Abstract
Fungus-derived indole-3-acetic acid (IAA), which is involved in development of ectomycorrhiza, affects both partners, i.e., the tree and the fungus. The biosynthesis pathway, excretion from fungal hyphae, the induction of branching in fungal cultures, and enhanced Hartig net formation in mycorrhiza were shown. Gene expression studies, incorporation of labeled compounds into IAA, heterologous expression of a transporter, and bioinformatics were applied to study the effect of IAA on fungal morphogenesis and on ectomycorrhiza. Tricholoma vaccinum produces IAA from tryptophan via indole-3-pyruvate, with the last step of this biosynthetic pathway being catalyzed by an aldehyde dehydrogenase. The gene ald1 was found to be highly expressed in ectomycorrhiza and induced by indole-3-acetaldehyde. The export of IAA from fungal cells is supported by the multidrug and toxic extrusion (MATE) transporter Mte1 found in T. vaccinum. The addition of IAA and its precursors induced elongated cells and hyphal ramification of mycorrhizal fungi; in contrast, in saprobic fungi such as Schizophyllum commune, IAA did not induce morphogenetic changes. Mycorrhiza responded by increasing its Hartig net formation. The IAA of fungal origin acts as a diffusible signal, influencing root colonization and increasing Hartig net formation in ectomycorrhiza.
INTRODUCTION
Fungi, mainly basidiomycetes, form a mutually beneficial symbiotic association, commonly known as ectomycorrhiza, with the roots of woody plants (1). After establishing contact with the host, the fungus initially grows around the roots to form a mass of mycelium called the fungal mantle. From there, some hyphae penetrate the roots to grow between cortical cells to form the Hartig net, which acts as a surface for the exchange of nutrients and signals between the two symbiotic partners (1). Variation in host specificity occurs within ectomycorrhizal fungi: some have a broad host range, whereas others form host-specific ectomycorrhiza. In contrast to ectomycorrhizal fungi such as Pisolithus tinctorius and Paxillus involutus, Tricholoma species often show host specificity; their fruiting bodies are found only below compatible host trees. For example, Tricholoma vaccinum fruiting bodies occur only near spruce, which is the compatible host (2). Under laboratory conditions, Tricholoma species form ectomycorrhiza with nonhost trees, but the mycorrhization process in such low-compatibility interactions requires more time and features spotty Hartig net formation (3). Thus, T. vaccinum, a slow-growing, late-stage, and highly host-specific mycorrhizal fungus was chosen for the investigation of fungus-derived phytohormone effects on both partners. These interactions were expected to show the importance of indole-3-acetic acid (IAA) in the establishment and functioning of the symbiosis. We hypothesized that a long period of incubation might be necessary to observe changes in the morphogenetic responses that are not easily visualized with fast-growing, early-stage mycorrhiza. In order to generalize our findings, we included experiments with other mycorrhizal fungi in our analysis of IAA formation by basidiomycetes.
Indole-3-acetic acid (IAA) was hypothesized, by proponents of the so-called “auxin theory” (4), to regulate ectomycorrhiza formation through the IAA produced by ectomycorrhizal fungi (5, 6). Mostly, biosynthesis takes place from tryptophan. In accordance with this hypothesis, we carried out a cytological examination of mycorrhiza between an IAA-overproducing transformant of Hebeloma cylindrosporum and Pinus pinaster seedlings and found a hypertrophic Hartig net (7). The authors suggested that fungal IAA affects the physiology of the host, thereby inducing the formation of the Hartig net.
Some studies have supported the role for IAA in mycorrhiza (6, 8, 9), whereas others have refuted it (10–12). This controversy remains unresolved, and explanations of the mechanisms involved remain elusive. It has been hypothesized that IAA acts as a signal to induce the expression of genes involved in mycorrhiza differentiation (13). Gea et al. (7) speculated that IAA facilitates the loosening of plant cell walls, allowing the fungus to enter the host root and to manipulate the host physiology to form a Hartig net. We propose that the phytohormone, in addition to influencing the morphogenesis of the host root, also affects the morphogenesis of the fungal partner.
Although many ectomycorrhizal fungi have been shown to produce IAA, the biosynthetic pathways and enzymes involved have not been investigated (14). Two different auxin biosynthetic pathways have been described for plants. One produces the phytohormone via indole-3-glycerophosphate; the other, which is tryptophan dependent, has branches that differ in the intermediates indole-3-pyruvate (IPA) (15–19), tryptamine (TAM) (20), indole-3-acetaldoxime (IAOx) (21), and indole-3-acetamide (IAM) (22–24). A route converting indole-3-acetonitrile (IAN) into IAA has been proposed (21) but is controversial (25). In pathogenic bacteria, IAA production seems to be realized via IAM, whereas plant growth-promoting bacteria prefer the IPA pathway (26–28). In fungi, IAA synthesis was shown for the basidiomycetes Ustilago maydis (15, 29, 30), Rhizoctonia (31), and Piriformospora indica (32) and for the ascomycetes Colletotrichum gloeosporioides (33) and Fusarium proliferatum (34).
Here, we aimed to comprehensively investigate IAA involvement in ectomycorrhizal morphogenesis, following our hypothesis that IAA is a crucial component in ectomycorrhizal signaling between fungus and tree. The ability of T. vaccinum to produce IAA was verified, possible biosynthetic pathways were determined, and the involvement of a previously identified aldehyde dehydrogenase gene, ald1, was shown (3, 35). Indeed, the gene is strongly upregulated in T. vaccinum colonizing spruce seedling roots. IAA export from fungal cells was linked to transport via a transporter of the multidrug and toxic compound extrusion (MATE) family.
MATERIALS AND METHODS
Culture conditions.
Fungal isolates used in this study are T. vaccinum GK6514 (FSU 4731; Jena Microbial Resource Collection, Jena, Germany), Armillaria mellea MG091013_14, Heterobasidion annosum MG091028_02, Leucoagaricus leucothites MG090924_04, Lyophyllum loricatum MG091108_01, Paxillus involutus MG091013_13, Schizophyllum commune 1-116, S. commune 12-43 (ura−), and T. vaccinum 1.16 (35). Fungi were cultured as described previously (3).
To test for IAA production, Pachlewski medium (36) (0.25 g of di-NH4-tartrate, 0.5 g of KH2PO4, 0.25 g of MgSO4 · 7H2O, 2.5 g of maltose, 5 g of glucose, 42 μl of thiamine hydrochloride [1.2 mg ml−1], 1 μg of FeCl3 · 3H2O, pH 7, 1 ml of trace element solution) (37) was used to exclude potential IAA sources in the medium. To quantify IAA biosynthesis by T. vaccinum and investigate the effect of IAA on fungal growth and hyphal ramification, the MMNb medium (38) was supplemented with tryptophan (Trp), indole-3-acetaldehyde (IAD), indole-3-pyruvate (IPA), indole-3-acetamide (IAM), tryptamine (TAM), indole-3-acetonitrile (IAN), tryptophol (TOL), or IAA in concentrations of 0.05 to 2.5 mM. The IAA transport inhibitor 2,3,5-triiodobenzoic acid (TIBA; Sigma-Aldrich, Taufkirchen, Germany) was applied (0.01 mM) to test for IAA functions.
