetine, 40mg vortioxetine, 20mg paroxetine or placebo for three consecutive days in four different periods with at least three weeks between them. Polysomnography and blood sampling for pharmacokinetic analysis were performed on the pre-dose night and nights 1 and 3 of dosing in each period. Plasma concentrations of vortioxetine and paroxetine during the polysomnography measurement were used to estimate SERT occupancies using published relationships in healthy subjects.
All three active treatments significantly increased REM onset latency and decreased time spent in REM sleep. In the pharmacokinetic/pharmacodynamics analysis significant relationships were found between REM onset latency and time spent in REM sleep and vortioxetine/paroxetine exposure. The relation between REM suppression parameters and SERT occupancy was significantly different between vortioxetine and paroxetine, despite the same SERT occupancy. This indicates that vortioxetine has a different clinical pharmacological profile from paroxetine, which may explain the differences in adverse effect profile of the two drugs, for instance the lower incidence of nausea, weight gain and sexual dysfunction with vortioxetine.
Keywords: Sleep, serotonin, vortioxetine
Introduction
Vortioxetine is a multimodal antidepressant working through several serotonergic targets. It is a 5-HT3, 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist and inhibitor of the 5-HT transporter (SERT) in vitro, displaying highest affinities for SERT and 5-HT3 (Bang-Andersen et al., 2011) . In vivo, this profile leads to increases in acetylcholine, dopamine and noradrenaline levels in both the frontal cortex and ventral hippocampus (Mørk et al., 2012). Vortioxetine is active in a number of preclinical behavioural models predictive of antidepressant and anxiolytic activity and is an efficacious antidepressant in clinical studies (Berhan and Barker, 2014; Fu and Chen, 2015; Guilloux et al., 2013). Since vortioxetine has a novel pharmacological profile in animal models it would be of benefit to be able to demonstrate this in humans. This study set out to evaluate, using sleep architecture in healthy humans, the brain effects of vortioxetine, compared with a potent serotonin reuptake inhibitor (SRI), paroxetine, for which a considerable amount of sleep data were available. At the same time the plasma concentration of the two drugs was measured and related to the SERT occupancy estimated from previous PET studies (Areberg et al., 2012a; Meyer et al., 2004) so that the observed effects on sleep could be related to plasma levels and SERT occupancy.
The structure of human sleep is well described and is a sensitive measure of drug effects in the brain, particularly those of antidepressants, where it is possible to identify differences between compounds with different pharmacologic profiles (Paterson et al., 2011). In particular SRIs exhibit acute and easily detectable changes in REM sleep; these effects are dose-related in both patients and healthy subjects and consist of a reduction in the overall amount of REM sleep during the night, and a delay in the first entry into REM sleep (increased REM onset latency (ROL)) (Wilson and Argyropoulos, 2005). REM sleep suppression after SRI administration is likely to result from increased levels of synaptic 5-HT and may be mediated through the 5-HT1A receptor. This observation is based on both preclinical and human studies; Monaca et al. (2003) showed that the REM sleep suppressing effect of citalopram was absent in 5-HT1A knockout mice, while in humans selective 5-HT1A receptor agonist drugs are strongly REM sleep suppressing (Driver et al., 1995; Wilson et al., 2005).
The effects of the other receptor interactions of vortioxetine are less well understood. 5-HT3 antagonists have been shown to shorten REM latency whereas 5-HT3 agonists may lengthen it, both in rodents and in humans (Adrien et al., 1992; Monti and Jantos, 2008; Rothe et al., 1994; Staner et al., 2001). A 5-HT7 antagonist suppressed REM sleep and increased ROL in both rodents and humans (Bonaventure et al., 2012).
Changes in sleep initiation and sleep continuity after short-term administration of SSRIs are also similar in healthy subjects and depressed patients, consisting of increased light (stage 1) sleep, an increased number of arousals from sleep, and an increased time spent awake at night. Collectively these changes in sleep continuity are described as sleep fragmentation. Fragmentation of sleep is probably not explained by stimulation of the 5-HT1A receptors responsible for REM sleep effects, as potent 5-HT1A receptor agonists do not have such marked sleep-fragmenting effects.
