Abstract
The cholera toxin genes of Vibrio cholerae are encoded by CTXΦ, a lysogenic bacteriophage. Infection with this phage plays a determinant role in toxigenicity conversion and the emergence of new clones of pathogenic V. cholerae. Multiple phage alleles, defined by sequence types of the repressor gene rstR, have been found, showing the divergence of phage genomes. Pre-CTXΦ, which is characterized by the absence of toxin genes, is predicted to be the precursor of CTXΦ. We have found a new pre-CTXΦ prophage genome (named pre-CTXZJΦ for its novel rstR allele) in nontoxigenic V. cholerae O1 isolates that were obtained during surveillance of the estuary water of the Zhujiang River. A novel hybrid genome of the helper phage RS1 was identified in an environmental strain carrying pre-CTXZJΦ in this study. The chromosomal integration and genomic arrangement of pre-CTXZJΦ and RS1 were determined. The RS2 of pre-CTXZJΦ was shown to have a function in replication, but it seemed to have lost its ability to integrate. The RstR of pre-CTXZJΦ exerted the highest repression of its own rstA promoter compared to other RstRs, suggesting rstR-specific phage superinfection immunity and potential coinfection with other pre-CTXΦ/CTXΦ alleles. The environmental strain carrying pre-CTXZJΦ could still be infected by CTXETΦ, the most common phage allele in the strains of the seventh cholera pandemic, suggesting that this nontoxigenic clone could potentially undergo toxigenicity conversion by CTXΦ infection and become a new toxigenic clone despite already containing the pre-CTXΦ prophage.
INTRODUCTION
Vibrio cholerae is primarily an inhabitant of estuarine water, particularly in estuary waters (1). More than 200 serogroups of V. cholerae have been recognized (2), although only serogroups O1 and O139 have caused epidemics (3). V. cholerae has caused seven pandemics historically. The ongoing seventh pandemic is caused by V. cholerae O1 biotype El Tor.
It has been proposed that the sixth and seventh pandemic strains evolved from nontoxigenic environmental strains (4). Whole-genome comparisons have shown that horizontal gene transfer plays critical roles in the emergence of toxigenic strains and the divergence of epidemic strains (5, 6). Epidemic strains of V. cholerae are characterized by the production of cholera toxin (CT) and toxin-coregulated pilus (TCP) (7). The CT gene cluster ctxAB is located in the genome of the lysogenic bacteriophage CTXΦ, which is integrated into the chromosome of toxigenic V. cholerae and may be transferred from toxigenic to nontoxigenic strains (8, 9). After induction, CTXΦ can infect V. cholerae by using TCP as its receptor and by interacting with its major subunit, TcpA; it then integrates into the chromosome at the attB site (10–12). The TCP gene cluster resides in the Vibrio pathogenicity island (VPI), an essential virulence gene cluster of V. cholerae (13). The acquisition of the VPI gene cluster by horizontal gene transfer is generally thought to be a requirement for conversion from nontoxigenic to pathogenic strains due to the production of the TCP, which mediates infection with CTXΦ (14, 15).
The typical CTXΦ prophage genome is composed of RS2 and the core region (9). The core region contains the genes involved in phage morphogenesis and the ctxAB genes. CTX prophages that are devoid of the ctxAB genes have also been identified (16, 17) and are named pre-CTXΦ; these prophages are predicted to be precursors of CTXΦ. RS2 is the regulation region of CTXΦ and contains the rstR, rstA, and rstB genes and two intergenic regions, ig-1 and ig-2. RstA and RstB are required for CTXΦ replication and integration, and RstR is a repressor that inhibits the transcription of rstA and rstB genes (18). Certain V. cholerae El Tor strains also carry RS1, which is absent in the classical biotype and integrated upstream and/or downstream of the CTX element. RS1 is similar to RS2 except that it contains an additional gene, rstC, and is considered to be a satellite phage of CTXΦ (19, 20). The rstC gene, which encodes an anti-repressor of RstR, may promote the propagation and transmission of RS1/CTXΦ (19).
Within the CTX and pre-CTX prophage genomes, the sequence of the rstR gene showed high divergence. The rstR genes are preliminarily designated rstRclass, rstRET, or rstRcalc based on nucleotide sequence variation from serogroup O1 biotype classical (class) and El Tor (ET) strains and some serogroup O139 strains (e.g., strain O139 Calcutta [calc]) (21, 22). Accordingly, CTXΦs are classified as CTXclassΦ, CTXETΦ, and CTXcalcΦ by their rstR sequences. Other rstR alleles, including rstR-4* (23), rstR-4** (23), rstR-5 (23), rstR6 (24, 25), rstR-232 (26) and rstRZJ (27), are found in nontoxigenic environmental strains of O1 and O139 and non-O1/non-O139 serogroups. RstR may provide immunity to recurrent infection by an identical CTXΦ (21, 22, 28, 29). This type of CTX immunity is rstR allele specific and is determined by the RstR sequence and its binding site in ig-2 (21, 22, 28). Moreover, CTX prophages with different rstR sequence types in certain V. cholerae strains have been reported (21, 30–32).