Norway spruce (Picea abies) and Scots pine (Pinus sylvestris) seeds (Thüringer Forstamt Schmalkalden, Germany) were cultivated after surface sterilization with 30% hydrogen peroxide for 1.5 h to achieve axenic cultures for ectomycorrhization in petri dishes (39) or hydroponically (40) on MMNa medium (38) using a 12-h day-night regime at 23/17°C and 80% relative humidity.
Fungal growth, morphogenesis, and mycorrhiza formation.
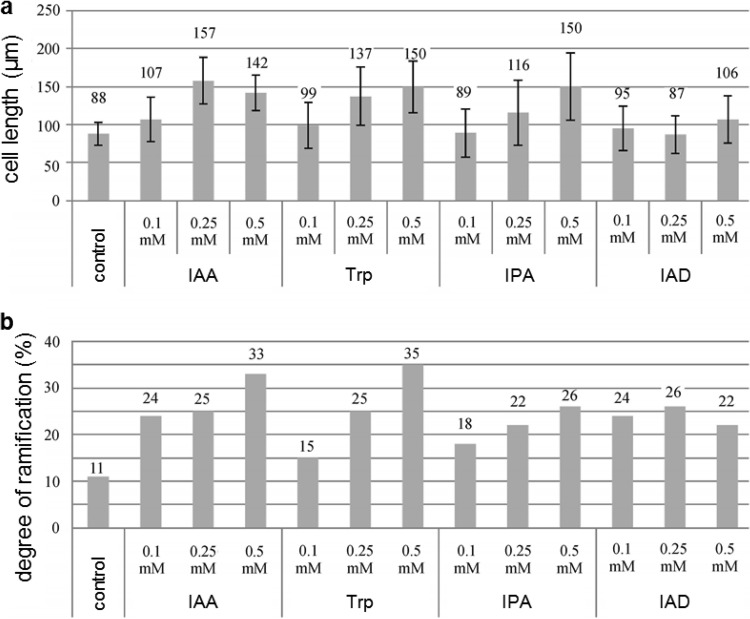
The effect of IAA on fungal growth was investigated in fungal cultures established on MMNb plates containing tryptophan (0.5 mM), IAA (0.1 mM), and/or TIBA (10 μM). Mycelial radial growth was quantified by measuring the diameter of fungal colonies in biological triplicates starting after 1 week, when aerial mycelium had begun to form, and continuing up to 4 weeks. To investigate the effect of the phytohormone on fungal morphogenesis, fungal cultures were grown for 4 weeks (T. vaccinum, A. mellea, and P. involutus) or for 2 weeks (S. commune, L. leucothites, L. loricatum, and H. annosum) with Trp, IPA, IAD, and IAA (0.1, 0.25, and 0.5 mM). Fungal mycelium was observed in three to five replicates under an Axioplan 2 microscope (Zeiss, Jena, Germany), and cell lengths and ramifications were quantified in 100 cells each. The length of each cell was measured (SPOT Advanced; Diagnostic Instruments, Sterling Heights, MI, USA), and the number of branches per hypha was microscopically counted.
Chitin was stained with 1 mM calcofluor (Sigma-Aldrich, Taufkirchen, Germany), nucleic acids were stained with 1 mM 4′,6-diamidino-2-phenylindole (DAPI; Roth, Karlsruhe, Germany) solution, and immunofluorescence was performed using anti-actin (rabbit) and Cy3-labeled goat-anti rabbit or anti-α-tubulin (mouse) and fluorescein (FITC)-labeled goat-anti-mouse as described previously (41). Fluorescence microscopy using filter sets 02 for calcofluor and DAPI, 15 for Cy3, and 10 for FITC was performed, and images were processed with SPOT Advanced software (Diagnostic Instruments, Sterling Heights, MI, USA).
Fungal mantle development around mycorrhized roots, as the first evidence of mycorrhiza formation, was monitored using a binocular microscope (KL 1500; Zeiss, Jena, Germany). Microtome sections (6 to 8 μm) were obtained from lateral roots of three trees for biological replicates. After 4 months in coculture, multiple root tips were analyzed for each replicate (rotation microtome HM 355; Microm, Walldorf, Germany), using embedding medium (Technovit 7100; Heraeus Kulzer, Hanau, Germany) and toluidine blue O (0.1%, wt/vol, in H2O) for observing Hartig net development (41), which was visualized with a confocal laser scanning microscope (LSM 780; Zeiss, Jena, Germany), with tile scanning and three-dimensional stack optimization.
Analysis of gene expression in mycorrhizal cultures.
The genomic sequence of ald1 (GenBank accession number HM363121) was used for expression analyses. Competitive PCR was performed on mycorrhiza tissues from different hosts and after the mycelium mantle and the root parts were microdissected to account for ald1 expression in the mantle and Hartig net. Total RNA was extracted from lyophilized material, and competitive PCR was performed with tef1 (42) as a control in cDNA (Oligotex mRNA Batch Protocol [Qiagen, Hilden, Germany], Superscript II RNase H Reverse Transcriptase [Invitrogen, Darmstadt, Germany], or iScript cDNA synthesis kit [Bio-Rad, Munich, Germany]) with the respective controls and using the same kit in each experiment. Degenerate primers tef0 (AAG AAG GTY GGN TAY AAY) and tef3 (GTY CTR CAV ATG TTC TAR) were designed, and the resulting PCR products from fungus (240 bp) and plant (204 bp) were compared to define the amounts of fungal cDNA in the mycorrhiza samples.
For competitive PCR, primers rf13a (GCA AGA AAG GCA TAC AAA ACT) and rf13b (GCG TCG CTG GTG AAA AT) were used to simultaneously amplify an 826-bp cDNA fragment and a 1,297-bp competitor fragment (a fragment comprised of genomic DNA with seven introns carried by a plasmid). The signal intensities of the resulting PCR products were monitored after agarose gel electrophoresis to estimate the relative amount of the transcript. Real-time quantitative PCR (qPCR) was performed using SYBR green/6-carboxy-X-rhodamine (ROX) qPCR master mix (Thermo Scientific, Waltham, MA, USA) with primers aldRTPCRF (GAA AGC TCT TGG AGC AGG TG) and aldRTPCRR (TGG ACT GTA GCA CCC TCC TT) in a MiniOpticon cycler (Bio-Rad, Munich, Germany) from RNA isolated with an RNeasy Plant Kit (Qiagen, Hilden, Germany). Subsequent cDNA synthesis from 0.5 μg of RNA was performed using an iScript cDNA synthesis kit (Bio-Rad, Munich, Germany) or QuantiTect reverse transcription kit (Qiagen, Hilden, Germany).
The accumulation of ald1 mRNA after cells were fed IAA precursors was determined in absolute terms by comparing transcript levels to a standard curve generated by using ald1 cDNA clones in pDrive. For the ald1-overexpressing transformant, expression was normalized to the reference genes act1, cis1, and tef1 coding for actin, citrate synthetase, and the translation elongation factor EF1α, respectively. Three biological and two technical replicates were performed in each case.