It was expected that the pharmacologic profile of vortioxetine as an SRI and an antagonist at various 5-HT receptors would mean that its effect on sleep would differ from those of a pure reuptake inhibitor. We measured sleep after three days’ dosing with 20 or 40mg/day of vortioxetine, to approximate the steady state conditions reached clinically with 10 and 20mg, respectively, and compared this with three days’ dosing of paroxetine, for which we had data from a previous study (Wilson et al., 2004). There were no sleep data for vortioxetine in humans available at the time of the study, so we estimated that 24 participants would be appropriate based on REM and sleep fragmentation results from this study of two SRIs.
Method
This was a randomised, double-blind, four-way crossover, placebo controlled, active comparator polysomnographic study in healthy young men. The study was conducted at Hammersmith Medicines Research, London, United Kingdom, and regulatory approval was obtained from the National Research Ethics Service (Edinburgh Independent Ethics Committee for Medical Research) and the EudraCT National Competent Body (MHRA). It was conducted according to good clinical practice guidelines (The Medicines for Human Use (Clinical Trials) Regulations, 2004).
Study design
Twenty-four healthy subjects were recruited. The eligibility of the subjects was initially assessed at a screening visit (day -21 to day -3). They were randomly allocated to one of 24 treatment sequences (1 subject per treatment sequence). Each treatment sequence consisted of administration of vortioxetine 20mg, vortioxetine 40mg, paroxetine 20mg or placebo for three consecutive days, and each treatment was separated by a washout period of three weeks. Actigraphy was recorded during a minimum of five consecutive days leading up to day -2 and for the entire duration of the washout periods for the purpose of excluding subjects with irregular sleep-wake rhythms. Eligible subjects were admitted to the research centre on day -2, and were dosed at 09:00 on days 1, 2 and 3 of each treatment period (periods 1–4). Sleep recordings (polysomnography, PSG) were performed in each treatment period on day -2 (familiarisation with procedure), day -1(baseline) and day 3. At predetermined time points, blood samples were drawn for drug concentration analysis of vortioxetine and paroxetine. Safety and tolerability were assessed throughout the study.
Participants
Participants were healthy, drug-free young men between the ages of 20 and 34 years with a BMI between 20 and 29 kg/m2. They were required to have a regular sleep pattern with habitual bedtime between 22:00 and 00:00 and a rising time between 06:00 and 08:00. This was checked when actigraphs were downloaded upon admission at each visit, and the visit was postponed if they had been non-compliant in the previous five days. Alcohol breath test and drugs of abuse urine tests were conducted at each visit. A blood sample for genotyping for polymorphisms in cytochrome P450 (CYP 2D6, 2C9, 2C19) was obtained at screening. CYP 2D6 is the main isozyme involved in the metabolism of paroxetine and vortioxetine, although the other two are also important for the latter.
Polysomnography (PSG)
For each night with PSG assessments, (days -2, -1, 3) subjects were set up for measurements of PSG sleep parameters according to standard methods, using an Embla PSG system with N7000 amplifiers. Silver-silver chloride electrodes were placed according to the International 10–20 system and the recommendations of Rechtschaffen and Kales (1968) to ensure standardisation, comparability, and replicability of EEG signals, and included electrooculogram and electromyograms from chin and leg (anterior tibialis) muscles. In addition, on day -2 in period 1, respiration was monitored using respiratory bands and a thermistor, arterial oxygen saturation was measured using a pulse oximeter, and the subject was recorded on video overnight. This was to exclude the possibility of a sleep disorder and video recordings were deleted after subject eligibility had been verified.
Subjects went to bed and got up at their usual time as noted at screening and were required to maintain this routine throughout the study. Recordings commenced after calibration and continued throughout the night for eight hours or until the subject woke up (whichever was first). The data were analysed and scored according to Rechtschaffen and Kales’ (1968) criteria by an experienced sleep scorer blind to treatment condition. Measures derived from the PSG data included: staging time (time between eyes closed at bedtime and eyes open at rising time), sleep onset latency (from beginning of staging time to beginning of the first continuous 2-minute period not classified as awake or stage 1 sleep), total sleep time (TST), sleep efficiency, wake after sleep onset (WASO), number of awakenings (of at least 15 seconds in length), number of shifts to stage 1 or waking, REM onset latency (ROL; time from sleep onset to beginning of first REM period), time spent in each stage of sleep.
Blood sampling for analysis of drug concentration
Blood samples for vortioxetine and paroxetine analysis were drawn at five time points on day 1 (pre-dose, 2, 4, 8 and 12 hours post-dose), at one time point on day 2 (time-matched to day 1 pre-dose), at two time points on day 3 (time-matched to day 1 pre-dose and 12 hours) and at one time point on day 4 (time-matched to day 1 pre-dose).
