Abstract
The chemotherapeutic drug Gemcitabine, 2′,2′-difluoro-2′-deoxycytidine, has long been the standard of care for a number of cancers. Gemcitabine’s chemotherapeutic properties stem from its 2′,2′-difluoro-2′-deoxyribose sugar, which mimics the natural nucleoside, but also disrupts nucleic acid synthesis, leading to cell death. As a result, numerous analogues have been prepared to further explore the biological implications for this structural modification. In that regard, a thieno-expanded guanosine analogue was of interest due to biological activity previously observed for the tricyclic heterobase scaffold. Several analogues were prepared, including the McGuigan ProTide, however the parent nucleoside exhibited the best chemotherapeutic activity, specifically against breast cancer cell lines (89.53% growth inhibition).
Keywords: Tricyclic, Nucleosides, Expanded guanosine, 2′, 2′-Difluoro-2′-deoxycytidine, Cancer
Graphical Abstract
2′-Fluorinated nucleosides have proven to be effective therapeutic candidates against cancers and viruses; 2′,2′-difluoro-2′-deoxycytidine, or Gemcitabine (Figure 1), has been FDA approved for the treatment of a broad spectrum of solid tumors. More recently, Sofosbuvir (Figure 1), another 2′-F nucleoside, was been approved to treat hepatitis C.1,2 Fluorine substitutions on nucleosides were initially pursued due to fluorine’s multi-faceted chemical properties: due to its high electronegativity and low polarizability, fluorines can imitate a hydrogen atom from a size perspective, a hydroxyl group from a polarity perspective, and can also act as a hydrogen bond acceptor.3–5 Moreover, nucleosides endowed with a fluorine at C-2′ are much more stable to enzymatic cleavage.4 Gemcitabine’s mechanism of action is two-fold; not only does Gemcitabine inhibit DNA and RNA synthesis, but it also inhibits ribonucleotide reductase, which further inhibits DNA synthesis.4,6,7
Figure 1.
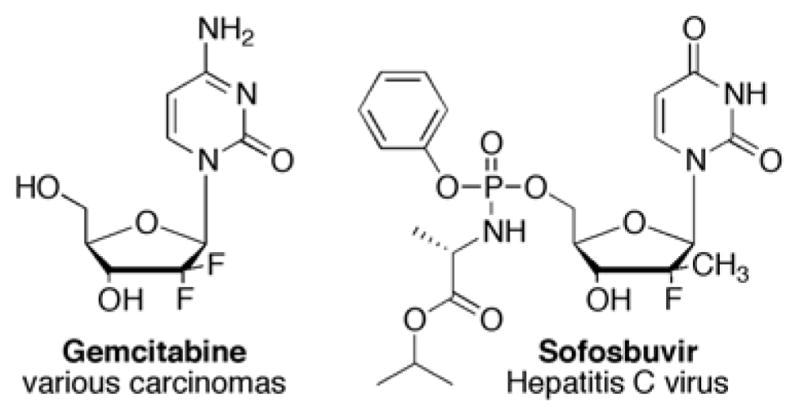
Gemcitabine and Sofosbuvir.
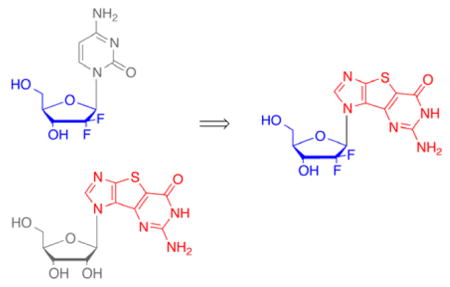
Although Gemcitabine is marketed as a cytidine nucleoside analogue, the guanosine version was also synthesized and showed similar activity profile.7 Our group has long been interested in modified purine nucleoside analogues and has previously synthesized various tricyclic thieno-expanded purine nucleosides (Figure 2) that possess a thiophene spacer ring between the imidazole and pyrimidine moieties of the purine.8–14 These novel nucleosides were designed to further explore the biological potential for bioprobes such as Nelson Leonard’s benzene expanded adenosine nucleosides.15–19 A number of his benzo-expanded nucleosides exhibited interesting therapeutic activities but occupied a larger spatial footprint that limited their potential.15–19 Our thieno-expanded purine nucleosides retain the essential binding elements of the parent nucleosides but also increase the aromaticity and polarizability of the base due to the thiophene spacer.8–14 The thiophene spacer also decreases the spatial implications of the expanded purine scaffold as compared to Leonard’s benzene spacer, thus allowing it to base pair more readily.20
Figure 2.
