Abstract
Truffles (Tuber spp.) are ascomycete subterraneous fungi that form ectomycorrhizas in a symbiotic relationship with plant roots. Their fruiting bodies are appreciated for their distinctive aroma, which might be partially derived from microbes. Indeed, truffle fruiting bodies are colonized by a diverse microbial community made up of bacteria, yeasts, guest filamentous fungi, and viruses. The aim of this minireview is two-fold. First, the current knowledge on the microbial community composition of truffles has been synthesized to highlight similarities and differences among four truffle (Tuber) species (T. magnatum, T. melanosporum, T. aestivum, and T. borchii) at various stages of their life cycle. Second, the potential role of the microbiome in truffle aroma formation has been addressed for the same four species. Our results suggest that on one hand, odorants, which are common to many truffle species, might be of mixed truffle and microbial origin, while on the other hand, less common odorants might be derived from microbes only. They also highlight that bacteria, the dominant group in the microbiome of the truffle, might also be the most important contributors to truffle aroma not only in T. borchii, as already demonstrated, but also in T. magnatum, T. aestivum, and T. melanosporum.
INTRODUCTION
Microbes can be found almost everywhere on our planet. They colonize many different types of habitats, among them living organisms, such as plant roots or insect and human guts. Classical microbiological methods have long offered a spotlight view on microbial diversity. Recent high-throughput molecular techniques have revolutionized the field of microbial ecology by unraveling an enormous microbial diversity in numerous organisms and highlighting the deep impact of microbiomes of their host physiology and behavior (1, 2). Truffle fungi are no exception, since they are colonized by a complex microbial community made up of bacteria, yeasts, guest filamentous fungi, and viruses (3–14).
Truffles are subterranean ascomycete fungi that form ectomycorrhizas in symbiotic relationship with plant roots (15). Their fruiting bodies are appreciated for their distinctive aroma, which is partially derived from microbes (6, 14, 16). The aim of this minireview is to synthesize the current knowledge on the composition of the microbial community of truffles and discuss their potential role in truffle aroma formation, specifically focusing on volatiles that are responsible for human-perceived truffle aroma (defined as odorants).
TRUFFLE MICROBIOMES
Truffles are colonized by microbes at all stages of their life cycle, which include a symbiotic stage in association with a host plant (ectomycorrhiza), a sexual stage (fruiting bodies), and a free-living mycelial stage, which might serve an exploratory purpose in the soil. To date, microbes and microbial communities have been characterized in truffles with culture-dependent and -independent techniques in >15 papers (3–14, 17–21). Various life cycle stages of four commercially relevant Tuber species have been investigated: the white truffles T. magnatum and T. borchii and the black species T. melanosporum and T. aestivum. Similarities and differences in the compositions of the microbial community of truffle species are highlighted here for bacteria, fungi (yeast and filamentous), and viruses.
BACTERIAL COMMUNITIES
Most studies investigating microbes in truffles have been performed on bacteria. These bacteria can heavily colonize the inner and outer parts of truffle fruiting bodies, as their densities range from a million to a billion cells per gram (dry weight) of fruiting bodies (4, 5, 19–22). The aims of these studies ranged from the characterization of taxonomic and/or functional community composition to the influence of specific variables. Indeed, bacterial community composition has been investigated in relation to fruiting body maturation, aging, season or life cycle (i.e., mycorrhizas versus fruiting body), and tissue specificity (the gleba [the inner part of the fruiting bodies] versus the peridium [the outer protective layer]).
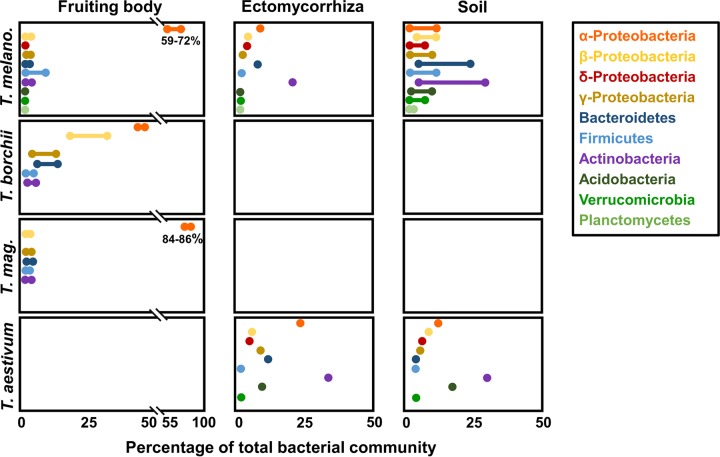
Combinations of culture-dependent and -independent methods have demonstrated that all truffle species analyzed so far are colonized by complex bacterial communities made mostly of Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria (Fig. 1) (4, 5, 12–14, 19). Similarities among fruiting bodies of the three truffle species investigated to date include a dominance of Alphaproteobacteria and a relative paucity of Firmicutes and Actinobacteria. On the contrary, differences among truffle species might exist for Betaproteobacteria, Gammaproteobacteria, and Bacteroidetes, which might be more abundant in T. borchii than in T. melanosporum and T. magnatum (Fig. 1). As a matter of fact, a Bacteroidetes strain might even coexist inside T. borchii mycelia grown under axenic laboratory conditions (18), suggesting a possible tight association between bacteria and truffles. The occurrence of endosymbionts has not been described so far in other truffle species.
FIG 1.
Bacterial communities in fruiting bodies, ectomycorrhizas, and soil. The most abundant bacterial communities associated with four truffle species based on culture-independent methods are shown. The bars represent the minimum and maximum values reported in the literature, whereas the points display a single literature value (T. aestivum, reference 12; T. magnatum [T. mag.], reference 5; T. borchii, references 4 and 14; and T. melanosporum [T. melano.], reference 24 [and for the period from December to January, reference 13 for the gleba). Cells for which no literature data were available were left empty.