It has been strongly suggested that the ctxAB genes are acquired subsequently to the development of the pre-CTXΦ (9, 16, 17), whereas the biological significance of pre-CTXΦ and its infection of V. cholerae is unclear. The identification of new CTX prophage genomes will provide more evidence for exploring the evolution of the CTXΦ family and lineages of toxigenic V. cholerae. During our surveillance of O1/O139 V. cholerae in the estuarine water of the Zhujiang River in Guangzhou, China, a higher frequency of strains carrying pre-CTXΦ was found in the nontoxigenic environmental strains (27). Eleven nontoxigenic O1 strains carrying a novel pre-CTXZJΦ with unique rstRZJ and tcpA genes were isolated in successive months and became the dominant environmental strains carrying pre-CTXΦ in the Zhujiang River (27). In this study, we unraveled the genomic structure of this prophage and the potential immunity of this novel RstR to other CTXΦ alleles. Considering the prevalence of these nontoxigenic strains in the environment and the fact that they encode a TCP that is similar to those encoded by the epidemic strains, we also explored the possibility that these strains could acquire CTXETΦ and found that they had the potential to become toxigenic.
MATERIALS AND METHODS
Strains and growth conditions.
The V. cholerae strains and Escherichia coli strains used in this study are described in Table 1. Strains were grown in Luria broth (LB) with agitation (200 rpm) at 37°C unless otherwise indicated. When necessary, the culture medium was supplemented with ampicillin (Amp; 100 μg/ml), tetracycline (Tet; 30 μg/ml), chloramphenicol (Cm; 30 μg/ml for E. coli or 15 μg/ml for V. cholerae), IPTG (isopropyl-β-d-thiogalactopyranoside; 20 μg/ml), or X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 20 μg/ml).
TABLE 1.
Characterization of strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source and/or reference(s) |
|---|---|---|
| V. cholerae strains | ||
| VC06-18 | O1, pre-CTXZJΦ, ΔctxAB, tcpA+ | Water (27) |
| VC06-41 | O1, Δzot, ΔctxAB, tcpA+ | Water |
| 1119 | O1, classical, Inaba, ΔCTXETΦ, CTXclassΦ+, tcpA+ | 33 |
| N16961 | O1, El Tor, CTXETΦ, ctxAB+, tcpA+ | Patient |
| 86015 | O1, El Tor, CTXETΦ, ctxAB+, tcpA+ | Water |
| N-Φc | N16961 ctxAB::cat | 33 |
| SCE264 | O42, RS1+, ΔCTXΦ, ΔctxAB, tcpA+ | Water (23) |
| MS6 | O1, El Tor, CTXETΦ, ctxAB+, tcpA+ | Patient (34, 35) |
| 234-93 | O141 | Patient (3) |
| E. coli strains | ||
| JM109 | recA1 supE44 endA1 hsdR17 thiΔ(lac-proAB)F′[traD36 proAB+ lacIqZΔM15] | TaKaRa |
| SM10λpir | supE recA::RP4-2-Tc::Mu λpir; Kmr | Our laboratory |
| Plasmids | ||
| pRL-null | rluc reporter gene | Promega |
| pACYC184 | orip15A, Cmr, Tcr | NEB |
| pRS591 | oriColE1, Apr, Tcr | 36 |
| pACYC184-rstRZJ | pACYC184 containing rstRZJ, Tcr | This study |
| pACYC184-rstRclass | pACYC184 containing rstRclass, Tcr | This study |
| pACYC184-rstRET | pACYC184 containing rstRET, Tcr | This study |
| pACYC184-rstRcalc | pACYC184 containing rstRcalc, Tcr | This study |
| pACYC184-rstR6 | pACYC184 containing rstR6, Tcr | This study |
| pACYC184-rstR-232 | pACYC184 containing rstR-232, Tcr | This study |
| pRS591-rstAZJ | pRS591 containing rstAZJ promoter and rluc, Apr, Tcr | This study |
| pRS591-rluc | pRS591 containing rluc without promoter, Apr, Tcr | This study |
| pKTN701 | Suicide plasmid vector, mobRP4 oriR6K, Cmr | 37 |
| pKTN701-RS2ZJ | pKTN701 containing RS2 of pre-CTXZJΦ, Cmr | This study |
| pKTN701-RS2ET | pKTN701 containing RS2 of CTXETΦ, Cmr | This study |
Ap, ampicillin; Cm, chloramphenicol; Tc; tetracycline.
PCR amplification, sequencing, and sequence alignment.
V. cholerae genomic DNA was extracted using a NucleoSpin tissue kit (Macherey-Nagel, USA) according to the instructions of the manufacturer. We amplified the entire pre-CTXΦ genome and its chromosomal integration sites using different combinations of the primers zot1, zot2, ctxAB1, ctxAB2, orfU1, orfU2, rstC1, rstC2, inA, inB, inI, and inJ (see Table S1 in the supplemental material). PCR amplification was performed using Taq DNA polymerase or LA Taq DNA polymerase (TaKaRa). All amplicons were sequenced using the primers listed in Table S1. BioEdit was used to perform multiple alignments of predicted amino acid sequences. All GenBank accession numbers are listed in Table S2.
Construction of plasmids.
