Abstract
Objective
As antiretroviral treatment (ART) expands for HIV-infected children, it is important to determine its impact on growth. We quantify growth and its determinants following ART in resource-limited (RLS) and developed settings (DS).
Design
Systematic review and meta-analysis.
Methods
We searched publications reporting growth [weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) Z-scores] in HIV-infected children following ART through August 2014. Inclusion criteria: 1) <18 years; 2) ART; 3) sample ≥20; 4) growth at ART; 5) post-ART growth. Standardized and overall weighted mean differences were calculated using random-effects-models.
Results
Sixty-seven articles were eligible (RLS=54; DS=13). Mean age was 5.8 years, and comparable between settings (P=0.90). Baseline growth was substantially lower in RLS versus DS (WAZ −2.1 vs. −0.5; HAZ −2.2 vs. −0.9; both P<0.01). Rate of weight but not height reconstitution during 12- and 24-months was higher in RLS (12-month WAZ change 0.84 vs. 0.17, P<0.01). Growth deficits persisted in RLS after 2-years ART (P=0.04). Younger cohort age was associated with greater growth reconstitution. PI and NNRTI regimens yielded comparable growth. Adjusting for age and setting, cohorts with nutritional supplements had greater growth gains (24-month rate difference: WAZ 0.55, P=0.03; HAZ 0.60, P=0.007). Supplement benefits were attenuated after adjusting for baseline cohort growth.
Conclusions
RLS children had substantial growth deficits compared to DS counterparts at ART; growth shortfalls in RLS persisted despite reconstitution. Earlier age and nutritional supplementation at ART may improve growth outcomes. Scant data on supplementation limits evaluation of impact and underscores need for systematic data collection regarding supplementation in pediatric ART programs/cohorts.
Keywords: growth, antiretroviral therapy, pediatric HIV, nutritional supplementation, systematic review, meta-analysis
Introduction
Antiretroviral therapy (ART) has substantially decreased morbidity and mortality in HIV-infected children [1-5]. ART also results in marked improvements in weight and height in children. Of the 3.3 million children living with HIV, more than 90% reside in resource-limited settings (RLS) [6]. In these settings, malnutrition is prevalent and children may be delayed in receiving ART due to late diagnosis [5, 7]. Both of these issues contribute to suboptimal growth reconstitution. Given prevalent nutritional needs, pediatric HIV treatment programs have varied approaches to nutritional supplementation during ART; with some empirically providing supplementation at initiation of ART [8-10]. It remains unclear whether ART alone or combined with nutritional supplements at initiation is better for long-term growth. It is well established, however, that suboptimal growth is associated with increased risk of mortality, repeat infections, and poor cognitive development [11].
Marked growth reconstitution occurs following ART. Previous reviews of pediatric ART outcomes have focused primarily on immunologic and virologic response, and have been limited to sub-Saharan Africa [3, 12] or resource-limited settings [13]. In a review by Peacock-Villada [14], we noted marked differences in baseline CD4% and viral load, and post-ART mortality in HIV-infected children in resource-limited versus developed countries. We performed a systematic review and meta-analysis to aggregate and compare growth outcomes following ART initiation among HIV-infected children in RLS and DS and to assess determinants of growth in these settings, including the role of nutritional supplementation in RLS.
Methods
Data search
A systematic literature search was conducted for all peer-reviewed literature published in English reporting growth outcomes (weight, length, height, weight-for-age, height-for-age, length-for-age, and weight-for-height Z-scores) in HIV-1 infected children following ART initiation. We searched PubMed, Cochrane, Embase, and Global Health Host through August 2014, using combinations of the following search terms: growth, length, height, weight, Z-score, outcomes, children, pediatric, paediatric, antiretroviral therapy, ART, HAART, HIV. Bibliographies of relevant articles were also examined.
Study selection and inclusion/exclusion criteria
The following inclusion criteria were used to select publications: 1) patients <18 years-old; 2) patients initiating ART (≥3 drugs); 3) sample size ≥20 patients; 4) growth measures at ART initiation; and 5) growth measures following 6, 12, or 24-months of ART. Outcome measures included the following growth parameters: weight-for-age (WAZ), weight-for-height (WHZ), and height-for-age (HAZ) Z-scores.
Studies reporting only clinical outcomes (CD4 count and HIV-1 RNA), limited to growth measures either prior to or following ART only, or lacking stated outcomes of interest were excluded. Manuscripts were also excluded if the outcome of interest was analyzed in terms of weight (kilograms or pounds) and height (centimeters or inches) without Z-score standardization.
Data extraction
Standardized data collection was used to extract all data, including: pre- and post-ART Z-scores, nutritional supplementation, study objective, site, sample size, ART regimen, time on ART, and disease severity at initiation. Articles were classified as having been conducted in a “Resource-limited Setting” (RLS) or “Developed Setting” (DS) based on the United Nations Statistics Division [15], and further subcategorized by geographic location. Studies reporting results from the same cohort or treatment program were compared, and only the most recent publication with relevant growth outcomes was included.
Statistical Analysis
Primary outcomes of interest were change in Z-scores (WAZ, WHZ, and HAZ) at 6, 12, or 24-months post-ART. Data on mean or median change in growth post-ART were either collected directly or calculated from reported results. Change in Z-score [or standard deviation (SD)] was calculated using the following methods: 1) difference between the baseline and 6, 12, or 24-month value; 2) overall rate of change during 6, 12, or 24-month interval, as reported, or 3) monthly rate of change in Z-score as reported from longitudinal analyses, specifically mixed-effects models for longitudinal data. The monthly rate of change estimations differ based on follow-up time as growth reconstitution generally peaks early followed by smaller incremental changes with longer duration on ART. Therefore, studies presenting changes in growth for 6-months may have a greater monthly change than those with 12-months follow-up (averaged over time). For studies reporting follow-up time in weeks, we included 24, 48 and 96 weeks as 6, 12, and 24-month growth outcomes.
