Abstract
Background
Accurate measurement of total cholesterol (TC) is important for cardiovascular disease risk management. The US Centers for Disease Control and Prevention (CDC) and Cholesterol Reference Method Laboratory Network (CRMLN) perform Abell–Levy–Brodie–Kendall (AK) reference measurement procedure (RMP) for TC as a secondary reference method, and implement Certification Protocol for Manufacturers. Japanese CRMLN laboratory at Osaka performed the AK RMP for 22 years, and conducted TC certification for reagent/calibrator/instrument systems of six Japanese manufacturers every 2 years for 16 years. Osaka TC performance was examined and compared to CDC’s reference values.
Methods
AK RMP involved sample hydrolysis, cholesterol extraction, and determination of cholesterol levels by spectrophotometry. The Certification Protocol for Manufacturers includes comparison with AK RMP using at least 40 fresh specimens. Demonstration of average bias ≤3% and total coefficient of variation ≤3% qualified an analytical system for certification.
Results
In the AK RMP used in the Osaka CRMLN laboratory, the regression equation for measuring TC was y (Osaka) = 1.000x (CDC) + 0.032 (n = 619, R2 = 1.000). Six Japanese manufacturers had allowable performance for certification.
Conclusions
The AK RMP for TC measurement was accurate, precise, and stable for 22 years. Six Japanese manufacturers were certified for 16 years.
Keywords: TC, AK RMP, Certification, CRMLN, CDC
1. Introduction
The association between elevated total cholesterol (TC), due to increased low-density lipoprotein cholesterol concentrations, and the risk of premature coronary heart disease (CHD) has been well documented [1–3]. CHD is the major cause of death in developed countries; accurate and reproducible TC measurements are of particular importance for correctly and consistently classifying individuals who are at increased risk for this disease, as is outlined in the clinical guidelines for the diagnosis, treatment, monitoring, and prevention of dyslipidema [4–7].
In 1988, the US Laboratory Standardization Panel [8,9] recommended that cholesterol measurements be standardized so that values are traceable to the US Centers for Disease Control and Prevention (CDC) reference measurement procedure (RMP) for cholesterol, which is a modification of Abell–Levy–Brodie–Kendall (AK) method [10,11]. As a result cholesterol tests performed in patient care as well as in clinical studies used to define clinical decision levels are standardized to the same AK RMP. This enabled the correct interpretation of cholesterol values and efficient implementation of clinical guidelines and public health efforts.
The AK RMP is linked to the National Institute of Standards and Technology (NIST) method for total serum cholesterol, which involves gas chromatography-isotope dilution mass spectrometry (GC-IDMS) [12–15]. The GC-IDMS RMP has higher specificity and selectivity than the AK RMP. Thus, results obtained with this method are not interchangeable with results from the AK RMP [10,14]. Because AK RMP-based clinical decision levels are currently being used in patient care, CDC continues to operate the AK RMP and standardizes clinical tests to this method. At the same time, it established a GC-IDMS RMP [14] to meet the increasing need for more specific and selective clinical measurements.
Epidemiological and large-scale clinical studies have been performed in Japan to investigate the risk of cardiovascular disease (CVD) using lipid measurements similar to studies conducted in Europe and the United States. The limitations of lipid measurement in Japan have historically been the comparability and accuracy of the assayed results. To overcome this limitation and to achieve traceable, accurate, and stable lipid measurements over time, an epidemiological study group at Osaka Medical Center has participated in the World Health Organization (WHO)-CDC Cooperative Cholesterol-Triglyceride Standardization Program since April 1975 [15–17]. The standardization of TC measurement at Osaka was achieved through the CDC-NHLBI Lipid Standardization Program in the 1970s and 1980s using Zak assays [18,19] and enzymatic methods [20,21], which are routinely used to analyze cholesterol levels in clinical laboratories in Japan. In 1991, the AK RMP for cholesterol was introduced to the epidemiological laboratory at Osaka, and it was standardized through the Cholesterol Reference Method Laboratory Network (CRMLN) from July 1992 to July 2014. TC certification has been performed by the CDC and CRMLN for reagent manufacturers using the Total Cholesterol Certification Protocol for Manufacturers [22]. For clinical laboratories, TC certification has been performed using the Certification Protocol for Clinical Laboratories [23]. As a result, most Japanese manufacturers and many clinical laboratories standardized TC measurements to provide traceability to the CDC’s AK RMP. In 2002, the Osaka laboratory established a GC-IDMS method similar to CDC’s RMP [24]. The AK RMP and GC-IDMS method have both been used continuously and simultaneously through regular CRMLN surveillance under the same measurement conditions since July 2012 to the present.