Statistical methods (PSG)
For each of the PSG variables, a mixed model analysis of the raw, post-dose values was performed. The model included treatment and period as fixed factors. The covariance matrix across treatments within subject was unstructured, and the Kenward-Roger method for adjustment of the degrees of freedom was used. Based on this mixed model analysis, the treatment-specific least squares (LS) means and the pairwise treatment contrasts were estimated with 95% confidence limits and, for the pairwise comparisons, the associated p-value for no difference.
A corresponding set of sensitivity analyses was performed for the change from baseline (day -1) for the post-dose values. The model included treatment and period as fixed factors and the baseline was included as a covariate. The covariance matrix within subject was unstructured across treatments, and the Kenward-Roger method for adjustment of the degrees of freedom was used. Based on this mixed model analysis, the baseline-adjusted treatment-specific LS mean change from baseline (day -1) and the pairwise treatment contrasts were estimated with 95% confidence limits and, for the pairwise comparisons, the associated p-value for no difference (no adjustment for multiple tests). Since 5 of the 24 subjects dropped out, an additional set of sensitivity analyses of the raw values was performed to preclude bias due to carry-over effects. The same approach as in the original analyses was used, except that the systematic effects consisted of the three factors: the (current) treatment, the period and the treatment (40mg vortioxetine, 20mg vortioxetine, 20mg paroxetine, placebo or none) in the preceding period.
Pharmacokinetic/pharmacodynamic modelling
The individual exposure of vortioxetine during the EEG measurements (i.e. the night between day 3 and day 4), denoted Cav,sleep, was estimated by non-linear mixed effect analysis using a previously developed population pharmacokinetic model for meta-analysis based upon the data obtained in healthy subjects (Areberg et al., 2012b). Due to the very sparse sampling schedule, all parameters were fixed to the previous model results. The individual exposure to paroxetine during the EEG measurements was estimated as the average of the day 3 and pre-dose day 4 values. The following PSG variables were used as pharmacodynamic (PD) parameters in the pharmacokinetic/pharmacodynamics (PK/PD) analysis: wake after sleep onset (WASO), sleep stage 1 (S1), total sleep time (TST), REM onset latency (ROL) and total time spent in REM sleep (TREM). The influence of a potential periodic effect was assessed by applying a mixed model for each PSG parameter, with treatment, sequence and period (1–4) as fixed effects and subject as a random effect. If period was significant it was included as a covariate in the further PK/PD modelling. The relationship, if any, between Cav,sleep and the main PSG parameters was investigated by non-linear mixed effect analysis using an Emax or a simple linear model. The Emax model for baseline corrected PSG parameters (Δpar) for subject i used for the PK/PD analysis was
where C is the plasma concentration of vortioxetine/paroxetine, E0 is the effect at C = 0 (i.e. placebo treatment), Emax the maximum effect, EC50 the plasma concentration of vortioxetine/paroxetine required for half the maximum effect, γ the Hill factor and εi the residual error. The relationship was regarded significant if the 95% confidence interval for Emax did not include zero. The linear model was of the form
In addition, uncorrected PSG values (i.e. day 3 only) were analysed using the same models and the same parameters. No covariate analysis was performed. Nonlinear and linear mixed effect modelling was performed with the NONMEM® software (ICON Development Solutions), version 7. The first-order conditional error with interaction (FOCE INTER) minimisation method was used.
SERT occupancy data
The expected SERT occupancies at the time of the EEG measurements were estimated by translating the Cav,sleep values to SERT occupancy using the published PK/PD (occupancy) relationships for vortioxetine (Areberg et al., 2012a) and paroxetine (Meyer et al., 2004).
Results
Twenty-four subjects were randomised to treatment and 19 subjects completed the study. Five subjects withdrew from the study: two were withdrawn because actigraphy measurements showed that they were repeatedly non-compliant with regard to sleep-wake routine; one was withdrawn at the decision of the physician (non-compliance with actigraph procedure on two occasions); one withdrew consent; one was withdrawn because of taking a prohibited concomitant medication (chlorpheniramine).
The Cav,sleep for vortioxetine was 13.8 ng/mL for 20mg and 29.5 ng/mL for 40mg. The Cav,sleep for paroxetine 20mg was 11.7 ng/mL.