Identification of genes for IAA synthesis.
Candidate genes for IAA synthesis were identified in silico from T. vaccinum genomic DNA Illumina reads (GATC, Constance, Germany) after conceptual translation and alignment (using MAFFT; for alignments and citations, see Fig. S1 in the supplemental material). The genes tam1 (GenBank accession number KP096350), ipd1 (GenBank accession number KP096351), and iah1 (GenBank accession number KP096352) were identified. For homologous transformation and overexpression, ald1 was introduced by Agrobacterium tumefaciens-mediated transformation using the constitutive glyceraldehyde-3-phosphate dehydrogenase promoter (Pgpd) of Agaricus bisporus on the pBGgHg plasmid backbone (35).
Production of IAA by T. vaccinum.
IAA concentrations were determined after T. vaccinum was cultivated in the dark at room temperature for about 4 weeks. Culture filtrates from three or four individual cultures, supplemented with labeled precursors, were sampled weekly and assessed by colorimetric quantification of IAA using a Salkowski assay (43) and gas chromatography-mass spectrometry (GC-MS) (44).
Indole derivatives were analyzed using thin-layer chromatography (Kieselgel 60 F254; Merck, Darmstadt, Germany), and compounds were separated in a solvent system using 2 parts H2O and 8 parts propanol. Spots were detected using UV at 254 and 366 nm, followed by immersion in Salkowski solvent. MMNb medium and 1 mM malt extract were checked to control for potential IAA contamination. For IAA, a retention coefficient of 0.89 was determined. The stability of IAA, IPA, and IAD was measured from 1 to 5 weeks and at 55°C; after 5 weeks, measurements revealed only slightly reduced IPA and IAD concentrations due to degradation.
Mass spectrometry analysis of tryptophan metabolites.
Samples were extracted, esterified by diazomethane (CH2N2), and analyzed by using a GC coupled to an ITQ 900 instrument (Thermo Finnigan, San Jose, CA, USA). An RTX-200MS column (30 m by 0.25 mm, 0.25-μm coating; Restek, Bad Homburg, Germany) was used to separate the compounds. Helium, at a constant rate of 40 cm s−1, served as the carrier gas, and samples (1 ml) were introduced in the splitless mode by an auto-injector. The GC injector, transfer line, and ion source were set at 220, 300, and 280°C, respectively. Spectra were taken in the total-ion-current mode (TIC) at 70 eV. The separation of the compounds was achieved with a temperature program from 60 to 140°C at 15°C min−1, at 5°C min−1 to 210°C, and at 15°C min−1 to 300°C. The final temperature was maintained for 2 min prior to cooling. Compounds were identified by comparing their mass spectra and retention times to those of authentic standards.
Transport of IAA.
To test whether the identified transporter protein accepts IAA as a substrate, drop tests were performed using yeast heterologously expressing the gene from T. vaccinum (for a complete description of vector construction, transformation, drop test, etc., see reference 45). The yeast strains used show sensitivity to external IAA (0.025 to 0.1 mM IAA), which was alleviated by the transporter. Saccharomyces cerevisiae BY4741, the empty vector control transformant PYES carrying the plasmid without insert, and Mte1 containing the mte1 gene of T. vaccinum were compared to prove a capacity for IAA transport by the heterologous gene. For the drop test, an optical density at 600 nm (OD600) of 1 was established in overnight cultures, and a dilution series (up to 10−4) was plated on synthetic dextrose (SD) medium or SD medium supplemented with Ura (SD Ura+). Two biological replicates were incubated for 2 to 10 days at 29°C in five repetitions.
Statistical analyses.
Statistical analysis was carried out using Origin, version 7.0 (OriginLab, Northampton, MA, USA) or SPSS (version 20.0, 2011; IBM Corp., Aromonk, NY, USA). Data were subjected to an analysis of variance (ANOVA), and, where significant, treatments were separated by a Tukey test (P < 0.05).
Nucleotide sequence accession numbers.
Sequence data for tam1, ipd1, and iah1 have been deposited in GenBank under accession numbers KP096350, KP096351, and KP096352, respectively.
RESULTS
IAA biosynthesis in T. vaccinum.
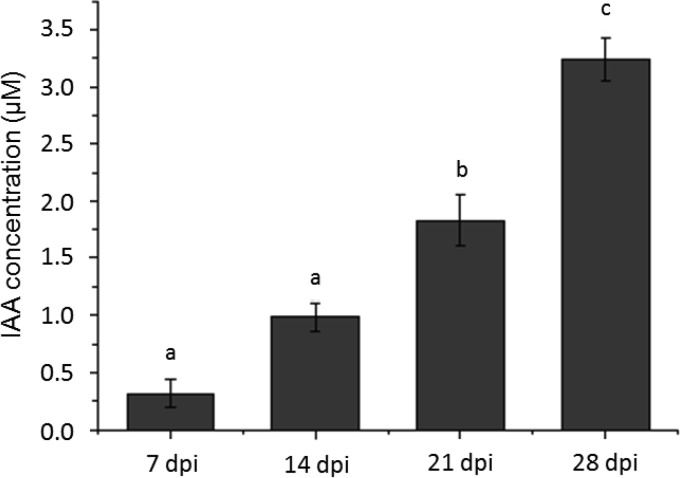
The fungal biosynthesis of IAA was investigated using labeled precursors. Mass spectrometry verified the results of thin-layer chromatography and Salkowski assays, namely, that there was no detectable production of IAA in pure cultures of T. vaccinum. Considerable amounts of [13C6]IAA with concentrations higher than 3 μM were found following supplementation of the medium with 13C6-labeled l-tryptophan after 1 month of incubation (Fig. 1).
FIG 1.
GC-MS quantification of IAA from T. vaccinum cultures supplemented with labeled tryptophan. IAA was purified weekly over a period of 4 weeks and analyzed by GC-MS. IAA peaks were obtained by tracing [13C6]IAA. The IAA amounts shown represent IAA only from labeled tryptophan. Bars denote standard errors, and letters indicate significant differences in IAA amounts (P < 0.05). dpi, days postinoculation.
These data were further verified by feeding cells 2H5-labeled precursors, showing a time-dependent conversion. One week after the addition of [2H5]tryptophan (0.5 mM), traces of unlabeled IAA and [2H5]IAA were released to the culture medium. Mass spectrometry revealed that the released IAA consisted of approximately 50% natural IAA and 50% labeled [2H5]IAA. After 2 weeks, [2H5]IAA prevailed (73%), while natural, unlabeled IAA dropped to 23% ± 4%. After 4 weeks, the ratio of [2H5]IAA to natural IAA approached 4:1.
