Abstract
Aim:
The aim of this study was to evaluate the antimicrobial and antioxidant effects of Crinum jagus (J. Thomps.) Dandy methanolic bulb extract in wound healing.
Materials and Methods:
Phytochemical screening revealed the presence of alkaloids, glycosides, tannins, and saponins in the extract. In vitro antimicrobial activity of the extract was determined by agar well diffusion method. In vivo antimicrobial activity of the extract was determined by microbial assay of excision wound in rats contaminated with Staphylococcus aureus, Bacillus subtilis, Pseudomonas areuginosa, and Candida albicans and treated with 300 mg/kg body weight (bw) of 10 and 5% methanolic C. jagus bulb extract ointment (MCJBEO), respectively. Enzymatic antioxidant effect of the extract was determined in vivo by assaying superoxide dismutase (SOD) and catalase (CAT) activity, and malondialdehyde (MDA) level in excision wound biopsies of rats treated with 10 and 5% MCJBEO, respectively, following standard methods. Non-enzymatic antioxidant effect of the extract was determined in vitro using diphenylpicrylhydrazyl (DPPH) method following standard procedure.
Results:
The extract exhibited in vitro antimicrobial effect in a concentration-dependent manner with one hundred (100) mg/ml concentration of the extract having the highest inhibitory zone diameter for B. subtilis (25 mm), S. aureus (21 mm), and C. albicans (14 mm) followed by the 50, 25 and 12.5 mg/ml concentrations, respectively. B. subtilis, S. aureus, and C. albicans were not isolated from wounds of animals treated with both extract concentrations 10% and 5% MCJBEO, and reference drug (framycetin sulfate/clotrimazole). Activities of the enzymatic antioxidants SOD and CAT in wound biopsies treated with 10% MCJBEO were significantly (P < 0.05) higher when compared with those treated with 5% MCJBEO. Significantly (P < 0.05) decreased MDA level of wound biopsies from extract-treated rats was observed. The extract exhibited non-enzymatic antioxidant (DPPH) effect in a concentration-dependent manner.
Conclusion:
This study has shown that an anti-microbial and antioxidant effects could possibly be part of mechanism by which C. jagus bulb extract promote wound healing process.
KEY WORDS: Antimicrobial, antioxidant, Crinum jagus, in vivo, wound healing
INTRODUCTION
Wound healing is the process of re-establishing the integrity of damaged skin [1-3]. It is an orderly intricate process initiated by a damaged tissue itself, and it involves complex mechanisms which include: Hemostasis, inflammation, proliferation, and remodeling [1,4,5]. Each of these mechanisms requires several biochemical substances to occur [4,6-8]. Thromboxane A2 and plasminogen activator inhibitor Type 1 ensures hemostasis, heme and heme proteins trigger expression of adhesion molecules, leukocytic infiltration, and release of reactive oxygen species (ROS) also called toxic free radicals or oxidants. The oxidants are detrimental to wound contaminating microorganisms and to the skin tissue itself especially when in excess [4,6,8]. Therefore, hemeoxygenase-1 elicit antioxidant effect and scavenge (mop-up) the toxic free radicals, while matrix metalloproteinase ensures remodeling of the extracellular matrix [9]. The length of time it takes for wound healing to be optimum and complete is determined by factors such as availability of the needed biochemical substances, presence or absence of contaminating microorganism(s), and the toxic free radicals in the wound bed [10,11].
A myriad of wound contaminating organisms including bacteria (both Gram-positive e.g., Staphylococcus spp., Streptococci, Bacillus spp., etc. and Gram-negative e.g., Pseudomonas aeruginosa, Escherichia coli, Proteus, etc.) and fungi (both yeast e.g., Candida albicans, and mold e.g., Aspergillus) impede natural wound healing process [12-15]. They produce substances (enzymes, toxins, and free toxic radicals such as hydrogen peroxide [H2O2]) that degrade biochemical substances and destroy cellular components needed for wound healing [13-15]. Majority of wound contaminating microorganisms have developed resistance to most of the commonly used orthodox antimicrobial agents and this has garnered public attention [16-20].
Toxic free radicals (oxidants) such as superoxide (SO2−) and hyrdroxyl (OH−) anions, and hydrogen peroxide (H2O2) causes oxidative stress [21]. These oxidants are produced by infiltrating phagocytes (during inflammation stage of healing), contaminating microbes in wound bed, and the skin itself following ultraviolet exposure [21,22]. Oxidative stress further damages wounded skin tissue by lipid peroxidation (caused by lipid peroxidase and evidenced by increased malondialdehyde [MDA] level in wound tissue), and destruction of proteins and extracellular matrix [23] - this further impedes natural wound healing process. Substances that elicit antimicrobial and antioxidant effects are often used in wound management in orthodox medicine [24,25]. These substances are used to control the growth of contaminating organisms and mop-up (scavenge) toxic free radicals to achieve optimum healing [11,16]. The radical scavenging activity of natural enzymatic antioxidants (such as superoxide dismutase [SOD], catalase [CAT], glutathione dismutase, thioredoxin reductase, etc.) produced by the skin is augmented by the orthodox medicines to promote wound healing process [21]. Reports have shown that these orthodox agents are often costly [17], and often times elicit side effects which are detrimental to the recipient [11]. Therefore, there is need for cheaper and safe alternative or complementary substances that could elicit both antimicrobial and antioxidant effects to enhance natural wound healing process.
Crinum jagus (J. Thomps.) Dandy popularly called St. Christopher or Harmattan lily, Frest crinum or Poison bulb is widely used in form of decoction by traditional practitioners in Africa, including Southeastern Nigeria, for treatment of skin wounds and several other ailments [26,27,28-32] some of which have been scientifically validated [33,34,35]. Chemical investigations revealed that it contained high amount of phenolic compounds including crinamine, lycorine, psuedolycorine, 6-hydroxycrinamine, hamayne, tetrahydro-1, 4-oxazine (morpholine), bowdensine, and demethoxy-bowdensine [36,37,38,39]. It also contained saponins, tannins, calcium oxalate, and calcium tetrata [37,38]. Phenolic compounds in plant extracts exhibited enzymatic and non-enzymatic antioxidant effect [40,41]. Plant extracts containing flavonoids, triterpernoids, and tannins exhibited antimicrobial and antioxidant effects [40,42]. Therefore, C. jagus which contains some of these bioactive substances could possibly promote wound healing process by eliciting antimicrobial and/or antioxidant effects.