Sleep architecture
There was a significant reduction in REM sleep and increase in ROL after all drugs compared with placebo; REM sleep suppression after vortioxetine was dose-related, and there was no significant difference between vortioxetine 40mg and paroxetine on ROL or TREM (see Table 1). Stage 1 sleep was also significantly increased after all drugs, and WASO was significantly increased after vortioxetine 40mg (Table 1).
Table 1.
PSG sleep parameters (mean ± SD).
| Night | Placebo |
Vortioxetine 20mg |
Vortioxetine 40mg |
Paroxetine 20mg |
||||
|---|---|---|---|---|---|---|---|---|
| −1 | 3 | −1 | 3 | −1 | 3 | −1 | 3 | |
| Total sleep time | 435 | 413 | 442 | 403 | 430 | 393* | 442 | 399* |
| ±20 | ±29 | ±15 | ±44 | ±28 | ±51 | ±17 | ±44 | |
| Sleep onset latency | 21 | 38 | 20 | 49 | 29 | 42 | 19 | 44 |
| ±14 | ±25 | ±15 | ±36 | ±20 | ±29 | ±16 | ±30 | |
| REM onset latency | 61 | 63 | 64 | 127***# | 61 | 231*** | 58 | 261*** |
| ±11 | ±18 | ±14 | ±45 | ±18 | ±65 | ±15 | ±98 | |
| Total REM sleep | 117 | 108 | 115 | 63***# | 113 | 41*** | 118 | 39*** |
| ±24 | ±21 | ±16 | ±20 | ±19 | ±22 | ±20 | ±22 | |
| Wake after sleep onset | 28 | 31 | 22 | 31 | 27 | 45* | 23 | 37 |
| ±13 | ±18 | ±10 | ±15 | ±16 | ±31 | ±15 | ±26 | |
| Stage 1 sleep | 40 | 43 | 44 | 51* | 45 | 68*** | 44 | 56** |
| ±14 | ±16 | ±11 | ±19 | ±14 | ±29 | ±16 | ±22 | |
| Stage 2 sleep | 133 | 121 | 142 | 146 | 127 | 140 | 134 | 148 |
| ±30 | ±25 | ±35 | ±35 | ±35 | ±42 | ±30 | ±30 | |
| Slow wave sleep | 145 | 141 | 141 | 143 | 146 | 147 | 146 | 155 |
| ±30 | ±23 | ±28 | ±29 | ±25 | ±25 | ±28 | ±29 | |
Significantly different from placebo: *p < 0.05, **p < 0.01, ***p < 0.001.
Significantly different from paroxetine: #p < 0.001.
Pharmacokinetic/pharmacokinetic (PK/PD) analysis
No significant period effect for the PSG parameters was found. The popPK model used for estimations of Cav,sleep for vortioxetine was predictive and reliable.
REM onset latency (ROL)
Clear and significant relationships were found for both vortioxetine and paroxetine plasma concentration for ROL using an Emax model (Table 2; Figures 1 and 2).
Table 2.
Parameter values for the plasma concentrations versus ΔROL (change from baseline) relationship for vortioxetine and paroxetine.
| Parameter | Vortioxetine |
Paroxetine |
||
|---|---|---|---|---|
| Population mean (RSEa) | Inter-individual variability (%) | Population mean (RSEa) | Inter-individual variability (%) | |
| Emax (min) | 250 (7.4) | 29 | 297 (5.8) | 3.1 |
| EC50 (ng/mL) | 21 (6.1) | – | 6.2 (15) | 34 |
| E0 (min) | 2.4 (135) | – | -2.8 (132) | – |
| Γ | 2.5 (22) | 74 | 2.3 (18) | 1.1 |
| ε (SD) (ng/mL) | 15 (35) | – | 15 (35) | – |
The models were of the form .
Relative standard error (RSE) expressed as percentage of SE/mean; 95% CIs for Emax are 216 to 284 (vortioxetine) and 263 to 331 (paroxetine).
Figure 1.