In this study, we present the accuracy and imprecision of the AK RMP obtained at Osaka during the course of 22 years. Moreover, we outline an evaluation of the accuracy, precision, and total error of reagent/ calibrator/instrument systems of 6 Japanese reagent manufacturers that participated in the TC certification program for manufacturers [22] every 2 years for 16 years.
2. Materials and methods
2.1. Materials
In the CRMLN survey, all standardization pools for TC were created using sera that were prepared according to the Clinical and Laboratory Standards Institute Document C37-A [25], which defines blood collection, clotting and processing conditions. This suggests that no preservatives or additives were added nor was the material lyophilized. CRMLN survey pools include round robin samples that were provided from a participating CRMLN laboratory, and which included native specimens from patients and 12.1% (75 of 619 runs) of all samples. All survey pools were blinded to the CRMLN laboratories. Samples were shipped frozen from the CDC, and stored at −70 °C for subsequent analysis.
In the TC Certification Protocol for Manufacturers [22], participating manufacturers collected 40 or more fresh specimens from individual fasting donors. The cholesterol concentration of these specimens were distributed over a clinically meaningful range, as close as possible to the following target distribution: 20% of samples from 120 mg/dL to 180 mg/dL, 30% of samples from 181 mg/dL to 220 mg/dL, 30% of samples from 221 mg/dL to 260 mg/dL, and 20% of samples from 261 mg/dL to 400 mg/dL. The minimum amount of serum needed per sample for the AK RMP analysis is 1.5 mL. Manufacturers analyzed the specimens using their reagent/calibrator/instrument system over 20 runs, with 2 samples per run. To estimate imprecision, manufacturers should provide quality control (QC) single data obtained from 20 separate runs. The recommended concentration range for the QC material is 200 mg/dL to 240 mg/dL.
In the Certification Protocol for Clinical Laboratories [23], clinical laboratories analyzed two fresh samples in each of the three concentration regions; namely region 1: 100 mg/dL and 200 mg/dL, region 2: 200 mg/dL and 240 mg/dL and region 3: >240 mg/dL. The samples were assayed using the AK RMP at Osaka.
TC measurements were conducted at the Osaka Medical Center for Cancer and Cardiovascular Diseases between July 1997 and June 2001, at the Osaka Medical Center for Health Science and Promotion between July 2001 and March 2012, and at the National Cerebral and Cardiovascular Center at Osaka continuously since April 2012 (all laboratories are referred to as the Osaka laboratory).
2.2. Methods
2.2.1. AK RMP for TC at the Osaka CRMLN laboratory
The AK RMP for TC measurement is a modification of the extraction procedure by Abell et al. [10] and the original method was improved at the CDC laboratory [11]. We used a Digifiex (ICN, Biomedicals, Inc.) automatic pipettor for aspirating and dispensing standard solution, sample, and reagent. Current RMP consists of saponification of a 0.250 mL serum sample with alcoholic potassium hydroxide at 50 °C for 1 h, extraction for 20 min with hexane using a mechanical shaker in a horizontal position, evaporation of an aliquot of extract connected with a vacuum oven, and color development with Liebermann–Burchard reagent (mixed reagent of acetic anhydride, glacial acetic acid, and concentrated sulfuric acid) at 620 nm using a spectrophotometer (Beckman DU600 and DU800). The AK RMP is calibrated using the NIST Standard Reference Material (SRM) of pure unlabeled cholesterol (SRM 911c). The working standard solutions of cholesterol in alcohol consist of 25.0, 50.0, 100.0, 200.0, 300.0, and 400.0 mg/dL concentrations.