Weighted baseline characteristics were compared between RLS and DS using a two-sample t-test assuming unequal variances, as appropriate. All means were weighted based on the reported sample size within the individual studies. When growth parameters were reported for only a subset of children with follow-up data, the weight for that parameter was adjusted based on reported size of the subset. Standardized mean differences were calculated for individual studies and overall weighted mean differences were calculated by setting (RLS versus DS) and displayed graphically in a Forest Plot. Forest plots display both the weighted mean difference and confidence interval for each study, as shown by a black box and corresponding horizontal line, and the pooled weighted mean difference across all studies, depicted with a diamond. Using methods described in Hozo et al [16], the median, range, and sample size were used to estimate mean and variance where this information was not reported or available. We observed similar meta-analysis results when including means alone versus combined means and medians; thus, results presented include both means and estimations of the mean using medians. Heterogeneity between studies was tested using X2 tests and the summary I-squared statistic [17]. Random-effects meta-analysis regression models were conducted to account for heterogeneity between studies and to assess the effect of setting and cohort age on growth rate post-ART. Cohort age was defined as the mean or median for each study and categorized as <4 years, 4-6 years, and >6 years at ART. All analyses were conducted using STATA 13.0 (StataCorp LP, College Station, Texas, USA).
Results
Study Selection and Characteristics
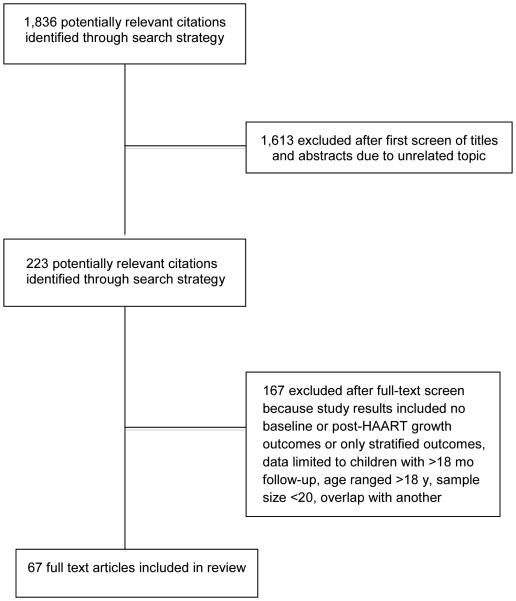
Our search strategy identified 1,836 potentially relevant articles. After review of abstracts, 223 articles were selected for full article review. Of these articles, 167 were excluded based on reasons outlined in Figure 1. The remaining 67 articles fulfilled the inclusion criteria and were included for at least one of the primary growth analyses (RLS=54, total N=25,927; and DS=13, total N=1,810). Table 1 describes characteristics of the included cohorts.
Fig 1.
Study flow chart describing the selection of publications for review
Table 1.
Characteristics of studies included in a systematic review of pediatric growth following ART
| Study authors (Year) | Location | N | Age, mean (a) or median (b) |
Study details, protease inhibit-based (a) or NNRTI-based (b) ART |
Months follow-up, mean (a), median (b) or total (c) |
|---|---|---|---|---|---|
| RLS (n=54) | |||||
|
| |||||
| Nacro et al. [18] (2011) | Burkino Faso | 51 | 6.8 yearsa | ANRS 12103, phase-II pediatric ART, EFVb | 12c |
|
| |||||
| De Beaudrap et al. [19] (2008) | Cote d'Ivoire | 177 | 5.8 yearsb | ANRS 1244/1278, protease inhibitor, 74%; NNRTI, 26% | 30a |
|
| |||||
| Fassinou et al. [2] (2004) | Cote d'Ivoire | 60 | 7.2 yearsb | ANRS 1244, NFVa, 78%; EFVb, 22% | 21a |
|
| |||||
| Hagströmer et al. [8] (2013) | Ethiopia | A: 658 | 52% <6 years | Pediatric HIV Care: Hospital, NR | 37b |
|
| |||||
| B: 230 | 52% <6 years | Health Centre Clinics, NR | 26b | ||
|
| |||||
| Taye et al. [20] (2010) | Ethiopia | 475 | NR | Malnutrition and mortality study, NVPb | 12b |
|
| |||||
| Benki-Nugent et al. [21] (2014) | Kenya | 73 | 3.7 monthsb | OPH03, <5 months at ART, LPV/ra, 38%; NVPb, 62% | 6c |
|
| |||||
| McGrath et al. [22] (2011) | Kenya | 169 | 4.7 yearsb | Treatment-naive, NNRTI | 19b |
|
| |||||
| Song et al. [23] (2007) | Kenya | 29 | 8.5 yearsa | Treatment-naive, NVPb | 15c |
|
| |||||
| Wamalwa et al. [5] (2007) | Kenya | 52 | 4.4 yearsb | First-line ART, EFVb, 27%; NVPb, 69% | 9b |
|
| |||||
| Kim et al. [24] (2012) | Malawi | 55 | 1.6 yearsa | Nutritional therapy at ART, NVPb | 6c |
|
| |||||
| Weigel et al. [25] (2010) | Malawi | 419 | 8.0 yearsb | 77% severely immunosuppressed, NVPb | 23b |
|
| |||||
| Marazzi et al. [10] (2014) | 17 sites Malawi, Mozambique, Guinea |
2215 | 4 yearsb | DREAM Cohort, LPV/ra, 3%; NVPb, 97% | 16b |
|
| |||||
| Chhagan et al. [26] (2012) | South Africa | 151 | 5.1 yearsb | Pediatric ART programme, LPV/ra, 24%; NVPb, 76% | 24c |
|
| |||||
| Cotton et al. [27] (2013) | South Africa | A: 126 | 7.4 weeksb | CHER Trial: ART 40 weeks, LPV/ra | 57b |
|
| |||||
| B: 126 | 7.5 weeksb | ART 96 weeks, LPV/ra | 57b | ||