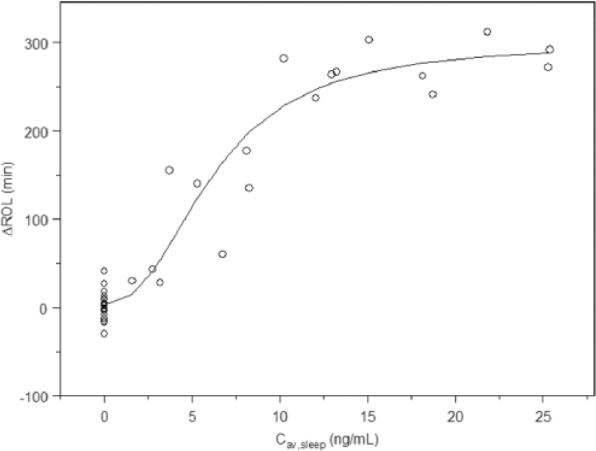
Plasma concentrations versus ΔROL (change from baseline) for paroxetine.
Figure 2.
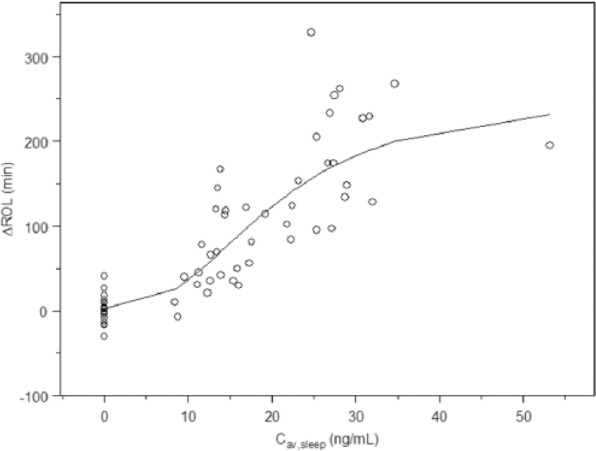
Plasma concentrations versus ΔROL (change from baseline) for vortioxetine.
Time spent in REM sleep (TREM)
Clear and significant relationships were found for both vortioxetine and paroxetine using an Emax model (Table 3).
Table 3.
Parameter values for the plasma concentrations versus ΔTREM (change from baseline) relationship for vortioxetine and paroxetine.
| Parameter | Vortioxetine |
Paroxetine |
||
|---|---|---|---|---|
| Population mean (RSEa) | Inter-individual variability (%) | Population mean (RSEa) | Inter-individual variability (%) | |
| Emax (min) | −149 (38) | – | −119 (22) | – |
| EC50 (ng/mL) | 36 (65) | 48 | 7.1 (57) | 34 |
| E 0 (min) | −9.0 (51) | – | −8.9 (51) | – |
| Γ | – | – | – | – |
| ε (SD) (ng/mL) | 19 (25) | – | 20 (37) | – |
The models were of the form .
Relative standard error (RSE) expressed as percentage of SE/mean; 95% CIs for Emax are -298 to -38 (vortioxetine) and -170 to -68 (paroxetine).
Intra-sleep wakening (WASO)
Emax models did not converge but linear models revealed a weak but significant relationship for vortioxetine, both for baseline corrected and uncorrected data. For paroxetine, neither Emax models nor linear models gave a significant relationship (Table 4).
Table 4.
Parameter values for ΔWASO PK/PD models.
| Parameter | Vortioxetine |
|
|---|---|---|
| Population mean (RSEa) | Inter-individual variability (%) | |
| k (min/ng/mL) | 0.48 (30) | 27 |
| E0 (min) | 1.6 (233) | 215 |
| ε (SD) (ng/mL) | 18 (35) | – |
The models were of the form .
Relative standard error (RSE) expressed as percentage of SE/mean.
Total sleep time (TST)
No significant relationship was observed for vortioxetine or paroxetine using either Emax or linear models, regardless of whether corrected or uncorrected values were used.
Sleep stage 1 (S1)
Linear models revealed a significant relationship for uncorrected data (but not for baseline corrected data) for both vortioxetine and paroxetine. Emax modelling did not converge (Table 5).
Table 5.
Parameter values for S1 PK/PD.
| Parameter | Vortioxetine |
Paroxetine |
||
|---|---|---|---|---|
| Population mean (RSEa) | Inter-individual variability (%) | Population mean (RSEa) | Inter-individual variability (%) | |
| k (min/ng/mL) | 0.72 (29) | 85 | 1.2 (33) | 36 |
| E0 (min) | 42 (8.5) | 30 | 41 (9.0) | 30 |
| ε SD (ng/ml) | 10 (21) | – | 10 (40) | – |
The models were of the form .
Relative standard error (RSE) expressed as percentage of SE/mean.