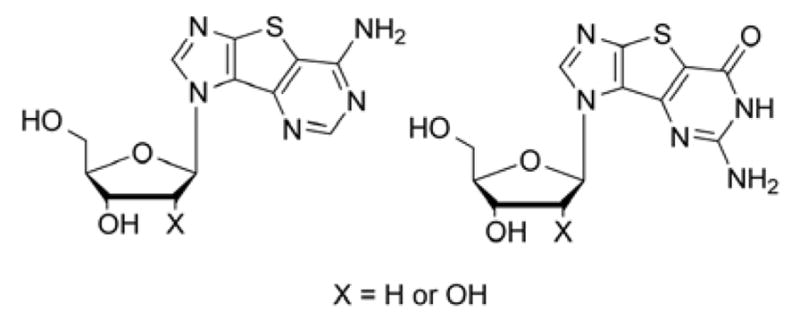
Tricyclic “thieno-expanded” purine nucleosides.
The thiophene-expanded analogues were shown to be recognized by nucleoside and nucleobase transporters, to exhibit activity against certain cancers, as well as to exhibit activity against hepatitis C.10,11,13,21,22 As a result, additional structural modifications were pursued to further explore the potential therapeutic profile of the tricyclic expanded guanosine base. In that regard, the 2′,2′-difluoro-2′-deoxyribose sugar of Gemcitabine was of interest (Figure 3, 1) as well as the corresponding McGuigan ProTide (Figure 3, 2). McGuigan’s ProTides have had a major impact on the nucleoside field since many inactive nucleosides that were subsequently converted to their corresponding Protide form show potent activity due to the ability to overcome the rate limiting step of monophosphorylation.23–27
Figure 3.
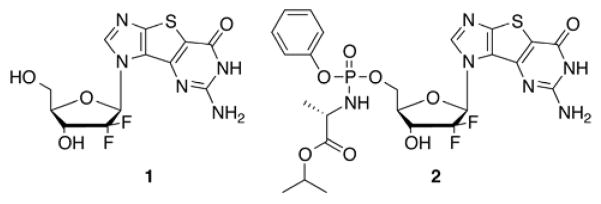
Target compounds 1 and 2.
Although there are numerous ProTides to choose from, the combination of the L-alanine (L-Ala) amino acid group, the phenyl aryl group and the iPrO ester group (Figure 3, 2) was selected due to its success with similar nucleosides.28
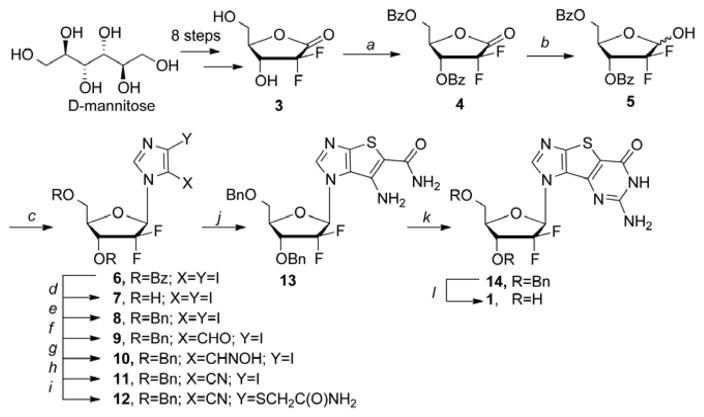
The 2′,2′-difluoro-2′-deoxyribose sugar was synthesized following a known route.29,30 Starting with D-mannitose, the fluorinated pentose sugar 3 can be achieved in 8-steps.
