Abstract
Objective:
Crinum jagus (J. Thomps.) Dandy commonly called Harmattan or St. Christopher’s lily belonging to the family Liliaceae is widely used traditionally in Southeastern Nigeria for treatment of skin sores. This study investigated the wound healing potentials of methanolic C. jagus bulb extract (MCJBE) using incision, excision, and dead space wound healing models.
Materials and Methods:
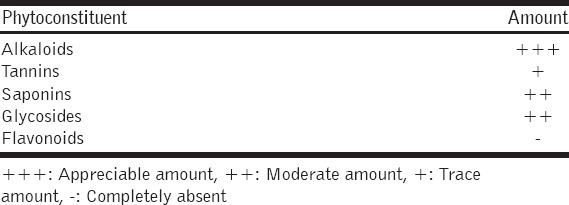
Phytochemical screening showed the presence of alkaloids, glycosides, tannins, saponins in the extract, but absence of flavonoids. In the incision and dead space wound models, rats were dosed orally with 300 mg/kg body weight (bw) of 10 and 5% of MCJBE solution, respectively, while in the excision wound model, rats were treated topically with 10 and 5% MCJBE ointments (MCJBEO), respectively.
Result:
The 10% MCJBE gave significantly (P < 0.05) highest percentage rate of wound contraction, shortest re-epithelialization and complete healing time when compared with 5% MCJBE and reference drug, framycetin sulfate. The extract of C. jagus showed significant (P < 0.05) concentration-dependent wound healing activity in incision, dead space and excision wound models. No contaminating microbial organism was isolated from wound sites of the rats dosed and treated with MCJBE throughout the study period. At day 7, post infliction of excision wound, histomorphological, and histochemical studies revealed more fibroblasts and Type 1 collagen deposits in wound site sections of rats treated with both 10 and 5% MCJBEO while those of the control showed more inflammatory cells and fewer Type 1 collagen deposits. At day 14 post infliction of excision wound, more epithelial regeneration with overlying keratin were seen in the histological sections of wounds of rats treated with both 10 and 5% MCJBEO, while histochemical study showed more Type 1 collagen deposits in wound site sections of rats in 10% MCJBEO treated group.
Conclusion:
This study established that methanolic C. jagus bulb extract potentiates wound healing. The study thus validated the folkloric use of C. jagus bulb in the management of skin sores and boils.
KEY WORDS: Crinum jagus, wound healing, methanolic extract, ointment
INTRODUCTION
Wound is interruption in the integrity of a tissue. Causes of cutaneous wound include: surgical, traumatic, toxic, or infectious or others [1]. To re-establish the integrity of a damaged tissue, an orderly intricate process which involve progression of events called wound healing is initiated by the damaged tissue itself from the moment an injury occurs [2,3]. Wound healing process involves complex mechanisms which involves hemostasis, inflammation, proliferation, and remodeling [1,4,5]. In each of these mechanisms, different biochemical substances are recruited to enhance the healing process. Vasoconstriction initiated by conversion of prostaglandin H2 into thromboxane A2 by thromboxane synthase results in hemostasis [6]. Intrinsic fibrinolytic factors that tend to prevent hemostasis are inhibited by plasminogen activator inhibitor Type 1 [7]. Inflammation results in accumulation of heme and heme proteins which have pro-oxidative and pro-inflammatory activities accumulate in the site of the wound to induce expression of adhesion molecules, subsequently resulting in vascular permeability, and infiltration leukocytic [4]. The infiltrating neutrophils release free radicals that kill any contaminating organism that may want to colonize the wound [8,9]. Overexpression of heme-oxygenase-1 (HO-1) which has anti-inflammatory and antioxidant (by converting heme into biliverdin/bilirubin, iron and carbon monoxide) activities accelerate wound healing process such as amelioration of the inflammation, proliferation of epithelial cells (epithelialization) and endothelial cells (angiogenesis/neovascularization), and protection of endothelial cell apoptosis [10]. Expression of matrix metalloproteinases promotes remodeling of the extracellular matrix [11]. Overall, measurable phenomena involved in wound healing include: Wound contraction, epithelialization, and granulation tissue formation [5]. The contributions to wound healing by these phenomena depend on the type of wound. Wound contraction and epithelialization play significant roles in healing of excision wound while granulation tissue formation contributes in healing of dead space and re-sutured incision wounds [2,12]. Hence, the need for using different wound healing models in evaluation of substances for potential wound healing activity.
The necessary biochemical substances need to be available for wound healing to occur. The length of time it takes for wound healing process to be optimum and complete is directly dependent on the rate of availability of biochemical substances required for each mechanism and phenomenon to occur [13]. Wound contaminating organisms often alter or lengthen the duration of wound healing process by production of biochemical substances (enzymes) that may further destroy the wounded tissues and/or degrade the biochemical substances that enhance wound healing. Therefore, medical professionals use drugs that may be applied topically, orally or systemic to shorten the duration, minimize complications such as overwhelming microbial wound contamination of natural wound healing and achieve optimum healing [13]. In orthodox medicine, wound healing is achieved by using drugs that promote wound healing process. However, these drugs are usually costly [14] and often times they elicit side effects which are detrimental to the recipient. Hence the need for cheaper and safe alternative or complementary substances that could promote wound healing.