Plasmid pACYC184-rstRZJ containing the rstRZJ gene and its promoter was constructed by amplifying the rstRZJ coding and promoter regions using primers rstRZJ1 and rstRZJ2 (see Table S1 in the supplemental material) and genomic DNA from strain VC06-18 (Table 1). Next, the PCR product was cloned into TA cloning vector pMD18T (TaKaRa) and sequenced. An EcoRI fragment containing the insertion was then ligated to EcoRI-digested and -dephosphorylated pACYC184 to generate pACYC184-rstRZJ. The plasmids pACYC184-rstRclass, pACYC184-rstRET, pACYC184-rstRcalc, pACYC184-rstR6, and pACYC184-rstR-232, which contain the rstRclass, rstRET, rstRcalc, rstR6, and rstR-232 genes and their promoters, respectively, were constructed using the same strategy. To construct pRS591-rstAZJ, the rstAZJ promoter region and the Renilla luciferase reporter gene (rluc) were amplified using primers rstAZJP1/rstAZJP2 (see Table S1) and genomic DNA from strain VC06-18 and using primers RlucZJ1/Rluc2 (see Table S1) and plasmid pRL-null, respectively. Next, the overlapping fragment containing the rstAZJ promoter and rluc reporter was amplified with primers rstAZJP1/Rluc2. To construct pRS591-rluc, the rluc reporter gene was amplified with primers Rluc1/Rluc2 (see Table S1). Following subcloning of these PCR products into pMD18T and sequencing, EcoRI fragments containing the insertion were ligated into EcoRI-digested and -dephosphorylated pRS591 to generate pRS591-rstAZJ and pRS591-rluc, respectively. Plasmids pKTN701-RS2ZJ and pKTN701-RS2ET were constructed by amplifying the region of partial zot-RS2-psh-cep-partial gIIICTX with primers RS-F and RS-R (see Table S1) and genomic DNA from strain VC06-18 or strain 86015 (Table 1). Next, the PCR products were cloned into pMD18T and sequenced. The SalI/EcoRI fragments containing the insertion were then ligated to SalI/EcoRI-digested pKTN701 to generate pKTN701-RS2ZJ and pKTN701-RS2ET.
Quantitative real-time PCR and calculation of the zot gene copy numbers.
Quantitative real-time PCR was performed using primers zotF1/zotR1 (see Table S1 in the supplemental material) targeting the zot gene and ThyA1/ThyA2 (see Table S1) targeting the thymidylate synthase (thyA) gene, fluorescent dye SYBR green I (TaKaRa), and a LightCycler 2.0 (Roche Diagnostics, USA) system. Melting curve analysis was performed for each reaction to confirm the specificity of the PCR. The number of PCR threshold cycles (CT) required for the fluorescent intensities to exceed a threshold just above the background level was calculated for each reaction. The 2−ΔΔCT method was used to calculate the copy number of the zot gene as previously described (38, 39). The average copy number and 95% confidence interval (CI) of the zot gene were calculated for six experiments by the use of SPSS 10.0.
Renilla luciferase luminescence assay.
JM109-derived strains containing different combinations of pACYC184 and pRS591 derivatives were incubated at 37°C with shaking. The optical density at 595 nm (OD595) of triplicate bacterial suspensions was measured every 30 min. Simultaneously, 95-μl culture samples were mixed with 5 μl of 1.2 mM ViviRen live cell substrates (Promega, USA) in triplicate and incubated for 10 min at room temperature. Luminescence was then measured using a luminometer (Tecan). The combination of pACYC184 and pRS591-rstA served as a positive control, while the combination of pACYC184 and pRS591-rluc without the promoter served as a negative control. To calculate the repression multiple of the novel rstA promoter mediated by RstR, as a positive control, the luminescence of the test strain at the same OD points was calculated by constructing the bivariate scatter plots with luminescence-OD values. The repression multiple of every RstR was obtained by dividing the luminescence of the positive control by the corresponding luminescence of the test. The repression curve of the rstA promoter was constructed with the OD value on the x axis and the repression multiple along the y axis.
In vitro and in vivo CTXETΦ infection assays.
Cm-resistant CTXETΦ (CTXΦc) was isolated from strain N-Φc as described previously (33, 40). For in vitro infection assays, V. cholerae strains were incubated without shaking in colonization factor broth (0.15% yeast extract, 1% casein hydrolysate, 50 ppm MgSO4, 5 ppm MnCl2, pH 6.8) at 30°C for 18 to 24 h, as described previously (33, 40). Control strain 1119, which is commonly used to validate the infectivity of CTXΦ (33, 40), and test strain VC06-18 were mixed with CTXΦc in triplicate and incubated without shaking at 30°C for 3 h, and the cells were then spread onto plates containing Cm. The in vivo CTXΦc infection was conducted as described previously (33). Briefly, as parallel controls, two New Zealand White rabbits (2 to 2.5 kg of body weight) were fasted for 24 h prior to surgery. Rabbits were anesthetized with ether. The small intestines were separated under sterile conditions and tied into 4-to-5-cm-long loops separated by 2 cm of interloops. Each loop was inoculated with the mixture of recipient V. cholerae and donor CTXΦc particles in triplicate. The V. cholerae strain and CTXΦc particles alone served as negative controls. The intestines were returned to the peritoneal cavity. The rabbits were sacrificed 16 to 18 h later. The small intestine was dissected, and each loop was washed with 1 ml of 0.9% saline solution. The number of CTXΦ-infected cells and the total number of cells were obtained by plating the ileal loop fluid onto LB agar plates containing Cm and onto antibiotic-free LB agar plates. Each colony was confirmed with antisera diagnostic for V. cholerae. Both experiments were performed three times.
Nucleotide sequence accession number.
Sequence data for V06-18 have been deposited in GenBank under accession number KP768424.