The bacterial community composition of truffle fruiting bodies might evolve over time and in relation to the physiology of the truffle host. Indeed, truffle fruiting bodies mature as their inner part (gleba) undergoes melanization due to the spore-forming process taking place inside the fungal asci. This maturation/melanization process generally lasts a few months and occurs in late autumn/winter for T. borchii, T. melanosporum, and T. magnatum harvested in Europe. Using fluorescence in situ hybridization (FISH) in the latter species, a slight but significant decrease in total bacterial count was observed with increasing maturity; nevertheless, no difference in the relative community composition was detectable for Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Bacteroidetes, Firmicutes, or Actinobacteria (5). A different pattern was observed in T. melanosporum using high-throughput sequencing methods (13). The composition of the bacterial community present inside the gleba and in the peridium significantly changed along the course of the maturation of the ascocarps. The community composition in the peridium was very close to that of the soil community in young ascocarps but strongly diverged from the soil community in mature ascocarps. The differences were mainly in the peridium, due to a progressive increase in the abundance of Bacteroidetes and Alphaproteobacteria, while the abundance of Betaproteobacteria members decreased. In contrast, the glebal bacterial community was dominated very early by Alphaproteobacteria. Moreover, this dominance kept increasing with the maturity level, just as it did in the peridium. All together, these data prompted Antony-Babu et al. (13) to propose the following model: soil bacteria would colonize truffle primordia before the differentiation of ascocarpic tissues would occur. Next, the bacteria would be trapped in the gleba and partly protected from soil exchanges by the warted peridium. Because of this compartmentalization, bacterial community composition would mainly evolve in response to changes in the physiology of the maturing ascocarp. In contrast, the peridium would remain in contact with the soil all along the development process of the ascocarp, due to cracks that open during growth of the ascocarp (13).
In addition to natural variations, the harvest of truffle fruiting bodies is likely to induce changes in the composition of the associated bacterial community. This might be due to modifications in physicochemical parameters, such as temperature and CO2 level (23). For example, Splivallo et al. (14) observed the appearance of colonies belonging to Firmicutes and Actinobacteria, while the abundance of members of Alphaproteobacteria and Betaproteobacteria decreased in fruiting bodies of T. borchii after 6 days of postharvest storage at room temperature (14).
The composition of bacterial communities associated with truffles is influenced not only by the stage of maturity of the fruiting bodies but also by the stage of the life cycle of the fungus. Comparative analysis of the bacterial communities associated with fruiting bodies and ectomycorrhizae (EcM) of T. melanosporum showed striking differences, suggesting that the fungus might provide two different habitats to bacteria. For example, Actinobacteria are dominant in EcM but rare in fruiting bodies of T. melanosporum (13). Interestingly, enrichment in several genera of Actinobacteria has also been demonstrated for specific zones within orchards of T. melanosporum, referred to as brûlés (24), which are especially rich in truffle mycelia (25).
Overall, these observations demonstrate that truffles provide several habitats to complex bacterial communities. Among the Alphaproteobacteria, members of the Bradyrhizobiaceae and Rhizobiaceae families mainly seem to form the core component of these communities, whatever the truffle species considered. The parameters that control the selection of this very specific community are still to be discovered. A tempting hypothesis is that truffle fruiting bodies would be more than a habitat for bacteria and that mutualistic interactions might occur between the fungi and their microbiota. Some members of the Rhizobiales order are well known for their ability to fix atmospheric nitrogen either as free-living organisms or in symbiosis with plants (26). Barbieri et al. (27) demonstrated that nitrogen fixation occurs inside fruiting bodies of the white truffle T. magnatum. Nif genes encoding the enzymes responsible for nitrogen fixation were also detected in bacteria associated with T. melanosporum (13). Thus, it is tempting to speculate that part of the nitrogen captured by bacteria in fruiting bodies might benefit the host fungus. However, it remains to be demonstrated that the nitrogen fixed by bacteria inside truffle fruiting bodies is indeed transferred to the fungus.
YEAST COMMUNITIES
Besides bacteria, yeasts are ubiquitous organisms that occupy most terrestrial ecological niches. Yeast community composition has been investigated in fruiting bodies (T. aestivum, T. melanosporum, and T. magnatum), ectomycorrhizas, and truffle orchard soil (T. aestivum) (3, 6, 21). These studies were based on culture-dependent methods and might hence miss the real diversity that exists; nevertheless, they also do provide useful insights. In a study comparing yeast distribution within an orchard of T. aestivum, Zacchi et al. (3) demonstrated that yeasts were enriched on truffle ectomycorrhizas and fruiting bodies, reaching up to 3 × 107 CFU/g of fruiting bodies (dry weight) compared to that in bulk soil (1 × 102 CFU/g of fruiting bodies [dry weight]). The total yeast diversity was made of five species, namely, Cryptococcus albidus, Cryptococcus humicola, Rhodotorula mucilaginosa, Debaryomyces hansenii, and Saccharomyces paradoxus (3). Interestingly, Cryptococcus spp., R. mucilaginosa, D. hansenii, and Saccharomyces spp. were also isolated by others (6, 21) from T. melanosporum, T. magnatum, or T. aestivum and might therefore be common to distinct truffle species. Yeast density might also vary between the peridium and gleba. Indeed, based on culture-dependent methods, yeasts were isolated only from the peridium of T. aestivum and T. melanosporum (103 to 104 CFU/g of fruiting bodies [fresh weight]) but not from the gleba of intact truffles (21).