Although Adesanya et al. [37] reported anti-staphylococcal activity of C. jagus bulb, the study was not conducted in a wound healing model; and Staphylococcus is one of the numerous potential wound contaminants that impede wound healing process. Ode et al. [35] and Nwaehujor et al. [36] reported non-enzymatic in vitro free radical scavenging of diphenylpicrylhydrazyl (DPPH) by C. jagus bulb extract which is not related to wound healing. The antimicrobial effect of C. jagus bulb extract on common potential wound contaminating microorganisms and its enzymatic antioxidant effect in wound healing have not been evaluated. The objective of this study was to determine if C. jagus methanolic bulb extract (CJMBE) could possibly exhibit antimicrobial and/or antioxidant effect in wound healing.
MATERIALS AND METHODS
The experimental protocols used in this study was approved by the Ethics Committee of the University of Nigeria, Nsukka and conforms with the guide to the care and use of animals in research and teaching of University of Nigeria, Nsukka, Enugu State Nigeria.
Animals
A total of 97, 8-week-old male albino Wistar rats weighing between 220 and 229 g were obtained from the laboratory animal unit, Faculty of Veterinary Medicine, University of Nigeria, Nsukka. They were fed on commercial growers mash (Top feeds®) and water was provided ad libitum. These rats were acclimatized for 2 weeks in the animal house at the Department of Veterinary Surgery, University of Nigeria, Nsukka.
Plant Collection and Identification
Fresh C. jagus bulbs were collected from Amokwe town in Udi Local Government Area Enugu State, Nigeria, in the month of May, 2014 and were identified at the International Center for Ethnomedicine and Drug Development (InterCEDD), Nsukka, by a plant taxonomist, Mr. A. Ozioko. A voucher specimen (number FRMPC/05/14) was deposited in the center’s herbarium.
Extraction
A kg of the C. jagus (J. Thomps.) Dandy bulbs were sliced into smaller pieces; air dried at room temperature for 2 weeks and then pulverized using the laboratory grinding machine at the Department of Crop Science, University of Nigeria, Nsukka. The pulverized bulbs were macerated in 80% methanol for 48 h with intermittent vigorous shaking at every 2 h. After 48 h, the mixture was filtered and the extract concentrated using a rotary evaporator set at 40°C. The dried CJMBE was weighed and the percentage yield calculated. The extract was then stored at 4°C in a refrigerator until needed.
Acute Toxicity Test
Totally, 25 adult rats were randomly divided into five groups of five animals per group. The animals were deprived water for 16 h before administration of the extract. The increasing doses of the extract 250, 500, 1000, 2000, and 5000 mg/kg body weight (bw) suspended in dimethyl sulfoxide (DMSO) was administered orally to the test groups, respectively, using a ball-tipped intubation needle fitted onto a syringe. The last group received 1 ml/kg bw of sterile distilled water and served as the control. The rats were allowed access to food and water ad libitum and were observed for 48 h for behavioral changes and death. The time of onset, intensity, and duration of these symptoms, if any, was recorded.
Phytochemical Analysis for Bioactive Substances
The extract was screened for the presence of bioactive components tannins, saponins, glycosides, flavonoids, and alkaloids following the methods of Trease and Evans [43].
Preparation of Ointments
The method of Okore et al. [44] was adapted in preparation of two herbal ointments containing 10 and 5% w/w of the extract in sterile soft white paraffin. Immediately after preparation, the ointments were aseptically transferred into sterile cream tubes and sealed until further needed.
Pathogens and Preparation of Inocula
The bacterial (Pseudomonas aeruginosa, Stapylococcus aureus, and Bacillus subtilis) and fungal (C. albicans) organisms used in this study were collected from the Department of Pharmaceutics, University of Nigeria, Nsukka. They were clinical wound isolates from patients in Nsukka, Nigeria, fully identified and maintained on nutrient agar slope at 4°C at the Department of Pharmaceutical Microbiology Laboratory, University of Nigeria, Nsukka. Prior to use, the bacterial organisms were sub-cultured on sterile nutrient agar plate, incubated aerobically at 37°C for 24 h, while the C. albicans was sub-cultured on sterile Sabouraud dextrose agar, incubated at 25°C for 48 h. Colonies of each organism were homogenized in sterile phosphate buffered saline (PBS) and the turbidity adjusted to correspond to 0.5 McFarland’s turbidity standard (equivalent to 1 × 108 cfu/ml). The standardized broth cultures were kept at 4°C until needed.
Preparation of Extract Concentrations
A 100 mg/ml stock concentration was prepared by dissolving 1 g of the extract in 10 ml of DMSO. Then, 2-fold dilutions were made from the stock concentration to obtain concentrations of 50 mg/ml, 25 mg/ml, 12.5 mg/ml, and 6.25 mg/ml.
Determination of In vitro Antimicrobial Effect of C. jagus Bulb Extract
The inhibitory zone diameter (IZD) of each of the extract concentrations for each test organism was determined by agar-well diffusion method [45]. Briefly, the standardized broth cultures of the bacterial organisms were incubated at 37°C for 10 min, and then inoculated on sterile Mueller-Hinton agar plates using sterile swab sticks. Five holes of 6 mm diameter were bored into the agar plates at strategic points using sterile cork borer and labeled to correspond to the extract concentrations. Then each of the holes was filled with 50 µl of the extract concentration. The plates were allowed on the bench to ensure complete diffusion of the extract into the agar and then incubated accordingly as above. For the fungal organism, broth culture was incubated at 25°C for 10 min before inoculating sterile Sabouraud dextrose agar. Same procedure as above was undertaken and then the plates were incubated at 25°C for 48 h. After incubation, the zone of inhibition around each well was measured with a meter rule. Each test for each organism was performed in triplicate and the mean IZD calculated to the nearest whole millimeters.