RESULTS AND DISCUSSION
Zhujiang estuarine strain VC06-18 carries a novel hybrid RS1.
We identified a pre-CTXZJΦ genome from 11 V. cholerae O1 nontoxigenic strains isolated during our Zhujiang estuarine water surveillance (27). The pre-CTXZJΦ carries a hybrid RS2 (rstRZJ rstA232 rstB232). The satellite phage RS1, which encodes an additional rstC gene compared to RS2, is also found in some V. cholerae strains (27). Here, we confirmed the presence of RS1 in the 11 strains carrying pre-CTXZJΦ by PCR and by sequencing rstC with the primers rstC1 and rstC2 (see Table S1 in the supplemental material) and the regions between pre-CTX ZJΦ and its integration site in the chromosome with combinations of primers rstRZJ1/rstRZJ2/zot1/zot2 targeting the rstRZJ and zot genes and InI/InJ/inA/inB targeting the integration site. Only strain VC06-18 has the RS1 region (data not shown), although the 11 strains have the same rstRZJ, rstA232, and rstB232 genes in the RS2 region (data not shown). Subsequently, we explored the organization of this RS1. Combinations of primers located in the rstRZJ, rstC, and zot genes (see Table S1) were used to amplify RS1 from strain VC06-18. A 4.3-kb fragment was amplified with primers rstC1 and zot1 (see Table S1) and sequenced. Based on the open reading frame (ORF) prediction and comparison, this RS1 was composed of ORFs rstR1, rstA1, rstB1, rstU, and rstC (Fig. 1B), and RstR1, RstA1, and RstB1 showed 21.4%, 11.6%, and 9.7% amino acid identity with RstR, RstA, and RstB of RS2 in its host VC06-18 strain (see Fig. S1, S2, and S3 in the supplemental material), respectively. We found that the amino acid sequence of RstC was highly conserved in all searched V. cholerae strains (see Fig. S4). RstA1 and RstB1 of VC06-18 RS1 showed 94.2% and 100% amino acid identity, respectively, with those of V. cholerae O1 El Tor strain MS6, which was isolated from Thailand-Myanmar in 2008 and carries CTXETΦ (34, 35), and 72.5% and 64.5% amino acid identity with those of O42 strain SCE264, which contains RS1 but lacks the CTX element (23) (see Fig. S1 and S2). In addition, no nucleotide or amino acid sequences similar to sequences of the rstA and rstB genes of VC06-18 were found by BLASTN or BLASTP in the whole-genome shotgun sequencing results seen with toxigenic V. cholerae O141 strain 234-93 (isolated in India in 1998), for which annotated rstA and rstB genes are unavailable (41). RstR1 of VC06-18 RS1 has 95.3% amino acid identity with a hypothetical helix-turn-helix family protein of strain 234-93, but it showed 20.4% identity with RstR1 of MS6 and also very little identity with other RstR and RstR1 sequences (see Fig. S3). In addition, a putative 117-amino-acid (aa) ORF located between rstB1 and rstC showed 100% nucleotide identity with the gene encoding fumarate reductase subunit D, which resides in the region between rstB1 and rstC of MS6 (data not shown). However, this is only a predicted ORF, and there is no other information about its function, but we have provisionally designated this ORF rstU (Fig. 1B). These analyses indicated that rstR1 and the rstA1, rstB1, and rstU genes of RS1 in strain VC06-18 derived from different V. cholerae strains, and even different serogroups, and formed a new RS1 variant with a conserved rstC gene.
FIG 1.
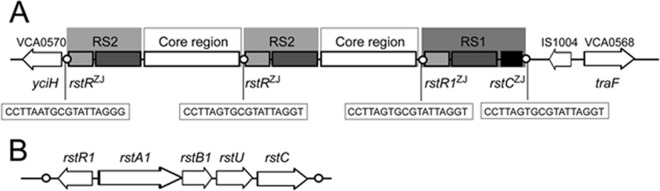
Genetic organization of pre-CTXZJΦ (A) and RS1 (B) in the small chromosome of strain VC06-18 (not drawn to scale). Circles represent integration site attB of pre-CTXΦ, and the sequences are shown in the rectangles.
Two copies of the pre-CTXZJΦ integrated in a tandem manner in the small chromosome of VC06-18.
CTXΦ/pre-CTXΦ integrates between the toxin-linked cryptic (TLC) element and RTX toxin loci in the large chromosome of epidemic strains or between the traF (VCA0570) and yciH (VCA0568) loci in the small chromosome of classical strains and in certain clinical El Tor strains (20, 42–45). To identify the integration site of pre-CTXZJΦ in strain VC06-18, PCR and sequencing were performed using primers InI/InJ and inA/inB (see Table S1 in the supplemental material), which span the attB sites in the small and large chromosomes of V. cholerae, respectively. An empty attB site was detected in the large chromosome but was absent from the small chromosome, implying that the pre-CTXZJΦ integrated into the small chromosome of strain VC06-18.