These observations suggest that, as with bacteria, yeast community composition might vary with tissues, and a “core yeast community” might exist among truffle species. Culture-independent techniques will nevertheless be necessary to confirm these hypotheses and get a better view of the variability in space and time of the yeast communities associated with truffles.
GUEST FILAMENTOUS FUNGI AND VIRUSES IN TRUFFLES
Besides being colonized by yeasts and bacteria, truffles may also be colonized by filamentous fungi (guest filamentous fungi as opposed to the host truffle mycelia) and viruses. As in the case of yeasts, only a few reports exist on the occurrence of viruses in truffles. Guest filamentous fungi, mostly ascomycetes, have been isolated from the Tuber species T. rufum, T. brumale, T. magnatum, T. melanosporum, T. nitidum, T. excavatum, T. aestivum, T. borchii, and T. puberulum (7). However, their occurrence in fruiting bodies might be seldom, since guest filamentous fungi were isolated from only 26% of all truffles (n = 30), suggesting a loose association. The density of guest filamentous fungi might vary between the gleba and peridium. In T. melanosporum and T. aestivum, corresponding with what has been observed for yeasts, guest filamentous fungi (ascomycete molds) predominantly colonized the peridium, with a density of 102 CFU/g of fruiting bodies (fresh weight), but they seem to be absent from the gleba (21). Similarly, a recent report described the occurrence of viruses (Totivirus, Mitovirus, and Endornavirus from T. aestivum and Mitovirus from T. excavatum) without, however, addressing their frequency of occurrence within fruiting bodies or in orchards (8–11). Some authors have also suggested viral gene integration in the genome of T. melanosporum (28). Surely, guest filamentous fungi and viruses might interact with truffles in nature; however, additional ecological data are needed at this stage to understand how frequently they might occur and to assess how relevant they are in the microbiome of truffles.
INVOLVEMENT OF MICROBES IN PRODUCTION OF AROMA THAT HUMANS PERCEIVE AS THE TRUFFLE SMELL
Unique and delightful aromas are partially responsible for the high demand of truffles in the world market. The particular aromas of truffles are made up of a mixture of various volatiles, namely alcohols, esters, ketones, aldehydes, and aromatic and sulfur compounds. To date, the number of identified volatiles from various truffle species is >200; however, only a small fraction of these, the so-called odorants, are responsible for what humans perceive as the truffle smell (16, 29, 30).
Historically, the aroma of the white truffle T. magnatum was the first one characterized and ascribed to a single sulfur compound (2,4-dithiapentane) (31). In the 1980s, a mixture of two constituents, 2-methylbutanal and dimethyl sulfide, were patented to reproduce the smell of the Périgord truffle T. melanosporum (32). Essentially, due to increasingly sensitive techniques in sensory science, the number of key odorants in T. melanosporum was recently revised to >15 volatiles (29). A comparable number of odorants (about 10 to 20) have also been described in four black truffle Tuber species (T. aestivum, T. himalayense, T. indicum, and T. sinense [29, 30]) and in the white truffle T. borchii (16). Interestingly, most of these odorants are common to almost all truffle species (i.e., methylthiomethane, 3-methyl-1-butanol, and oct-1-en-3-ol), and only a few are species specific or occur in a rather small number of species (i.e., thiophene derivatives and 2,4-dithiapentane) (Fig. 2). The exact origin of truffle volatiles and specifically of most odorants reported in Fig. 2 is unclear. It has been speculated that truffle aroma might result from the intimate interaction of truffles and their microbiomes (6, 33, 34). Indeed, some volatiles might be produced by both truffles and microbes, while others might be derived from a single player (i.e., yeasts, bacteria, or truffles). Only recently has the role of bacteria in the formation of thiophene derivatives, odorants unique to T. borchii, been demonstrated. In the latter species, only bacteria and not truffles metabolize a precursor of unknown origin into volatile thiophene derivatives (14). As the matter of fact, the biosynthetic pathway leading to thiophene derivatives remains elusive (14), and this is also the case for 2,4-dithiapentane, the major odorant of T. magnatum. In contrast, based on the genome of T. melanosporum, pathways leading to odorants commonly produced by yeasts and bacteria most likely exist in truffles as well (34, 35). This is the case, for example, for the Ehrlich pathway, which consists of the catabolism of specific amino acids and results in dimethyl sulfide, 2-phenylethanol, 2- and 3-methylbutanol, and numerous other volatiles common to microbes and truffles (35). The Ehrlich pathway consists of a three-step process involving the initial transamination of an amino acid, followed by decarboxylation and reduction steps (36). Indeed, enzymes fulfilling these steps most likely exist in T. melanosporum (34, 35); their functions have nevertheless not yet been demonstrated. At this stage, however, genomes provide limited insights on the possible identity of the producer of specific odorants, because either the pathways leading to those odorants are highly conserved among yeasts, bacteria, and truffles (i.e., the Ehrlich pathway) or the biosynthetic pathways are not known (i.e., thiophene derivatives and 2,4-dithiapentane).
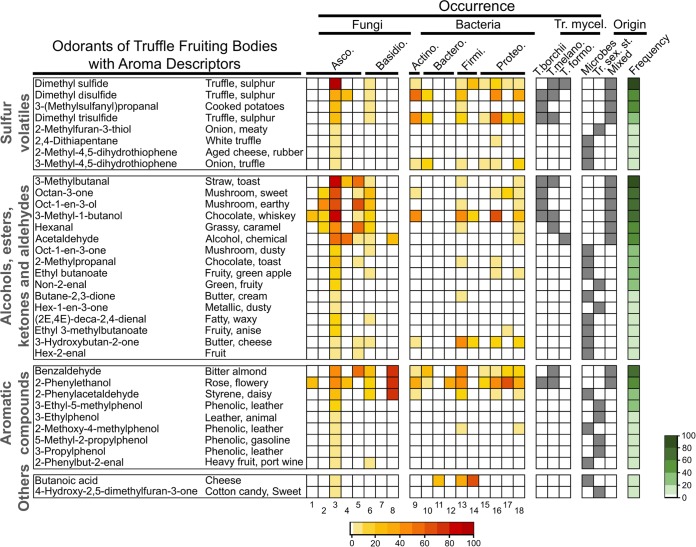
FIG 2.