Determination of In vivo Antimicrobial Effect of C. jagus Bulb Extract
Creation and contamination of excision wound with test microorganisms
Thirty six rats were anesthetized with 10 and 50 mg/kg bw of xylazine hydrochloride and ketamine hydrochloride, respectively. Their dorsum was shaved and disinfected with 70% alcohol. Then, full thickness (480 mm2) circular excision wounds were created following the method described by Morton and Malone [46]. Post-wounding, the rats were randomly assigned into 4 groups of 9 animals per group. Then, using sterile Pasteur pipettes, wound on each animal was contaminated by flooding with 1 ml of standardized broth culture of each test organism. To minimize further microbial contamination of wound, each animal was carefully placed individually in disinfected cages kept in a disinfected, clean and dust-free animal house in the Department of Veterinary Surgery, University of Nigeria, Nsukka. The wounds were not treated for 24 h post-contamination to ensure colonization and establishment of infection [11].
Treatment of Infected Excision Wound
Treatment of contaminated animal wound commenced 48 h post-contamination. Four treatment groups consisting of 9 animals each were treated as follows: Groups A and B were treated topically with 10% and 5% w/w methanolic CJMBE ointment (MCJBEO), respectively, Group C was treated with sterile normal saline (negative control), while Group D was treated with framycetin sulfate/clotrimazole (Sofradex-f®) (positive control), respectively. Treatment of the animals continued until complete healing occurred.
Isolation of Infective Pathogen from Contaminated Excision Wound
At days 3, 7, and 14 post-infection (p.i.), wound swabs from 3 animals in each treatment group was taken in duplicate using sterile swab sticks. The swabs were inoculated into sterile nutrient and Sabouraud dextrose broths, incubated at 37°C for 24 h and 25°C for 48 h, for bacterial and fungal isolation, respectively. The broth cultures were observed for microbial growth (cloudiness/turbidity) and if any, a loopful of the broth culture was sub-cultured on appropriate sterile agar and incubated accordingly. Isolates of different colonial types, if any, were purified on appropriate fresh media and incubated accordingly. Morphological characteristics of pure colonies of the isolates were noted and appropriately described. Then, pure colonies of the isolates were gram stained and subjected to biochemical tests such as catalase, hemolysis and coagulase, for identification following standard biochemical methods. The number of animals from which each organism was isolated was appropriately recorded and the percentage calculated.
Determination of Enzymatic Antioxidant Effect of C. jagus Bulb Extract in Wound Tissue
A total of 36 rats were anesthetized and full thickness (480 mm2) circular excision wounds were created as described above. Four treatment groups consisting of 9 animals per group were treated as follows: Groups I and II were treated topically with 10 and 5% w/w MCJBEO, respectively, while Groups III and IV were treated with sterile white soft paraffin (negative control) and framycetin sulfate/clotrimazole (positive control), respectively. Then, the wound on each of the animal was carefully bandaged using sterile gauze and adhesive tape was placed over the gauze. The animals were placed individually in a clean disinfected metal cage after grouping to avoid them biting each other’s wound. At days 3, 7, and 14 post-treatment, wound biopsy specimen was taken from 3 animals in each group and the bandages were changed. Immediately after collection of wound biopsy, specimen was placed in 10% PBS and used for biochemical assay of SOD and CAT activities, and MDA level. The SOD activity was determined following the method described by Sun et al. [47], catalase activity was determined following the procedure described by Sinha [48], while the MDA level was determined according to the method described by Draper and Hardley [49].
Determination of In vitro Non-enzymatic Antioxidant Effect of C. jagus Bulb Extract
Free radical scavenging activity of the extract of C. jagus was determined using DPPH assay as described by Brand-Williams et al. [50]. Briefly, 2 ml of various concentrations (10, 50, 100, 200, and 400 µg/ml) of the Crinum jagus bulb extract was added to 1 ml of DPPH (0.5 mM in 95% methanol) in a cuvette. The mixture was shaken and incubated at 30°C for 30 min in the dark. Then, the absorbance was taken at 517 nm using a spectrophotometer. Ascorbic acid at doses lower than that which have been reported to act as pro-oxidant [51,52] were used as a standard compound (control) in this assay. For each extract concentration, the experiment was performed in triplicate and the mean absorbance calculated. The percentage scavenging activity was calculated as follows:
Scavenging effect (%) = (control absorbance − sample absorbance/[control absorbance]) × 100
Statistical Analysis
Data obtained for in vivo antimicrobial effect were expressed in percentages, while data obtained for in vivo and in vitro antioxidant effects were summarized as mean ± standard error of mean. Mean values of SOD and CAT activities and MDA levels for different groups were compared using one-way Analysis of Variance. Duncan multiple range test was used to separate variant means. P < 0.05 was considered significant.
RESULTS
Extraction
The CJMBE had an aromatic smell and was brownish in color. The percentage yield was 14.7% w/w material.
Acute Toxicity Test
Administration of MCJBE extract in DMSO to rats even at the highest dose of 2000 mg/kg bw did not produce any death in the treated groups. No sign of acute toxicity was also observed.
Phytochemical Analysis
Preliminary phytochemical analysis of CJMBE MCJBE qualitatively revealed the presence of alkaloids, tannins, saponins, and glycosides [Table 1].
Table 1.
Phytochemical analysis of methanolic C. jagus bulb extract

In vitro Antimicrobial Effect of Crinum jagus Bulb Extract
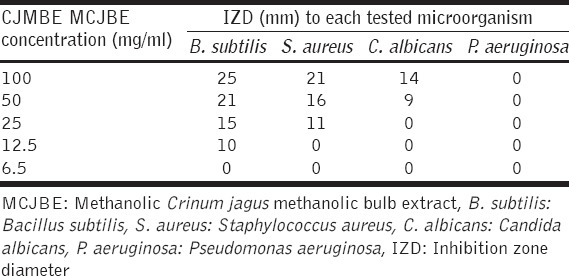
The 100 mg/ml concentration of the MCJBE gave the highest IZD for B. subtilis (25 mm), S. aureus (21 mm), and C. albicans (14 mm) [Table 2]. The 50 mg/ml concentration gave IZD for B. subtilis (21 mm), S. aureus (16 mm), and C. albicans (9 mm). The 25 mg/ml concentration gave IZD for B. subtilis (15 mm) and S. aureus (10 mm) while the 12.5 mg/ml concentration gave IZD for only B. subtilis (10 mm). None of the tested concentrations of the extract inhibited the growth of P. aeruginosa.