In traditional medicine, medicinal plants are used in the preparation of decoctions, which are applied topically to skin wounds to enable healing [14]. The efficacies of these plants in wound healing have been experienced and passed on from one generation to the other [13]. One of these medicinal plants widely used traditionally in Africa including Southeastern Nigeria in treating wounds is Crinum jagus (J. Thomps.) Dandy (Family Lilliaceae, formerly in family Amaryladiaceae). Its common names include Harmattan lily, St. Christopher’s lily, Frest crinum or Poison bulb [15,16]. In Nigeria, it is popularly called Bush onions [16]. It is widely distributed in tropical and sub-tropical regions [17]. It is a tender perennial bulb with tulip-like (showy) white flowers, which bloom in clusters in dry season atop leafless stalks typically growing up to about 1metre tall from a clump of strap-shaped green leaves [18]. It is used in horticulture as ornamental plant [19]. Traditional practitioners in Africa including those in Southeast Nigeria, claim that the bulbs of C. jagus are used in form of poultices and decoctions to treat different ailments such as pain, asthma, cough, ear ache, constipation, inflammatory swellings, memory loss and skin sores, wounds, and boils [15,17,20-24]. For the treatment of wounds, the bulb is prepared in form of decoction and externally applied [20]. Scientific investigations have validated some of these claims such as antibacterial [25], antivenomous [26], antihemorrhagic, and antioxidant [27], and hepatoprotective [24] activities of C. jagus bulb extracts. However, the wound healing activity of C. jagus bulb has not been evaluated.
Phytochemical screening of extracts of C. jagus bulb showed that it contains alkaloids, polyphenols, and triterpenoids [16]. Specific compound contained in C. jagus bulb extracts as revealed by chemical investigation included crinamine, lycorine, psuedolycorine, 6-hydroxycrinamine, hamayne, tetrahydro-1, 4-oxazine (morpholine), calcium oxalate, calcium tetrata, bowdensine, and demethoxy-bowdensine [16,25,28]. Some of the bioactive compounds contained in C. jagus bulb extracts have been shown to exhibit properties that may promote wound healing mechanisms. For instance, crinamine from C. jagus bulb has been reported to exhibit strong antibacterial activity against common wound contaminant, Staphylococcus aureus [25]. Plant extracts that contain crinamine exhibited wound healing activity [29]. Extract of C. jagus bulb was shown to possess antioxidant [24,27] and hemostatic effects [27] which may promote wound healing [5]. Plants that contain phenolic compounds exhibited antioxidant activity [5,13,30]. Wound healing activity was exhibited by plants that contain alkaloids [5,13]. Alkaloids in Crinum species have been reported to be associated with polyphenols and resins, which exhibit anti-inflammatory and immunostimulating effects [31] which could enhance wound healing. Therefore, the presence of these compounds that exhibited mechanism involved in wound healing in C. jagus bulb, may contribute to its wound healing activity in humans and animals as claimed by the traditional practitioners. The objective of this study therefore was to evaluate the wound healing activity of C. jagus methanolic bulb extract in rats.
MATERIALS AND METHODS
The experimental protocols used in this study were approved by the Ethics Committee of the University of Nigeria, Nsukka and conforms with the guide to the care and use of animals in research and teaching of University of Nigeria, Nsukka, Enugu State Nigeria.
Animals
A total of 125 8-week-old male albino Wistar rats weighing between 120 and 198 g were obtained from the laboratory animal unit, Faculty of Veterinary Medicine, University of Nigeria, Nsukka. They were fed on commercial growers mash (Vital feeds®) and water was provided ad libitum. These rats were acclimatized for 2 weeks in the animal house at the Department of Veterinary Surgery, University of Nigeria, Nsukka.
Plant Collection and Identification
Fresh C. jagus bulbs (J. Thomps.) Dandy were collected from Amokwe town in Udi Local Government Area Enugu State, Nigeria, in the month of May, 2014 and were identified at the International Center for Ethnomedicine and Drug Development (InterCEDD), Nsukka, by a plant taxonomist, Mr. A. Ozioko. Samples were registered with a voucher specimen number FRMPC/05/14 and deposited in the center’s herbarium.
Extraction
A kilogram of the C. jagus bulbs were sliced into smaller pieces, air dried at room temperature for 2 weeks, and then pulverized using the laboratory grinding machine at the Department of Crop Science, University of Nigeria, Nsukka. The pulverized bulbs were macerated in 80% methanol for 48 h with intermittent vigorous shaking at every 2 h. After 48 h, the mixture was filtered and the extract concentrated using a rotary evaporator set at 40°C. The dried extract was weighed and the percentage yield calculated. The extract was then stored at 4°C in a refrigerator before use.
Acute Toxicity Test
Twenty five adult rats were randomly divided into five groups of five animals per group. The animals were deprived water for 16 h before administration of the extract. The increasing doses of the extract 250, 500, 1000, and 2000 mg/kg body weight (bw) suspended in 10% Tween 20 was administered orally to the test groups, respectively, using a ball-tipped intubation needle fitted onto a syringe. The last group received 1 ml/kg of sterile distilled water and served as the control. The rats were allowed access to food and water ad libitum and were observed for 48 h for behavioral changes and death. The time of onset, intensity, and duration of these symptoms, if any, was recorded.
Phytochemical Analysis for Bioactive Substances
The methanolic C. jagus bulb extract (MCJBE) was screened for the presence of bioactive components following the methods of Trease and Evans [32].
Preparation of Ointments
The method of Okore et al. [33] was adopted in preparation of two herbal ointments containing 10% w/w and 5% w/w of the extract in sterile soft white paraffin. Immediately after preparation, the ointments were aseptically transferred into sterile cream tubes and sealed.