2.2.2. TC performance criteria applied to the CRMLN laboratory using AK RMP
TC performance criteria applied to the CRMLN lipid reference laboratory are summarized in Table 1. Precision was evaluated in terms of co-efficient of variation (CV, %), and accuracy (%bias versus CDC reference value) was evaluated in terms of deviation (%) from the CDC reference value.
Table 1.
TC performance criteria applied to CRMLN lipid reference laboratory using AK RMP.
| Lipid | Precision criterion | Accuracy criterion |
|---|---|---|
| TC | Coefficient of variation ≤ 1% | Bias (deviation from CDC reference value) ± 1% |
CRMLN: Cholesterol Reference Method Laboratory Network. AK RMP: Abell–Levy–Brodie–Kendall Reference Measurement Procedure. TC: total cholesterol.
CDC: Centers for Disease Control and Prevention.
2.2.3. Statistical criteria of TC certification for manufacturers
Statistical criteria of TC certification for manufacturers are summarized in Table 2A. As a reference, statistical criteria of TC certification for clinical laboratories are summarized in Table 2B.
Table 2A.
Statistical criteria of TC certification for manufacturer.
| Parameter | Criterion |
|---|---|
| r2 | >0.975 |
| Bias at 200 mg/dl | ≤3% |
| Bias at 240 mg/dl | ≤3% |
| Average % bias | ≤3% |
| Average absolute % bias | ≤3% |
| Among-run CV | ≤3% |
| Z-test of bias | Not significant at α = 5% |
| Within-method outliers | 1 allowed |
| Between-method outliers | None allowed, but may eliminate one sample |
Table 2B.
Statistical criteria of TC certification for clinical laboratory.
| Parameter | Criterion |
|---|---|
| r2 | >0.975 |
| Bias at 200 mg/dl | ≤3% |
| Bias at 240 mg/dl | ≤3% |
| Average % bias | ≤3% |
| Average absolute % bias | ≤3% |
| Among-run CV | ≤3% |
| t-test of bias | Not significant at α = 5% |
| Within-method outliers | 1 allowed |
| Between-method outliers | None allowed, but may eliminate one sample |
2.3. Statistical analysis
We used the protocol of NCCLS guideline EP9-A from the Clinical and Laboratory Standards Institute for bias estimation [26] and the STATA12 analysis program for all other calculations [27,28].
3. Results
3.1. Regression, accuracy and precision of TC by AK RMP at Osaka laboratory over time
In the AK RMP for TC measurement at Osaka, the CDC pooled sera with 219 different concentrations (lots) were analyzed among 619 survey samples with 165 survey runs, in which each survey run consisted of 3 to 4 different pools. There was one native sample from patients with low TC concentration ranged 27.7 to 83.4 mg/dL in the three to four provided pools: these samples were for the purpose of measuring the accuracy and precision of high-density lipoprotein cholesterol. The TC concentration ranged from 27.7 to 383.7 mg/dL: concentrations were analyzed during the course of 22 years between July 1992 and July 2014.
In the scatter plots of %bias between Osaka (y) and CDC (x), y = 1.000x + 0.032 (n = 619, R2 = 1.000). This means that 200 mg/dL at the CDC corresponds to 200.03 mg/dL at Osaka. The p-value and 95% confidence interval (CI) for the slope were p < 0.001 and (1.000, 1.001), respectively. The p-value and 95% CI for the intercept were p = 0.502 and (−0.061, 0.124), respectively (Table 3).
Table 3.
Regression, accuracy and precision of TC by Osaka AK RMP over time.
| Parameter | TC method | Number of samples | Pass rate | Slope (95%CI) | Intercept (95%CI) | R2 | Time period |
|---|---|---|---|---|---|---|---|
| Regression | AK RMP | 619 | 1.000 (1.000, 1.001) p < 0.001 |
0.032 (−0.061, 0.124) p = NS |
1.000 | July 1992 to July 2014 (22 y) | |
| Accuracy (as %Bias vs. CDC) | AK RMP | 619 | 99.0% | −0.001 (−0.001, −0.000) p < 0.001 |
0.141 (0.086, 0.195) p < 0.001 |
0.022 | July 1992 to July 2014 (22 y) |
| Precision (as CV) | AK RMP | 619 | 99.2% | −0.001 (−0.001, −0.001) p < 0.001 |
0.321 (0.286, 0.357) p < 0.001 |
0.093 | July 1992 to July 2014 (22 y) |
AK RMP: Abell–Brodie–Levy–Kendall Reference Measurement Procedure. TC: total cholesterol.