Table 2.
IZD of test concentrations of extract to each tested microorganism
In vivo Antimicrobial Effect of Crinum jagus Bulb Extract in Wound
The result of frequency of reisolation of infective microorganism from wounds of animals in Groups A (treated with 10% MCJBEO), B (treated with 5% MCJBEO), C (treated with sterile normal saline), and D (treated with framycetin sulfate/clotrimazole) are presented in Figures 1-4, respectively. B. subtilis and S. aureus were not reisolated from wound of any animal in Groups A, B, and D at days 3, 7, and 14 post-infection. P. aeruginosa was reisolated from wound of all the animals in all the groups throughout the experiment. C. albicans was not reisolated from wound of any animal in Groups A, B, and D throughout the experiment, but was reisolated from wound of all the animals in Group C throughout the experiment.
Figure 1.
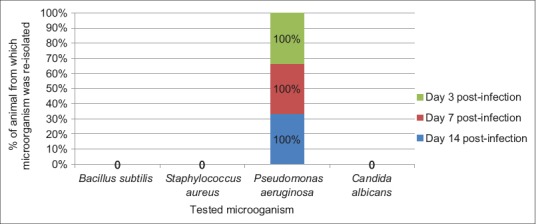
Frequency of reisolation of infective microorganism from animals in Group A treated with 10% methanolic Crinum jagus bulb methanolic extract ointment
Figure 2.
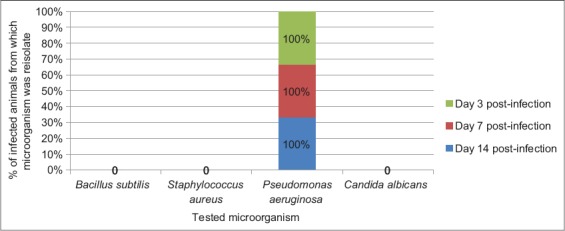
Frequency of reisolation of infective microorganism from animals in Group B treated with 5% methanolic Crinum jagus methanolic bulb extract ointment
Figure 3.
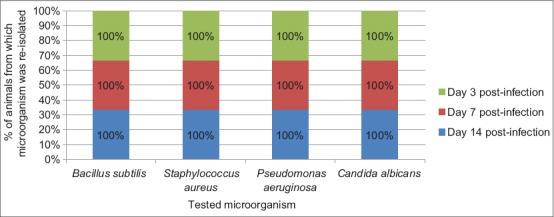
Frequency of reisolation of infective microorganism from animals in Group C treated with sterile normal saline (negative control)
Figure 4.
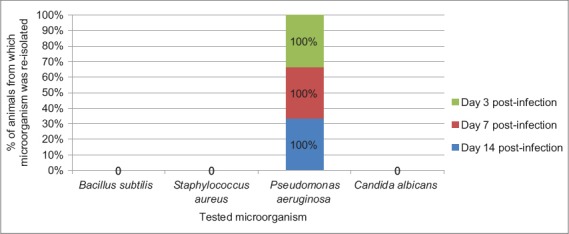
Frequency of reisolation of infective microorganisms from animals in Group D treated with framycetin sulfate/clotrimazole (Sofradex-f®)
Antioxidant Effect of C. jagus Bulb Extract in Wound Tissue
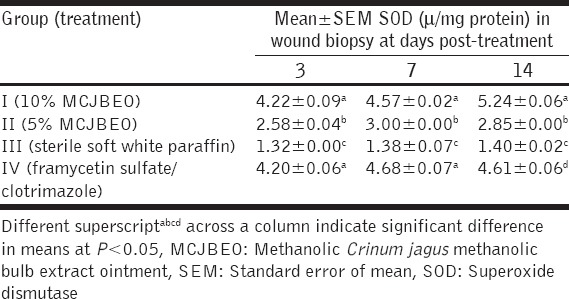
At day 3 post-treatment, SOD activity of Group I (treated with 300 mg/kg of 10% MCJBEO) significantly (P < 0.05) increased when compared with Groups II (treated with 300 mg/kg of 5% MCJBEO) and III (treated with sterile normal saline) [Table 3]. No significant (P > 0.05) difference existed in SOD activity between Groups I and IV (treated with framycetin sulfate/clotrimazole). SOD activity of Group II was significantly (P < 0.05) higher compared with Group III throughout the experiment but significantly lower compared with the Group IV. Similar trend was observed at day 7 post-treatment At day 14 post-treatment, SOD activity of Group I was significantly (P < 0.05) higher than those of the other groups.
Table 3.
SOD activity in wound biopsy of animals treated with MCJBEO
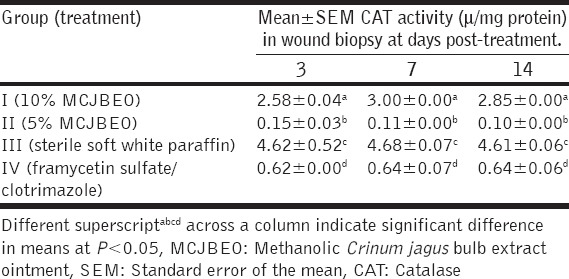
There were significant differences (P < 0.05) in catalase activity of wound biopsy among all the groups at day 3 post-treatment [Table 4]. There was significant (P < 0.05) increase in CAT activity of Group III when compared with the other groups. Increased significant difference (P < 0.05) existed between CAT activity of Groups I, II and III. Similar trend was observed at days 7 and 14 post-treatment.
Table 4.