Wound Healing Studies
Incision wound model
Thirty rats were anesthetized by injecting intramuscularly with 10 and 50 mg/kg bw of xylazine hydrochloride and ketamine hydrochloride, respectively. Incision wound was created following the procedure described by Rathi et al. [34]. Briefly, under general anesthesia, dorsum of the animals were shaved thoroughly and prepared for aseptic surgery. Paravertebral skin incisions (5 cm in length) were made on the animals using sterile scalpel blade. The incisions were sutured using size 2/0 silk thread. Then, the rats were randomly assigned into three treatment groups consisting of 10 animals per group and treated as follows: Groups A and B were dosed orally with 300 mg/kg bw of 10% and 5% of the MCJBE once daily, respectively, while Group C was similarly given 1 ml/kg bw of sterile distilled water. The animals were treated daily for a period of 7 days. Sutures were removed at day 8 post-wounding (pw). The wound tissue breaking strength was determined at day 10 pw using the constant water flow technique described by Morton and Malone [35].
Dead Space Wound Model
Thirty rats were randomly assigned into 3 groups of 10 animals per group. They were anaesthetized by injecting intramuscularly with 10 and 50 mg/kg bw of xylazine hydrochloride and ketamine hydrochloride, respectively. Dead space wound was created following the procedure described by Rathi et al. [34]. Briefly, under general anesthesia, subcutaneous dead space wound were created in the region of the axilla by making a pouch through a small nip in the skin. Granulation tissue formation was induced by implanting one 30 mg sterile cotton pellets in each axilla. The wounds were sutured using size 2/0 silk and mopped with alcoholic swab. The animals were grouped and then placed individually in a clean and disinfected metal cage to avoid them licking or biting each other’s wound. Groups C and D were administered orally with 300 mg/kg bw of 10% and 5% of the MCJBE once daily, respectively, while Group E was similarly given 1 ml/kg of sterile distilled water for 8 days. At day 10 pw, rats were euthanized, the cotton pellets together with the granulation tissues carefully dissected out, dried in a hot air oven at 60°C for 24 h and weighed. Weight of the granulation tissue was obtained by subtracting the post-drying weight from the pre-implantation weight.
Excision Wound Model
Totally, 40 rats were anaesthetized by injecting intramuscularly 10 and 50 mg/kg bw of xylazine hydrochloride and ketamine hydrochloride, respectively. Under general anaesthesia, dorsum of the rats were shaved and disinfected. Then, full thickness 20 mm circular wound was made on the dorsal thoracic region as per Dash et al. [36]. Post wounding, the rats were randomly assigned into 4 groups of 10 animals per group and treated as follows: Groups I and II were treated topically with 10% and 5% MCJBE ointment (MCJBEO), respectively, while Groups III and IV were treated with framycetin sulfate (Steritin tulle®) (reference drug) and sterile distilled water, respectively. The animals were housed individually to avoid wound biting and/or licking and treated daily with their respective ointments until complete healing occurred.
Assessment of Wound Healing Post Infliction of Excision Wound
Percentage wound contraction
Percentage wound contraction of the excision wound was determined following the procedure described by Chah et al. [37]. Briefly, at day 1 post infliction of excision wound, wound diameter were manually traced on a transparent white tracing paper by outlining the wound edge with a fine-tip permanent marker, and recorded as the initial wound diameter. The wound diameters were re-measured at days 7, 14, and 21 pw. After the tracing of wound diameter of each animal, the tracing paper was appropriately labeled with the group number, rat identity and date. The area within the lines of each tracing was determined by placing the tracing paper on a 1 mm2 graph sheet and traced out. The squares were counted and the area recorded. The degree of wound contraction was determined by subtracting the total wound area of each tracing day from the area of the initial tracing. Percentage wound contraction was then calculated as described by Chah et al. [37].
Wound epithelialization
Period of wound epithelialization was calculated as the number of days required for the scar to fall off leaving no raw wound [38]. The mean days for complete healing (i.e., full wound epithelialization) when epithelium covered the entire wound and hairs appear in the wound site was calculated for each group following the procedure described by Okoli et al. [39].
Wound Microbial Assay
Wound swabs were taken from all the animals in each group at days 3, 7, 14 and 21 using sterile swab stick moistened with sterile normal saline. Swabs were inoculated in brain heart infusion (BHI) broth (Oxoid®) and incubated at 37°C for 24 h aerobically. The broth cultures were observed for microbial growth (cloudiness/turbidity), and if any, a loopful of the broth cultures was sub-cultured on blood agar and incubated at 37°C for 24 h. Isolates of different colonial types, if any, were purified on fresh media, incubated and then used for identification following standard biochemical methods.
Histomorphological and Histochemical Examination
At days 7 and 14 post infliction of excision wound, the animals were euthanized and wound biopsies taken for histomorphological and histochemical examinations. The biopsies were fixed in 10% buffered formalin, dehydrated in graded alcohol and embedded in paraffin wax. Then, 5 µm thick sections were stained with hematoxylin and eosine (H and E) for general histomorphological analysis. Vangieson stain was used for the demonstration of Type 1 collagen (histochemical analysis) in the dermis. Semi-quantitative evaluation of the wound slides for re-epithelialization, polymorphonulcear leukocytes, tissue macrophages (TM), fibroblasts, neovascularization, collagen formation and Type 1 collagen was done using subjective scoring method on a 5-point scale from 0 to 4 by 2 independent observers blinded to the treatment protocol [40].
Statistical Analysis
Data obtained were summarized as mean ± standard error of mean. Mean values of wound breaking strength, granulation tissue weight, percentage wound contraction, wound epithelialization and time for complete healing for different groups were compared using one-way Analysis of Variance. Duncan multiple range test was used to separate variant means. P < 0.05 was considered significant.