For pass rate, TC accuracy criterion as %bias is ±1% vs. CDC target value and TC precision criterion as CV is ≤1%.
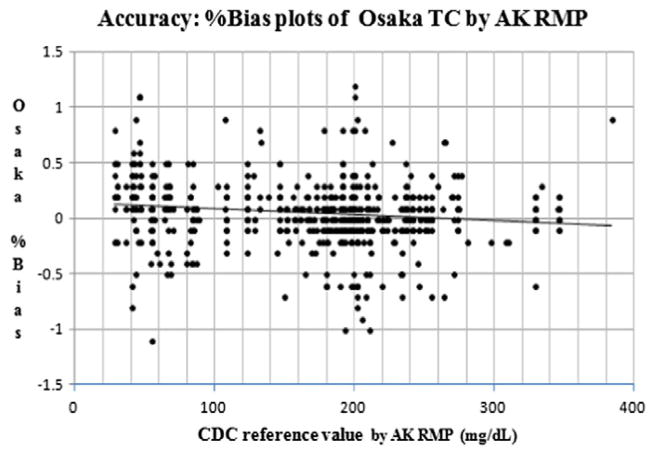
In the scatter plots of accuracy, %bias vs. CDC at Osaka, y (Osaka) = −0.001 × (CDC reference value) + 0.141 (n: 619, R2 = 0.022). The p-value and 95% CI for the slope were p < 0.001 and (−0.001, −0.000), respectively. The p-value and 95% CI for the intercept were p < 0.001 and (0.086, 0.195), respectively (Table 3). The Osaka laboratory met the acceptable accuracy goals within ± 1% compared to the CDC reference values for 99.0% of the samples (613 of 619) (Fig. 1., Table 3). The maximum %bias at Osaka AK RMP was +1.2% and the minimum was −1.1% among all 619 samples. The %bias between the reference values of the CDC and the measurements of the Osaka laboratory at a medical decision point of 200 mg/dL was only −0.06% at Osaka.
Fig. 1.
Accuracy: %Bias plots of Osaka TC by AK RMP. Y-axis indicates the %bias vs. CDC reference value of Osaka TC by AK RMP and x-axis indicates CDC reference value by AK RMP (mg/dL). CDC: the US Centers for Disease Control and Prevention. AK RMP: Abell–Kendall reference measurement procedure. TC: total cholesterol.
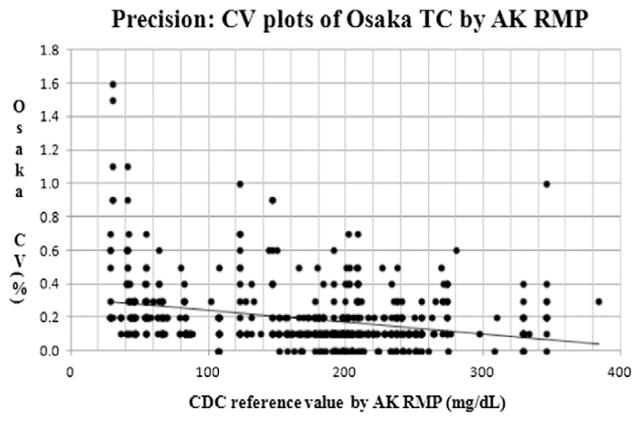
In the scatter plots of precision, CV(%) at Osaka, y (Osaka CV%) = −0.001 × (CDC reference value) + 0.321 (n: 619, R2 = 0.093). The p-value and 95% CI for the slope were p < 0.001 and (−0.001, −0.001), respectively. The p-value and 95% CI for the intercept were p < 0.001 and (0.286, 0.357), respectively (Table 3). The Osaka laboratory met acceptable precision goals < 1% at CV for 99.2% of the samples (614 of 619). The maximum CV at the Osaka AK RMP was 1.6% (Fig. 2.).
Fig. 2.