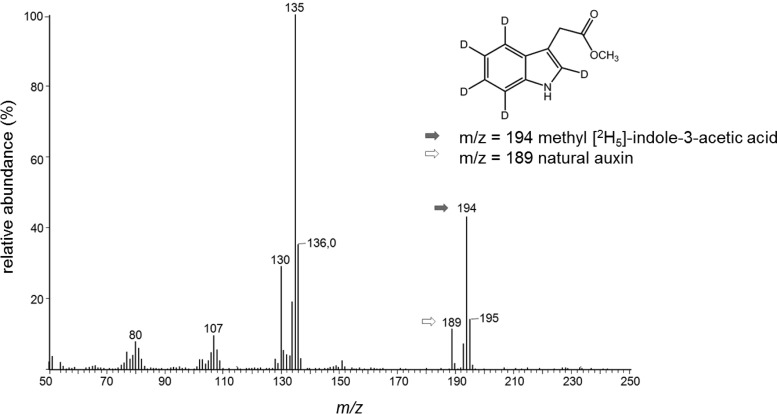
Labeled intermediates en route from tryptophan to IAA, easily recognized by an intense methyl-indole fragment at m/z 130, were not observed. The administration of other IAA precursors (0.25 mM), such as TAM, IAM, IAN, or TOL, did not enhance the production of IAA. Experiments with IPA remained inconclusive as the precursor proved to be unstable and generated IAA spontaneously (46, 47). Interestingly, the incubation with 0.2 mM IAD yielded the largest amount of IAA, 140.39 μM. Figure 2 shows the mass spectra (methyl ester) of natural and labeled IAA determined by GC-MS after 4 weeks. After extraction and esterification with diazomethane, both compounds displayed an intense molecular ion (m/z 189 for natural IAA and m/z 194 for [2H5]IAA) and a base peak at m/z 130 or m/z 135, corresponding to the methylindole cation after the loss of O=C-OCH3. The fragments at m/z 130 and m/z 135 or m/z 136 result from an indole-typical loss of HCN or 2HCN, respectively. Aromatic core fragments at m/z 77 and m/z 81 indicate that the labeled fragment still contains four of the five deuterium atoms of the administered [2H5]tryptophan.
FIG 2.
Feeding cells l-[2H5]tryptophan resulted in increased IAA concentrations, as documented by mass spectrometry after 4 weeks. Fragment ions of pure substances of [2H5]IAA (m/z 77, 107, 108, 134, 135, and 194) and of l-[2H5]tryptophan (m/z 77, 91, 165, 181, 197, 209, and 210) were used for identification.
In order to check whether IAA is produced only by ectomycorrhizal fungi, five additional fungi were investigated: one fast-growing, early-stage mycorrhizal species and four nonmycorrhizal species. Supernatants of three samples for each fungus were analyzed with a Salkowski assay after treatment with 0.1 mM tryptophan to evaluate the amount of IAA produced that might induce morphogenetic changes in fungi and/or trees. In addition to T. vaccinum, which produced approximately 7 μM IAA in this experiment, the mycorrhizal fungus P. involutus produced 17 μM IAA. Orthologs to the biosynthesis genes in the IPA pathway could be identified from S. commune (see Table S1 in the supplemental material), and IAA production was shown for single isolates of that fungus (48). However, under growth conditions similar to those used for T. vaccinum, none of the phytopathogenic or saprobic basidiomycetes, H. annosum, S. commune, L. leucothites, and L. loricatum, produced IAA, with the exception of 4.1 μM measured for A. mellea.
Regulation of ald1 in IAA synthesis and mycorrhization.
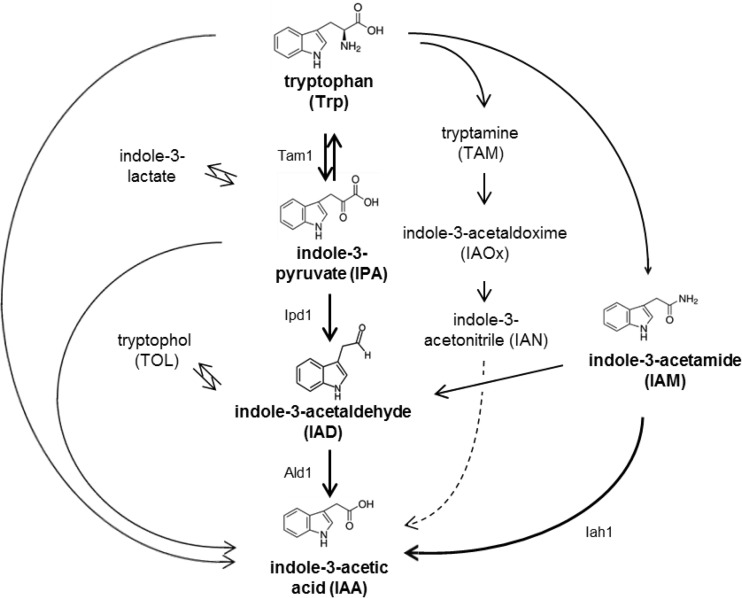
The pathways of IAA synthesis via IPA and conversion of IAD to IAA, and likewise via conversion of IAM to IAA, are based on enzymatic activity. From the genome of T. vaccinum, genes related to both synthesis pathways could be deduced (Fig. 3). The genes tam1, ipd1, and ald1 were identified. Additionally, iah1 was identified, a gene that is thought to be involved in IAA production from indole-3-acetamide. An ortholog to iah1 was also found in the closely related Tricholoma matsutake (see Table S1 in the supplemental material). However, the synthesis of the substrate IAM, catalyzed by the gene product of a tryptophan 2-monooxygenase, could not be verified in silico. No homolog to plant or bacterial enzymes is present in the T. vaccinum genome.
FIG 3.
Tryptophan-dependent biosynthesis pathways for IAA production (59). The biosynthetic pathway used by T. vaccinum (boldface) was confirmed by feeding cells labeled precursors (for which structural formulas are given). Genes coding for the enzymes involved were identified from T. vaccinum genomic sequences: tam1 (coding for a tryptophan aminotransferase; GenBank accession no. KP096350), ipd1 (coding for an indole-3-pyruvic acid decarboxylase; GenBank accession no. KP096351), ald1 (coding for an aldehyde dehydrogenase; GenBank accession no. HM363121), and iah1 (coding for an indole-3-acetamide hydrolase; GenBank accession number KP096352). Conversion of IAN to IAA has not yet been fully clarified; thus this potential route is indicated as a dotted line.
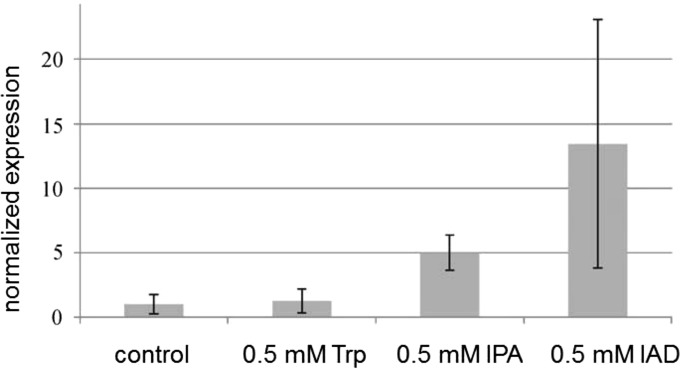
After the treatment of axenically grown mycelium with IAA precursors, the regulation of ald1 was tested. We hypothesized that the final step of IAA biosynthesis might be prone to experiencing upregulation, and indeed upregulation was shown by qPCR (Fig. 4). Equal concentrations of all precursors were used for standardization. In addition, the gene ald1 was found to be induced about 30-fold in mycorrhiza after 3 weeks and 45-fold after 3 months, while expression in low-compatibility interactions with pine roots was induced 30-fold after 3 months (Table 1; see also Fig. S2 in the supplemental material). The expression was induced in compatible interactions in both the mantle (50-fold) and Hartig net (40-fold).