RESULTS
Extraction
The MCJBE had an aromatic smell and was brownish in colour. The percentage yield was 13.6% w/w material.
Acute Toxicity Test
Administration of MCJBE extract suspended in 10% Tween 20 to rats even at the highest dose of 2000 mg/kg bw did not produce any death in the treated groups. No sign of acute toxicity was also observed except transient dullness and weakness which disappeared in few minutes.
Phytochemical Analysis
Preliminary phytochemical analysis of MCJBE qualitatively revealed the presence of alkaloids, tannins, saponins, glycosides but absence of flavonoids [Table 1].
Table 1.
Phytochemical analysis of methanolic Crinum jagus bulb extract
Incision Wound Model
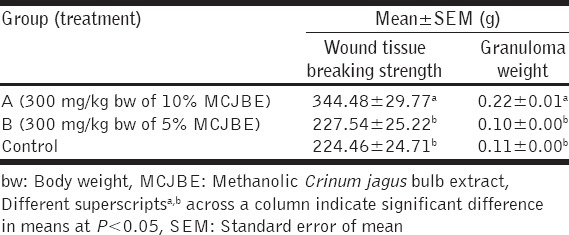
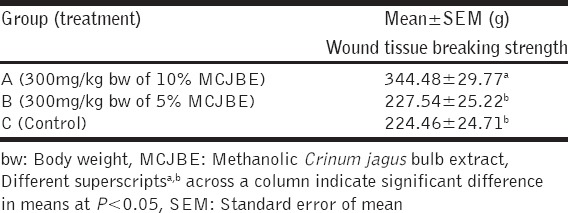
The result of the effect of MCJBE on breaking strength of the healed wound showed significantly (P < 0.05) higher wound breaking strength in animals in Group A (300 mg/kg bw of 10% MCJBE) when compared with those in Group B (300 mg/kg bw of 5% MCJBE) and the control [Table 2]. There was no significant difference (P > 0.05) in wound breaking strength of animals in Group B and the control.
Table 2.
Wound tissue breaking strength and granuloma weight in rats post infliction of incision and dead space wounds
Dead Space Wound Model
The result of the effect of MCJBE on granulation tissue weight showed that dry granulation tissue weight of animals in Group A was significantly (P < 0.05) higher when compared against that of animals in Group B and the control [Table 3]. There was no significant difference (P > 0.05) in granulation tissue weight of animals in Groups B and the control.
Table 3.
Wound tissue breaking strength in rats post infliction of incision wound
Excision Wound Model
Percentage wound contraction
The percentage rate of wound contraction in Group I (10% MCJBEO treated) at day 7 pw, was significantly (P < 0.05) higher compared to Group III (framycetin sulfate treated) and the control [Table 4]. Wound contraction in both MCJBEO treated groups (I and II) did not differ significantly (P > 0.05). Wound contraction in Group 2 did not vary significantly (P > 0.05) when compared with Group III. Similar trends were observed at day 14 pw. At day 21 wound contraction in both MCJBEO treated groups (I and II) and the control significantly (P < 0.05) increased when compared against Group III. No significant difference (P > 0.05) existed between the MCJBEO treated groups throughout the study period. For all groups, most wound contraction occurred between days 7 and 21 pw [Table 5].
Table 4.
Granulation tissue weight in rats post infliction of incision wound
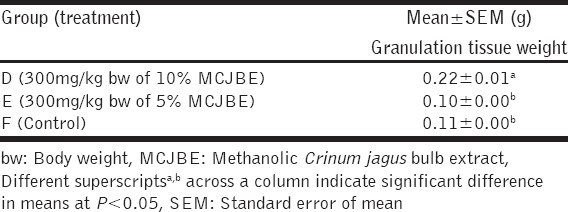
Table 5.
Percentage rate of wound contraction in rats post infliction of excision wound
Wound Epithelialization and Complete Wound Healing Time
Epithelialization occurred between days 11 and 17 pw [Table 6]. Epithelialization time was significantly (P < 0.05) shorter in animals in Groups I and II and the control compared with those in Group III. There was no significant difference (P > 0.05) in epithelialization time of wound between animals in Groups I-III. Mean value of epithelialization time of Group I revealed that animals in the group had the shortest wound epithelialization time. Similar trends were observed for complete healing time among the groups. Complete wound healing occurred significantly (P < 0.05) earlier among animals in Group I and II when compared with those in Group III.
Table 6.
Wound epithelialization and complete wound healing time of rats post infliction of excision wound
Wound Microbial Assay
No wound contaminating bacterial organism was isolated from the wound sites of animals in Groups I-III throughout the study period, while few colonies of Bacillus species was isolated from the wound sites of animals in the control group at days 7 and 14 post infliction of excision wound.