Precision: coefficient of variation plots of Osaka TC by AK RMP. Y-axis indicates co-efficient of variation (CV, %) of Osaka TC by AK RMP and x-axis indicates CDC reference value by AK RMP (mg/dL). CDC: the US Centers for Disease Control and Prevention. AK RMP: Abell–Kendall reference measurement procedure. TC: total cholesterol.
3.2. Accuracy, precision and total error of Japanese manufacturers conducted by the TC Certification Protocol for Manufacturers
Six reagent manufacturers in Japan were evaluated between 1996 and 2012 eight times according to the TC Certification Protocol for Manufacturers [22], with regard to their analytical systems consisting of a reagent/calibrator/instrument. Their accuracy (mean %bias versus reference value by AK RMP), precision (among-run CV), and total error (absolute mean %bias + 1.96 among-run CV) are presented in Table 4A, Table 4B, and Table 4C, respectively.
Table 4A.
Accuracy (mean %bias) of 6 Japanese manufacturers conducted by TC Certification Protocol for Manufacturers.
| Manufacturer | Certification year for TC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1996 | 1997 | 1998 | 2002 | 2004 | 2006 | 2008 | 2010 | 2012 | |
| A | 0.6 | −0.8 | −0.4 | −0.1 | 1.0 | −0.3 | −0.3 | 1.3 | |
| B | −1.2 | −0.6 | 1.6 | −0.4 | 1.4 | 1.0 | −0.3 | −0.1 | |
| C | 1.0 | −1.3 | −1.0 | 0.6 | −0.4 | 1.1 | −0.1 | −0.9 | |
| D | 2.1 | 1.2 | −1.1 | −0.1 | 0.7 | 0.1 | −0.5 | 0.5 | |
| E | −2.2 | 0.2 | 0.2 | −0.2 | 1.8 | 0.5 | −0.1 | ||
| F | 1.2 | 0.4 | −0.4 | 0.0 | 0.5 | 0.3 | 0.0 | ||
Accuracy criterion: mean % bias ≦3% unit: %.
Table 4B.
Precision (among-run CV) of 6 Japanese manufacturers conducted by TC Certification Protocol for Manufacturers.
| Manufacturer | Certification year for TC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1996 | 1997 | 1998 | 2002 | 2004 | 2006 | 2008 | 2010 | 2012 | |
| A | 0.5 | 0.6 | 0.6 | 0.6 | 0.8 | 0.4 | 0.5 | 0.5 | |
| B | 0.7 | 0.7 | 0.7 | 0.7 | 1.2 | 0.6 | 0.6 | 0.7 | |
| C | 0.6 | 0.7 | 0.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | |
| D | 0.6 | 0.7 | 0.5 | 0.4 | 0.3 | 0.4 | 0.4 | 0.4 | |
| E | 0.4 | 0.5 | 0.7 | 0.5 | 0.4 | 0.8 | 1.0 | ||
| F | 1.5 | 0.8 | 0.7 | 0.5 | 0.5 | 0.4 | 1.0 | ||
Precision criterion: among-run CV ≦3% unit: %.
Table 4C.
Total error of 6 Japanese manufacturers conducted by TC Certification Protocol for Manufacturers.
| Manufacturer | Certification year for TC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1996 | 1997 | 1998 | 2002 | 2004 | 2006 | 2008 | 2010 | 2012 | |
| A | 1.5 | 2.0 | 1.5 | 1.3 | 2.6 | 1.1 | 1.2 | 2.3 | |
| B | 2.6 | 2.1 | 3.0 | 1.7 | 3.9 | 2.3 | 1.5 | 1.4 | |
| C | 2.2 | 2.7 | 2.0 | 1.8 | 1.4 | 2.3 | 1.0 | 1.8 | |
| D | 3.3 | 2.7 | 2.2 | 0.8 | 1.4 | 0.9 | 1.3 | 1.3 | |
| E | 3.0 | 1.3 | 1.6 | 1.3 | 2.6 | 2.1 | 2.1 | ||
| F | 4.2 | 1.9 | 1.8 | 1.0 | 1.5 | 1.1 | 2.0 | ||
Total error (absolute mean %bias + 1.96 among-run CV) criterion: ≦8.9% unit: %.