FIG 4.
Effect of IAA precursors on ald1 gene expression. Expression was tested in three biological replicates by qPCR and normalized relative to a standard curve. Bars denote standard errors.
TABLE 1.
Relative amounts of ald1 transcript in mycorrhiza, determined by competitive PCR
| Type of T. vaccinum interaction (time posttreatment) | Fold increase in ald1 expressiona |
|---|---|
| Control | 1 |
| Mycorrhizal stage of compatible interaction | |
| Spruce (3 weeks) | 30 |
| Spruce (3 mo) | 45 |
| Mycorrhizal tissues of compatible interaction | |
| Hartig net | 40 |
| Fungal mantle | 50 |
| Low-compatible interaction | |
| Pine (3 mo) | 30 |
Relative amount of the transcript using 1 ng of cDNA.
IAA increases ramification of T. vaccinum hyphae.
To investigate the possible role of IAA in fungal morphogenesis, IAA precursors were exogenously applied, and fungal morphology was monitored. Inoculation with the IAA precursors Trp and IPA resulted in slightly elongated hyphal compartments and a higher hyphal ramification at higher precursor and tryptophan concentrations in three biological replicates and with 100 hyphal tips being counted (Fig. 5; see Fig. S3 in the supplemental material). Thus, IAA induced hyphal elongation as well as hyphal branching. Growth was not affected by the exogenous application of 100 μM IAA to fungal cultures (see Fig. S4). However, supplementation of fungal medium with 10 μM TIBA, either alone or simultaneously with 100 μM IAA, drastically reduced fungal radial growth, indicating that IAA supports T. vaccinum growth (Fig. S4). The same observations were made when the experiment was carried out using 0.5 mM Trp instead of IAA. The observed changes in fungal growth did not result in visible modifications of the cytoskeleton in chitin, actin, or tubulin structure as assayed by (immuno)fluorescence microscopy of T. vaccinum (see Fig. S5 in the supplemental material).
FIG 5.
T. vaccinum cell length and ramification in response to feeding cells external IAA or precursors at different concentrations. A total of 100 cells were counted (n = 3 replicates). Bars denote standard errors.
Changes in cell length and hyphal ramification were also found with the ectomycorrhizal fungus P. involutus; in contrast, the nonmycorrhizal species A. mellea, H. annosum, S. commune, L. leucothites, and L. loricatum did not respond to IAA. Thus, IAA was seen to have a signaling function in ectomycorrhizal fungi such as T. vaccinum and to affect hyphal morphology and growth, connections which have not previously been observed.
Ectomycorrhiza development is supported by IAA secretion from the fungus.
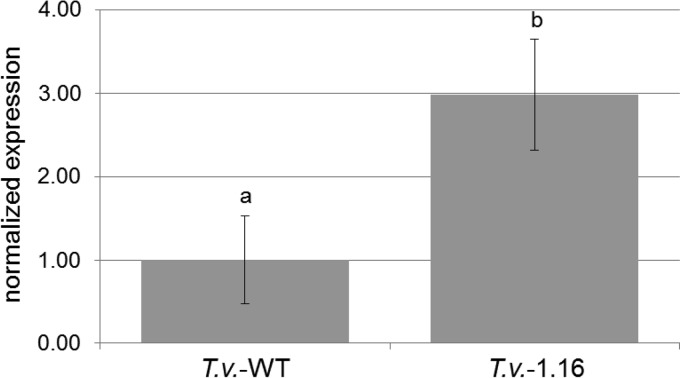
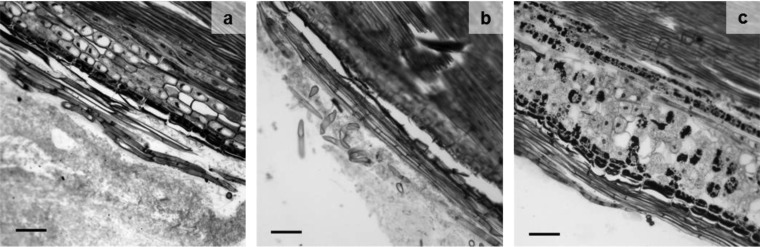
To examine the effect of IAA in symbiotic interactions, homologous transformation of ald1 was performed, and ald1 overexpression was established in the transformant T. vaccinum 1.16 (Fig. 6 gives qPCR data). The strain was used to form ectomycorrhizas, which in turn formed an enhanced Hartig net and a thicker hyphal mantle (Fig. 7). Thus, an effect of Ald1, likely through IAA formation, was verified.
FIG 6.
Expression of ald1 in T. vaccinum wild-type (T.v.-WT) and the ald1-overexpressing strain T. vaccinum 1.16 (T.v.-116). The regulation was evaluated in three biological replicates by qPCR and normalized to three reference genes (act1, cis1, and tef1). Bars denote standard errors, and letters indicate significantly different expression levels (P < 0.05).
FIG 7.
Effect of IAA on ectomycorrhiza development in microtome sections of 4-month-old spruce ectomycorrhiza with T. vaccinum in hydroponic culture. The ald1-overexpressing T. vaccinum strain 1.16 (a), the transformation recipient (b), and a nonmycorrhizal short root of P. abies (c) are shown. Scale bar, 100 μm.
For IAA to influence the morphogenesis of both plants and fungi, it must be secreted to allow a transkingdom signaling function. Involvement of a multidrug and toxic compound extrusion (MATE) transporter, mte1 of T. vaccinum, was tested since, for this transporter gene, mycorrhiza-specific expression had been established (3). We tested the hypothesis that Mte1-overexpressing, heterologous yeast transformants should show better growth in the presence of an inhibitory high concentration of IAA. Indeed, mte1 rescued growth of yeast transformants (Fig. 8). Our results showed that IAA was indeed a substrate for the tested transporter. Taking these observations together, the production, potential secretion, and effect of IAA on the fungus as well as on the tree partners were shown, and genes crucial for this mycorrhiza-specific IAA signaling between both partners were identified.
FIG 8.
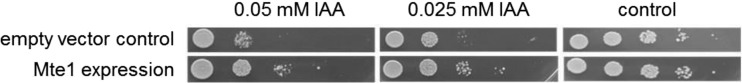
Drop test of Saccharomyces cerevisiae expressing the empty vector or mte1 showing the response to IAA applied at two concentrations (0.025 and 0.05 mM).