The CTXΦ/pre-CTXΦ element may be present in more than one copy in the V. cholerae chromosomes (46). We determined the copy number of the pre-CTXZJΦ element in strain VC06-18 with quantitative real-time PCR using the zot core region gene of CTXΦ/pre-CTXΦ and the thyA housekeeping gene of V. cholerae as the indicators. A single copy of thyA exists in V. cholerae based on the analysis of seven complete genomes (NC_016445, NC_016944, NC_017270, NC_012578, NC_012668, NC_002505, and NC_012582) and 159 shotgun whole genomes (data not shown). El Tor strain N16961, which contains one copy of the zot gene in its chromosome, was used as a control. The data were analyzed as described in Materials and Methods. The average copy number of the zot gene of strain VC06-18 was calculated to be 2.1 (95% confidence interval, 1.91 to 2.17); therefore, VC06-18 has two copies of zot and two copies of core regions of pre-CTXZJΦ.
Subsequently, the RS1, core, and RS2 regions of the pre-CTXZJΦ genome and the junction regions between the chromosome and the pre-CTXΦ genome/RS1 were amplified with combinations of primers located in these regions (see Table S1 in the supplemental material), and all amplicons were sequenced. By sequence comparison and assembly, we obtained the following information regarding the arrangement of pre-CTXZJΦ-RS1 in the chromosome of VC06-18. (i) The pre-CTXZJΦ-RS1 genome was integrated into the intergenic region between VCA0570 and VCA0568 in the small chromosome, with two tandem copies in the arrangement (Fig. 1A). (ii) The RS2 region is composed of rstRZJ, rstA232, and rstB232, as described in our previous report (27), and its core region contains the psh, cep, gIIICTX, ace, and zot genes and is thus quite similar to the core region of CTXETΦ. (iii) The region downstream of RS1 is adjacent to the second copy of pre-CTXZJΦ. We drew the physical map of pre-CTXZJΦ-RS1 with the pattern (rstRZJ-rstA232-rstB232-core region without ctxAB)2-rstR1-rstA1-rstB1-rstU-rstC in strain V06-18 (Fig. 1), which can be simplified as (RS2-core)2-RS1.
RS2 of pre-CTXZJΦ has replication but no integration function.
The rstA and rstB genes in the RS2 region are responsible for the replication and integration of CTXΦ (18). To study the replication and integration functions of the RS2 region of pre-CTXZJΦ, we constructed plasmids pKTN701-RS2ZJ (containing the RS2 region of pre-CTXZJΦ) and pKTN701-RS2ET (containing the RS2 region of CTXETΦ) as a control. Among the environmental V. cholerae strains isolated from the estuary waters of the Zhujiang River (27), strain VC06-41 (Table 1) has no CTX or pre-CTX element, has empty CTXΦ integration sites on both chromosomes, and carries the same tcpA gene as strain VC06-18 (detected by PCR and sequencing; data not shown). This strain was isolated from the same estuarine site as V06-18. Therefore, VC06-41 was chosen to be the donor strain for this experiment. Plasmids pKTN701-RS2ZJ and pKTN701-RS2ET were introduced into strain VC06-41 by conjugation. Sixty-five colonies of Cmr transconjugants were picked randomly and confirmed to contain pKTN701-RS2ZJ by PCR with primers RS1/RS2 and plasmid extraction. However, no pKTN701-RS2ZJ was found to integrate in the large and/or small chromosomes of VC06-41 by PCR with primers inI/inJ and inA/inB and by sequencing. In contrast, chromosomal integration of pKTN701-RS2ET in the transconjugants was confirmed. Among 65 randomly selected transconjugants, 16, 40, and 9 demonstrated pKTN701-RS2ET integration in the large and/or small chromosomes. This result demonstrated that RS2 of pre-CTXZJΦ can mediate replication but not integration. It seems that the integration function of RS2 in pre-CTXZJΦ is lost. Whether rstB in this pre-CTXZJΦ is dysfunctional or the integration is recipient dependent still needs to be defined. In comparison to the results seen with CTXETΦ, infection of the recipient V. cholerae strain with pre-CTXZJΦ is less stable because the pre-CTXZJΦ remains in plasmid form rather than undergoing chromosomal integration.
Repression of the rstA promoter in pre-CTXZJΦ has an rstR allele specificity.
In CTXΦ, repression of rstAB transcription by RstR is biotype specific (22, 28), which confers immunity to CTXΦ superinfection. In this study, we investigated whether the expression of rstAB of VC06-18 could be repressed by other RstR alleles. pRS591-rstAZJ, which contains an rluc transcriptional fusion under the control of the rstA promoter of pre-CTXZJΦ, was constructed as the reporter plasmid. Six pACYC184 plasmid derivatives containing rstR genes (rstRclass, rstRET, rstRcalc, rstR6, rstR-232, and rstRZJ) and their promoters were constructed. Each of these six plasmids was combined with pRS591-rstAZJ and introduced into E. coli JM109. Luminescence activity was measured, and the curves of luminescence activity relative to the OD for each plasmid combination showed that the activity of the pACYC184-rstRZJ/pRS591-rstAZJ combination was lower than that of all other combinations tested except for the negative-control pACYC184/pRS591-rluc combination (Fig. 2A). RstRZJ repressed the expression of rluc in pRS591-rstAZJ by nearly 3.5-fold, but the other five alleles of RstR did not clearly repress the expression of rluc (Fig. 2B). This result indicated that RstRZJ strongly represses only the transcription of its own rstAB and that other RstRs with different sequence types have a quite diminished capacity to inhibit the transcription of RstAZJ. It can be deduced that pre-CTXZJΦ or CTXZJΦ can still infect the V. cholerae strains carrying a pre-CTX/CTX element with other sequence types of rstR.
FIG 2.