|
| |||||
| C: 125 | 7.1 weeksb | Deferred ART (not included), LPV/ra | 57b | ||
|
| |||||
| Coovadia et al. [28] (2010) | South Africa | 99 | 11 monthsb | NEVEREST: <2 years and NVP-exposed, LPV/ra (control) | 12c |
|
| |||||
| Davies et al. [29] (2009) | South Africa | 2966 | 3.6 yearsb | IeDEA, treatment-naïve, LPV/ra, 52%; EVFb, 29% | 36c |
|
| |||||
| Eley et al. [30] (2006) | South Africa | 409 | 1.9 yearsb | 63% severe clinical disease, protease inhibitor, 51%; NNRTI, 49% | 12c |
|
| |||||
| Jaspan et al. [31] (2008) | South Africa | A: 126 | 2.2 yearsb | LPV/ra or RTVa | 24c |
|
| |||||
| B: 146 | 2.2 yearsb | NVPb or EFVb | 24c | ||
|
| |||||
| Kruger et al. [32] (2013) | South Africa | 53 | 6.8 yearsa | Dietary iron intake and iron status, EFVb | 18c |
|
| |||||
| Meyers et al. [33] (2011) | South Africa | 1734 | 4.3 yearsb | Large public clinic, LPV/ra <3 years at ART; NVPb ≥3 years | 17b |
|
| |||||
| Purchase et al. [34] (2012) | South Africa | 94 | 8.6 monthsb | <1 year at ART, LPV/ra, 79%; NVPb, 4% | 18c |
|
| |||||
| Reddi et al. [35] (2007) | South Africa | 151 | 5.7 yearsb | Pediatric programme, LPV/ra <3 years, 15%; EFVb ≥3 years, 66% |
8b |
|
| |||||
| Reitz et al. [36] (2010) | South Africa | 254 | 8.8 monthsb | ART Strategy Trial, LPV/ra, 72%; RTVa, 28% | 9c |
|
| |||||
| Mwiru et al. [37] (2014) | Tanzania | 2133 | 51% <6 years | Growth post-ART, NVPb <3 y; EFVb or NVPb ≥3 y | 17b |
|
| |||||
| Kabue et al. [38] (2008) | Uganda | 749 | 7.5 yearsa | Pediatric treatment programme, NR | 6a |
|
| |||||
| Kekitiinwa et al. [39] (2008) | Uganda | A: 853 | 7.6 yearsb | Mulago Cohort, EFVb or NVPb, 98% | 12c |
|
| |||||
| UK & Ireland | B: 436 | 5.0 yearsb | CHIPS, LPV/ra or NFVa, 29%; EFVb or NVPb, 63% | 12c | |
|
| |||||
| Barlow-Mosha et al. [40] (2012) | Uganda | 104 | 5.4 yearsb | Adult fixed-dose ART, NVPb (Triomune) | 22c |
|
| |||||
| Musiime et al. [41] (2012) | Uganda | A: 449 | 11.9 yearsa | Urban, EFVb or NVPb | 33b |
|
| |||||
| B: 499 | 11.4 yearsa | Rural, EFVb or NVPb | 33b | ||
|
| |||||
| Musoke et al. [42] (2010) | Uganda | 124 | 5.0 yearsb | Adult fixed-dose ART, NVPb (Triomune) | 11c |
|
| |||||
| Prendergast et al. [43] (2011) | Uganda & Zimbabwe |
1168 | 6.0 yearsb | ARROW Trial, EFVb or NVPb | 6c |
|
| |||||
| Bolton-Moore et al. [1] (2007) | Zambia | 1926 | 6.8 yearsb | Pediatric ART programme, EFVb, 11%; NVPb, 88% | 12b |
|
| |||||
| Sutcliffe et al. [44] (2011) | Zambia | 119 | 2.9 yearsb | 65% ART-naïve, EFVb or NVPb | 13b |
|
| |||||
| van Dijk et al. [45] (2013) | Zambia | A: 39 | 17.4 monthsb | EFVb-based ART and anti-TB treatment | 17b |
|
| |||||
| B: 58 | 20.2 monthsb | NVPb-based ART | 13b | ||
|
| |||||
| Devi et al. [46] (2011) | India | 49 | 6.2 yearsb | TB Research Centre, NR | 12c |
|
| |||||
| Kumarasamy et al. [47] (2009) | India | 67 | 6.3 yearsa | Treatment-naïve, EFVb, 58%; NVPb, 21% | 12c |
|
| |||||
| Lodha et al. [48] (2005) | India | 26 | 5.7 yearsa | Tertiary care hospital, IDVa, 3%; NVPb, 97% | 20a |
|
| |||||
| Parakh et al. [49] (2009) | India | 30 | 7 yearsb | First-line ART in treatment-naive, NVPb | 36c |
|
| |||||
| Palumbo et al. [50] (2010) | India & sub- Saharan Africa |
A: 82 | 0.7 yearsb | P1060 cohort 1 (prior NVP): LPV/ra | 22c |
|
| |||||
| B: 82 | 0.7 yearsb | NVPb | 22c | ||
|
| |||||
| Violari et al. [51] (2012) | India & sub- Saharan Africa |
A: 140 | 1.7 yearsb | P1060 (no prior NVP): LPV/ra | 16b |
|
| |||||
| B: 147 | 1.8 yearsb | NVPb | 16b | ||
|
| |||||
| Isaakidis et al. [52] (2010) | Cambodia | 220 | 6 yearsb | Long-term ART outcomes, NVPb | 24b |
|
| |||||
| Janssens et al. [53] (2007) | Cambodia | 212 | 6.0 yearsb | Split fixed-dose combination, NVPb | 17b |
|
| |||||
| Sophan et al. [54] (2010) | Cambodia | 23 | 5.5 yearsb | Modified directly observed therapy, NVPb | 18c |
|
| |||||
| Zhang et al. [55] (2007) | China | A: 51 | 10 yearsb | ART-naive, NVPb | 12c |
|
| |||||
| B: 32 | 12 yearsb | ART-experienced, NVPb | 12c | ||
|
| |||||
| Zhao et al. [56] (2013) | China | A: 302 | 2.0 yearsb | National data: <36 months at ART, EFVb, 8%; NVPb, 92% | 24b |
|
| |||||
| B: 366 | 4.1 yearsb | 36-59 months at ART, EFVb, 30%; NVPb, 70% | 24b | ||
|
| |||||
| C: 1150 | 8.8 yearsb | >59 months at ART, EFVb, 28%; NVPb, 72% | 24b | ||
|
| |||||
| Aurpibul et al. [57] (2009) | Thailand | 225 | 7.2 yearsa | Growth post-ART, EFVb: 62%; NVPb: 38% | 50b |