Ability of microbes to produce typical odorants of truffle fruiting bodies. List of odorants and aroma descriptors from T. melanosporum (T. melano.) and T. aestivum (29), T. indicum, T. himalayense, and T. sinense (30), T. borchii (16), and T. magnatum (31). Occurrences in fungal and bacterial phyla/classes are derived from the mVOC database (37) and the data from a review on fungal volatiles (38). They are shown as a heatmap representing the percent occurrence in each class, with n being the total number of organisms in each class (for Ascomycetes [Asco.]: 1, Dothideomycetes [n = 4]; 2, Eurotiomycetes [n = 29]; 3, Pezizomycetes [n = 26]; 4, Saccharomycetes [n = 4]; and 5, Sordariomycetes [n = 47]; for Basidiomycetes [Basidio.]: 6, Agaricomycetes [n = 135]; 7, Exobasidiomycetes [n = 3]; and 8, Pucciniomycetes [n = 4]; for Actinomycetes [Actino.]: 9, Actinobacteria [n = 62]; for Bacteroidetes [Bactero.]: 10, Bacteroidetes [n = 17]; 11, Bacteroidia [n = 24]; and 12, Flavobacteria and Sphingobacteria [n = 3]; for Firmicutes [Firmi.]: 13, Bacilli [n = 55]; and 14, Clostridia [n = 10]; for Proteobacteria [Proteo.]: 15, Alphaproteobacteria [n = 25]; 16, Betaproteobacteria [n = 43]; 17, Deltaproteobacteria [n = 16]; and 18, Gammaproteobacteria [n = 61]). Occurrence in axenic cultures of truffle (Tr. mycel.) is shown as the presence/absence for T. borchii (33, 40, 48), T. melanosporum (41), T. formosanum (T. formo.) (42). Origin refers to the speculative origin of the odorants in truffle fruiting bodies, where some odorants could be produced by microbes only (microbes), by truffle only at its sexual stage (Tr. sex. st.), or by both microbes and truffles (mixed). The frequency represents the percent occurrence of each odorant in fruiting bodies of 13 truffle species (T. aestivum, T. brumale, T. himalayense, T. indicum, T. sinense, T. melanosporum, Tuber mesentericum, T. borchii, T. excavatum, T. magnatum, Tuber oligospermum, Tuber panniferum, and T. rufum [16, 29–31, 37]).
By combining knowledge about the structure of truffle microbiomes (Fig. 1) with literature data on the ability of specific microbes to produce odorants, we speculate here on the origin of these volatiles in truffles. For this purpose, we first established a list of all odorants described in truffles and reported in four publications (16, 29–31). We then used the mVOC database (37) and the data from a review on fungal volatiles (38) to understand which organisms had the ability to produce those volatiles, specifically focusing on the phyla and classes reported in Fig. 1. For the purpose of this review, volatile occurrence is expressed for bacterial and fungal phyla/classes and presented as a heatmap in Fig. 2.
SULFUR-CONTAINING VOLATILES
Sulfur-containing volatiles (sulfur volatiles) represent the most important group of odorants in truffles, since they confer the typical garlicky and sulfurous notes that characterize all truffle species (see the aroma descriptors in Fig. 2). The most common sulfur-containing volatile in truffle fruiting bodies is dimethyl sulfide, which has been detected in 85% of the species investigated to date (Fig. 2). Along with dimethyl disulfide, dimethyl trisulfide, and 3-(methylsulfanyl)propanal, dimethyl sulfide might be derived from the catabolism of methionine through the Ehrlich pathway (36, 39, 48). According to the mVOC database on microbial volatiles (37), the last four volatiles occur in the fungal classes of Pezizomycetes (i.e., truffles) and Agaricomycetes and in eight bacterial classes (Fig. 2). Since most of these volatiles are also produced by axenic cultures of truffle mycelia (40–42, 48), they might be synthesized in truffle fruiting bodies by both bacteria and truffle mycelia. Of special interest is dimethyl sulfide, since it might be produced by some Alphaproteobacteria and Betaproteobacteria (Fig. 2), which are also dominant in truffle fruiting bodies (Fig. 1).
In contrast to the relatively common sulfur volatiles just described, other sulfur odorants might be more specific (i.e., specific to a single or a limited number of species). Four sulfur volatiles, namely, 2-methyl-4,5-dihydrothiophene, 3-methyl-4,5-dihydrothiophene, 2,4-dithiapentane, and 2-methylfuran-3-thiol, occur in one or two truffle species only (Fig. 2). As for the common sulfur-containing volatiles, they might be derived from methionine; however, this has not yet been appropriately demonstrated (i.e., through feeding with labeled precursors). Interestingly, none of these specific sulfur odorants have been reported in axenic cultures of truffle mycelia, but some microbes have the ability to produce them (Fig. 2). Based on this observation, 2,4-dithiapentane might be produced by Betaproteobacteria in T. magnatum fruiting bodies. Interestingly, this might be a case similar to the one of thiophene derivatives, which were recently shown to originate from bacteria inhabiting T. borchii (14). 2-Methylfuran-3-thiol has been reported in fruiting bodies of T. melanosporum and T. aestivum (29), but this volatile has been not detected in either axenic mycelial cultures or microbes (Fig. 2). Its origin, therefore, remains elusive; nevertheless, it can be speculated that the latter odorant might be specifically produced during the sexual stage of truffles.