3.3. %Bias plots of TC by Osaka AK RMP at each run over time
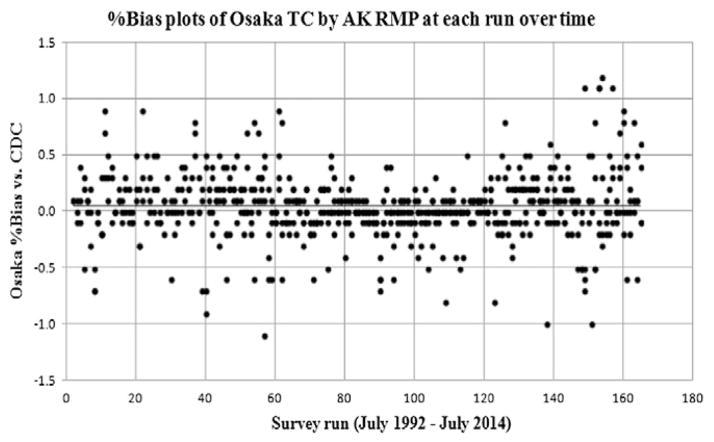
Fig. 3 shows the %bias compared to the CDC reference value plots of the Osaka AK RMP at each run from a total of 165 runs for 22 years. The minimum value of the %bias was −1.1%, whereas the maximum value was 1.2%. The x-axis indicates the survey run number every 20 runs, and the y-axis indicates the %bias versus CDC reference value of the Osaka AK RMP. The acceptable criterion for the accuracy of TC was within ± 1.0% compared to the reference value of CDC. Each survey run consisted of three to four CDC pools, including round robin samples provided from the CRMLN laboratory for TC analysis.
Fig. 3.
%Bias plots of Osaka TC by AK RMP at each run over time. Y-axis indicates Osaka %bias vs. CDC reference value and x-axis indicates each survey run from total 165 runs (July 1992 - July 2014). CDC: The US Centers for Disease Control and Prevention. AK RMP: Abell–Kendall reference measurement procedure. TC: total cholesterol.
4. Discussion
The CRMLN maintains robust reference measurement systems with high quality reference materials, measurement procedures, and continuous monitoring. Established protocols and guidelines within CDC’s CRMLN certification program allows manufacturers to perform measurement comparisons between the test method and the reference method to assess performance accuracy and imprecision. As shown in earlier reports, CRMLN successfully applies this principle to complex analytes such as HDL- and LDL-cholesterol as well as to analytes such as total cholesterol. Reliable data allows for consistent patient monitoring, treatment management, and improved worldwide public health efforts for CVD.
The AK RMP as operated at the CDC Laboratory and the Osaka laboratory is highly accurate and reliable for over 20 years. Measurement results obtained by both laboratories show excellent agreement, precision and stability. This ensures assay manufacturers calibrated by the Osaka CRMLN laboratory can produce measurement results that are highly accurate and comparable over time.
The main purpose of the CRMLN laboratories is to work with manufacturers to certify the accuracy and precision of cholesterol measurements of reagent/calibrator/instrument systems used in clinical laboratories [29]. This is in agreement with the US Laboratory Standardization Panel, which suggests that standardization is most effectively achieved through the manufacturers of analytical instruments and reagents [8,9]. Between 1996 and 2012, six reagent manufacturers in Japan were certified eight times with regard to their analytical systems consisting reagent/calibrator/instrument [22]. Standardization of 2,122 Japanese clinical laboratories has been performed by the Certification Protocol for Clinical Laboratories [23] between 1993 and 2014, and 98.2% of these participants met the certification criteria, which were derived from clinical needs. This high pass rate with clinical laboratories suggests that calibration of assay manufacturers by CRMLN laboratories is highly successful and effective. Lipid standardization activities have improved the accuracy of TC measurements [30]. All manufacturers and clinical laboratories with current certification are listed on CDC’s-CRMLN web site (http://www.cdc.gov/labstandards/crmln.html).
The AK RMP was proposed more than 60 years ago and is considered the secondary reference method for analyzing cholesterol. It has been used internationally as a reference procedure for measuring cholesterol at CDC, CRMLN laboratories, and in many epidemiological research institutes worldwide. Despite its widespread use, the AK method has limitations such as complex operations, the requirement of skilled technicians, use of hazardous reagents, and interferences related to the measurement of reactive substances other than cholesterol. These limitations can be minimized with current GC-IDMS RMPs.
