Abstract
Objective:
The objective of this study was to profile over a 24 h period the prolactin responses of exercise-trained men on a day involving rest with no exercise in comparison to a day involving exercise training sessions.
Methods:
This is a quasi-experimental design study using repeated measures determination of 24 h prolactin responses in exercise-trained men (n = 16; age = 27.3± 3.3 years (mean ± standard deviation)). Blood samples were taken hourly over a 24 h period on a day involving two intensive exercise training sessions (ED), and on a separate control day (CD) with no exercise activity. The order of the ED and CD was randomized. Physical activity and diet were controlled and replicated for the ED and CD. Blood specimens were handled, prepared and analyzed utilizing appropriate standard clinical practices. The data were analyzed with the Friedman analysis of variance and Nemenyi post hoc statistical procedure for repeated measures.
Results:
On the CD, prolactin displayed a typical circadian rhythm with daytime values of the hormone being less than the nocturnal rise once sleep had begun (p < 0.05; 16:00–20:00 h > all other times). On the ED, prolactin responses were noticeably different from those of the CD. The morning and afternoon exercise sessions included significant increases in prolactin immediately at the end of the exercise sessions, being greater than corresponding CD time points (p < 0.01; 01:00 h and 10:00 h); also for the second hour (2 h) following the morning exercise session. On the ED there was a displayed circadian nocturnal response in the hormone with 16:00–24:00 h being elevated above the all nonexercise effected values for that specific day (p < 0.01). Finally, the ED nocturnal elevation for prolactin for 16:00–24:00 h was significantly greater than the same respective hours on the CD (p < 0.05).
Conclusion:
Findings clearly demonstrated that nocturnal prolactin responses are augmented in exercise-trained men on days when they perform exercise. The mechanisms inducing this adaptive response are unclear but warrant further investigation.
Keywords: exercise, hormones, physical activity, prolactin, stress
Introduction
Regular exercise is a critical behavioral component to improving health and physical capacity in individuals. Such physical exercise is a powerful stimulus to the neuroendocrine system, resulting in large and dramatic changes in the circulating levels of hormones [Hackney, 2006].
Many of the hormonal responses to exercise have been well profiled by a multitude of research studies. However, many of these studies have used very limited blood sampling protocols, resulting in a less than complete picture of exercise changes in hormones over a 24 h period [Reilly, 1990]. This latter point is important due to the circadian and diurnal variations and rhythms observed in many hormones [Reilly, 1990].
One hormone that has been studied to a lesser degree than most in relation to exercise responses is prolactin. This is regrettable since prolactin, a critical hormone in male and female reproduction, is associated with slow wave sleep development, and the upregulation of necessary temporal circadian immune function changes. All of these effects are factors that can influence the quality of life in an individual, and in the case of athletes, impact on the rest recovery process which has important implications for their health [Freeman et al. 2000]. This lack of an emphasis in exercise studies on prolactin has occurred because most exercise-related endocrinology research focuses upon metabolic (e.g. glucoregulatory) or anabolic hormones [Hackney, 2006; Viru and Smirnova, 1995]. Of those studies that have profiled the prolactin response to exercise, many have suffered from methodology limitations (i.e. lack of controls for prior diet or physical activity, prior sleep, emotional stress, and use of blood sampling protocols with insufficient sampling frequencies) [Hackney and Viru, 2008]. In light of the importance of prolactin and the restricted aspects of its profiling in response to exercise, and the limitations in existing research, the current study was undertaken. The purpose being to profile over a 24 h period the prolactin responses of exercise-trained men on a day involving rest with no exercise in comparison to a day involving exercise training sessions.
Methodology
Exercise-trained male subjects were recruited to participate in this study (n = 16). All signed an informed consent statement in accordance with the Helsinki Declaration prior to participation. Inclusion criteria for entry into the study included the subjects having been engaged in exercise training and sports competition (running) for a minimum of 5 years. At the time of the study no participants were consuming any prescription drugs and medical screening revealed no endocrine abnormalities.
Maximal oxygen uptake (VO2max; treadmill, Astrand protocol) and physical characteristics were obtained on all the subjects using procedures that have previously been described in detail elsewhere [Daly et al. 2005; Hackney and Viru, 1999]. The VO2max results were used to calculate the workload for the exercise sessions denoted below [Hackney and Viru, 1999]. The VO2max exercise testing occurred approximately 2 weeks prior to the experimental conditions explained below.