Histomorphological Findings
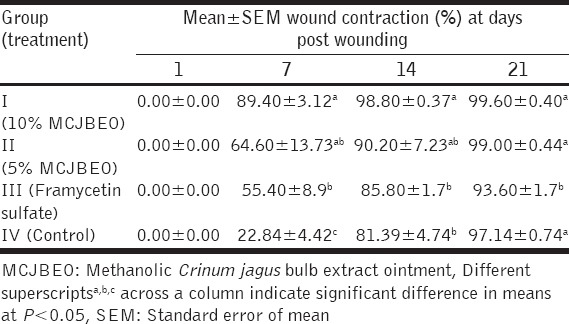
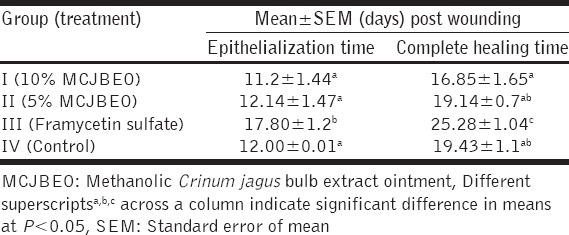
At day 7 post infliction of excision wound, wound sections of animals in the control group revealed more inflammatory cells, that of animals in Groups I (10% MCJBEO treated) and II (5% MCJBEO treated) revealed more fibroblasts [Figure 1a-c] while those of animals in group III (framycetin sulfate treated) showed in addition to more fibroblasts, complete layer of epithelial regeneration [Figure 1d]. At day 14 pw, epithelial regeneration with overlying keratin was observed to be more in wound sections of animals in Group I-III than in wound sections of animals in the control group [Figure 2a-d].
Figure 1.
Photomicrographs of wound site sections at day 7 post infliction of excision wound showing moderate inflammatory cell infiltrates (arrow) and more fibroblasts in wound sections of animals in Groups I (10% methanolic Crinum jagus bulb extract ointment [MCJBEO] treated) (a) and II (5% MCJBEO treated) (b), more inflammatory cell infiltrates in wound section of animals in Group IV (control) (c) and complete layer of epithelial regeneration (R) with more fibroblasts in wound section of animals in Group III (framycetin sulfate treated) (d). H and E ×400
Figure 2.
Photomicrograph of wound site sections at day 14 post infliction of excision wound showing a complete layer of regenerated epithelium (R) with overlying keratin (arrow) which was greater in wound sections of animals in Groups I (10% methanolic Crinum jagus bulb extract ointment [MCJBEO] treated) (a), II (5% MCJBEO treated) (b) and III (framycetin sulfate treated) (c) than in wound sections of animals in the control group (d). H and E ×400
Histochemical Findings
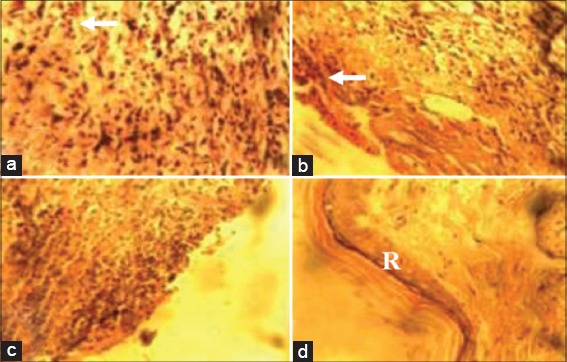
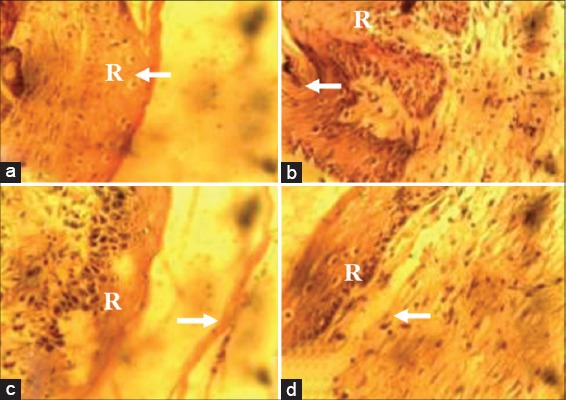
At day 7 pw, there were more Type 1 collagen deposits in wound sections of animals in Groups I-III compared with that of animals in the control group [Figure 3a-d]. At day 14 pw, wound sections of animals in Group I revealed more Type 1 collagen deposits when compared with wound sections of animals in Groups II-IV [Figure 4a-d].
Figure 3.
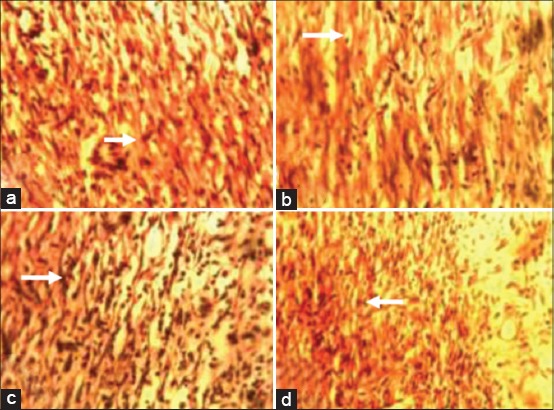
Photomicrograph of wound site sections at day 7 showing greater Type 1 collagen positive tissues (arrow) in wound sections of animals in Groups I (10% methanolic Crinum jagus bulb extract ointment [MCJBEO] treated) (a), II (5% MCJBEO treated) (b) and III (framycetin sulfate treated) (d) compared to wound section of animals in the control (c). Vangieson ×400.
Figure 4.