|
| |||||
| Bunupuradah et al. [58] (2011) | Thailand | 107 | 6.2 yearsb | NNRTI study, EFVb: 30%; NVPb: 70% | 22c |
|
| |||||
| Hansudewechakul et al. [59] (2012) | Thailand | 410 | 8.6 yearsb | Community-based pediatric HIV care network, EFVb: 13%; NVPb: 83%; PI: 4% |
28b |
|
| |||||
| Phongsamart et al. [60] (2014) | Thailand | 1139 | 7.1 yearsb | Pediatric database, PI: 4%; NNRTI: 74% | 35b |
|
| |||||
| Puthanakit et al. [61] (2009) | Thailand | 26 | 9.8 monthsb | ≤2 years at ART, NVPb | 22c |
|
| |||||
| Puthanakit et al. [62] (2012) | Thailand & Cambodia |
A: 149 | 6.4 yearsb | PREDICT Trial: Early ART, LPV/ra, 5%; NVPb, 94% | 33c |
|
| |||||
| B: 150 | 6.5 yearsb | Deferred ART (not included), LPV/ra, 2%; NVPb, 41% | 33c | ||
|
| |||||
| Hansudewechakul et al. [63] (2010) |
5 Asian countries |
1189 | 7.0 yearsb | TApHOD: PI, 6%; NVPb, 93% | 35b |
|
| |||||
| George et al. [64] (2007) | Haiti | 163 | 6.3 yearsb | GHESKIO Centers, treatment-naïve, EFVb, 68%; NVPb, 24% | 20b |
|
| |||||
| Pierre et al. [65] (2008) | Jamaica | 197 | 5.0 yearsb | 38% Treatment-naïve, NVPb, 85% | 23b |
|
| |||||
| Diniz et al. [66] (2011) | Brazil | 196 | 3.4 yearsb | Treatment-naïve, NFVa or LPV/ra, 68%; NNRTI, 32% | 33c |
|
| |||||
| DEVELOPED SETTINGS (n=13) | |||||
|
| |||||
| Babiker et al. [67] (2011) | Europe, N. & S. America |
A: 131 | 7.1 yearsb | PENPACT 1 Trial: LPV/ra or NFVa | 48c |
|
| |||||
| B: 132 | 6.4 yearsb | EFVb or NVPb | 48c | ||
|
| |||||
| Aboulker et al. [68] (2004) | 5 European sites |
20 | 2.5 monthsb | PENTA 7: <3 months at ART, NFVa | 22b |
|
| |||||
| Buchacz et al. [69] (2001) | US | 544 | 3 months to 18 years |
PACTG 219: NFVa, 43%; RTVa, 38%; IDVa, 11%; SQVa 11% | 24a |
|
| |||||
| Chadwick et al. [70] (2011) | 17 sites US & Brazil |
21 | <6 months | IMPAACT-P1030: Cohort 2, LPV/ra | 28b |
|
| |||||
| Dreimane et al. [71] (2001) | US | 27 | 6.5 yearsa | Protease inhibitor added to ART, NR | 20a |
|
| |||||
| Miller et al. [72] (2001) | US | 45 | 6.8 yearsa | NFVa or RTVa or IDVa or SQVa | 5b |
|
| |||||
| Nachman et al. [73] (2005) | US | 192 | 4 months to 17 years |
PACTG 377, RTVa, 21%; NFVa, 79% | 22c |
|
| |||||
| Faye et al. [74] (2002) | France | 31 | 3.7 monthsb | French Perinatal Cohort, RTVa, 32%; NFVa, 68% | 24c |
|
| |||||
| Thuret et al. [75] (1999) | France | 22 | 6.6 yearsb | Protease inhibitor study, RTVa | 15b |
|
| |||||
| Scherpbier et al. [76] (2007) | Netherlands | 36 | 6.6 yearsb | NNRTI-based ART, EFVb | 16b |
|
| |||||
| van Rossum et al. [77] (2003) | Netherlands | 27 | 5.5 yearsb | Growth recovery, IDVa, 89%; NFVa, 11% | 11c |
|
| |||||
| Verweel et al. [78] (2002) | Netherlands | 24 | 5.2 yearsb | Growth and ART, IDVa, 83%; NFVa, 17% | 22c |
|
| |||||
| Guillen et al. [79] (2007) | Spain | 122 | 6.0 yearsb | 39% treatment-naive, NR | 71b |
N is the total number of children initiating ART with at least 1 anthropometric measure and not always representative of the cohort with follow up growth measures. ANRS, French National Agency for AIDS Research; ARROW, Antiretroviral Research for Watoto; ART, antiretroviral therapy; CHER, Children with HIV Early Antiretroviral; CHIPS, Collaborative HIV Paediatric Study; DREAM, Drug Resource Enhancement against AIDS and Malnutrition; EFV, efavirenz; IeDEA, International Epidemiologic Databases to Evaluate AIDS; IDV, indinavir; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group; LPV/r, lopinavir/ritonavir; NEVEREST, Nevirapine Resistance Study; NFV, nelfinavir; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NR, not reported; NVP, nevirapine; OPH03, Optimizing Pediatric HAART 03; PENTA, Paediatric European Network for Treatment of AIDS; PREDICT, Pediatric Randomized Early vs. Deferred Initiation in Cambodia and Thailand; RLS, resource-limited settings; RTV, ritonavir; SQV, saquinavir; TApHOD, TREAT Asia Pediatric HIV Observational Database; TB, tuberculosis.
Baseline Mean Age and Growth
The mean baseline age at ART initiation was 5.8 years (range, 0.1 to 16 years) in RLS and 6.6 years (range, 0 to 18 years) in DS (P=0.90) (Supplemental Table 1). Baseline growth parameters were well below average (Z-score=0) in RLS children initiating ART. Mean baseline WAZ was −2.1 in RLS compared to −0.5 in DS (P<0.01). Mean WHZ was lower in RLS versus DS (−1.5 and 0.3, respectively, P=0.05). Mean HAZ was −2.2 in RLS versus −0.9 in DS (P<0.01).