Occupancy
By translating vortioxetine/paroxetine Cav,sleep values to estimated SERT occupancy, the effects of vortioxetine and paroxetine on ΔROL were clearly separated. Also at a given SERT occupancy, vortioxetine affected REM sleep to a lesser degree than paroxetine. Linear regression analysis of SERT occupancy versus ΔROL showed that both the intercepts and slopes were statistically significantly (p < 0.01) different between vortioxetine and paroxetine (Figure 3). For clarification of this we plotted plasma levels of the drugs versus SERT occupancy and plasma levels of the drugs versus REM suppression (Figure 4). For paroxetine these were very similar but for vortioxetine they were clearly different.
Figure 3.
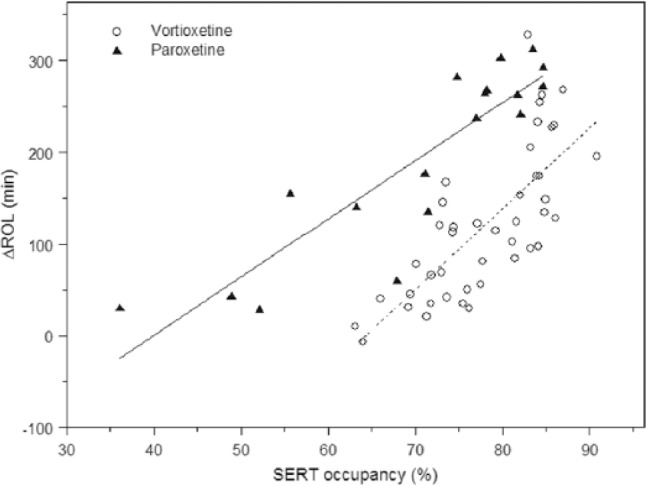
Estimated SERT occupancy versus ΔROL.
Figure 4.
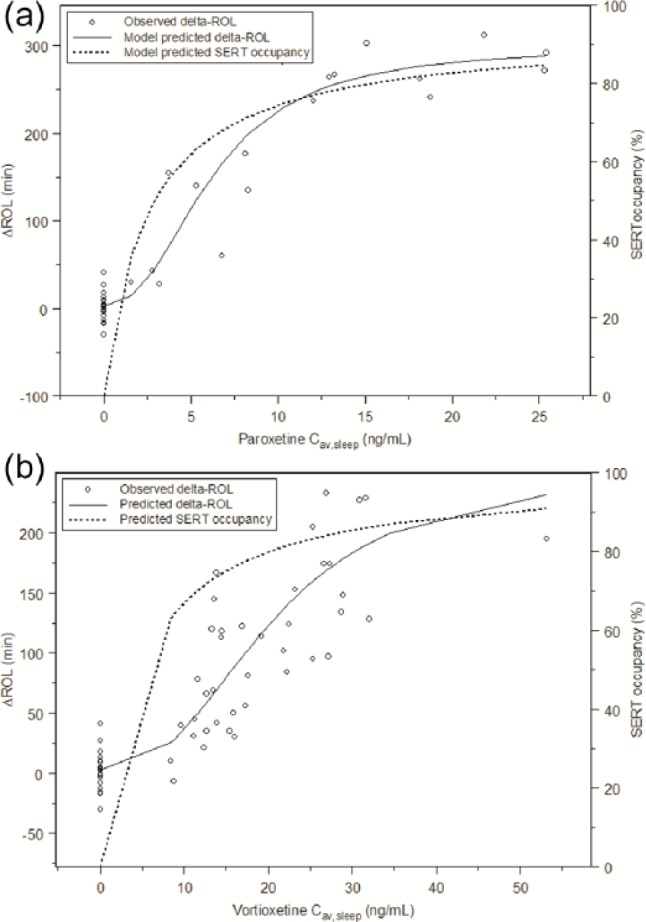
(a) Models for predicted ΔROL and SERT occupancy versus plasma concentrations (paroxetine). (b) Models for predicted ΔROL and SERT occupancy versus plasma concentrations (vortioxetine).
Discussion
We found as expected that both antidepressants had significant effects on suppression of REM sleep and that these effects were closely related to plasma levels, and to the estimated SERT occupancy. The relationship of REM variables to plasma level has been shown for fluoxetine (Feige et al., 2002) in humans and to plasma levels and SERT occupancy in rodents (Geldof et al., 2007), but this is the first time that the relationship between REM suppression and SERT occupancy has been reported in humans. Importantly, this relationship was different for vortioxetine and paroxetine.