Current clinical and public health decision points are based on measurements standardized to the AK RMP. Because results obtained with this RMP are not interchangeable with results obtained with GC-IDMS RMPs, and a reliable relationship between both types of RMPs has not been established yet. The AK RMP continues to be used to standardize tests in patient care and public health. At the same time, CDC together with CRMLN laboratories including Osaka laboratory is working to further establish mass spectrometry-based RMPs for TC and other lipid and lipoproteins [14,31–33]. Mass spectrometry-based RMPs for TC and triglycerides are already part of the CRMLN. This will help the clinical and public health communities to generate new data and clinical decision points that are linked to new more specific mass spectrometry-based RMPs.
5. Conclusions
We presented the performance of TC measurements using the AK RMP over the past 22 years and demonstrated the accuracy, precision, and long-term stability of this method. As examples of AK RMP application, we presented six reagent manufacturers in Japan that successfully certified their reagent/calibrator/instrument systems and participated in the TC Certification Protocol for Manufacturers for 16 years. The clinical laboratories in Japan demonstrated high achievement rates for TC certification. This accomplishment is one example on how CRMLN is improving clinical testing of cholesterol and other blood lipids not only in Japan but worldwide. These data as well as data shown in previous reports demonstrate that the same standardization principles can be applied to analytes such as TC as well as highly complex analytes such as HDL- and LDL-cholesterol.
Acknowledgments
Sources of funding
No declared.
This work was supported by a Health and Labour Sciences Research Grant, Japan (Comprehensive Research on Lifestyle-Related Diseases Including Cardiovascular Diseases and Diabetes Mellitus) from the Ministry of Health, Labour, and Welfare of Japan. The authors would like to thank Dr. Katsuyuki Nakajima and Dr. Ikunosuke Sakurabayashi for their valuable comments and discussion, and Ms. Yukari Ichikawa for her excellent help in providing the references and manuscript.
Footnotes
Disclaimer: The results and conclusions in this study are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Conflict of interest
No authors have any financial, personal or professional relationships associated with other people or organizations.
References
- 1.Lipid Research Clinics Program. The Lipid Research Clinics Primary Prevention Trial results. I: reduction in incidence of coronary heart disease. II: the relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:351–75. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 2.The Expert Panel. Report of the National Cholesterol Education Program Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults. Arch Intern Med. 1988;148:36–9. [PubMed] [Google Scholar]
- 3.Kitamura A, Sato S, Kiyama M, et al. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003, The Akita-Osaka Study. J Am Coll Cardio. 2008;52:71–9. doi: 10.1016/j.jacc.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Warnick GR, Myers GL, Cooper GR, et al. Impact of the third cholesterol report from the adult treatment panel of the national cholesterol education program on the clinical laboratory. Clin Chem. 2002;48:11–7. [PubMed] [Google Scholar]
- 6.European guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 7.Japan Atherosclerosis Society. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases. 2012;2012 [PubMed] [Google Scholar]
- 8.Current status of blood cholesterol measurement in clinical laboratories in the United States: a report from the laboratory standardization panel of the National Cholesterol Education Program. Clin Chem. 1988;34:193–201. [PubMed] [Google Scholar]
- 9.NIH Publication No. 90–2964. Feb, 1990. Recommendations for improving cholesterol measurement. A Report From The Laboratory Standardization Panel Of The National Cholesterol Education Program. [Google Scholar]
- 10.Abell LL, Levy BB, Brodie BB, et al. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–66. [PubMed] [Google Scholar]
- 11.Clinical Chemistry Division 1982. Center For Environmental Health, Centers For Disease Control; 1982. The procedure for the proposed cholesterol reference method. [Google Scholar]