The 2′,2′-difluoro-2′-deoxyribonolactone 3 was then benzoyl-protected before reducing the ketone to give 5 (Scheme 1). Next, 4,5-diiodoimidazole was coupled to the modified sugar 5 to give both the α and β isomers of 6. The benzoyl protecting groups were removed and replaced with more robust benzyl groups 8, so as to withstand the rigorous conditions employed while synthesizing the tricyclic base, and the α and β isomers were separated. Construction of the tricyclic ring system followed our previously reported route.8–13 Finally, benzyl deprotection of 14 yielded the desired nucleoside 1.
Scheme 1.
Reagents and conditions: (a) BzCl, Py, DCM, 0°C, 10 min, then rt, 30 min; (b) LiAl(OtBu)3H, THF, −20°C, 6 h;(c) 4,5-diiodoimidazole, Ph3P, DIAD, THF, 0°C to rt, 24 h; (d) NaOMe, MeOH, 0°C, 1 h; (e) (i) NaH, 0°C, 2 h; (ii) BnBr, TBAI, DMF, rt, 4 h; (f) (i) EtMgBr, THF, 0°C, 30 min; (ii) anhydrous DMF, rt, overnight; (g) NH2OH•HCl, NaHCO3, EtOH, reflux, 5 h; (h) CDI, THF, reflux, 8 h; (i) NH2C(O)CH2SH, K2CO3, DMF, 65°C, 24 h; (j) EtONa, EtOH, reflux, 2 h; (k) (i) NaOH, CS2, MeOH, 150°C, 18 h; (ii) H2O2, MeOH, 0°C, 2 h; (iii) NH3/MeOH, 130°C, 12 h; (l) BF3•Et2O, EtSH, DCM, rt, 72 h.
The McGuigan ProTide of 1 was then synthesized following literature procedures (Scheme 2) to give 2 as well as and the bis-ProTide 15.31–35
Scheme 2.
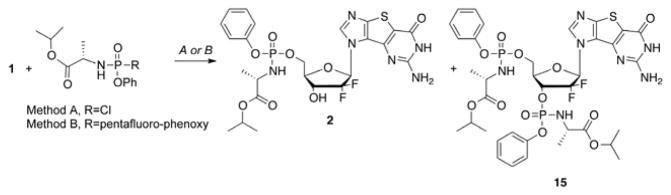
Reagents and conditions: (A) tBuMgCl, DMF, −78 °C, 1h, then r.t., 4 h; (B) tBuMgCl, DMF, 0 °C, 1 h, then rt, overnight.
Compounds 1, 2 and 15 were submitted to NCI for screening in their 60 cell line assay system. As shown below in Table 1, the compounds showed inhibitory activity against the MOLT-4 (leukemia), T-47D (breast cancer), LOX IMVI (melanoma) and NCI-H522 (non-small cell lung cancer) cell lines at 15μg/mL.
Table 1.
Growth percent of cancer cell lines MOLT-4, T-47D, LOX IMVI and NCI-H522 at 15μg/mL.
| Compound | Cell Line (percent growth) | |||
|---|---|---|---|---|
| MOLT-4 | T-47D | LOX IMVI | NCI-H522 | |
| 1 | 13.94 | 10.47 | 25.60 | 33.95 |
| 2 | 93.98 | 64.50 | 74.06 | 65.63 |
| 15 | 109.04 | 87.15 | 101.93 | 77.66 |
The results show that the best activity was observed for compound 1 against the breast cancer cell line T-47D (10.47%). Surprisingly, the McGuigan ProTides 2 and 15 did not demonstrate superior inhibition as compared to 1 as would have been expected. In fact, the therapeutic activity worsened with a second ProTide moiety present. Subsequent to our synthesis, a report appeared in the literature that ultimately confirmed the biological results: Slusarczyk et al. found that the selected combination of the L-Ala amino acid group, the phenyl aryl group and the iPrO ester led to a decrease in the activity of Gemcitabine.27 As a result, future efforts will focus on the ProTide combination they found to be best for the gemcitabine modification: a L-Ala amino acid group, a phenyl aryl group and an OBn ester.27
The tricyclic thiophene-expanded guanine base was successfully combined with the 2′,2′-difluoro-2′-deoxyribose sugar of Gemcitabine. In addition the corresponding McGuigan ProTide was also made and all three compounds were screened. The synthetic approach was nontrivial as both the sugar and base had to be constructed in a linear fashion, thus the overall yield was quite low. As a result, only preliminary biological testing could be undertaken. While the results from the NCI cancer screen showed promising results against leukemia, melanoma and breast cancer, the McGuigan ProTides were less active as compared to the parent nucleoside. Current efforts are underway to optimize the synthesis of the parent nucleoside 1 so further testing can be undertaken. The results of those efforts will be reported as they become available.