Change in WAZ at 6, 12 and 24-Months post-ART
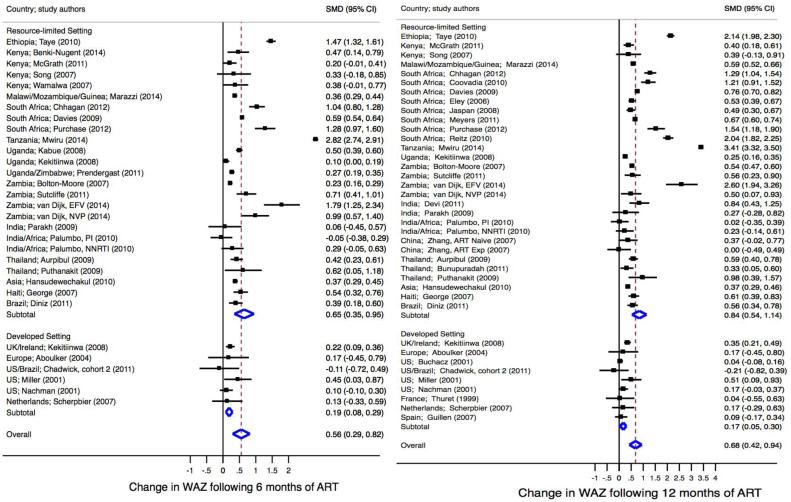
Twenty-seven studies (RLS=33, N=16,841; and DS=6, N=713) reported WAZ at 6-months post-ART (Table 2). Gains in WAZ ranged from 0.01 to 2.19 in RLS and −0.10 to 0.51 in DS. Twenty-nine studies (RLS=23, N=14,032; and DS=6, N=713) with data on mean or median WAZ were included in the estimate of the pooled mean difference (Figure 2a). At 6-months post-ART, children in RLS gained 0.65 (95% CI 0.35-0.95) in WAZ compared to a gain of 0.19 (95% CI 0.08-0.29) in children in DS (P=0.095). After adjusting for cohort age, the 6-monthly rate of increase in WAZ did not differ between RLS and DS (0.32 SD; 95% CI −0.09-0.73; P=0.12).
Table 2.
Change in growth from baseline at 6, 12, and 24-months following ART in HIV-infected children in resource-limited and developed settings.
| N | Change in WAZ at months | Change in HAZ at months | WHZ at months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Country (study authors) | base | 6 | 12 | 24 | base | 6 | 12 | 24 | base | 6 | 12 | 24 | |
| RLS (n=44) | |||||||||||||
|
| |||||||||||||
| Burkino Faso (Nacro et al. [18]) | 51 | −2.01a | - | 0.63a | - | −2.12a | - | 0.57a | - | - | - | - | - |
|
| |||||||||||||
| Cote d’Ivoire (De Beaudrap et al. [19]) | 177 | −2.37a | - | 0.61%a | - | −2.07a | - | 0.38%a | - | - | - | - | - |
|
| |||||||||||||
| Cote d'Ivoire (Fassinou et al. [2]) | 60 | −2.02a | - | - | 0.63a,c | −2.03a | - | - | 0.20a,c | - | - | - | - |
|
| |||||||||||||
| Ethiopia (Hagströmer et al. [8]) | |||||||||||||
|
| |||||||||||||
| Hospital | 658 | −2.50b | 0.65b | 0.90b | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Health Centers | 230 | −2.50b | 0.60b | 0.80b | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Ethiopia (Taye et al. [20]) | 475 | −2.40b | 0.60b | 0.80b | 1.00b | −2.10b | −0.11b | 0.00b | 0.10b | −0.99b | 0.90b | 1.26b | 1.48b |
|
| |||||||||||||
| Kenya (Benki-Nugent et al. [21]) | 73 | −2.00b | 0.70b | - | - | −1.90b | −0.20b | - | - | −0.60b | 0.00b | - | - |
|
| |||||||||||||
| Kenya (McGrath et al. [22]) | 169 | −1.98a | 0.30d | 0.60d | 1.20d | −2.09a | 0.18d | 0.36d | 0.72d | −0.96a | 0.36d | 0.72d | 1.44d |
|
| |||||||||||||
| Kenya (Song et al. [23]) | 29 | −1.61a | 0.42a | 0.49a | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Kenya (Wamalwa et al. [5]) | 52 | −2.30b | 0.63b | - | - | −2.54b | 0.37 b | - | - | - | - | - | - |
|
| |||||||||||||
| Malawi (Kim et al. [80]) | 55 | - | - | - | - | −3.60a | - | - | - | −1.49a | 1.80a | - | - |
|
| |||||||||||||
| Malawi (Weigel et al. [25]) | 419 | −2.10b | - | - | 0.70b | −2.60b | - | - | 0.80 b | - | - | - | - |
|
| |||||||||||||
| Malawi/Mozam/Guinea (Marazzi et al. [10]) | 1226 | −2.16b | 0.44a | 0.70a | - | −2.58b | 0.07a | 0.09a | - | −0.74b | 0.57a | 0.74a | - |
|
| |||||||||||||
| South Africa (Chhagan et al. [26]) | 151 | −1.26b | 0.22d | 0.43d | 0.86d | −2.05b | 0.34d | 0.69d | 1.37d | - | - | - | - |
|
| |||||||||||||
| South Africa (Coovadia et al. [28]) | 99 | −2.23a | - | 1.84a,c | - | −3.14a | - | - | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Cotton et al. [27]) | |||||||||||||
|
| |||||||||||||
| 40 weeks | 126 | −0.80b | - | - | 0.47: 4.8 years |
- | - | - | - | - | - | - | - |
|
| |||||||||||||
| 96 weeks | 126 | −0.70b | - | - | 0.02: 4.8 years |
- | - | - | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Davies et al. [29]) | 2966 | −1.81b | 0.81b | 1.06b | 1.08b | −2.34b | 0.28b | 0.47b | 0.80b | −0.39b | 0.97b | 1.13b | 0.91b |
|
| |||||||||||||
| South Africa (Eley et al. [30]) | 409 | −2.17b | - | 1.24b | - | −2.51b | - | 0.59b | - | −0.63b | - | 1.06b | - |
|
| |||||||||||||
| South Africa (Jaspan et al. [31]) | |||||||||||||
|
| |||||||||||||
| Protease inhibitor | 126 | −2.80b | - | 1.70b | 2.20b | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| NNRTI | 146 | −2.40b | - | 1.57b | 1.70b | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Kruger et al. [32]) | 53 | - | - | - | - | −1.70a | 0.00a | 0.20a | - | −0.40a | 0.30a | 0.00a | - |
|
| |||||||||||||