SSRIs have been shown to suppress REM sleep (decrease total REM sleep and increase ROL) in several studies, both in healthy subjects and depressed patients. REM sleep suppression after SSRI administration is probably caused by increased synaptic 5-HT levels. Assuming a direct relationship between SERT occupancy and 5-HT level, one would expect to find the two relationships (plasma levels of the SSRI versus SERT occupancy and plasma levels of the SSRI versus REM suppression) to be very similar. This is what was found for paroxetine in this study, but not for vortioxetine. This indicates that the two compounds are clearly distinguishable, implying that vortioxetine has a different clinical pharmacological profile compared with the SSRI paroxetine, which is most probably related to its interactions with 5-HT receptors.
Since SRIs are thought to suppress REM via stimulation of 5-HT1A receptors, and vortioxetine is both an SRI and a 5-HT1A agonist, it might have been expected that REM suppression would be substantially more with vortioxetine than paroxetine. However, this was not the case. As far as we are aware there has been no previous human study of an SRI plus a 5-HT1A agonist, in comparison with SRI alone, on sleep to determine if there is an additive effect.
It has been suggested that 5-HT3 receptor agonism might suppress REM in rodents (Monti and Jantos, 2008; see also companion paper Leiser et al., this issue) and humans (Staner et al., 2001) and that 5-HT3 receptor antagonism may decrease ROL in humans (Rothe et al., 1994). These effects in humans are modest but most likely contribute to our results, as the very high affinity of vortioxetine for the 5-HT3 receptor means that even at low plasma concentrations this receptor is likely to be saturated (Bang-Andersen et al., 2011). As SERT occupation increases then synaptic 5-HT levels will also increase, offsetting the actions of the 5-HT3 receptor blockade. The other receptor interactions are less likely explanations as both 5-HT1A receptor partial agonism and 5-HT7 receptor antagonism tend to increase REM latency and decrease TREM (Bonaventure et al., 2012; Wilson et al., 2005).
We used the average overnight plasma concentration of the drugs as the PK variable in the PK/PD analysis of sleep variables, and of course this is not optimal; however, any attempt to do more frequent sampling would have disrupted sleep. In fact, the plasma concentrations of the drugs remained fairly stable overnight, with an approximately 20–25% drop from evening to morning, and no difference between the two drugs. The SERT occupancy estimates used here were measured in the striatum for paroxetine, and in the raphe for vortioxetine, but the SERT occupancy versus plasma exposure relationships were very similar between raphe nuclei and striatum (J. Areberg, unpublished results).
Other known effects of SRIs that we found were increased stage 1 sleep, increased intra-sleep wake and decreased TST. These were seen with all active treatments and there was no significant difference between vortioxetine and paroxetine. There was a weak positive relationship (using Emax model) between intra-sleep wake time and plasma levels of vortioxetine. The fact that these effects on sleep maintenance are similar between the two drugs is interesting in the light of the lower incidence of subject-reported sleep disturbance in clinical trials with vortioxetine compared with those with SSRIs (Sanchez et al., 2015). However, because of the loading dose regime we used it is difficult to draw any useful conclusions from our data regarding clinical implications.
This study is the first to explore the impact of the multimodal antidepressant vortioxetine on sleep parameters and in comparison with paroxetine, a pure SRI. We found a clear suppression of REM sleep with both drugs, consistent with increased synaptic 5-HT concentration. However, the relationships between the SERT occupancies (calculated from PET in human brain for each drug) and the extent of REM suppression, showed different profiles, with vortioxetine producing a different effect on REM sleep from that expected for an SRI which also has 5-HT1A agonist properties. The most probable explanation is that the 5-HT3 receptor antagonism of vortioxetine partly offsets REM suppression produced by 5-HT reuptake blockade and 5-HT1A agonism, because of its very much higher affinity for 5-HT3 receptors than 5-HT1A (Sanchez et al., 2015). These findings provide evidence that vortioxetine has a multimodal action in humans.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was sponsored by H. Lundbeck A/S as part of a joint clinical development programme with the Takeda Pharmaceutical Company, Ltd.