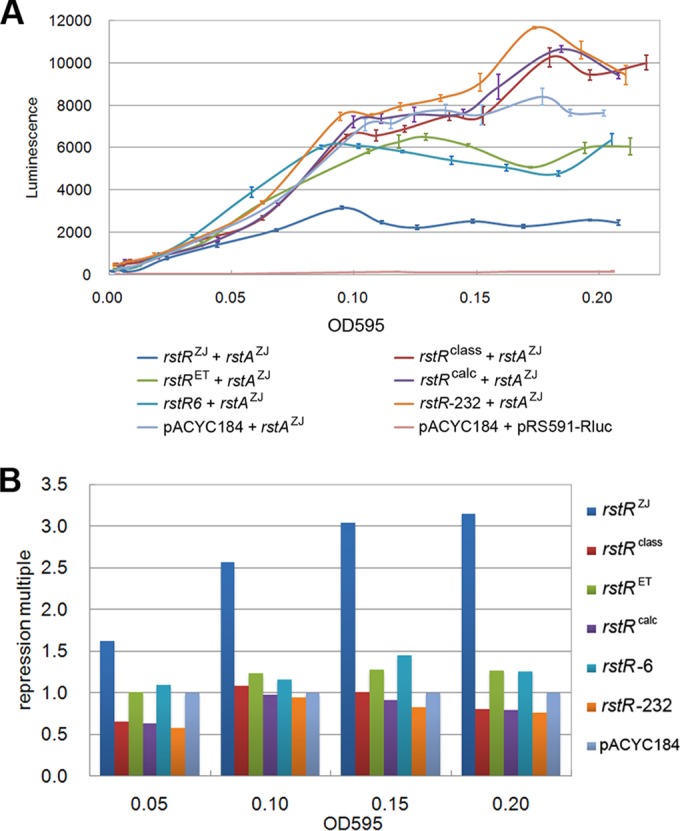
Specific repression of the rstAZJ promoter by RstRZJ. (A) Luminescence values generated under the control of the rstAZJ promoter combined with different rstRs during culture with different OD595 values. (B) Repression presented with alternation of repression multiples under conditions of different OD595 values. Each value represents the mean of the results from six independent experiments. Error bars denote the standard deviation.
CTXETΦ converts pre-CTXZJΦ lysogen VC06-18 into a toxigenic strain.
Infection and lysogenic conversion by CTXΦ may result in the emergence of new toxigenic strains or clones, and the reversion of live-attenuated V. cholerae vaccine strains to toxigenicity is a possibility (22). Lysogenic strains with pre-CTXZJΦ, such as VC06-18, were more frequently isolated during our environmental surveillance. Whether such strains could be converted to toxigenicity by CTXΦ should be considered. The seventh-pandemic El Tor strains and toxigenic O139 strains carried CTXETΦ, the most common prophage of the current epidemic strains; therefore, we experimentally tested whether VC06-18 could be infected by this phage. In the in vitro experiments, strain VC06-18 was not found to be infected by CTXΦc, which is consistent with other El Tor strains (33), while the average infection rate (average number of CTXΦ-infected cells/100 total cells) of control strain 1119 was 3.09 × 10−5 with a 95% confidence interval (CI) of 1.09 × 10−4 to 8.75 × 10−6. When these strains were tested in rabbit ileal loops, CTXΦc could infect VC06-18 with an average infection rate of 2.6 × 10−7 (95% CI, 2.56 × 10−6 to 7.29 × 10−8) and strain 1119 with a rate 4.68 × 10−4 (95% CI, 2.56 × 10−4 to 5.84 × 10−5), showing that VC06-18 could still be infected by El Tor type CTXΦ in vivo even though it carries the prophage pre-CTXZJΦ. These results may also suggest that RstR of pre-CTXZJΦ does not effectively inhibit the transcription of rstA and rstB of CTXETΦ and permits the infection of the latter. Strains carrying pre-CTXZJΦ are predominant in estuarine water (27); therefore, it could be predicted that, during the coexistence of this type of strain and toxigenic El Tor (or O139) strains in a specific microenvironment such as a biofilm on plankton or in human intestines, these strains could obtain CTXETΦ released from the toxigenic strains and could become toxigenic, thus generating a new pathogenic or epidemic clone.
In conclusion, we describe a pre-CTXZJΦ genome with a novel hybrid RS1. Divergence of CTXΦ/pre-CTXΦ genomes showed dynamic genomic recombination in the evolution of the CTXΦ family. Because phage immunity to CTXΦ superinfection mediated by RstR is limited by its sequence, pre-CTXΦ alleles can play roles in the complexity of the pre-CTXΦ family through coinfection and subsequent genomic recombination among the different alleles, cause the emergence of new CTXΦ alleles, and further increase the complexity of toxigenic V. cholerae clones. Pre-CTXZJΦ is not rare, because environmental strains carrying this prophage were isolated from different surveillance months in the Zhujiang estuary. The strain carrying pre-CTXZJΦ has no resistance to infection by CTXETΦ. Therefore, toxigenic conversion of these strains should be surveyed, although they have already integrated the pre-CTXΦ allele. In addition, based on the unique or uncommon prophage arrays of CTXΦ and pre-CTXΦ and their rstR sequences, these structures or sequence types may also be used as epidemiological genetic markers to trace the spread of specific V. cholerae clones.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Priority Project on Infectious Disease Control and Prevention (2012ZX10004215 and 2008ZX10004-008) of the Ministry of Health, China, the National Basic Research Priorities Program (2009CB522604), the Key Project of National Natural Science Foundation of China (NSFC; 30830008) and the NSFC project (30872260).