CAT activity in wound biopsy of animals treated with MCJBEO
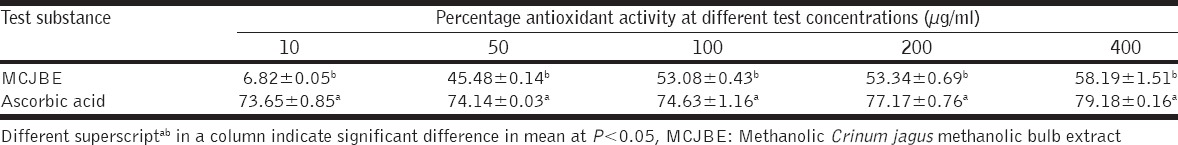
At day 3 post-treatment, MDA level in wound biopsies of animals in Group I significantly (P < 0.05) decreased when compared with the other groups [Table 5]. No significant difference (P > 0.05) occurred in MDA levels between Groups I and IV. Similar trends were observed at days 7 and 14 post-treatment At concentration of 10 µg/ml, the non-enzymatic free radical scavenging activity of CJMBE decreased significantly (P < 0.05) when compared with the control (ascorbic acid) [Table 6]. Similar trend was observed at concentrations of 50, 100, 200, and 400 µg/ml. Mean values of the antioxidant activity of the extract showed increasing activity with an increase in concentration.
Table 5.
MDA level in wound biopsy of animals treated with MCJBEO
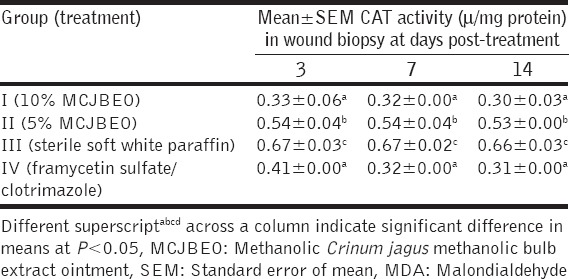
Table 6.
Free radical scavenging activity of MCJBE
Bacteriological Assay of Excision Wound Post-Contamination
Cultures of wound swabs from animals in Groups III yielded heavy growth of all the infective organisms (S. aureus, B. subtitlis, P. aeruginosa, and C. albicans) throughout the study period, whereas cultures of wound swabs from animals in Group I, II, and IV yielded scanty growth of only P aeruginosa at days 3, 7, and 14 post-treatment.
DISCUSSION
In this study, the antimicrobial and antioxidant effects of CJMBE in wound healing was evaluated. Microbial wound contamination alters healing process and result in complication such as bacteremia and/or septicemia following wound invasion [53,54]. These complications are often common when resistant organisms constitute the contaminants. Ability of a substance to control the growth of a microorganism is ascertained by observing the extent to which the growth of the organism is inhibited by the substance. In the present study, in vitro antimicrobial study revealed that C. jagus bulb extract inhibited the growth of bacteria - B. subtilis and S. aureus and a yeast C. albicans. This indicates that the plant extract exhibited antibacterial and antifungal activities. The fact that there was decreased antimicrobial activity with decrease in concentration of the extract, suggests that the antimicrobial effect of the C. jagus bulb extract is concentration-dependent. The 100 mg/ml concentration of the extract gave the highest IZD for the 3 inhibited organisms (B. subtilis [25 mm], S. aureus [21 mm], and C. albicans [14 mm]) which suggests that C. jargus jagus bulb extract exhibits the best antimicrobial effect at this (100 mg/ml) concentration. The 50 mg/ml concentration gave IZD for B. subtilis (21 mm), S. aureus (16 mm), and C. albicans (9 mm). The IZD for each organism at 50-6.25 mg/ml concentration is lower when compared with those of 100 mg/ml concentration. This result may suggest that the more the concentration of phytochemicals responsible for the antimicrobial activity, the better the effect. The fact that none of the tested concentrations of the extract inhibited the growth of P. aeruginosa indicates that C. jagus do not exhibit anti-pseudomonal effect. This suggests that the P. aeruginosa isolate used was resistant to the extract. The antimicrobial activity of C. jagus could be attributed to its alkaloids, tannins, and saponin content as revealed by the phytochemical analysis. Studies have reported antibacterial effect of plant extract containing tannins, alkaloids, and saponins [11,17,40]. Crinamine, a phenolic alkaloid contained in C. jagus bulb have been reported to exhibit antibacterial activity [37]. The anti-candidal effect of C. jagus bulb extract observe in this study may be related to its high tannin content (as revealed by the result of phytochemical screening). Plant extracts containing tannins have been widely reported to inhibit the growth of Candida [55,56,57]. Moreover, inhibition of growth of S. aureus by the C. jagus bulb extract in this study corroborates the report of Adesanya et al. [37]. Failure to inhibit the growth of P. aeruginosa could be attributed to its inherent resistance to most antibacterial agents [58]. It could also be that the P. aeruginosa strain is a highly-resistant isolate having being isolated from septic wound.
The result of the in vitro antimicrobial studies (highest IZD produced by 100 and 50 mg/ml) prompted us to prepare the 10 and 5% C. jagus extract ointments for the in vivo wound healing studies. Failure to re-isolate B. subtilis, S. aureus, and C. albicans from infected wound of any animal in Groups A (treated with 10% MCJBEO), B (treated with 5% MCJBEO) and D (treated with framycetin sulfate/clotrimazole) throughout the study period is attributable to the antimicrobial effect of both concentrations of the extract and of course the reference antimicrobial agent, framycetin sulfate/clotrimazole. Re-isolation of P. aeruginosa from all the animals in all the groups throughout the course of the experiment further suggests that the organism was resistant to the extract irrespective of the concentration and to the reference drug. The result of the in vivo antimicrobial studies further supports the antimicrobial effect of the extract on the tested microorganisms except P. aeruginosa.