| South Africa (Meyers et al. [33]) | 1734 | −2.40a | - | 1.00a | - | −2.69a | - | 0.43a | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Purchase et al. [34]) | 94 | −2.70a | 2.19a | 2.65a | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Reddi et al. [35]) | 151 | −1.90b | - | 1.00b,c | - | −2.20b | - | 0.40b,c | - | - | - | - | - |
|
| |||||||||||||
| South Africa (Reitz et al. [36]) | 254 | −2.38a | - | 3.20a,c | - | −3.45a | - | - | - | - | - | - | - |
|
| |||||||||||||
| Tanzania (Mwiru et al. [37]) | 2133 | −2.60a | 0.80a | 0.92a | 1.12a | −2.19a | 0.02a | 0.09a | 0.17a | −1.78a | 0.80a | 0.97a | 1.11a |
|
| |||||||||||||
| Uganda (Barlow-Mosha et al. [40]) | 104 | −1.20a | - | 1.48a,c | 2.14a,c | −1.96a | - | 1.55a,c | 2.81a,c | ||||
|
| |||||||||||||
| Uganda (Kabue et al. [38]) | 749 | −3.20a | 1.10a | - | - | −2.70a | 0.30a | - | - | −1.50a | 1.30a | - | - |
|
| |||||||||||||
| Uganda (Kekitiinwa et al. [39]) | 853 | −2.80b | 0.19b | 0.49b | - | −2.85b | −0.11b | 0.06b | - | - | - | - | - |
|
| |||||||||||||
| Uganda (Musiime et al. [41]) | |||||||||||||
|
| |||||||||||||
| Urban | 449 | −4.90a | - | - | 3.4: 2.8 years |
−7.30a | - | - | 5.0: 2.8 years |
- | - | - | - |
|
| |||||||||||||
| Rural | 499 | −4.60a | - | - | 4.2: 2.8 years |
−5.70a | - | - | 5.1: 2.8 years |
- | - | - | - |
|
| |||||||||||||
| Uganda (Musoke et al. [42]) | 124 | −1.14a | - | 0.54a,c | - | −2.06b | - | 1.65a,c | - | - | - | - | - |
|
| |||||||||||||
| Uganda/Zimbab (Prendergast et al. [43]) | 1168 | −2.10b | 0.40b | - | - | −2.40b | 0.00b | - | - | −0.50b | - | - | - |
|
| |||||||||||||
| Zambia (Bolton-Moore et al. [1]) | 1926 | −2.20a | 0.40a | 0.60a | 0.70a | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Zambia (Sutcliffe et al. [44]) | 119 | −2.40a | 1.10a | 0.90a | 0.70a | −3.50a | 0.40a | 0.90a | 1.40a | - | - | - | - |
|
| |||||||||||||
| Zambia (van Dijk et al. [45]) | |||||||||||||
|
| |||||||||||||
| EFV | 39 | −2.60a | 1.30a | 1.70a | 2.20a | −2.30b | 0.12a | 0.25a | - | - | - | - | - |
|
| |||||||||||||
| NVP | 58 | −1.40b | 0.60a | 0.30a | 0.70a | −2.30b | 0.21a | 0.43a | - | - | - | - | - |
|
| |||||||||||||
| India (Devi et al. [46]) | 49 | −2.84b | - | 0.66b | - | −2.02b | - | −0.25b | - | −2.41b | - | 1.33b | - |
|
| |||||||||||||
| India (Kumarasamy et al. [47]) | 67 | - | - | - | - | - | - | - | - | 0.53a | - | 0.05a | - |
|
| |||||||||||||
| India (Lodha et al. [48]) | 26 | −2.46b | - | - | 0.70b,c | −2.48b | - | - | 1.49b,c | −1.01b | - | - | 1.01b,c |
|
| |||||||||||||
| India (Parakh et al. [49]) | 30 | −1.98b | 0.12b | 0.55b | 0.16b | −1.75b | −0.36b | −0.30b | −0.86b | - | - | - | - |
|
| |||||||||||||
| India/Africa (Palumbo et al. [50]) | - | - | - | - | |||||||||
|
| |||||||||||||
| Protease inhibitor | 82 | −1.10b | 0.01b | 0.01b | 0.00b,e | −1.00b | −0.20b | 0.10b | 0.30b,e | - | - | - | - |
|
| |||||||||||||
| NNRTI | 82 | −1.30b | 0.30b | 0.70b | 1.40b,e | −1.50b | 0.30b | 0.40b | 0.60b,e | - | - | - | - |
|
| |||||||||||||
| India/Africa (Violari et al. [51]) | - | - | - | - | |||||||||
|
| |||||||||||||
| Protease inhibitor | 140 | −2.70b | 0.78a | 1.04a | - | −2.30b | 0.12a | 0.25a | - | - | - | - | - |
|
| |||||||||||||
| NNRTI | 147 | −2.60b | 1.03a | 1.36a | - | −2.30b | 0.21a | 0.43a | - | - | - | - | - |
|
| |||||||||||||
| Cambodia (Isaakidis et al. [52]) | 220 | −2.65b | - | - | 1.88b | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Cambodia (Janssens et al. [53]) | 212 | - | - | - | - | - | - | - | - | −1.59a | - | 0.81a | - |
|
| |||||||||||||
| Cambodia (Sophan et al. [54]) | 23 | −2.97a | 0.45b | 0.60b | 0.78b,c | −3.32a | 0.10b | 0.60b | 0.48b,c | −1.57a | - | - | 0.64b,c |
|
| |||||||||||||
| China (Zhang et al. [55]) | |||||||||||||
|
| |||||||||||||
| ART naive | 51 | −1.90b | - | 0.30b | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| ART experienced | 32 | −1.90b | - | 0.00b | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| China (Zhao et al. [56]) | - | - | - | - | |||||||||
|
| |||||||||||||
| <36 months at ART | 302 | −1.50a | 0.70a | 1.00a | 1.00a | −1.90a | 0.00a | 0.30a | 0.70a | - | - | - | - |
|