The subjects were asked to report to our laboratory on two occasions: a control day (CD) with no exercise, and an exercise day (ED) involving moderate- to high-intensity exercise sessions. The order of these treatment conditions was randomized (approximately 1 week apart, and occurred in the same season of the year, late spring). All procedures and steps during each of the two treatment conditions were identical, except for the periods during which exercise was, or was not, performed. For the CD, the subjects performed two supine resting sessions in the morning and the afternoon, in which no physical activity or exercise occurred at all. For the ED the subjects performed a 60 min continuous aerobic exercise bout (treadmill running at ~100 ± 5% of individual ventilatory threshold) [Daly et al. 2005] in the morning and a 45 min continuous aerobic bout (same running speed as morning) in the afternoon. Subjects were allowed, however, to request the treadmill speed be reduced if they felt they could not complete the full exercise session time period. The exercise protocol of using two bouts within the same day was chosen in an attempt to mimic what competitive athletes might do in their regular training regimens [Viru and Smirnova, 1995]. The rest periods on the CD occurred at the same time of day as the aerobic exercise on the ED and were matched on durations.
On the CD and ED, the following procedures were carried out and replicated by the subjects. They awoke between 05:30 and 06:00 h (24 h clock time) and consumed a light standardized meal. They then reported to the laboratory at 06:30 h where an indwelling venous catheter was placed in an antecubital vein, followed by a 20–30 min rest. At 07:00 h (±5 min; denoted as 00:00 h on 24 h clock used as reference here), the first blood sample was taken, and the subjects began the first experimental session (control or exercise) activity (supine rest, exercise). At the end of the experimental session activity, the next blood sample was taken (01:00 h). The second session activity within a given day was begun at approximately 09:00 h and was identical to the session performed in the morning, only shorter in duration (45 min). Blood samples were taken hourly between the two sessions as well as immediately at the end of the second session. Starting at 14:00 h until 24:00 h (07:00 next day), blood samples were only taken every 2 h. The less frequent blood sampling during the night was due to constraints issued by the institutional ethical review board. The subjects consumed additional standardized meals at 06:00 and 13:00 h, and were allowed to consume fluid ad libitum throughout the day. Additionally, in between blood sampling times, the subjects were allowed to walk freely in the laboratory area and perform routine daily activities (e.g. read, watch television). For the 24 h before each day of testing, the subjects were asked to perform minimal physical activity and to consume a normal diet. These conditions were replicated before each day of testing.
Analytical methods
At each blood sampling, 2 ml of whole blood was collected into EDTA-treated tubes. The tubes were immediately placed on ice until processed. Plasma was collected after centrifugation of the tubes (3000 × g for 15 min at 4°C). Separated plasma was placed in cryofreeze tubes and stored at −50°C for later analysis. The plasma samples were analyzed for prolactin using radioimmunoassay techniques (Diagnostic Products Corporation, Los Angeles, CA, USA). All plasma samples were analyzed in duplicate. The average within-assay and between-assay coefficients of variation were less than 10%. A single subject’s plasma samples were analyzed in entirety in an attempt to minimize within-subject variability.
Statistical methods
Statistically, the data were analyzed with the Friedman analysis of variance (ANOVA) procedure using the repeated measures design feature. The procedures recommended by Linton and colleagues [Linton et al. 1975] were used to correct for missing data (i.e. for some subjects not all blood samples were obtained, less than 5% of total samples), and the ANOVA results were adjusted in degrees of freedom for missing data to ensure a ‘conservative’ statistical approach and to minimize the possibility of type I error. The Nemenyi post hoc procedure was used after the ANOVA to determine significant differences between means [Linton et al. 1975]. The probability level for statistical significance was set at 0.05 or lower.
Results
The physical characteristics of the subjects are given in Table 1. Based upon their VO2max responses, the subjects were classified as having a high level of cardiovascular fitness [Thompson et al. 2009]. This point is substantiated by the fact that all subjects were able to complete both exercise sessions on the ED without undue distress or excessive fatigue.
Table 1.
Physical characteristics and exercise-training history of subjects.
| Measure | Mean | SD |
|---|---|---|
| Age (years) | 27.3 | 3.3 |
| Mass (kg) | 71.9 | 4.7 |
| Height (cm) | 177.8 | 6.9 |
| VO2max (ml/kg/min) | 62.0 | 4.9 |
| Training (years) | 9.7 | 4.1 |
VO2max, maximal oxygen uptake.