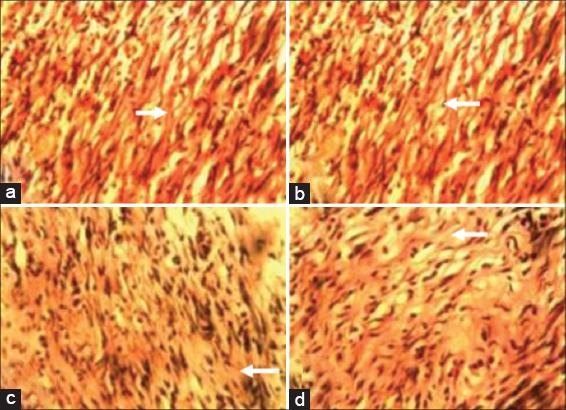
Photomicrograph of wound site sections at day 14 post infliction of excision wound showing more of Type 1 collagen positive tissues (arrow) in wound sections of animals in Group I (10% methanolic Crinum jagus bulb extract ointment [MCJBEO] treated) (a) than in wound sections of animals in Groups II (5% MCJBEO treated) (b), the control Group IV (c) and Group III (framycetin sulfate treated) (d). Vangieson ×400
DISCUSSION
In this study, three wound models - incision, excision and dead space were used to investigate the effect of MCJBE on various events of wound healing. To ascertain the rate of healing following creation of excision wounds, rates of wound contraction and epithelialization as well as time of complete healing were assessed. The fact that animals in Group I (treated with 10% MCJBEO) had the highest percentage rate of wound contraction and shortest period of wound re-epithelialization when compared with their counterparts in the other groups, suggest that the 10% MCJBEO promoted wound healing better than at the lower concentration (5% MCJBEO) and the reference drug (framycetin sulfate) used in this study. The result also suggests that wound healing effect of C. jagus bulb extract occurs in a concentration-dependent manner. The shortest healing time of wounds recorded among animals in the Group I (treated with 10% MCJBEO) could be attributed to the more rapid epithelialization and wound contraction observed among animals in the group. Wound contraction occurs following increased stimulation of interleukin-8 (an inflammatory chemokine) which affects the function and recruitment of various inflammatory cells, fibroblasts and keratinocytes thereby resulting in rapid maturation of granulation tissue [41]. Therefore, it is possible that the 10% MCJBEO promoted this mechanism more than the lower concentration and the reference drug used in this study. The shortest epithelialization time recorded among animals in the 10% MCJBEO treated group could be due to enhancement of collagen deposition by the extract which occurred better at a higher concentration [42]. This further suggests a concentration-dependent manner of activity exhibited by the extract.
Granulation tissue formed in a dead space wound comprises of an accumulation of modified macrophages, histological giant cells and undifferentiated connective tissue which consist largely of collagen [43,44]. Increase in granulation tissue in dead space wound is associated with enhanced collagen maturation and increased protein content as well as angiogenesis in the wound [45]. As observed in this study, the dry granulation tissue weight from dead space wounds of animals in Group D (dosed with 300 mg/kg bw of 10% MCJBE) was significantly highest when compared with Group E (dosed with 300 mg/kg bw of 5% MCJBE) and the control. This result suggests more rapid collagen maturation in the healing wounds of animals in Group D. This finding is further collaborated by the results of the histologic and histochemical studies of the excision wounds which revealed marked increase in TM, fibroblasts, collagen content, and neovascularization in wound sections of animals in Group I (10% MCJBEO treated). However, the non-significant difference between the granulation tissue weight of animals in Group E (dosed with 300 mg/kg bw of 5% MCJBE) and the control, suggests that the lower concentration of C. jagus bulb extract may not elicit wound healing effect better than what occurs in natural healing. Therefore, it may not be useful to use lower concentration below 10% of the C. jagus bulb extract in management of wound.
The tensile strength of a healing wounded tissue is dependent on the amount of collagen content and stabilization of the fibres [46]. According to Omale and Isaac [2], collagen the major component which strengthens and supports extracellular tissue is composed of amino acids and hydroxyl proline which are used as a biochemical marker for tissue collagen. Therefore, the observed significant increase in tensile strength of wounds among animals in Group A (dosed with 300 mg/kg bw of 10% MCJBE) when compared with Group B (dosed with 300 mg/kg bw of 5% MCJBE) and the control, may not only be due to increased collagen synthesis, but also due to its proper deposition and alignment [47]. The significantly higher wound breaking strength among animals in Group A when compared with their counterparts in the other groups, further suggests that the C. jagus bulb extract exhibited its wound healing effect in a concentration-dependent manner.
In the present study, preliminary phytochemical analysis of C. jagus bulb extract revealed the presence of alkaloid, tannins, saponins, and glycosides. This finding is in agreement with the report of Ode et al. [27] except that they reported negative saponin content. Studies with other plant extracts showed that plants containing alkaloids [5,13,48], triterpenoids [49], and tannins [50] promoted wound healing process. Therefore, higher concentration of the bioactive substances in the 10% MCJBE might have contributed to the significantly faster tissue approximation and increased tensile strength of wounds observed among animals in the groups treated with the concentration.
Non isolation of microbial organisms from the wounds in both MCJBEO treated Groups (I and II) and Group III (framycetin sulfate treated) suggests that the extract was able to prevent microbial contamination of the wounds. This may suggest that the extract induced migration of phagocytes (responsible for killing contaminating microbes in wound) to the wound site. This is supported by the histological findings of increased infiltration of phagocytic cells in wound sites of animals in the MCJBEO treated groups. Absence of microbial isolation from wounds of animals in the extract treated groups may also suggest that the C. jagus extract could have elicited antimicrobial effect similar to the reference drug, framycetin sulphate which is an antibiotic. Adesanya et al. [25] reported antistaphylococcal activity of C. jagus bulb extract. Plant extracts containing alkaloids and saponins have been reported to exhibit antimicrobial activity [48]. Plant extracts containing these phytochemicals including C. jagus bulb extract have been shown to exhibit antioxidant activity [24,27]. And wound healing properties of plants, in most cases, are associated with their significant antioxidant activities [5,30]. Therefore, the absence of microbial contamination of wound in this study may be attributed to the presence of alkaloids and saponnins in the extract. Nevertheless, isolation of Bacillus species from wounds of animals in the control group in this study may be because Bacillus is ubiquitous and the wounds were not treated with any agent that could have elicited antimicrobial effect.