We are grateful to Pengcheng Du for assistance in sequence analysis of the thyA gene.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01742-15.
REFERENCES
- 1.Faruque SM, Albert MJ, Mekalanos JJ. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 62:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M, Shimada T, Morris JG Jr, Sulakvelidze A, Sozhamannan S. 2002. Evidence for the emergence of non-O1 and non-O139 Vibrio cholerae strains with pathogenic potential by exchange of O-antigen biosynthesis regions. Infect Immun 70:2441–2453. doi: 10.1128/IAI.70.5.2441-2453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JG Jr, Takeda T, Tall BD, Losonsky GA, Bhattacharya SK, Forrest BD, Kay BA, Nishibuchi M. 1990. Experimental non-O group 1 Vibrio cholerae gastroenteritis in humans. J Clin Invest 85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaolis DK, Lan R, Reeves PR. 1995. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol 177:3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon YS, Kim DW, Lee JH, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, Croucher NJ, Choi SY, Harris SR, Lebens M, Niyogi SK, Kim EJ, Ramamurthy T, Chun J, Wood JL, Clemens JD, Czerkinsky C, Nair GB, Holmgren J, Parkhill J, Dougan G. 2011. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477:462–465. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med 168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faruque SM, Asadulghani MN, Saha MN, Alim AR, Albert MJ, Islam KM, Mekalanos JJ. 1998. Analysis of clinical and environmental strains of nontoxigenic Vibrio cholerae for susceptibility to CTXPhi: molecular basis for origination of new strains with epidemic potential. Infect Immun 66:5819–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldor MK, Mekalanos JJ. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 10.Faruque SM, Asadulghani AR, Alim AR, Albert MJ, Islam KM, Mekalanos JJ. 1998. Induction of the lysogenic phage encoding cholera toxin in naturally occurring strains of toxigenic Vibrio cholerae O1 and O139. Infect Immun 66:3752–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber KE, Waldor MK. 2002. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 417:656–659. doi: 10.1038/nature00782. [DOI] [PubMed] [Google Scholar]
- 12.Lazar S, Waldor MK. 1998. ToxR-independent expression of cholera toxin from the replicative form of CTXphi. Infect Immun 66:394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A 84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaolis DK, Johnson JA, Bailey CC, Boedeker EC, Kaper JB, Reeves PR. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci U S A 95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovach ME, Shaffer MD, Peterson KM. 1996. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology 142:2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 16.Boyd EF, Heilpern AJ, Waldor MK. 2000. Molecular analyses of a putative CTXΦ precursor and evidence for independent acquisition of distinct CTXΦs by toxigenic Vibrio cholerae. J Bacteriol 182:5530–5538. doi: 10.1128/JB.182.19.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kan B, Qi G, Liu Y, Liu C, Gao S. 1999. Genome of bacteriophage CTXΦ without the presence of ctxAB exists in ctxAB− strains of Vibrio cholerae. Chin J Microbiol Immunol 3:175–179. [Google Scholar]
- 18.Waldor MK, Rubin EJ, Pearson GD, Kimsey H, Mekalanos JJ. 1997. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol 24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 19.Davis BM, Kimsey HH, Kane AV, Waldor MK. 2002. A satellite phage-encoded antirepressor induces repressor aggregation and cholera toxin gene transfer. EMBO J 21:4240–4249. doi: 10.1093/emboj/cdf427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis BM, Moyer KE, Boyd EF, Waldor MK. 2000. CTX prophages in classical biotype Vibrio cholerae: functional phage genes but dysfunctional phage genomes. J Bacteriol 182:6992–6998. doi: 10.1128/JB.182.24.6992-6998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BM, Kimsey HH, Chang W, Waldor MK. 1999. The Vibrio cholerae O139 Calcutta bacteriophage CTXΦ is infectious and encodes a novel repressor. J Bacteriol 181:6779–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimsey HH, Waldor MK. 1998. CTXΦ immunity: application in the development of cholera vaccines. Proc Natl Acad Sci U S A 95:7035–7039. doi: 10.1073/pnas.95.12.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay AK, Chakraborty S, Takeda Y, Nair GB, Berg DE. 2001. Characterization of VPI pathogenicity island and CTXΦ prophage in environmental strains of Vibrio cholerae. J Bacteriol 183:4737–4746. doi: 10.1128/JB.183.16.4737-4746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Kotetishvili M, Chen Y, Sozhamannan S. 2003. Comparative genomic analyses of the vibrio pathogenicity island and cholera toxin prophage regions in nonepidemic serogroup strains of Vibrio cholerae. Appl Environ Microbiol 69:1728–1738. doi: 10.1128/AEM.69.3.1728-1738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rui Y, Kan B, Gao S, Liu Y, Qi G. 2006. Cloning, sequencing and analysis of CTXETΦ and nct-CTXnewO139Φ genomes of Vibrio cholerae JS9803. J Trop Med 6:861–864. [Google Scholar]