- 12.Pelletier O, Wright LA, Breckenridge WC. Isotope dilution/mass spectrometry of serum cholesterol with [3,4-13C]cholesterol: proposed definitive method. Clin Chem. 1987;33:1403–11. [PubMed] [Google Scholar]
- 13.Ellerbe P, Meiselman S, Sniegoski LT, et al. Determination of serum cholesterol by a modification of the isotope dilution mass spectrometric definitive method. Anal Chem. 1989;61:1718–23. doi: 10.1021/ac00190a025. [DOI] [PubMed] [Google Scholar]
- 14.Edwards SH, Kimberly MM, Pyatt SD, et al. Proposed serum cholesterol reference measurement procedure by gas chromatography-isotope dilution mass spectrometry. Clin Chem. 2011;57:614–22. doi: 10.1373/clinchem.2010.158766. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GR, Smith SJ, Duncan IW, et al. Interlaboratory testing of the transferability of a candidate reference method for total cholesterol in serum. Clin Chem. 1986;32:921–9. [PubMed] [Google Scholar]
- 16.Cooper GR, Myers GL, Smith SJ, et al. Standardization of lipid, lipoprotein, and apoli-poprotein measurements. Clin Chem. 1988;34:B95–105. [PubMed] [Google Scholar]
- 17.Myers GL, Cooper GR, Winn CL, et al. The Center for Disease Control, National Heart, Lung, and Blood Institute Lipid Standardization Program: an approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–35. [PubMed] [Google Scholar]
- 18.Zak B, Artiss JD. Some observations on cholesterol measurement in the clinical laboratory [Review] Microchem J. 1990;41:251–70. [Google Scholar]
- 19.Zak B. Cholesterol methodologies: a review. Clin Chem. 1977;23:1201–14. [PubMed] [Google Scholar]
- 20.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–6. [PubMed] [Google Scholar]
- 21.Allain CC, Poon LS, Chan CSG, et al. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 22.Cholesterol Reference Method Laboratory Network for the National Reference System for Cholesterol. October 2004. Total cholesterol certification protocol for manufacturers — revised. [Google Scholar]
- 23.Certification Protocol for Clinical Laboratories. Cholesterol Reference Method Laboratory Network For The National Reference System For Cholesterol. May 2004. [Google Scholar]
- 24.Koyama I, Iso H, Kiyama M, et al. Establishment of practical procedure for measurement of total cholesterol by isotope dilution/gas chromatography/mass spectrometry at the Osaka Medical Center for Health Science and Promotion (CRMLN lipid reference laboratory) Clin Chem. 2010;(Supplement 56):A186. [Google Scholar]
- 25.Clinical And Laboratory Standards Institute, Document C37-A. 1999. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guideline. [Google Scholar]
- 26.Method Comparison And Bias Estimation Using Patient Samples; Approved Guideline. NCCLS EP9-A. 1995 Dec;15(17) [Google Scholar]
- 27.Westgard JO, Hunt MR. Use and interpretation of common statistical tests in method-comparison studies. Clin Chem. 1973;19:49–57. [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. [PubMed] [Google Scholar]
- 29.Bennett ST, Eckfeldt JH, Belcher JD, et al. Certification of cholesterol measurements by the National Reference Method Laboratory Network with routine clinical specimens: effects of network laboratory bias and imprecision. Clin Chem. 1992;38:651–7. [PubMed] [Google Scholar]
- 30.Nakamura M, Sato S, Shimamoto T. Improvement in Japanese clinical laboratory measurements of total cholesterol and HDL-cholesterol by the US Cholesterol Reference Method Laboratory Network. J Atheroscler Thromb. 2003;10:145–53. doi: 10.5551/jat.10.145. [DOI] [PubMed] [Google Scholar]
- 31.Siekmann L. Reference methods for total cholesterol and total glycerol. Eur J Clin Chem Clin Biochem. 1991;29:277–9. doi: 10.1515/cclm.1991.29.4.277. [DOI] [PubMed] [Google Scholar]
- 32.Edwards SH, Stribling SL, Pyatt SD, et al. Reference measurement procedure for total glycerides by isotope dilution GC–MS. Clin Chem. 2012;58:768–76. doi: 10.1373/clinchem.2011.177063. [DOI] [PubMed] [Google Scholar]
- 33.Bernert JT, Jr, Bell CJ, McGuffey JE, et al. Determination of “free” glycerol in human serum reference materials by isotope-dilution gas chromatography-mass spectrometry. J Chromatogr. 1992;578:1–7. doi: 10.1016/0378-4347(92)80218-f. [DOI] [PubMed] [Google Scholar]