In this study, assay of SOD and CAT activities, and MDA level was performed to determine the superoxide (O2−) and hydrogen peroxide (H2O2) radical scavenging activity of Crinum jagus extract, and degree of lipid peroxidase activity (cellular lipid peroxidation), respectively. Superoxide radical is considered a major biological source of ROS [59]. Although superoxide anion is a weak oxidant, it gives rise to generation of powerful and dangerous hydroxyl radicals as well as singlet oxygen, both of which contribute to oxidative stress and tissue damage [60,61]. This radical is scavenged and by SOD, an enzyme that dismutates superoxide anions (O2−) to generate hydrogen peroxide (H2O2) [61] which is then detoxified by catalase to water and oxygen which are non-toxic to tissues [61,62]. Therefore, the significantly increased SOD of the extract treated Groups (I and II) when compared with Group III (treated with sterile soft white paraffin), suggests that the extract increased SOD activity in the wound biopsies. However, the fact that SOD activity of Group I (treated with 10% MCJBEO) was significantly higher than the other groups, may suggest that the extract exhibited a better superoxide radical scavenging at this concentration. This suggests that C. jagus bulb extract elicited superoxide radical scavenging in a concentration-dependent manner. Interestingly, the SOD activity of wound biopsies from animals in Group I was significantly higher than all the other groups, including those treated with the reference drug, framycetin sulfate/clotrimazole, an antibiotic/antifungal combination drug. This finding suggests that at 10% concentration, superoxide radical scavenging activity of C. jagus bulb extract was better sustained than at the 5% concentration, and of course, the controls. This may explain the observed faster wound healing in animals in the group, in contrast to their counterparts in the other groups. Ability of the C. jagus extract to increase the SOD activity in the wound bed could be related to its high tannin content [63] as revealed by the result of the phytochemical screening.
Hydrogen peroxide, a non-reactive compound is converted to free hydroxyl radical (OH−), which reacts with biomolecules to cause tissue damage and cell death [61]. It is scavenged by CAT which breaks it down into water and oxygen [62]. Significantly increased CAT activity in wound biopsies of animals in Group III (negative control) on comparison with their counterparts in the other groups may be attributed to the presence of the infective microorganisms present in the wound. Result of the post-contamination wound microbial assay revealed the presence of S. aureus and other infective contaminating microbes in wounds of animals in the negative control group. S. aureus is known to produce catalase which destroys phagocytic cells recruited to engulf them in the wound site [64]. Therefore, since the negative control group was neither treated with CJMBEO nor the reference drug, the isolation of microbes especially S. aureus in the wound of animals in the group was not surprising and hence, the observed significant increase in catalase activity. Nevertheless, significantly increased CAT activity of Group I as against Groups II, and IV suggests that C. jagus bulb extract exhibited H2O2 scavenging activity in a concentration-dependent manner. This result also suggests that H2O2 scavenging was better sustained by 10% concentration of the extract throughout the experiment. Free superoxide radical and H2O2 scavenging activity of plant phenolics especially tannins have been reported [61,63]. Interestingly, the significant increase in CAT activity of Group I on comparison with Group IV (treated with framycetin sulfate/clotrimazole) suggest that the extract enhanced catalase activity more than the reference drug. This finding is supported by the fact that infective pathogens were not isolated from wounds of animals in both groups, hence there was no microbe that could have produced catalase in Group I.
The consequence of decreased SOD and CAT activities is increased cellular lipid peroxidation and delayed wound healing [65]. Lipid peroxidation is caused by the activity of lipid peroxidase which is evidenced by the presence of MDA in tissues [65,66]. MDA is toxic and causes considerable changes in the structural organization and function of cell membrane making it to be porous [66]. In the present study, a significant decrease in MDA level in wound biopsies of animals in Group I compared with the other groups suggests that C. jagus exhibited anti-lipid peroxidation activity [65]. This finding also suggests that C. jagus bulb extract exhibited this activity in a concentration-dependent manner. None significant difference observed in MDA level between Groups I and IV (positive control), suggests that the extent at which the 10% MCJBEO prevented cellular lipid peroxidation is comparable to that of the reference drug, framycetin sulfate/clotrimazole. This finding conforms to Panneerselvam and Govindasamy [65] who reported that a significant increase in SOD and CAT activities results in decreased lipid peroxidase activity indicated by decreased concentration of MDA. Therefore, the results of this study suggests that 10% MCJBEO exhibited antioxidant activity, which resulted in low MDA level in the wound biopsies of animals in the group since increase in lipid peroxidation (MDA) level suggests increased generation of toxic free radicals [67].
In the present study, in vitro non-enzymatic free radical scavenging activity of C. jagus bulb extract was determined using DPPH method [41]. The method is based on scavenging of DPPH through the addition of a radical species or antioxidant that decolorizes the DPPH solution. The degree of color change is proportional to the concentration and potency of the antioxidants. A large decrease in the absorbance of the reaction mixture indicates significant free radical scavenging activity of the compound under test [41,68]. In the present study, the reference antioxidant (ascorbic acid) used showed significantly higher free radical scavenging activity than all the C. jagus bulb extract concentrations tested. However interestingly, the mean values of the antioxidant activity of the extract revealed that the extract exhibited free radical scavenging activity in a concentration-dependent manner. Optimum wound healing occurs when ROS are reduced to a level where oxidative stress is minimal [25]. Moreover, reduced ROS in wound biopsies is evidenced by increased antioxidant activities [25]. Many studies have shown that tannins contained in plant extracts exhibits antioxidant activity [41,69,70]. Therefore, the non-enzymatic antioxidant effects exhibited by the C. jagus bulb extract in this study could also be attributed to its high tannin content.