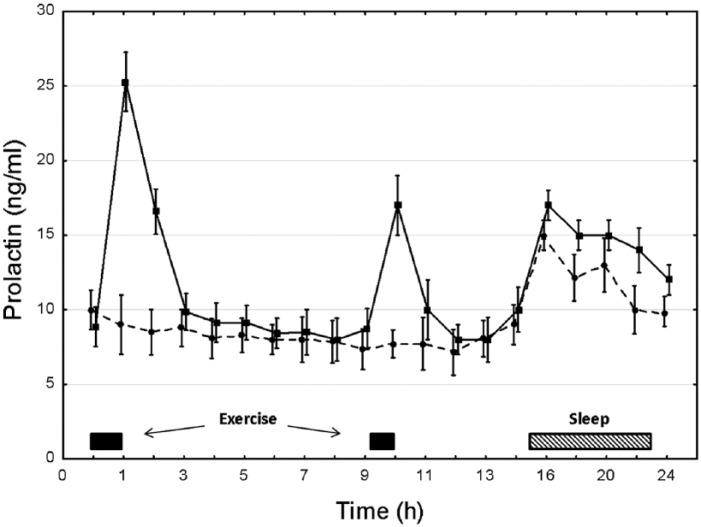
The prolactin responses for the CD and ED are depicted in Figure 1. For the CD prolactin displayed a typical circadian rhythm with daytime values of the hormone being less than the nocturnal rise once sleep had begun (p < 0.05; 16:00–20:00 h > all other times).
Figure 1.
The prolactin response over a 24 h period for exercise-trained men on a day with two training sessions (square symbol, solid line) and on a control day (round symbol, dashed line) with only rest and no exercise. Plotted values are mean ± standard error.
ED prolactin responses were substantially different from those of the CD. The morning and afternoon exercise sessions included significant increases in prolactin immediately at the end of the exercise sessions being greater than corresponding CD time points (p < 0.01; 01:00 h and 10:00 h); and also for the second hour (2 h) following the morning exercise session. The ED also induced a circadian nocturnal response in the hormone, with 16:00–24:00 h being elevated above the all nonexercise effected values for that specific day (p < 0.01). Additionally, the ED nocturnal elevation for prolactin for 16:00–24:00 h was significantly greater than the same respective hours on the CD (p < 0.05).
Discussion
The intent of this study was to examine the influence of exercise on the 24 h hormonal profile of prolactin. The results clearly demonstrate that the normal circadian occurrence of a nocturnal elevation in prolactin is augmented at night after daytime exercise.
The hormonal concentration variations across the CD were within the typical clinical reference ranges for prolactin [Burtis et al. 2012], and the ED responses to the exercise sessions (and in recovery from the exercise) agree with previous research findings [Rojas Vega et al. 2012]. However, the unique finding of augmented nocturnal prolactin responses following daytime exercise appears to be somewhat novel. Prior work from our laboratory had pointed to the potential for such an occurrence, but this earlier work was methodologically limited and viewed as highly preliminary in nature [Hackney et al. 1989]. To our knowledge this is the first systematic study to quantify in a highly accurate means the degree of this nocturnal augmentation following exercise. This finding is substantial and important as it emphasizes to researchers and clinicians that hormonal responses to exercise are not limited to just the immediate period of recovery (i.e. a few hours) from the end of an exercise session. The findings support the fact that more research studies are needed to investigate other hormones and the nocturnal responses to daytime exercise. In so doing, a more complete profile and understanding of the affects and influences of exercise on the human endocrinological system can be ascertained.
The physiological mechanism inducing the augmented nocturnal responses is unclear, but relative to exercise training several highly feasible possibilities exists. First, there is an enhanced glucocorticoid response with exercise activity which can stimulate prolactin release [Freeman et al. 2000]. A two- to fourfold increase in basal glucocorticoid (cortisol) concentrations is highly possible with intense and prolonged physical exercise such as was performed in this study [Hackney, 2006]. However, nocturnal cortisol responses are typically suppressed following daytime exercise [Kern et al. 1995]. Second, thyrotropin-releasing hormone (TRH) and vasoactive intestinal peptide (VIP) can stimulate prolactin release [Christian et al. 2007; Freeman et al. 2000]. Each of these agents can be increased with exercise, and some evidence suggests TRH may be elevated slightly at night following daytime exercise. However, the number of studies examining this is extremely limited and not conclusive, and more work is needed [Hackney et al. 1989, 2012].
Prolactin is unique in that it is under chronic dopaminergic inhibition at the pituitary, and subsequent removal of this inhibition allows levels to elevate in the circulation without a direct stimulating factor [Freeman et al. 2000; Rojas Vega et al. 2012]. Dopamine responses are typically acutely elevated during and following exercise [Meeusen and De Meirleir, 1995]. The influence of daytime exercise on nocturnal dopamine responses is currently unknown, but sleeplessness is highly associated with increased dopamine [Sadamatsu et al. 1995]. Our subjects, however, reported comparable sleep quantity (~7–8 h each night) and quality (no one indicated difficulties sleeping) on the CD and ED, but this is based on just verbal discussions with the subjects and not a rigorous evaluation. Nonetheless, daytime exercise is associated with increased nocturnal melatonin which can inhibit central dopamine release and could thus lead to greater prolactin release [Zisapel, 2001]. At this time our working hypothesis is that the augmented nocturnal prolactin response observed is due to an exercise-induced dopaminergic inhibition more so than an enhanced release of prolactin stimulatory factors such as cortisol, TRH or VIP. This hypothesis is a viable and plausible explanation for our findings, but at this point remains highly speculative.