Supplementary Material
Acknowledgments
This work was funded in part by the National Institutes of Health [R21AI097685 (K.S.R.) and T32GM066706 (K.S.R. and T.C.K.)].
Footnotes
Synthetic procedures and structural characterization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu P, Sharon A, Chu CK. J Fluorine Chem. 2008;129:743. doi: 10.1016/j.jfluchem.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang DY, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. New Engl J Med. 2013;368:1878. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 3.Gesto DS, Cerqueira NM, FSA, Fernandes PA, Ramos MJ. Curr Med Chem. 2012;19:1076. doi: 10.2174/092986712799320682. [DOI] [PubMed] [Google Scholar]
- 4.Pankiewicz KW. Carbohyd Res. 2000;327:87. doi: 10.1016/s0008-6215(00)00089-6. [DOI] [PubMed] [Google Scholar]
- 5.Hunter L. Beilstein J Org Chem. 2010;6 doi: 10.3762/bjoc.6.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plunkett W, Huang P, Gandhi V. Nucleosides Nucleotides. 1997;16:1261. [Google Scholar]
- 7.Gandhi V, Mineishi S, Huang P, Chapman AJ, Yang YD, Chen F, Nowak B, Chubb S, Hertel LW, Plunkett W. Cancer Res. 1995;55:1517. [PubMed] [Google Scholar]
- 8.Zhang ZB, Wauchope OR, Seley-Radtke KL. Tetrahedron. 2008;64:10791. doi: 10.1016/j.tet.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Wauchope OR, Seley-Radtke KL. Tetrahedron. 2008;64:10791. doi: 10.1016/j.tet.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wauchope OR, Tomney MJ, Pepper JL, Korba BE, Seley-Radtke KL. Org Lett. 2010;12:4466. doi: 10.1021/ol101482h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wauchope OR, Johnson C, Krishnamoorthy P, Andrei G, Snoeck R, Balzarini J, Seley-Radtke KL. Bioorg Med Chem. 2012;20:3009. doi: 10.1016/j.bmc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seley KL, Zhang L, Hagos A, Quirk S. J Org Chem. 2002;67:3365. doi: 10.1021/jo0255476. [DOI] [PubMed] [Google Scholar]
- 13.Seley KL, Januszczyk P, Hagos A, Zhang L, Dransfield DT. J Med Chem. 2000;43:4877. doi: 10.1021/jm000326i. [DOI] [PubMed] [Google Scholar]
- 14.Wauchope OR, Velasquez M, Seley-Radtke K. Synthesis. 2012;44:3496. doi: 10.1055/s-0032-1316791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard NL, Morrice AG, Sprecker MA. J Org Chem. 1975;40:356. doi: 10.1021/jo00891a021. [DOI] [PubMed] [Google Scholar]
- 16.Leonard NJ. Acc Chem Res. 1982;15:128. [Google Scholar]
- 17.Leonard NJ, Hiremath SP. Tetrahedron. 1986;42:1917. [Google Scholar]
- 18.Leonard NJ, Scopes DI, VanDerLijn P, Barrio JR. Biochemistry. 1978;17:3677. doi: 10.1021/bi00611a001. [DOI] [PubMed] [Google Scholar]
- 19.Leonard NJ, Sprecker MA, Morrice AG. J Am Chem Soc. 1976;98:3987. doi: 10.1021/ja00429a040. [DOI] [PubMed] [Google Scholar]