Overall, this suggests that common sulfur volatiles might be produced inside truffle fruiting bodies by both truffles and microbes (mixed origin), whereas more specific sulfur volatiles might be derived from truffles or microbes only.
ALCOHOLS, ESTERS, KETONES, AND ALDEHYDES
Another important group of truffle odorants is made of alcohols, esters, ketones, and aldehydes that are possibly derived from amino acid and fatty acid catabolism (36). As for sulfur volatiles, some commonly occur in numerous truffle species, while others are more specific (Fig. 2). Axenic cultures of truffle mycelia and numerous fungal and bacterial phyla are able to produce the most common volatiles (3-methylbutanal, octan-3-one, oct-1-en-3-ol, 3-methyl-1-butanol, hexanal, and acetaldehyde) which occur in >50% of all species. Interestingly enough, eight-carbon-containing volatiles (i.e., octan-3-one and oct-1-en-3-ol) were believed to be strictly of fungal origin, but Fig. 2 suggests that like 3-methylbutanal, 3-methyl-1-butanol, hexanal, and acetaldehyde, they might also be produced by specific bacterial classes. Eight-carbon-containing volatiles are important contributors to fungal aroma and have a characteristic mushroom flavor (43).
The remaining less common alcohol, ketone, ester, and aldehyde odorants found in truffle fruiting bodies have not been detected in truffle mycelia and are potentially produced only by guest filamentous fungi, yeasts, and/or bacteria. This is the case, for example, with 3-hydroxybutan-2-one, which potentially is produced by fungi of the Sordariomycetes class or the Betaproteobacteria class, which is a dominant group in the truffle microbiome (Fig. 1). Other rare volatiles might not be produced by microbes or by axenic cultures of truffle mycelia. It can be hypothesized that they are specific to the sexual stage of truffle fruiting bodies.
Similar to sulfur volatiles, the trend with alcohols, esters, ketones, and aldehydes is that common volatiles might be of mixed origins, while more specific ones might be produced either by microbes or truffles.
AROMATIC COMPOUNDS
Aromatic odorants produced by truffles include, for example, the volatile 2-phenylethanol with a characteristic rose smell, and benzaldehyde, an odorant with a characteristic bitter almond flavor (Fig. 2). Aromatic odorants might be derived from the catabolism of phenylalanine (36). Interestingly, with the exception of benzaldehyde and 2-phenylethanol, none of these volatiles have been detected in truffle mycelia (Fig. 2). These common aromatic odorants are potentially also synthesized by numerous fungal and or bacterial phyla and might therefore be of mixed origin (Fig. 2). The less common aromatic odorant 2-methoxy-4-methylphenol is potentially produced by the two bacterial classes Bacilli and Gammaproteobacteria, whereas some rare odorants might be derived from the sexual stage of truffles.
Overall, common aromatic odorants might be of either mixed fungal (truffle) or only microbial origin. The absence or rare occurrence in microbes of specific aromatic odorants suggests that they might be synthesized by truffles only and possibly during their sexual stage only.
OTHER VOLATILES
Butanoic acid and 4-hydroxy-2,5-dimethylfuran-3-one are odorants specific to T. melanosporum and T. aestivum (29). Based on what is seen in Fig. 2, they have been detected neither in truffle mycelia nor, in the case of 4-hydroxy-2,5-dimethylfuran-3-one, in microbes, which suggests that the latter volatile might be synthesized only during the sexual stage of truffles. Numerous microbes have the ability to produce butanoic acid, suggesting that it might be of microbial origin in truffle fruiting bodies (Fig. 2).
Overall, we are well aware that the absence of evidence is not evidence of absence. In other words, not having detected a volatile in a given organism does not demonstrate that the organism in question is not able to produce it under specific circumstances. For example, this might be the case with truffles, which might produce specific odorants during their sexual stage only (fruiting body) and not as free-living mycelia (axenic cultures). Our approach, nevertheless, allows the construction of a hypothesis on the identity of the possible producers of specific odorants. Demonstrating what produces what will not only require fully characterizing pathways leading to specific odorants in truffles and microbes but also microbe-free truffles to be obtained. This is an especially challenging task considering that to date, all truffle fruiting bodies harvested from the wild contain microbes, and microbe-free fruiting bodies cannot be obtained under axenic conditions.
DO TRUFFLES OR ACTUAL MICROBES ATTRACT ANIMALS?
Truffles are hypogeous fungi, meaning that they form their fruiting bodies below the soil surface. Since their belowground habitat prevents them from dispersing spores through the air/wind, truffles have developed intense aromas to attract small rodents and larger mammals. These animals eat fruiting bodies and subsequently disperse truffle spores through their feces. Mammals are not the only animals that are able to locate fruiting bodies belowground; a beetle (Leiodes cinnamomea Panzer) and a fly (Suillia pallida) can achieve the same. However, it remains unclear whether these insects participate in spore dispersal or whether they just feed on truffles (44, 45).