CONCLUSION
This study has shown that methanolic CJMBE exhibited antioxidant and antimicrobial effects against some common wound contaminating microorganism both in vitro and in vivo in a wound healing model. These effects could possibly be part of its mechanism in promoting wound healing, forming the basis for its use in wound management in folkloric medicine. However, further studies that would involve cell biology are recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Nguyen DT, Orgill DP, Murphy GF. Biomaterials for Treating Skin Loss. Cambridge/Boca Raton: Wood Head Publishing (UK/Europe) and CRC Press (US); 2009. The pathophysiologic basis for wound healing and cutaneous regeneration; pp. 25–57. [Google Scholar]
- 2.Omale J, Isaac AV. Excision and incision wound healing potential of Saba Florida (Benth) leaf extract in rattus novergicus Rattus novergicus. Int J Pharm Biochem Res. 2010;1:101–7. [Google Scholar]
- 3.Al-Henhena N, Mahmood AA, Al-Magrami A, Nor Syuhada AR, Zahra AA, Summaya MD, et al. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. J Med Plants Res. 2011;5:3660–6. [Google Scholar]
- 4.Pandith H, Zhang X, Liggett J, Min KW, Gritsanapan W, Baek SJ. Hemostatic and Wound Healing Properties of Chromolaena odorata Leaf Extract. ISRN Dermatol 2013. 2013:168269. doi: 10.1155/2013/168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsala DE, Nga N, Thiery BN, Bienvenue T, Theophile D. Evaluation of the antioxidant activity and the healing action of the ethanol extract of Calotropis procera bark against surgical wounds. J Intercult Ethnopharmacol. 2014;4:64–9. doi: 10.5455/jice.20141211071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezza R, Mezzasoma AM, Venditti G, Gresele P. Prostaglandin endoperoxides and thromboxane A2 activate the same receptor isoforms in human platelets. Thromb Haemost. 2002;87:114–21. [PubMed] [Google Scholar]
- 7.Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–66. doi: 10.2741/2285. [DOI] [PubMed] [Google Scholar]
- 9.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 10.Houghton PJ, Hylands PJ, Mensahb AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J Ethnopharmacol. 2005;100:100–7. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Udegbunam SO, Udegbunam RI, Muogbo CC, Anyanwu MU, Nwaehujor CO. Wound healing and antibacterial properties of methanolic extract of Pupalia lappacea Juss in rats. BMC Complement Altern Med. 2014;14:157. doi: 10.1186/1472-6882-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyles CL. Ames: Iowa University Press; 1997. Pathogenesis of Bacteria Infections in Animals; p. 164. [Google Scholar]
- 13.Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: The nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–49. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel PL, Komtebedde J, Hirsh DC, Kass PH. Wound contamination and antimicrobial susceptibility of bacteria cultured during total ear canal ablation and lateral bulla osteotomy in dogs. J Am Vet Med Assoc. 1999;214:1641–3. [PubMed] [Google Scholar]
- 15.Gales AC, Ronald NJ, Michael A, Pfaller A, Helie S. Sentry Study Group. Two year assessment of the pathogen frequency and antimicrobial resistance patterns among organisms isolated from skin and soft tissue infections in Latin American hospitals. Int J Infect Dis. 2000;4:75–6. doi: 10.1016/s1201-9712(00)90098-5. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Al-Basal MA. Healing potential of Rosmarinus officinalis L. on full thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/C mice. J Ethnopharmacol. 2010;131:443–50. doi: 10.1016/j.jep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Udegbunam SO, Nnaji TO, Udegbunam RI, Okafor JC, Agbo I. Evaluation of herbal ointment formulation of Milicia excelsa (Welw) C.C berg for wound healing. Afr J Biotechnol. 2013;12:3351–9. [Google Scholar]
- 18.Chah KF, Eze CA, Emuelosi CE, Esimone CO, Chah KF, Eze CA, et al. Antibacterial and Wound healing properties of Methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol. 2005;104:164–7. doi: 10.1016/j.jep.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 19.Mboto CL, Eja ME, Adegoke AA, Iwatt GD, Askong BE, Takon I, Udo SM, Akeh M. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medicinally important microorganisms. Afr J Microbiol Res. 2009;3:557–9. [Google Scholar]
- 20.Mahin T, Masood K, Yahid N, Heidar M. Evaluation of antibacterial activity and wound healing of Pistacia atlantica and Pistacia khinjuk. JMed Plants Res. 2011;5:4310–4. [Google Scholar]
- 21.Trenam CW, Blake DR, Morris CJ. Skin inflammation: Reactive oxygen species and the role of iron. J Invest Dermatol. 1992;99:675–82. doi: 10.1111/1523-1747.ep12613740. [DOI] [PubMed] [Google Scholar]
- 22.Darr D, Fridovitch L. Free radicals in cutaneous biology. J Invest Dermatol. 1994;102:671–5. doi: 10.1111/1523-1747.ep12374036. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese V, Scapagnini G, Catalano C, Dinotta F, Geraci D, Morganti P. Biochemical studies of a natural antioxidant isolated from rosemary and its application in cosmetic dermatology. Int J Tissue React. 2000;22:5–13. [PubMed] [Google Scholar]
- 24.Udupa AL, Kulkarni DR, Udupa SL. Effect of Tridax procumbans extracts on wound healing. Int J Pharmacogn. 1995;33:37–40. [Google Scholar]
- 25.Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. J Pharmacol Biophysic Res. 2011;24:113–26. doi: 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- 26.Olorode O. London: Longman Publishing Company; 1984. Taxonomy of West African Flowering Plants; p. 121. [Google Scholar]
- 27.Houghton PJ, Agbedahuns JM, Adegbulugbe A. Choline esterase inhibitory properties of alkaloids from two Nigerian Crinum species. Sci Direct. 2004;65:2893–6. doi: 10.1016/j.phytochem.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Kokwaro JO. Kenya: General Printers; 1976. Medicinal Plants of East Africa; p. 230. [Google Scholar]
- 29.Gill LS. Benin City, Nigeria: Uniben Press; 1992. Ethnomedicinal Uses of Plants in Nigeria; p. 27. [Google Scholar]
- 30.Sonibare MA, Gbile ZO. Ethnobotanical survey of anti-asthmatic plants in South Western Nigeria. Afr J Tradit Complement Altern Med. 2008;5:340–5. doi: 10.4314/ajtcam.v5i4.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idu M, Obaruyi GO, Erhabor JO. Ethnobotanical uses of plants among the binis in the treatment of ophthalmic and ENT (ear, nose and throat) ailments. Ethnobot Leafl. 2008;13:480. [Google Scholar]