CONCLUSION
The results of this study have established that the MCJBE potentiates wound healing partly by increasing collagen deposition and epithelialization. The wound healing potentials of the extract could be attributed to the presence of polyphenolic compounds including tannins, saponins, glycosides, and alkaloids in the extract. The extract elicited the best wound healing activity at a concentration of 10% and the activity was concentration-dependent. The use of C. jagus bulb in the management of skin sores and boils in folkloric medicine is thus validated by this study. However, further studies which would involve cell biology, immunology, and biochemistry to elucidate fully the process of wound healing by C. jagus bulb extract is recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Nguyen DT, Orgill DP, Murphy GF. Cambridge, Boca Raton: Wood Head Publishing, CRC Press; 2009. The pathophysiologic basis for wound healing and cutaneous regeneration, biomaterials for treating skin loss; pp. 25–57. [Google Scholar]
- 2.Omale J, Isaac AV. Excision and incision wound healing potential of Saba Florida (Benth) leaf extract in Rattus novergicus. Int J Pharm Biochem Res. 2010;1:101–7. [Google Scholar]
- 3.Al-Henhena N, Mahmood AA, Al-magrami A, Nor Syuhada AR, Zahra AA, Summaya MD, et al. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. J Med Plants Res. 2011;5:3660–6. [Google Scholar]
- 4.Pandith H, Zhang X, Liggett J, Min KW, Gritsanapan W, Baek SJ. Hemostatic and wound healing properties of Chromolaena odorata Leaf Extract. ISRN Dermatol 2013. 2013:168269. doi: 10.1155/2013/168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsala DE, Nga N, Thiery BN, Bienvenue T, Theophile D. Evaluation of the antioxidant activity and the healing action of the ethanol extract of Calotropis procera bark against surgical wounds. J Intercult Ethnopharmacol. 2014;4:64–9. doi: 10.5455/jice.20141211071136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezza R, Mezzasoma AM, Venditti G, Gresele P. Prostaglandin endoperoxides and thromboxane A2 activate the same receptor isoforms in human platelets. Thromb Haemost. 2002;87:114–21. [PubMed] [Google Scholar]
- 7.Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957–66. doi: 10.2741/2285. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–64. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Wagener FA, van Beurden HE, von den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102:521–8. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 11.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A. New Delhi: Vikas Publishing House PVT Limited; 2002. Wound healing and general management of wounds. Veterinary Surgical Technologies; pp. 151–61. [Google Scholar]
- 13.Udegbunam SO, Udegbunam RI, Muogbo CC, Anyanwu MU, Nwaehujor CO. Wound healing and antibacterial properties of methanolic extract of Pupalia lappacea Juss in rats. BMC Complement Altern Med. 2014;14:157. doi: 10.1186/1472-6882-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udegbunam SO, Nnaji TO, Udegbunam RI, Okafor JC, Agbo I. Evaluation of herbal ointment formulation of Milicia excelsa (Welw) C.C berg for wound healing. Afr J Biotechnol. 2013;12:3351–9. [Google Scholar]
- 15.Houghton PJ, Agbedahuns JM, Adegbulugbe A. Choline esterase inhibitory properties of alkaloids from two Nigerian Crinum species. Sci Dir. 2004;65:2893–6. doi: 10.1016/j.phytochem.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Shorinwa OA, Ebong OO, Obianime AW, Siminialayi IM. The effect of acetone extracts of Crinum jagus bulbs on the histology of the kidney, liver and testis of albino rats. Peak J Med Plant Res. 2013;2:38–44. [Google Scholar]
- 17.Sonibare MA, Gbile ZO. Ethnobotanical survey of anti-asthmatic plants in South Western Nigeria. Afr J Tradit Complement Altern Med. 2008;5:340–5. doi: 10.4314/ajtcam.v5i4.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olorode O. Vol. 121. London: Longman Publishing Company; 1984. Taxonomy of West African Flowering Plants. [Google Scholar]
- 19.Hannibal LS. California: Fair Oaks 95628; 1992. A systematic review of the genus Crinum (Amaryllidaceae) pp. 1–15. [Google Scholar]
- 20.Kokwaro JO. Kenya: General Printers; 1976. Medicinal Plants of East Africa; p. 230. [Google Scholar]
- 21.Gill LS. Benin City, Nigeria: Uniben Press; 1992. Ethnomedicinal Uses of Plants in Nigeria; p. 27. [Google Scholar]
- 22.Idu M, Obaruyi GO, Erhabor JO. Ethnobotanical uses of plants among the Binis in the treatment of ophthalmic and ENT (ear, nose and throat) ailments. Ethnobot Leaflets. 2008;13:480. [Google Scholar]
- 23.Ogunkunle AT, Olopade OR. Studies on the asthma coughs plant Crinum jagus L. (Amaryllidaceae) in Nigeria. Afr J Plant Sci. 2011;5:108–14. [Google Scholar]
- 24.Nwaehujor CO, Nwinyi FC, Ode JO. Liver protective activity of the methanol extract of Crinum jagus bulb against acetaminophen-induced hepatic damage in rats. Asian J Biochem. 2012;7:182–93. [Google Scholar]