Mammals are able to locate truffles belowground due to the dimethyl sulfide emitted by fruiting bodies (46). Dimethyl sulfide is obviously not the only volatile that animals can smell, since, for example, dogs, like humans, are able to distinguish between truffle species. Nevertheless, besides dimethyl sulfide, species-specific attractants have not been identified in truffles, and the structures of the compounds that attract flies and beetles to truffles are not known (44). The question of what actually produces these attractants raises interesting hypotheses about multitrophic interactions. Indeed, dimethyl sulfide might be of mixed fungal (truffle) and bacterial origin, since truffle mycelia and Alphaproteobacteria, which are dominant in fruiting bodies, are able to produce it. Assuming that dimethyl sulfide is partially derived from bacteria would imply that bacteria participate in attracting mammals and small rodents to truffles. A similar case has actually been demonstrated for the fruit fly, Drosophila melanogaster, which is not attracted by fruit volatiles but rather by microbial volatiles emitted by the yeasts that colonize the surface of the fruit (47). Finding the answer to what produces truffle attractants will require microbe-free truffles to be obtained, and this has not yet been achieved.
CONCLUSION
Understanding to what extent the microbiomes of truffles participate in truffle aroma formation promises to be a complex and challenging task. Literature data on the ability of organisms to produce volatiles suggests that truffles and microbes might be able to produce common truffle odorants, whereas more specific compounds might be of microbial origin only. Disentangling what produces what within truffle fruiting bodies will require elucidation of the biosynthetic pathways for specific odorants and the use of innovative techniques to follow the fate of aroma precursors in situ. Overall, truffles offer a unique opportunity to better understand the ecological function of microbes associated with fungi and their involvement in aroma formation.
ACKNOWLEDGMENTS
We thank T. Mello (CNR di Torino, Italy) and M. Gryndler (Academy of Sciences of the Czech Republic) for making accessible the raw data of their manuscripts published on truffle microbiomes. We acknowledge B. Piechulla and M. C. Lemfack (University of Rostock, Germany) for providing raw data from the mVOC database.
M.V. and R.S. are supported by the LOEWE research funding program of the government of Hessen, in the framework of the Integrative Fungal Research Cluster (IPF), and by German Research Foundation/Deutsche Forschungsgemeinschaft (DFG) grant 1191/4-1. A.D. is partially supported by a grant overseen by the French National Research Agency (ANR) as part of the “Investissements d'Avenir” program (ANR-11-LABX-0002-01, Lab of Excellence, ARBRE).
REFERENCES
- 1.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. 2014. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijden MGA, Schlaeppi K. 2015. Root surface as a frontier for plant microbiome research. Proc Natl Acad Sci U S A 112:2299–2300. doi: 10.1073/pnas.1500709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacchi L, Vaughan-Martini A, Angelini P. 2003. Yeast distribution in a truffle-field ecosystem. Ann Microbiol 53:275–282. [Google Scholar]
- 4.Barbieri E, Bertini L, Rossi I, Ceccaroli P, Saltarelli R, Guidi C, Zambonelli A, Stocchi V. 2005. New evidence for bacterial diversity in the ascoma of the ectomycorrhizal fungus Tuber borchii Vittad. FEMS Microbiol Lett 247:23–35. doi: 10.1016/j.femsle.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri E, Guidi C, Bertaux J, Frey-Klett P, Garbaye J, Ceccaroli P, Saltarelli R, Zambonelli A, Stocchi V. 2007. Occurrence and diversity of bacterial communities in Tuber magnatum during truffle maturation. Environ Microbiol 9:2234–2246. doi: 10.1111/j.1462-2920.2007.01338.x. [DOI] [PubMed] [Google Scholar]
- 6.Buzzini P, Gasparetti C, Turchetti B, Cramarossa MR, Vaughan-Martini A, Martini A, Pagnoni UM, Forti L. 2005. Production of volatile organic compounds (VOCs) by yeasts isolated from the ascocarps of black (Tuber melanosporum Vitt.) and white (Tuber magnatum Pico) truffles. Arch Microbiol 184:187–193. doi: 10.1007/s00203-005-0043-y. [DOI] [PubMed] [Google Scholar]
- 7.Pacioni G, Leonardi M, Aimola P, Ragnelli AM, Rubini A, Paolocci F. 2007. Isolation and characterization of some mycelia inhabiting Tuber ascomata. Mycol Res 111:1450–1460. doi: 10.1016/j.mycres.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Stielow B, Menzel W. 2010. Complete nucleotide sequence of TaV1, a novel totivirus isolated from a black truffle ascocarp (Tuber aestivum Vittad.). Arch Virol 155:2075–2078. doi: 10.1007/s00705-010-0824-8. [DOI] [PubMed] [Google Scholar]
- 9.Stielow B, Klenk H-P, Menzel W. 2011. Complete genome sequence of the first endornavirus from the ascocarp of the ectomycorrhizal fungus Tuber aestivum Vittad. Arch Virol 156:343–345. doi: 10.1007/s00705-010-0875-x. [DOI] [PubMed] [Google Scholar]