DISCUSSION
In this study, we identified the biosynthesis pathway for IAA production in the ectomycorrhizal fungus T. vaccinum and presented candidate genes for enzymes involved in this IAA biosynthesis pathway. Although ectomycorrhizal fungi have been reported to produce IAA, mainly with tryptophan as an IAA precursor (5, 14, 49), no single study has investigated the potential biosynthetic pathways and genes involved. Based on precursor feeding and isotope studies, we propose that T. vaccinum produces IAA through a tryptophan-indole pyruvate-indole acetaldehyde biosynthetic pathway. Free intermediates were not detected. Nevertheless, feeding cells intermediates resulted in labeled IAA being released from the fungal mycelium. The genes identified as involved in this biosynthetic pathway further support the findings for the IPA and IAM pathway available for T. vaccinum (see Fig. S1 and Table S1 in the supplemental material). Orthologs from Laccaria bicolor and T. matsutake were identified, thus supporting the notion that other ectomycorrhizal fungi also use IAA signaling in mycorrhiza formation.
One of the candidate genes for biosynthesis, the aldehyde dehydrogenase gene ald1, was identified previously in an earlier attempt to understand ectomycorrhiza-specific gene expression (3). Increased expression after cultures were fed precursors of the suggested pathway suggests that the enzyme might be involved in the oxidation of IAD to IAA, the last step in the proposed Trp-dependent biosynthetic pathway (19, 25). The variation in ald1 expression after feeding cells IAD compared to that after feeding cells IPA or tryptophan might result from the toxicity of the aldehyde that necessitates rapid detoxification by oxidation. Also, a spontaneous nonenzymatic breakdown cannot be ruled out. This pathway for IAA biosynthesis has been shown to be unlikely for plants (50) and might be specific to the fungal kingdom. Another basidiomycete, the smut fungus Ustilago maydis, has been shown to produce IAA via IPA (30). Interestingly, the product of ald1 has a significant amino acid identity of 58% to U. maydis indole-3-acetaldehyde dehydrogenase, iad1, especially in the conserved motifs (15, 35), supporting the role of ald1 in ectomycorrhizal IAA-dependent morphogenesis (51). Alternative pathways have been suggested for other fungi in different environmental settings (15, 33); however, these await detailed investigation.
In our study, the mycorrhizal fungi produced appreciable IAA amounts even when grown in axenic culture. T. vaccinum responded to IAA by increasing average cell length and ramification. In T. vaccinum, the addition of IAD did not result in cell elongation, perhaps because of the toxicity of the aldehyde and/or the involvement of other metabolic pathways. A reorganization of the cytoskeleton was not detected in our study after treatment with IAA precursors. However, a slight modification, as has been described for ectomycorrhiza (52), cannot be ruled out. For saprobic fungi, IAA production seems to be dispensable, or the concentrations necessary to exert a change in morphology might differ from those of mycorrhizal fungi. With saprobic fungi such as S. commune, IAA production is strain dependent (48) and depends on induction through interaction with other organisms or media.
The upregulation of ald1 in T. vaccinum ectomycorrhiza indicated that fungal IAA biosynthesis is relevant to the symbiosis. Comparing different mycorrhizal tissues, stages, and fungal-host compatibility, we did not observe significant differences in the expression of ald1, which confirms the hypothesis that IAA is involved in different steps of the mycobiont-plant interaction. At early stages of mantle formation, but also within the Hartig net, hyperbranching is necessary to form the pseudoparenchymatic tissues (53). By feeding T. vaccinum with IAA precursors and IAA, we showed that IAA increases fungal cell length and hyphal ramification. The lack of increased colony diameter either indicates sufficient IAA production by the fungus under in vitro conditions, where the fungus is not additionally stimulated by plant compounds, or is the result of a denser mycelium as a response to ramification.
The induction of fungal morphogenetic changes by IAA during the mycobiont-plant interaction supports the hypothesis that phytohormones play a role in enhancing ectomycorrhiza formation and efficiency (54). Our results, which show increased mantle and Hartig net formation using an ald1 overexpression strain, confirm that hypothesis and support the findings of Gay et al. (54), which show that IAA affects mycorrhiza morphology in pine when Hebeloma overproduces IAA. Additionally, the ald1-overexpressing strain, combined with feeding experiments, supported the hypothesis that ald1 catalyzes the last step in the IPA pathway in IAA biosynthesis in T. vaccinum. Knockout mutants, which would be desirable for future investigation, are not feasible since gene deletion is not available in the dikaryotic fungus T. vaccinum. Nor have gene knockout or knockdown techniques yet been established for this slow-growing fungus. With tryptophan a typical component of root exudates present at relevant concentrations (55), conversion into IAA can increase the speed and efficiency of mycorrhization. Indeed, root exudates were reported to stimulate the hyphal growth of ectomycorrhizal fungi (56). Additionally, auxin was thought to be involved in host recognition during the premycorrhizal phase (57). The functional complexity of IAA explains why the controversy over IAA involvement in ectomycorrhiza formation has continued for so long.
For IAA to act as a signaling compound between host and fungus, it must be exported by the fungus and imported into either organism. The phytohormone might act as a diffusible signal in the communication between the mycobiont and the plant (51). IAA transport mechanisms are known in plants, where TIBA acts as a polar auxin transport inhibitor and reverses the effects of IAA on spruce-Laccaria bicolor ectomycorrhiza (58). We observed similar TIBA effects although an ortholog to the plant transporters is not encoded within the T. vaccinum genome. Nevertheless, we cannot rule out the possibility that TIBA targets other mechanisms. Instead, we suggest that IAA is transported by Mte1, a transporter belonging to the multidrug and toxic compound extrusion (MATE) family and known to be upregulated in the compatible mycorrhiza of T. vaccinum (45). In this study, IAA was shown to play a role in ectomycorrhizal morphogenesis, linking Hartig net formation to the response of the fungus to IAA by increased hyphal ramification. Thus, IAA is a signal in the mycobiont-plant interaction, and fungal IAA can contribute to ectomycorrhizal functions. Genes involved in biosynthesis (such as ald1) and in transport (such as mte1) were indeed identified as mycorrhiza specific. This identification further contributes to the finding that IAA plays a versatile role in mycorrhiza, with the phytohormone regulating ectomycorrhiza formation and morphology.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Max Planck Society, Germany and by the Excellence Graduate School GSC 214 and the Research Training Group GRK 1257 funded by the German Science Foundation.
We thank Paulina Dabrowska and Anja David for assistance and Maritta Kunert, Ines Schlunk, and Elke-Martina Jung for support. Petra Mitscherlich is acknowledged for technical help. We thank Emily Wheeler for editorial assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01991-15.