Seldom with exercise training do physiological adaptions occur without there being some degree of a necessity, or utility aspect, of the specific accommodation to allow for a relative enhancement of performance. On this point, we must again speculate; however, in light of the critical role prolactin plays in cytokine and cytokine receptor expression, and growth survival factor (T cells, lymphocytes) upregulation, most certainly the augmented nocturnal elevations could be a means for providing a robust immune response during the recovery from exercise [Freeman et al. 2000; Rojas Vega et al. 2012].
Conclusion
We have demonstrated that nocturnal prolactin responses are augmented in exercise-trained men on days when they perform exercise. Whether this augmented response would be seen in men who are less exercise trained or when only a single daytime exercise session had been completed is unknown. Nonetheless, the current findings are a unique occurrence in the endocrinology of exercise and warrant further investigation.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Anthony C. Hackney, Endocrine Section, Applied Physiology Laboratory, Department of Exercise & Sport Science, University of North Carolina, Department of Nutrition, School of Public Health, University of North Carolina, CB # 8700 - UNC-CH, Chapel Hill, NC 27599, USA.
Hope C. Davis, Endocrine Section, Applied Physiology Laboratory, Department of Exercise & Sport Science, University of North Carolina, Chapel Hill, NC, USA
Amy R. Lane, Endocrine Section, Applied Physiology Laboratory, Department of Exercise & Sport Science, University of North Carolina, Chapel Hill, NC, USA Human Movement Science Curriculum, University of North Carolina, Chapel Hill, NC, USA.
References
- Burtis C., Ashwood E., Bruns D. (2012) Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. St Louis, MO: Saunders/Elsevier. [Google Scholar]
- Christian H., Chapman L., Morris J. (2007) Thyrotrophin releasing hormone, vasoactive intestinal peptide, prolactin-releasing peptide and dopamine regulation of prolactin secretion by different lactotroph morphological subtypes in the rat. J Neuroendocrinol 19: 605–613. [DOI] [PubMed] [Google Scholar]
- Daly W., Seegers C., Rubin D., Dobridge J., Hackney A. C. (2005) Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J Appl Physiol 93: 375–380. [DOI] [PubMed] [Google Scholar]
- Freeman M., Kanyicska B., Lerant A., Nagy G. (2000) Prolactin: structure, function, and regulation of secretion. Physiol Rev 80: 1523–1631. [DOI] [PubMed] [Google Scholar]
- Hackney A. C. (2006) Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab 1: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney A. C., Kallman A., Hosick K., Rubin D., Battaglini C. (2012) Thyroid hormonal responses to intensive interval versus steady-state endurance exercise sessions. Hormones (Athens) 11: 54–60. [DOI] [PubMed] [Google Scholar]
- Hackney A. C., Ness R., Schreiber A. (1989) Effects of endurance exercise on nocturnal hormone concentrations in males. Chronobiol Int 6: 341–346. [DOI] [PubMed] [Google Scholar]
- Hackney A. C., Viru A. (2008) Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train 43: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W., Perras B., Wodick R., Fehm H., Born J. (1995). Hormonal secretion during nighttime sleep indicating stress of daytime exercise. J Appl Physiol 79: 1461–1468. [DOI] [PubMed] [Google Scholar]
- Linton M., Gallo P., Logan C. (1975) The Practical Statistician: Simplified Handbook of Statistics. Monterey, CA: Brooks/Cole. [Google Scholar]
- Meeusen R., De Meirleir K. (1995) Exercise and brain neurotransmission. Sports Med 20: 160–188. [DOI] [PubMed] [Google Scholar]
- Reilly T. (1990) Human circadian rhythms and exercise. Crit Rev Biomed Eng 18: 165–180. [PubMed] [Google Scholar]
- Rojas Vega S., Hollmann W., Struder H. (2012) Influences of exercise and training on the circulating concentration of prolactin in humans. J Neuroendocrinol 24: 395–402. [DOI] [PubMed] [Google Scholar]
- Sadamatsu M., Kato N., Iida H., Takahashi S., Sakaue K., Takahashi K., et al. (1995) The 24-hour rhythms in plasma growth hormone, prolactin and thyroid stimulating hormone: effect of sleep deprivation. J Neuroendocrinol 7: 597–606. [DOI] [PubMed] [Google Scholar]
- Thompson W., Gordon N., Pescatello L. (2009) ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health. [Google Scholar]
- Viru A., Smirnova T. (1995) Health promotion and exercise training. Sports Med 19: 123–136. [DOI] [PubMed] [Google Scholar]
- Zisapel N. (2001) Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol 21: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]