- 10.Stielow B, Klenk H-P, Winter S, Menzel W. 2011. A novel Tuber aestivum (Vittad.) mitovirus. Arch Virol 156:1107–1110. doi: 10.1007/s00705-011-0998-8. [DOI] [PubMed] [Google Scholar]
- 11.Stielow JB, Bratek Z, Klenk H-P, Winter S, Menzel W. 2012. A novel mitovirus from the hypogeous ectomycorrhizal fungus Tuber excavatum. Arch Virol 157:787–790. doi: 10.1007/s00705-012-1228-8. [DOI] [PubMed] [Google Scholar]
- 12.Gryndler M, Soukupová L, Hršelová H, Gryndlerová H, Borovička J, Streiblová E, Jansa J. 2013. A quest for indigenous truffle helper prokaryotes: Tuber aestivum-associative prokaryotes. Environ Microbiol Rep 5:346–352. doi: 10.1111/1758-2229.12014. [DOI] [PubMed] [Google Scholar]
- 13.Antony-Babu S, Deveau A, Van Nostrand JD, Zhou J, Le Tacon F, Robin C, Frey-Klett P, Uroz S. 2014. Black truffle-associated bacterial communities during the development and maturation of Tuber melanosporum ascocarps and putative functional roles. Environ Microbiol 16:2831–2847. doi: 10.1111/1462-2920.12294. [DOI] [PubMed] [Google Scholar]
- 14.Splivallo R, Deveau A, Valdez N, Kirchhoff N, Frey-Klett P, Karlovsky P. 2014. Bacteria associated with truffle-fruiting bodies contribute to truffle aroma. Environ Microbiol doi: 10.1111/1462-2920.12521. [DOI] [PubMed] [Google Scholar]
- 15.Le Tacon F, Rubini A, Murat C, Riccioni C, Robin C, Belfiori B, Zeller B, De la Varga H, Akroume E, Deveau A, Martin F, Paolocci F. 2015. Certainties and uncertainties about the life cycle of the Périgord black truffle (Tuber melanosporum Vittad.). Ann For Sci doi: 10.1007/s13595-015-0461-1. [DOI] [Google Scholar]
- 16.Splivallo R, Ebeler SE. 2015. Sulfur volatiles of microbial origin are key contributors to human-sensed truffle aroma. Appl Microbiol Biotechnol 99:2583–2592. doi: 10.1007/s00253-014-6360-9. [DOI] [PubMed] [Google Scholar]
- 17.Citterio B, Cardoni P, Potenza L, Amicucci A, Stocchi V, Gola G, Nuti M. 1995. Isolation of bacteria from Sporocarps of Tuber magnatum Pico, Tuber borchii Vitt. and Tuber maculatum Vitt., p 241–248. In Stocchi V, Bonfante P, Nuti M (ed), Biotechnology of ectomycorrhizae. Plenum Press, New York, NY. [Google Scholar]
- 18.Barbieri E, Potenza L, Rossi I, Sisti D, Giomaro G, Rossetti S, Beimfohr C, Stocchi V. 2000. Phylogenetic characterization and in situ detection of a Cytophaga-Flexibacter-Bacteroides phylogroup bacterium in Tuber borchii Vittad. ectomycorrhizal mycelium. Appl Environ Microbiol 66:5035–5042. doi: 10.1128/AEM.66.11.5035-5042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sbrana C, Agnolucci M, Bedini S, Lepera A, Toffanin A, Giovannetti M, Nuti MP. 2002. Diversity of culturable bacterial populations associated to Tuber borchii ectomycorrhizas and their activity on T. borchii mycelial growth. FEMS Microbiol Lett 211:195–201. doi: 10.1111/j.1574-6968.2002.tb11224.x. [DOI] [PubMed] [Google Scholar]
- 20.Nazzaro F, Fratianni F, Picariello G, Coppola R, Reale A, Di Luccia A. 2007. Evaluation of gamma rays influence on some biochemical and microbiological aspects in black truffles. Food Chem 103:344–354. doi: 10.1016/j.foodchem.2006.07.067. [DOI] [Google Scholar]
- 21.Rivera CS, Blanco D, Oria R, Venturini ME. 2010. Diversity of culturable microorganisms and occurrence of Listeria monocytogenes and Salmonella spp. in Tuber aestivum and Tuber melanosporum ascocarps. Food Microbiol 27:286–293. doi: 10.1016/j.fm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Saltarelli R, Ceccaroli P, Cesari P, Barbieri E, Stocchi V. 2008. Effect of storage on biochemical and microbiological parameters of edible truffle species. Food Chem 109:8–16. doi: 10.1016/j.foodchem.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 23.Rivera CS, Blanco D, Salvador ML, Venturini ME. 2010. Shelf-life extension of fresh Tuber aestivum and Tuber melanosporum truffles by modified atmosphere packaging with microperforated films. J Food Sci 75:E225–E233. doi: 10.1111/j.1750-3841.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 24.Mello A, Ding G-C, Piceno YM, Napoli C, Tom LM, DeSantis TZ, Andersen GL, Smalla K, Bonfante P. 2013. Truffle brûlés have an impact on the diversity of soil bacterial communities. PLoS One 8:e61945. doi: 10.1371/journal.pone.0061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suz LM, Martín MP, Oliach D, Fischer CR, Colinas C. 2008. Mycelial abundance and other factors related to truffle productivity in Tuber melanosporum-Quercus ilex orchards. FEMS Microbiol Lett 285:72–78. doi: 10.1111/j.1574-6968.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho FM, Souza RC, Barcellos FG, Hungria M, Vasconcelos ATR. 2010. Genomic and evolutionary comparisons of diazotrophic and pathogenic bacteria of the order Rhizobiales. BMC Microbiol 10:37. doi: 10.1186/1471-2180-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbieri E, Ceccaroli P, Saltarelli R, Guidi C, Potenza L, Basaglia M, Fontana F, Baldan E, Casella S, Ryahi O, Zambonelli A, Stocchi V. 2010. New evidence for nitrogen fixation within the Italian white truffle Tuber magnatum. Fungal Biol 114:936–942. doi: 10.1016/j.funbio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Fu Y, Li B, Yu X, Xie J, Cheng J, Ghabrial SA, Li G, Yi X, Jiang D. 2011. Widespread horizontal gene transfer from circular single-stranded DNA viruses to eukaryotic genomes. BMC Evol Biol 11:276. doi: 10.1186/1471-2148-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culleré L, Ferreira V, Chevret B, Venturini ME, Sánchez-Gimeno AC, Blanco D. 2010. Characterisation of aroma active compounds in black truffles (Tuber melanosporum) and summer truffles (Tuber aestivum) by gas chromatography–olfactometry. Food Chem 122:300–306. doi: 10.1016/j.foodchem.2010.02.024. [DOI] [Google Scholar]