- 32.Ogunkunle ATJ, Olopade OR. Studies on the asthma coughs plant Crinum jagus L. (Amaryllidaceae) in Nigeria. Afr J Plant Sci. 2011;5:108–14. [Google Scholar]
- 33.Ode OJ, Asuzu IU. The anti-snake venom activities of the methanolic extract of the bulb of Crinum jagus (Amaryllidaceae) Toxicon. 2006;48:331–42. doi: 10.1016/j.toxicon.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Ode OJ, Nwaehujor CO, Onakpa MM. Evaluation of antihaemorrhagic and antioxidant potentials of Crinum jagus bulb. Int J Appl Biol Pharm Tech. 2010;1:1330–6. [Google Scholar]
- 35.Shorinwa OA, Ebong OO, Obianime AW, Siminialayi IM. The effect of acetone extracts of Crinum jagus bulbs on the histology of the kidney, liver and testis of albino rats. Peak J Med Plant Res. 2013;2:38–44. [Google Scholar]
- 36.Nwaehujor CO, Nwinyi FC, Ode JO. Liver protective activity of the methanol extract of Crinum jargus bulb against acetaminophen-induced hepatic damage in rats. Asian J Biochem. 2012;7:182–93. [Google Scholar]
- 37.Adesanya SA, Olugbade TA, Odebiyi OO, Aladesanmi AJ. Antibacterial alkaloids in Crinum jagus. Int J Pharmacol. 1992;30:303–7. [Google Scholar]
- 38.Edema MD, Okieimen FE. Chemical and anticonvulsant screening of Crinum jagus. Nig J Chem Res. 2002;7:25–8. [Google Scholar]
- 39.Tram NT, Titorenkova TV, Bankova VSH, andjieva NV, Popov S. Crinum L. (Amaryllidaceae) Fitoterapia. 2002;73:183–208. doi: 10.1016/s0367-326x(02)00068-0. [DOI] [PubMed] [Google Scholar]
- 40.Goncalves RS, Battistin A, Pauletti G, Rota L, Serafini LA. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev Bras Pl Med Botucatu. 2009;11:372–82. [Google Scholar]
- 41.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekren S, Yerlikaya O, Tokul HE, Akpınar A, Açu M. Chemical composition, antimicrobial activity and antioxidant capacity of some medicinal and aromatic plant extracts. Afr J Microbiol Res. 2013;7:383–8. [Google Scholar]
- 43.Trease EC, Evans WC. 12th ed. London: Bailliere and Tindall; 1983. Pharmacognosy; pp. 115–625. [Google Scholar]
- 44.Okore VC, Ibezim EC, Adikwu MU, Attama AA, Esimone CO, Uzuegbu BD, et al. 2nd ed. Nigeria: El'Demark Publishers; 2004. Laboratory Techniques in Pharmaceutics and Pharmaceutical Microbiology; pp. 1–20. [Google Scholar]
- 45.Perez C, Pauli M, Bazerque P. An antibiotic assay by the agar-well diffusion method. Acta Biol Med. 1990;15:113–5. [Google Scholar]
- 46.Morton JJ, Malone MM. Evaluation of vulnerary activity by open wound procedure in rats. J Trauma. 1992;20:323–4. [PubMed] [Google Scholar]
- 47.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 48.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 49.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 50.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- 51.Yen GC, Duh PD, Tsai UL. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–13. [Google Scholar]
- 52.Ling LT, Palanisamy UD, Cheng HM. Prooxidant/antioxidant ratio (ProAntidex) as a better index of net free radical scavenging potential. Molecules. 2010;15:7884–92. doi: 10.3390/molecules15117884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohan H. Textbook of Pathology. 5th ed. New Delhi: Ed Jaypee Brothers; 2005. Inflammation and healing; pp. 51–92. [Google Scholar]
- 55.Vasconcelos LC, Sampaio MC, Sampaio FC, Higino JS. Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses. 2003;46:192–6. doi: 10.1046/j.1439-0507.2003.00884.x. [DOI] [PubMed] [Google Scholar]
- 56.Vasconcelos LC, Sampaio FC, Sampaio MC, Pereira Mdo S, Higino JS, Peixoto MH. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz Dent J. 2006;17:223–7. doi: 10.1590/s0103-64402006000300009. [DOI] [PubMed] [Google Scholar]
- 57.Anibal PC, Peixoto IT, Foglio MA, Höfling JF. Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz J Microbiol. 2013;44:839–48. doi: 10.1590/S1517-83822013005000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li XZ, Livermore DM, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: Resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–41. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves CQ, David JM, David JP, Bahia MV, Aguiar RM. Methods for determination of in vitro antioxidant activity for extracts and organic compounds. Química Nova. 2010;33:2202–10. [Google Scholar]
- 60.Meyer AS, Isaksen A. Application of enzymes as food antioxidants. Trends Food Sci Tech. 1995;6:300–4. [Google Scholar]
- 61.Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J. 2012;6:12. doi: 10.1186/1752-153X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singleton P. 5th ed. Cheichester Sussex England: John Wiley and Sons; 2005. Bacteria in Microbiology, Biotechnology and Medicine; pp. 2–60. [Google Scholar]
- 63.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agri Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 64.Quinn PJ, Markey BK. 2nd ed. Oxford: Blackwell Publishing Limited; 2003. Concise Review of Veterinary Microbiology; pp. 15–7. [Google Scholar]
- 65.Panneerselvan SR, Govindasamy S. Effect of sodium molybdate on the status of lipids, lipid peroxidation and antioxidant systems in alloxan-induced diabetic rats. Clin Chim Acta. 2004;345:93–8. doi: 10.1016/j.cccn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Wall RK, Jaffe S, Kumar D, Songenete N, Kalra VK. Increased adherence of oxidant treated human and bovine erythrocyte to culture endothelial cells. J Cell Physiol. 1987;113:25–36. doi: 10.1002/jcp.1041330104. [DOI] [PubMed] [Google Scholar]
- 67.Woeff SP, Dean RT. Glucose anti oxidation and protein modification. The potential role of anti-oxidative glycosylation in diabetes. Biochem J. 1987;247:243–50. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnaiah D, Sarbatly R, Nithyanandam RR. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–33. [Google Scholar]
- 69.Zhang SJ, Lin YM, Zhou HC, Wei SD, Lin GH, Ye GF. Antioxidant tannins from stem bark and fine root of Casuarina equisetifolia. Molecules. 2010;15:5658–70. doi: 10.3390/molecules15085658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao S, Liu JY, Chen SY, Shi LL, Liu YJ, Ma C. Antioxidant potential of polyphenols and tannins from burs of Castanea mollissima Blume. Molecules. 2011;16:8590–600. doi: 10.3390/molecules16108590. [DOI] [PMC free article] [PubMed] [Google Scholar]