| |||||||||||||
| 36-59 months at ART | 366 | −1.20a | 0.32a | 0.30a | 0.38a | −1.97a | 0.19a | 0.17a | 0.44a | - | - | - | - |
|
| |||||||||||||
| >59 months at ART | 1150 | −1.40b | 0.20a | 0.30a | 0.35a | −2.10b | 0.00a | 0.05a | 0.20a | - | - | - | - |
|
| |||||||||||||
| Thailand (Aurpibul et al. [57]) | 225 | −2.02a | 0.48a,c | 0.66a,c | 0.86a,c | −2.22a | −0.05a,c | 0.22a,c | 0.55a,c | - | - | - | - |
|
| |||||||||||||
| Thailand (Bunupuradah et al. [58]) | 107 | −1.50b | - | 0.40b,c | 0.30b,c | −1.70b | - | 0.00b,c | 0.30b,c | −0.50b | - | 0.40b,c | 0.20b,c |
|
| |||||||||||||
| Thailand (Hansudewechakul et al. [59]) | 410 | −1.90b | - | - | 0.50b | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Thailand (Phongsamart et al. [60]) | 1139 | −1.80b | - | - | 0.80: 6.4 years |
−1.80b | - | - | 0.70: 6.4 years |
- | - | - | - |
|
| |||||||||||||
| Thailand (Puthanakit et al. [61]) | 26 | −2.49a | 0.86a | 1.40a | 1.89a | −2.19a | −0.31a | 0.38a | 1.11a | - | - | - | - |
|
| |||||||||||||
| Thailand/Cambodia (Puthanakit et al. [81]) | 149 | −1.30b | - | - | - | −1.60b | −0.06a,c | 0.01a,c | 0.07a,c | - | - | - | - |
|
| |||||||||||||
| Asia (Hansudewechakul et al. [63]) | 1189 | −2.15b | 0.45b | 0.59b | 0.67b | −2.35b | 0.10b | 0.25b | 0.45b | - | - | - | - |
|
| |||||||||||||
| Haiti (George et al. [64]) | 163 | −2.00b | 0.60b | 0.70b | 0.80b | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Jamaica (Pierre et al. [65]) | 197 | −0.86a | - | - | 0.16a | −0.48a | - | - | −0.55a | −1.58a | - | - | 1.71a |
|
| |||||||||||||
| Brazil (Diniz et al. [66]) | 196 | −1.62a | 0.48a | 0.70a | 0.87a | −1.88a | 0.22a | 0.45a | 0.89a | - | - | - | - |
|
| |||||||||||||
| DEVELOPED SETTINGS (n=14) | |||||||||||||
|
| |||||||||||||
| Europe/America (Babiker et al. [67]) | |||||||||||||
|
| |||||||||||||
| Protease inhibitor | 131 | −0.80a | - | - | 0.53: 4 years |
−1.00a | - | - | 0.61: 4 years |
- | - | - | - |
|
| |||||||||||||
| NNRTI | 132 | −0.80a | - | - | 0.77: 4 years |
−1.00a | - | - | 0.74: 4 years |
- | - | - | - |
|
| |||||||||||||
| Europe (Aboulker et al. [68]) | 20 | −1.00b | 0.51b,c | 0.53b,c | - | −1.40b | 0.83b,c | 0.60b,c | - | - | - | - | - |
|
| |||||||||||||
| United States (Buchacz et al. [69]) | 544 | −0.40a | - | 0.05a | 0.11a | −0.90a | NR | −0.01a | 0.11a | - | - | - | - |
|
| |||||||||||||
| United States/Brazil (Chadwick et al. [70]) | 21 | −0.80b | −0.10b,c | −0.20b,c | 0.40b,c | 0.70b | 0.10b,c | 0.70b,c | 0.60b,c | - | - | - | - |
|
| |||||||||||||
| United States (Dreimane et al. [71]) | 27 | −0.59a | - | - | 0.18a,c | −1.05a | - | - | 0.26a,c | - | - | - | - |
|
| |||||||||||||
| United States (Miller et al. [72]) | 45 | −0.67a | 0.45a,c | 0.51a,c | - | −1.11a | 0.15a,c | 0.26a,c | - | 0.25a | 0.50a,c | 0.43a,c | - |
|
| |||||||||||||
| United States (Nachman et al. [73]) | 192 | −0.16a | 0.11a,c | 0.20a,c | 0.37a,c | −0.57a | 0.11a,c | 0.19a,c | 0.40a,c | - | - | - | - |
|
| |||||||||||||
| France (Faye et al. [74]) | 31 | −0.50b | - | - | 0.70b | −0.30b | - | - | 0.90b | - | - | - | - |
|
| |||||||||||||
| France (Thuret et al. [75]) | 22 | −0.77b | - | 0.04b | - | - | - | - | - | - | - | - | - |
|
| |||||||||||||
| Netherlands (Scherpbier et al. [76]) | 36 | −0.60a | 0.20a,c | 0.27a,c | 0.27a,c | −1.20a | 0.30a,c | 0.40a,c | 0.41a,c | 0.28a | 0.10a,c | 0.09a,c | 0.07a,c |
|
| |||||||||||||
| Netherlands (van Rossum et al. [77]) | 27 | - | - | - | - | −1.30b | 0.00b | 0.30b | - | - | - | - | - |
|
| |||||||||||||
| Netherlands (Verweel et al. [78]) | 24 | −0.74b | - | - | 0.34b | −1.22b | - | - | 0.20b | - | - | - | - |
|
| |||||||||||||
| Spain (Guillen et al. [79]) | 122 | −0.29a | - | 0.12a | 0.30a | −0.50a | - | 0.11a | 0.25a | - | - | - | - |
|
| |||||||||||||
| UK/Ireland (Kekitiinwa et al. [39]) | 436 | −0.60b | 0.26b | 0.41b | - | −0.82b | 0.08b | 0.20b | - | - | - | - | - |
Increases in WAZ, WHZ, and HAZ are reported in difference in mean or median values from baseline to 6, 12, and 24 months. ART, antiretroviral therapy; HAZ, height-for-age; RLS, resource-limited setting; WAZ, weight-for-age; WHZ, weight-for-height.