- 25.Adesanya SA, Olugbade TA, Odebiyi OO, Aladesanmi JA. Antibacterial alkaloids in Crinum jagus. Pharm Biol. 1992;30:303–7. [Google Scholar]
- 26.Ode OJ, Azusu IU. The anti-venom activities of the methanolic extract of the bulb of Crinum jagus (Amaryllidaceae) Toxicon. 2006;48:331–42. doi: 10.1016/j.toxicon.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Ode OJ, Nwaehujor CO, Onakpa MM. Evaluation of antihaemorrhagic and antioxidant potentials of Crinum jagus bulb. Int J Appl Biol Pharm Tech. 2010;1:1330–6. [Google Scholar]
- 28.Edema MD, Okieimen FE. Chemical and anticonvulsant screening of Crinum jagus. Niger J Chem Res. 2002;7:25–8. [Google Scholar]
- 29.Shilpa K, Rajendra Y, Sanjeeva K, Kumar DV, Kumar RV, Gnananath K. Evaluation of wound healing potential in Crinum defixum Ker gawl bulbs. Asian J Pharm Clin Res. 2013;6:61–3. [Google Scholar]
- 30.Suntar I, Akkol K, Nahar L, Sarker SD. Wound-healing and antioxidant properties: Do they co-exist in plants?Free Radic Antioxid. 2012;2:2–7. [Google Scholar]
- 31.Thi Ngoc Tram N, Titorenkova TV, St, Bankova V, Handjieva NV, Popov SS. Crinum L. (Amaryllidaceae) Fitoterapia. 2002;73:183–208. doi: 10.1016/s0367-326x(02)00068-0. [DOI] [PubMed] [Google Scholar]
- 32.Trease EC, Evans WC. 12th ed. London: Bailliere and Tindall; 1983. Pharmacognosy; pp. 115–625. [Google Scholar]
- 33.Okore VC, Ibezim EC, Adikwu MU, Attama AA, Esimone CO, Uzuegbu BD, et al. 2nd ed. Nigeria: El'Demark Publishers; 2004. Laboratory Techniques in Pharmaceutics and Pharmaceutical Microbiology; pp. 1–20. [Google Scholar]
- 34.Rathi B, Patil PA, Baheti AM. Evaluation of aqueous extract and seeds of Moringa oleifera for wound healing in albino rats. J Nat Remedies. 2004;4:145–9. [Google Scholar]
- 35.Morton JJ, Malone MM. Evaluation of vulnerary activity by open wound procedure in rats. J Trauma. 1992;20:323–4. [PubMed] [Google Scholar]
- 36.Dash GK, Saresh P, Ganapaty S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J Nat Remedies. 2001;1:105–10. [Google Scholar]
- 37.Chah KF, Eze CA, Emuelosi CE, Esimone CO. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J Ethnopharmacol. 2006;104:164–7. doi: 10.1016/j.jep.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 38.Nayak BS, Pinto Pereira LM. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement Altern Med. 2006;6:41. doi: 10.1186/1472-6882-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okoli CO, Ezike AC, Akah PA, Udegbunam SO, Okoye TC, Mbanu TP, et al. Studies on wound healing and antiulcer activities of extract of aerial parts of Phyllanthus niruri L. (Euphorbiaceae) Am J Pharmacol Toxicol. 2009;4:118–26. [Google Scholar]
- 40.Gal P, Kilik R, Mokry M, Vidinsky B, Vasilenko T, Mozes S, et al. Simple method of open skin wound healing model in cortico-steroid-treated and diabetic rats: Standardization of semi-quantitative and quantitative histological assessments. Vet Med. 2008;53:652–9. [Google Scholar]
- 41.Moyer KE, Saggers GC, Allison GM, Mackay DR, Ehrlich HP. Effects of interleukin-8 on granulation tissue maturation. J Cell Physiol. 2002;193:173–9. doi: 10.1002/jcp.10160. [DOI] [PubMed] [Google Scholar]
- 42.Clark RA. Wound repair: Overview and general considerations. In: Clark RA, Henson PM, editors. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. p. 3. [Google Scholar]
- 43.Whaley K, Burt AD. Inflammation, healing and repair. In: Macsween RM, Whaley K, editors. Muir's Textbook of Pathology. 13th ed. London: Arnold; 1996. pp. 112–65. [Google Scholar]
- 44.Bairy KL, Rao CM. Wound healing profiles of Ginkigo biloba. J Nat Remedies. 2001;1:25–7. [Google Scholar]
- 45.Abu-Al-Basal MA. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol. 2010;131:443–50. doi: 10.1016/j.jep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Udupa SL, Shaila HP, Udupa AL, Ramesh KV, Kulkami DR. Wound healing properties of some medicinal plants. Biochem Arch. 1991;7:207–12. [Google Scholar]
- 47.Shivhare Y, Singour PK, Patil UK, Pawar RS. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb (fruits) in rats. J Ethnopharmacol. 2010;127:614–9. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Maatalah MB, Bouzidi NK, Bellahouel S, Merah B, Fortas Z, Soulimani R, et al. Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulate. J Biotech Pharm Res. 2012;3:54–7. [Google Scholar]
- 49.Scortichini M, Pia RM. Preliminary in-vitro evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora (Burril) J Appl Bacteriol. 1991;71:109–12. [Google Scholar]
- 50.Rane MM, Mengi SA. Comparative effect of oral administration and topical application of alcoholic extract of Terminalia arjuna bark on incision and excision wounds in rats. Fitoterapia. 2003;74:553–8. doi: 10.1016/s0367-326x(03)00118-7. [DOI] [PubMed] [Google Scholar]