- 30.Liu R-S, Li D-C, Li H-M, Tang Y-J. 2012. Evaluation of aroma active compounds in Tuber fruiting bodies by gas chromatography-olfactometry in combination with aroma reconstitution and omission test. Appl Microbiol Biotechnol 94:353–363. doi: 10.1007/s00253-011-3837-7. [DOI] [PubMed] [Google Scholar]
- 31.Fiecchi A, Kienle M, Scala A, Cabella P. 1967. Bis-methylthiomethane, an odorous substance from white truffle, Tuber magnatum Pico. Tetrahedron Lett 8:1681–1682. doi: 10.1016/S0040-4039(00)90698-1. [DOI] [Google Scholar]
- 32.Talou T, Delmas M, Gaset A. 1989. Black Périgord truffle: from aroma analysis to aromatizer formulation, p 715–728. In Charalambous G. (ed), Flavors and off-flavors '89: proceedings of the 6th International Flavor Conference, Rethymnon, Crete, Greece. [Google Scholar]
- 33.Splivallo R, Bossi S, Maffei M, Bonfante P. 2007. Discrimination of truffle fruiting body versus mycelial aromas by stir bar sorptive extraction. Phytochemistry 68:2584–2598. doi: 10.1016/j.phytochem.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Splivallo R, Ottonello S, Mello A, Karlovsky P. 2011. Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol 189:688–699. doi: 10.1111/j.1469-8137.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin F, Kohler A, Murat C, Balestrini R, Coutinho PM, Jaillon O, Montanini B, Morin E, Noel B, Percudani R, Porcel B, Rubini A, Amicucci A, Amselem J, Anthouard V, Arcioni S, Artiguenave F, Aury J-M, Ballario P, Bolchi A, Brenna A, Brun A, Buée M, Cantarel B, Chevalier G, Couloux A, Da Silva C, Denoeud F, Duplessis S, Ghignone S, Hilselberger B, Iotti M, Marçais B, Mello A, Miranda M, Pacioni G, Quesneville H, Riccioni C, Ruotolo R, Splivallo R, Stocchi V, Tisserant E, Viscomi AR, Zambonelli A, Zampieri E, Henrissat B, Lebrun M-H, Paolocci F, Bonfante P, Ottonello S, et al. 2010. Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464:1033–1038. doi: 10.1038/nature08867. [DOI] [PubMed] [Google Scholar]
- 36.Hazelwood LA, Daran J-M, van Maris AJ, Pronk JT, Dickinson JR. 2008. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B. 2014. mVOC: a database of microbial volatiles. Nucleic Acids Res 42:D744–D748. doi: 10.1093/nar/gkt1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiron N, Michelot D. 2005. Odeurs des champignons: chimie et rôle dans les interactions biotiques—une revue. Cryptogam Mycol 26:299–364. [Google Scholar]
- 39.Liu R-S, Zhou H, Li H-M, Yuan Z-P, Chen T, Tang Y-J. 2013. Metabolism of l-methionine linked to the biosynthesis of volatile organic sulfur-containing compounds during the submerged fermentation of Tuber melanosporum. Appl Microbiol Biotechnol 97:9981–9992. doi: 10.1007/s00253-013-5224-z. [DOI] [PubMed] [Google Scholar]
- 40.Tirillini B, Verdelli G, Paolocci F, Ciccioli P, Frattoni M. 2000. The volatile organic compounds from the mycelium of Tuber borchii Vitt. Phytochemistry 55:983–985. doi: 10.1016/S0031-9422(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 41.Li Y-Y, Wang G, Li H-M, Zhong J-J, Tang Y-J. 2012. Volatile organic compounds from a Tuber melanosporum fermentation system. Food Chem 135:2628–2637. doi: 10.1016/j.foodchem.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Du M, Huang S, Wang J. 2014. The volatiles from fermentation product of Tuber formosanum. Open J For 04:426–429. doi: 10.4236/ojf.2014.44047. [DOI] [Google Scholar]
- 43.Combet E, Eastwood DC, Burton KS, Combet E, Henderson J, Henderson J, Combet E. 2006. Eight-carbon volatiles in mushrooms and fungi: properties, analysis, and biosynthesis. Mycoscience 47:317–326. doi: 10.1007/S10267-006-0318-4. [DOI] [Google Scholar]
- 44.Hochberg ME, Bertault G, Poitrineau K, Janssen A. 2003. Olfactory orientation of the truffle beetle, Leiodes cinnamomea. Entomol Exp Appl 109:147–153. doi: 10.1046/j.1570-7458.2003.00099.x. [DOI] [Google Scholar]
- 45.Maser C, Claridge AW, Trappe JM. 2008. Trees, truffles, and beasts: how forests function. Rutgers University Press, New Brunswick, NJ. [Google Scholar]
- 46.Talou T, Gaset A, Delmas M, Kulifaj M, Montant C. 1990. Dimethyl sulphide: the secret for black truffle hunting by animals? Mycol Res 94:277–278. doi: 10.1016/S0953-7562(09)80630-8. [DOI] [Google Scholar]
- 47.Becher PG, Flick G, Rozpędowska E, Schmidt A, Hagman A, Lebreton S, Larsson MC, Hansson BS, Piškur J, Witzgall P, Bengtsson M. 2012. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol 26:822–828. doi: 10.1111/j.1365-2435.2012.02006.x. [DOI] [Google Scholar]
- 48.Splivallo R, Maier C. March 2015. Production of natural truffle flavours from truffle mycelium. EU patent EP 2448430.