REFERENCES
- 1.Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Academic Press, London, United Kingdom. [Google Scholar]
- 2.Mankel A, Krause K, Kothe E. 2002. Identification of a hydrophobin gene that is developmentally regulated in the ectomycorrhizal fungus Tricholoma terreum. Appl Environ Microbiol 68:1408–1413. doi: 10.1128/AEM.68.3.1408-1413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause K, Kothe E. 2006. Use of RNA fingerprinting to identify fungal genes specifically expressed during ectomycorrhizal interaction. J Basic Microbiol 46:387–399. doi: 10.1002/jobm.200610153. [DOI] [PubMed] [Google Scholar]
- 4.Slankis V. 1973. Hormonal relationships in mycorrhizal development, p 231–298. In Marks GC, Kozlowski TT (ed), Ectomycorrhizae: their ecology and physiology. Academic Press, London, United Kingdom. [Google Scholar]
- 5.Gay G, Debaud JC. 1987. Genetic study on indole-3-acetic acid production by ectomycorrhizal Hebeloma species: inter- and intraspecific variability in homo- and dikaryotic mycelia. Appl Microbiol Biotechnol 26:141–146. doi: 10.1007/BF00253898. [DOI] [Google Scholar]
- 6.Rudawska ML, Kieliszewska-Rokicka B. 1997. Mycorrhizal formation by Paxillus involutus strains in relation to their IAA-synthesizing activity. New Phytol 137:509–517. doi: 10.1046/j.1469-8137.1997.00838.x. [DOI] [PubMed] [Google Scholar]
- 7.Gea L, Normand L, Vian B, Gay G. 1994. Structural aspects of ectomycorrhiza of Pinus pinaster (Ait.) Sol. formed by an IAA-overproducer mutant of Hebeloma cylindrosporum Romagnési. New Phytol 128:659–670. doi: 10.1111/j.1469-8137.1994.tb04030.x. [DOI] [Google Scholar]
- 8.Herrmann S, Oelmüller R, Buscot F. 2004. Manipulation of the onset of ectomycorrhiza formation by indole-3-acetic acid, activated charcoal or relative humidity in the association between oak microcuttings and Piloderma croceum: influence on plant development and photosynthesis. J Plant Physiol 161:509–517. doi: 10.1078/0176-1617-01208. [DOI] [PubMed] [Google Scholar]
- 9.Niemi K, Vuorinen T, Ernstsen A, Häggman H. 2002. Ectomycorrhizal fungi and exogenous auxins influence root and mycorrhiza formation of Scots pine hypocotyl cuttings in vitro. Tree Physiol 22:1231–1239. doi: 10.1093/treephys/22.17.1231. [DOI] [PubMed] [Google Scholar]
- 10.Horan DP. 1991. The infection process in eucalypt mycorrhizas. Australian National University, Canberra, Australia. [Google Scholar]
- 11.Wallander BH, Nylund J-E, Sundberg B. 1994. The influence of IAA, carbohydrate and mineral concentration in host tissue on ectomycorrhizal development on Pinus sylvestris L. in relation to nutrient supply. New Phytol 127:521–528. doi: 10.1111/j.1469-8137.1994.tb03970.x. [DOI] [Google Scholar]
- 12.Wallander H, Nylund JE, Sundberg B. 1992. Ectomycorrhiza and nitrogen effects on root IAA: results contrary to current theory. Mycorrhiza 1:91–92. doi: 10.1007/BF00206142. [DOI] [Google Scholar]
- 13.Charvet-Candela V, Hitchin S, Ernst D, Sandermann H, Marmeisse R, Gay G. 2002. Characterization of an Aux/IAA cDNA upregulated in Pinus pinaster roots in response to colonization by the ectomycorrhizal fungus Hebeloma cylindrosporum. New Phytol 154:769–777. doi: 10.1046/j.1469-8137.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 14.Sukumar P, Legue V, Vayssieres A, Martin F, Tuskan GA, Kalluri UC. 2013. Involvement of auxin pathways in modulating root architecture during beneficial plant-microorganism interactions. Plant Cell Environ 36:909–919. doi: 10.1111/pce.12036. [DOI] [PubMed] [Google Scholar]
- 15.Basse CW, Lottspeich F, Steglich W, Kahmann R. 1996. Two potential indole-3-acetaldehyde dehydrogenases in the phytopathogenic fungus Ustilago maydis. Eur J Biochem 242:648–656. doi: 10.1111/j.1432-1033.1996.0648r.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooney TP, Nonhebel HM. 1989. The measurement and mass-spectral identification of indole-3-pyruvate from tomato shoots. Biochem Biophys Res Commun 162:761–766. doi: 10.1016/0006-291X(89)92375-9. [DOI] [PubMed] [Google Scholar]
- 17.Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. 2002. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 49:249–272. doi: 10.1023/A:1015298812300. [DOI] [PubMed] [Google Scholar]
- 18.Tam YY, Normanly J. 1998. Determination of indole-3-pyruvic acid levels in Arabidopsis thaliana by gas chromatography selected ion monitoring mass spectrometry. J Chromatogr A 800:101–108. doi: 10.1016/S0021-9673(97)01051-0. [DOI] [PubMed] [Google Scholar]
- 19.Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Ann Bot 95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter H. 1966. Effect of auxin and sugar on cell wall synthesis and growth of pea stem segments. Proc K Ned Akad Wet C C 69:64–72. [Google Scholar]
- 21.Zhao YD, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mano Y, Nemoto K, Suzuki M, Seki H, Fujii I, Muranaka T. 2010. The AMI1 gene family: indole-3-acetamide hydrolase functions in auxin biosynthesis in plants. J Exp Bot 61:25–32. doi: 10.1093/jxb/erp292. [DOI] [PubMed] [Google Scholar]
- 23.Oka M, Miyamoto K, Okada K, Ueda J. 1999. Auxin polar transport and flower formation in Arabidopsis thaliana transformed with indoleacetamide hydrolase (iaaH) gene. Plant Cell Physiol 40:231–237. doi: 10.1093/oxfordjournals.pcp.a029532. [DOI] [PubMed] [Google Scholar]
- 24.Pollmann S, Duchting P, Weiler EW. 2009. Tryptophan-dependent indole-3-acetic acid biosynthesis by “IAA-synthase” proceeds via indole-3-acetamide. Phytochemistry 70:523–531. doi: 10.1016/j.phytochem.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Mano Y, Nemoto K. 2012. The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872. doi: 10.1093/jxb/ers091. [DOI] [PubMed] [Google Scholar]
- 26.Kumavath RN, Ramana CV, Sasikala C. 2010. l-Tryptophan catabolism by Rubrivivax benzoatilyticus JA2 occurs through indole 3-pyruvic acid pathway. Biodegradation 21:825–832. doi: 10.1007/s10532-010-9347-y. [DOI] [PubMed] [Google Scholar]
- 27.Manulis S, Shafrir H, Epstein E, Lichter A, Barash I. 1994. Biosynthesis of indole-3-acetic-acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology 140:1045–1050. doi: 10.1099/13500872-140-5-1045. [DOI] [PubMed] [Google Scholar]
- 28.Patten CL, Glick BR. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bölker M, Basse CW, Schirawski J. 2008. Ustilago maydis secondary metabolism-from genomics to biochemistry. Fungal Genet Biol 45(Suppl 1):S88–S93. doi: 10.1016/j.fgb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW. 2008. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Pathol 9:339–355. doi: 10.1111/j.1364-3703.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa T, Koga J, Adachi T, Kishi K, Syono K. 1996. Efficient conversion of l-tryptophan to indole-3-acetic acid and/or tryptophol by some species of Rhizoctonia. Plant Cell Physiol 37:899–905. doi: 10.1093/oxfordjournals.pcp.a029037. [DOI] [Google Scholar]