- 26.Maiti D, Das B, Saha A, Nandy RK, Nair GB, Bhadra RK. 2006. Genetic organization of pre-CTX and CTX prophages in the genome of an environmental Vibrio cholerae non-O1, non-O139 strain. Microbiology 152:3633–3641. doi: 10.1099/mic.0.2006/000117-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Wang X, Li B, Deng X, Tan H, Diao B, Chen J, Ke B, Zhong H, Zhou H, Ke C, Kan B. 2014. High prevalence and diversity of pre-CTXPhi alleles in the environmental Vibrio cholerae O1 and O139 strains in the Zhujiang River estuary. Environ Microbiol Rep 6:251–258. doi: 10.1111/1758-2229.12121. [DOI] [PubMed] [Google Scholar]
- 28.Kimsey HH, Waldor MK. 2004. The CTXΦ repressor RstR binds DNA cooperatively to form tetrameric repressor-operator complexes. J Biol Chem 279:2640–2647. doi: 10.1074/jbc.M311109200. [DOI] [PubMed] [Google Scholar]
- 29.Liang W, Wang S, Yu F, Zhang L, Qi G, Liu Y, Gao S, Kan B. 2003. Construction and evaluation of a safe, live, oral Vibrio cholerae vaccine candidate, IEM108. Infect Immun 71:5498–5504. doi: 10.1128/IAI.71.10.5498-5504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya T, Chatterjee S, Maiti D, Bhadra RK, Takeda Y, Nair GB, Nandy RK. 2006. Molecular analysis of the rstR and orfU genes of the CTX prophages integrated in the small chromosomes of environmental Vibrio cholerae non-O1, non-O139 strains. Environ Microbiol 8:526–634. doi: 10.1111/j.1462-2920.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 31.Kimsey HH, Nair GB, Ghosh A, Waldor MK. 1998. Diverse CTXphis and evolution of new pathogenic Vibrio cholerae. Lancet 352:457–458. doi: 10.1016/S0140-6736(05)79193-5. [DOI] [PubMed] [Google Scholar]
- 32.Nusrin S, Khan GY, Bhuiyan NA, Ansaruzzaman M, Hossain MA, Safa A, Khan R, Faruque SM, Sack DA, Hamabata T, Takeda Y, Nair GB. 2004. Diverse CTX phages among toxigenic Vibrio cholerae O1 and O139 strains isolated between 1994 and 2002 in an area where cholera is endemic in Bangladesh. J Clin Microbiol 42:5854–5856. doi: 10.1128/JCM.42.12.5854-5856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Yan M, Liang W, Qi G, Liu Y, Gao S, Kan B. 2006. Resistance of the cholera vaccine candidate IEM108 against CTXΦ infection. Vaccine 24:1749–1755. doi: 10.1016/j.vaccine.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 34.Okada K, Roobthaisong A, Swaddiwudhipong W, Hamada S, Chantaroj S. 2013. Vibrio cholerae O1 isolate with novel genetic background, Thailand-Myanmar. Emerg Infect Dis 19:1015–1017. doi: 10.3201/eid1906.120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada K, Na-Ubol M, Natakuathung W, Roobthaisong A, Maruyama F, Nakagawa I, Chantaroj S, Hamada S. 2014. Comparative genomic characterization of a Thailand-Myanmar isolate, MS6, of Vibrio cholerae O1 El Tor, which is phylogenetically related to a “US Gulf Coast” clone. PLoS One 9:e98120. doi: 10.1371/journal.pone.0098120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons RW, Houman F, Kleckner N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 37.Nishibuchi M, Kumagai K, Kaper JB. 1991. Contribution of the tdh1 gene of Kanagawa phenomenon-positive Vibrio parahaemolyticus to production of extracellular thermostable direct hemolysin. Microb Pathog 11:453–460. doi: 10.1016/0882-4010(91)90042-9. [DOI] [PubMed] [Google Scholar]
- 38.Ingham DJ, Beer S, Money S, Hansen G. 2001. Quantitative real-time PCR assay for determining transgene copy number in transformed plants. Biotechniques 31:132–134, 136. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Liang W, Kan B, Qi G, Liu Y, Gao S. 2003. Induction of lysogenic bacteriophage CTXΦ in Vibrio cholerae O139 strain and its transduction. Chin J Microbiol Immunol 23:53–56. [Google Scholar]
- 41.Bishop-Lilly KA, Johnson SL, Verratti K, Luu T, Khiani A, Awosika J, Mokashi VP, Chain PS, Sozhamannan S. 2014. Genome sequencing of 15 clinical Vibrio isolates, including 13 non-o1/non-o139 serogroup strains. Genome Announc 2:e00893-14. doi: 10.1128/genomeA.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, Gill SR, Nelson KE, Read TD, Tettelin H, Richardson D, Ermolaeva MD, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann RD, Nierman WC, White O, Salzberg SL, Smith HO, Colwell RR, Mekalanos JJ, Venter JC, Fraser CM. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin W, Fullner KJ, Clayton R, Sexton JA, Rogers MB, Calia KE, Calderwood SB, Fraser C, Mekalanos JJ. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci U S A 96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nandi S, Maiti D, Saha A, Bhadra RK. 2003. Genesis of variants of Vibrio cholerae O1 biotype El Tor: role of the CTXΦ array and its position in the genome. Microbiology 149:89–97. doi: 10.1099/mic.0.25599-0. [DOI] [PubMed] [Google Scholar]
- 45.Rubin EJ, Lin W, Mekalanos JJ, Waldor MK. 1998. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Mol Microbiol 28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 46.Mekalanos JJ. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:253–263.46. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