References
- Adrien J, Tissier MH, Lanfumey L, et al. (1992) Central action of 5-HT3 receptor ligands in the regulation of sleep-wakefulness and raphe neuronal activity in the rat. Neuropharmacology 31: 519–529. [DOI] [PubMed] [Google Scholar]
- Areberg J, Luntang-Jensen M, Sogaard B, et al. (2012a) Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol 110: 401–404. [DOI] [PubMed] [Google Scholar]
- Areberg J, Naik H, Chen G, et al. (2012b)A population pharmacokinetic meta-analysis of vortioxetine (Lu AA21004) in healthy subjects. Poster (P.02) presented at the 12th International Forum on Mood and Anxiety Disorders (IFMAD), Barcelona, Spain, 7–9 November 2012. [Google Scholar]
- Bang-Andersen B, Ruhland T, Jørgensen T, et al. (2011) Discovery of 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54: 3206–3221. [DOI] [PubMed] [Google Scholar]
- Berhan A, Barker A. (2014) Vortioxetine in the treatment of adult patients with major depressive disorder: A meta-analysis of randomized double-blind controlled trials. BMC Psychiatry 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Dugovic C, Kramer M, et al. (2012) Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther 342: 429–440. [DOI] [PubMed] [Google Scholar]
- Driver HS, Flanigan MJ, Bentley AJ, et al. (1995) The influence of ipsapirone, a 5-HT1A agonist, on sleep patterns of healthy subjects. Psychopharmacol 117: 186–192. [DOI] [PubMed] [Google Scholar]
- Feige B, Voderholzer U, Riemann D, et al. (2002) Fluoxetine and sleep EEG: Effects of a single dose, subchronic treatment, and discontinuation in healthy subjects. Neuropsychopharmacology 26: 246–258. [DOI] [PubMed] [Google Scholar]
- Fu J, Chen Y. (2015) The efficacy and safety of 5mg/d vortioxetine compared to placebo for major depressive disorder: A meta-analysis. Psychopharmacology (Berl). 232: 7–16. [DOI] [PubMed] [Google Scholar]
- Geldof M, Freijer J, van Beijsterveldt L, et al. (2007) Population pharmacokinetic model of fluvoxamine in rats: Utility for application in animal behavioral studies. Eur J Pharm Sci 30: 45–55. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Mendez-David I, Pehrson A, et al. (2013) Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharm 73: 147–159. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Iglesias-Bregna D, Westrich L, Pehrson AL, Sanchez C. (2015) Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 2, pharmacological interactions in rodents suggest a role of 5-HT3 receptor antagonism. J Psychopharmacol. 29: 1092–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, et al. (2004) Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C]DASB positron emission tomography study. Am J Psychiatry 161: 826–835. [DOI] [PubMed] [Google Scholar]
- Monaca C, Boutrel B, Hen R, et al. (2003) 5-HT1A/1B receptor-mediated effects of the selective serotonin reuptake inhibitor, citalopram, on sleep: Studies in 5-HT1A and 5-HT1B knockout mice. Neuropsychopharm 28: 850–856. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. (2008) Activation of the serotonin 5-HT3 receptor in the dorsal raphe nucleus suppresses REM sleep in the rat. Prog Neuropsychopharmacol Biol Psychiatry 32: 940–947. [DOI] [PubMed] [Google Scholar]
- Mørk A, Pehrson A, Brennum LT, et al. (2012) Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340: 666–675. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Nutt DJ, Wilson SJ. (2011) Sleep and its disorders in translational medicine. J Psychopharmacol 25: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. (1968) A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Department of HEW. [DOI] [PubMed] [Google Scholar]
- Rothe B, Guldner J, Hohlfeldt E, et al. (1994) Effects of 5-HT3 receptor antagonism by tropisetron on the sleep EEG and on nocturnal hormone secretion. Neuropsychopharm 11: 101–106. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol Ther 145: 43–57. [DOI] [PubMed] [Google Scholar]
- Staner L, Linker T, Toussaint M, et al. (2001) Effects of the selective activation of 5-HT3 receptors on sleep: A polysomnographic study in healthy volunteers. Eur Neuropsychopharmacol 11: 301–305. [DOI] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S. (2005) Antidepressants and sleep: A qualitative review of the literature. Drugs 65: 927–947. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Bailey JE, Rich AS, et al. (2004) Using sleep to evaluate comparative serotonergic effects of paroxetine and citalopram. Eur Neuropsychopharmacol 14: 367–372. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Bailey JE, Rich AS, et al. (2005) The use of sleep measures to compare a new 5-HT1A agonist with buspirone in humans. J Psychopharmacol 19: 609–613. [DOI] [PubMed] [Google Scholar]