- 20.O’Daniel PI, Jefferson M, Wiest O, Seley-Radtke KL. J Biomol Struct Dyn. 2008;26:283. doi: 10.1080/07391102.2008.10507243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Jochmans D, Ku T, Paeshuyse J, Neyts J, Seley-Radtke KL. ACS Inf Dis. 2015 doi: 10.1021/acsinfecdis.5b00029. [DOI] [PubMed] [Google Scholar]
- 22.Wallace LJM, Candlish D, Hagos A, Seley KL, de Koning HP. Nucleosides, Nucleotides and Nucleic Acids. 2004;23:1441. doi: 10.1081/NCN-200027660. [DOI] [PubMed] [Google Scholar]
- 23.Cahard D, McGuigan C, Balzarini J. Mini-Rev Med Chem. 2004;4:371. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 24.McGuigan C, Shackleton JM, Tollerfield SM, Riley PA. Nucleic Acids Res. 1989;17:10171. doi: 10.1093/nar/17.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balzarini J, Karlsson A, Aquaro S, Perno CF, Cahard D, Naesens L, De Clercq E, McGuigan C. Proc Natl Acad Sci U S A. 1996;93:7295. doi: 10.1073/pnas.93.14.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuigan C, Harris SA, Daluge SM, Gudmundsson KS, McLean EW, Burnette TC, Marr H, Hazen R, Condreay LD, Johnson L, De Clercq E, Balzarini J. J Med Chem. 2005;48:3504. doi: 10.1021/jm0491400. [DOI] [PubMed] [Google Scholar]
- 27.Slusarczyk M, Lopez MH, Balzarini J, Mason M, Jiang WG, Blagden S, Thompson E, Ghazaly E, McGuigan C. J Med Chem. 2014;57:1531. doi: 10.1021/jm401853a. [DOI] [PubMed] [Google Scholar]
- 28.Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Wang P, Zhang HR, Bansal S, Espiritu C, Keilman M, Lam AM, Steuer HMM, Niu C, Otto MJ, Furman PA. J Med Chem. 2010;53:7202. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- 29.Hertel LW, Kroin JS, Misner JW, Tustin JM. J Org Chem. 1988;53:2406. [Google Scholar]
- 30.Wang P, Chun BK, Rachakonda S, Du J, Khan N, Shi J, Stec W, Cleary D, Ross BS, Sofia MJ. J Org Chem. 2009;74:6819. doi: 10.1021/jo901345j. [DOI] [PubMed] [Google Scholar]
- 31.McGuigan C, Daverio F, Najera I, Martin JA, Klumpp K, Smith DB. Bioorg Med Chem Lett. 2009;19:3122. doi: 10.1016/j.bmcl.2009.03.138. [DOI] [PubMed] [Google Scholar]
- 32.McGuigan C, Kelleher MR, Perrone P, Mulready S, Luoni G, Daverio F, Rajyaguru S, Le Pogam S, Najera I, Martin JA, Klumpp K, Smith DB. Bioorg Med Chem Lett. 2009;19:4250. doi: 10.1016/j.bmcl.2009.05.099. [DOI] [PubMed] [Google Scholar]
- 33.Perrone P, Daverio F, Valente R, Rajyaguru S, Martin JA, Leveque V, Le Pogam S, Najera I, Klumpp K, Smith DB, McGuigan C. J Med Chem. 2007;50:5463. doi: 10.1021/jm070362i. [DOI] [PubMed] [Google Scholar]
- 34.Perrone P, Luoni GM, Kelleher MR, Daverio F, Angell A, Mulready S, Congiatu C, Rajyaguru S, Martin JA, Leveque V, Le Pogam S, Najera I, Klumpp K, Smith DB, McGuigan C. J Med Chem. 2007;50:1840. doi: 10.1021/jm0613370. [DOI] [PubMed] [Google Scholar]
- 35.Ross BS, Reddy PG, Zhang HR, Rachakonda S, Sofia MJ. J Org Chem. 2011;76:8311. doi: 10.1021/jo201492m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.