- 32.Hilbert M, Voll LM, Ding Y, Hofmann J, Sharma M, Zuccaro A. 2012. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol 196:520–534. doi: 10.1111/j.1469-8137.2012.04275.x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M, Riov J, Sharon A. 1998. Indole-3-acetic acid biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol 64:5030–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsavkelova E, Oeser B, Oren-Young L, Israeli M, Sasson Y, Tudzynski B, Sharon A. 2012. Identification and functional characterization of indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal Genet Biol 49:48–57. doi: 10.1016/j.fgb.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Asiimwe T, Krause K, Schlunk I, Kothe E. 2012. Modulation of ethanol stress tolerance by aldehyde dehydrogenase in the mycorrhizal fungus Tricholoma vaccinum. Mycorrhiza 22:471–484. doi: 10.1007/s00572-011-0424-9. [DOI] [PubMed] [Google Scholar]
- 36.Duponnois R, Garbaye J. 1991. Techniques for controlled synthesis of the Douglas-fir-Laccaria-laccata ectomycorrhizal symbiosis. Ann For Sci 48:641–650. doi: 10.1051/forest:19910603. [DOI] [Google Scholar]
- 37.Fortin JA, Piche Y. 1979. Cultivation of Pinus strobus root-hypocotyl explants for synthesis of ectomycorrhizae. New Phytol 83:109–119. doi: 10.1111/j.1469-8137.1979.tb00732.x. [DOI] [Google Scholar]
- 38.Kottke I, Guttenberger M, Hampp R, Oberwinkler F. 1987. An in vitro method for establishing mycorrhizae on coniferous tree seedlings. Trees Struct Funct 1:191–194. [Google Scholar]
- 39.Asiimwe T, Krause K, Schlunk I, Kothe E. 2010. Ectomycorrhiza in sustainable ecosystem functioning: a closer look at the symbiotic association, p 113–120. In Behl RK, Kubat J, Kleynhans T (ed), Resource management towards sustainable agriculture and development. Agrobios, Jodhpur, India. [Google Scholar]
- 40.Henke C, Jung E-M, Kothe E. 21 March 2015. Hartig' net formation of Tricholoma vaccinum-spruce ectomycorrhiza in hydroponic cultures. Environ Sci Pollut Res Int doi: 10.1007/s11356-015-4354-5. [DOI] [PubMed] [Google Scholar]
- 41.Knabe N, Jung EM, Freihorst D, Hennicke F, Horton JS, Kothe E. 2013. A central role for Ras1 in morphogenesis of the basidiomycete Schizophyllum commune. Eukaryot Cell 12:941–952. doi: 10.1128/EC.00355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendland J, Kothe E. 1997. Isolation of tef1 encoding translation elongation factor EF1α from the homobasidiomycete Schizophyllum commune. Mycol Res 101:798–802. doi: 10.1017/S0953756296003450. [DOI] [Google Scholar]
- 43.Sosa-Morales ME, Guevara-Lara F, Martinez-Juarez VM, Paredes-Lopez O. 1997. Production of indole-3-acetic acid by mutant strains of Ustilago maydis (maize smut/huitlacoche). Appl Microbiol Biotechnol 48:726–729. doi: 10.1007/s002530051123. [DOI] [Google Scholar]
- 44.Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E. 2008. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 45.Schlunk I, Krause K, Wirth S, Kothe E. 8 January 2015. A transporter for abiotic stress and plant metabolite resistance in the ectomycorrhizal fungus Tricholoma vaccinum. Environ Sci Pollut Res Int doi: 10.1007/s11356-014-4044-8. [DOI] [PubMed] [Google Scholar]
- 46.Bentley JA, Farrar KR, Housley S, Smith GF, Taylor WC. 1956. Some chemical and physiological properties of 3-indolylpyruvic acid. Biochem J 64:44–49. doi: 10.1042/bj0640044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, DuBois J, Ballou D, Zhao Y. 2013. The biochemical mechanism of auxin biosynthesis by an arabidopsis YUCCA flavin-containing monooxygenase. J Biol Chem 288:1448–1457. doi: 10.1074/jbc.M112.424077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein E, Miles PG. 1967. Identification of indole-3-acetic acid in the basidiomycete Schizophyllum commune. Plant Physiol 42:911–914. doi: 10.1104/pp.42.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karabaghli C, Frey-Klett P, Sotta B, Bonnet M, Le Tacon F. 1998. In vitro effects of Laccaria bicolor S238 N and Pseudomonas fluorescens strain BBc6 on rooting of de-rooted shoot hypocotyls of Norway spruce. Tree Physiol 18:103–111. doi: 10.1093/treephys/18.2.103. [DOI] [PubMed] [Google Scholar]
- 50.Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development 140:943–950. doi: 10.1242/dev.086363. [DOI] [PubMed] [Google Scholar]
- 51.Podila GK. 2002. Signaling in mycorrhizal symbioses: elegant mutants lead the way. New Phytol 154:541–545. doi: 10.1046/j.1469-8137.2002.00434_1.x. [DOI] [PubMed] [Google Scholar]
- 52.Timonen S, Peterson RL. 2002. Cytoskeleton in mycorrhizal symbiosis. Plant Soil 244:199–210. doi: 10.1023/A:1020209213524. [DOI] [Google Scholar]
- 53.Sirrenberg A, Salzer P, Hager A. 1995. Induction of mycorrhiza-like structures and defense reactions in dual cultures of spruce callus and ectomycorrhizal fungi. New Phytol 130:149–156. doi: 10.1111/j.1469-8137.1995.tb01825.x. [DOI] [Google Scholar]
- 54.Gay G, Normand L, Marmeisse R, Sotta B, Debaud JC. 1994. Auxin overproducer mutants of Hebeloma-cylindrosporum romagnesi have increased mycorrhizal activity. New Phytol 128:645–657. doi: 10.1111/j.1469-8137.1994.tb04029.x. [DOI] [Google Scholar]
- 55.Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B. 2006. Organic acids, sugars, and l-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19:250–256. doi: 10.1094/MPMI-19-0250. [DOI] [PubMed] [Google Scholar]
- 56.Tagu D, Lapeyrie F, Martin F. 2002. The ectomycorrhizal symbiosis: genetics and development. Plant Soil 244:97–105. doi: 10.1023/A:1020235916345. [DOI] [Google Scholar]
- 57.Sarjala T, Niemi K, Häggman H. 2010. Mycorrhiza formation is not needed for early growth induction and growth-related changes in polyamines in Scots pine seedlings in vitro. Plant Physiol Biochem 48:596–601. doi: 10.1016/j.plaphy.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 58.Karabaghli-Degron C, Sotta B, Bonnet M, Gay G, Le Tacon F. 1998. The auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA) inhibits the stimulation of in vitro lateral root formation and the colonization of the tap-root cortex of Norway spruce (Picea abies) seedlings by the ectomycorrhizal fungus Laccaria bicolor. New Phytol 140:723–733. doi: 10.1046/j.1469-8137.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 59.Bartel B. 1997. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 48:51–66. doi: 10.1146/annurev.arplant.48.1.51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.