Mean.
Median.
Follow-up time varied slightly around 6, 12, and 24 months.
Estimates based on mean monthly change from longitudinal models.
<12 children at this time point.
Fig 2a. Standardized mean difference in WAZ at 6 months (left) and 12 months (right) post-antiretroviral therapy.
Meta-regression comparing SMD at 6 months in RLS vs. developed settings, P=0.12. Meta-regression comparing SMD at 12 months in RLS vs. developed settings, P=0.03. ART, antiretroviral therapy; RLS, resource-limited setting; SMD, standardized mean difference.
At 12-months post-ART, 45 studies (RLS=36, N=17,087; and DS=9, N=1,340) reported mean or median WAZ. Gains ranged from 0 to 3.20 in RLS and −0.20 to 0.53 in DS. Of the 36 studies (RLS=27, N=13,822; and DS=9, N=1,340) included in the pooled estimate (Figure 2a), children in RLS gained an average WAZ of 0.84 (95% CI 0.54-1.14) compared to 0.17 (95% CI 0.05-0.30) among children in DS (P=0.02). Twelve-month gains in WAZ remained greater in RLS than DS (0.56 SD; 95% CI 0.08-1.04; P=0.03) after adjusting for cohort age.
Thirty-four studies (RLS=26, N=11,104; and DS=8, N=789) reported WAZ at 24-months post-ART. Change in 24-month WAZ ranged from 0 to 2.20 in RLS and from 0.11 to 0.70 in DS. After pooling data from 29 studies (RLS=21, N=9,078; and DS=8, N=789), children in RLS experienced greater 24-month gains in WAZ as compared to children in DS (1.03, 95% CI 0.53-1.53; and 0.17, 95%CI 0.08-0.27; respectively, P=0.04) [data not shown]. After adjusting for cohort age, the 2-year rate of increase in WAZ remained higher in RLS (0.60 SD, 95% CI 0.03-1.17; P=0.04).
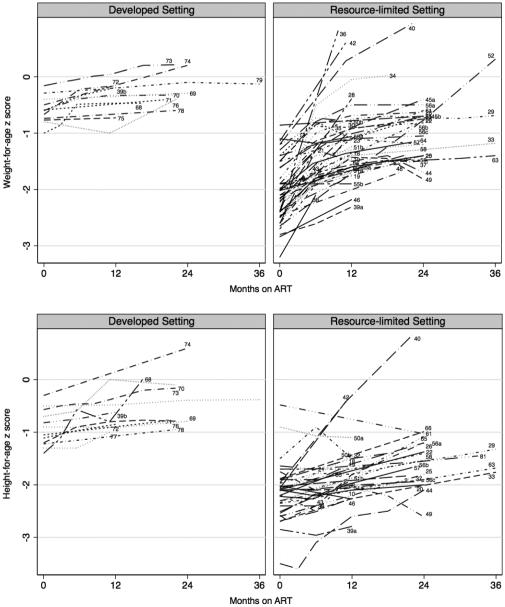
Figure 3 illustrates WAZ over time in 38 studies in RLS and 12 studies in DS.
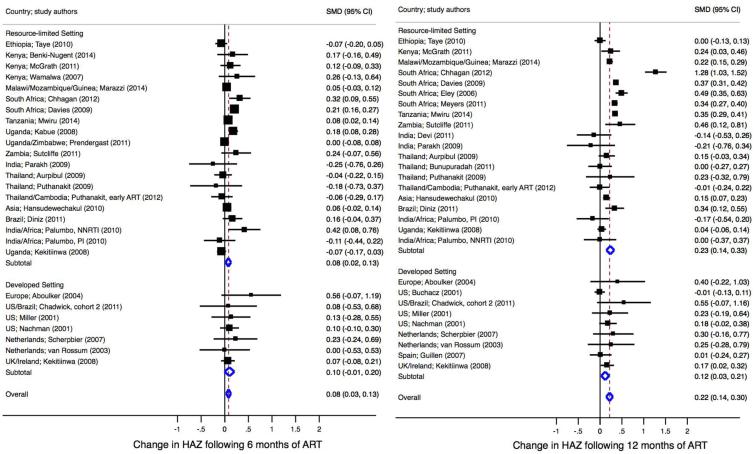
Fig 2b. Standardized mean difference in height-for-age at 6 months (left) and 12 months (right) post-antiretroviral therapy.
Meta-regression comparing SMD at 6 months in RLS vs. developed settings, p=0.62. Meta-regression comparing SMD at 12 months in RLS vs. developed settings, p=0.73. ART, antiretroviral therapy; RLS, resource-limited setting; SMD, standardized mean difference.
Change in HAZ at 6, 12 and 24-Months post-ART
Six-month post-ART growth was reported in 31 studies (RLS=24, N=13,693; and DS=7, N=681) [Table 2]. Change in 6-month HAZ ranged from −0.36 to 0.40 in RLS and from 0 to 0.83 in DS. Of the 26 studies (RLS=19, N=11,585; and DS=7, N=681) included in the pooled analysis (Figure 2b), children in RLS and DS experienced similar 6-month gains in height (0.08, 95% CI 0.02-0.13; and 0.10, 95% CI −0.01-0.20; respectively, P=0.62). After adjusting for cohort age, there was no difference in 6-month HAZ gains by setting.
Thirty-eight studies (RLS=29, N=13,603; and DS=9, N=1,295) reported HAZ at 12-months post-ART (Table 2). Change in HAZ ranged from −0.30 to 1.65 in RLS and −0.01 to 0.70 in DS. Twenty-eight studies were included in pooled analysis (RLS=19, N=10,930; and DS=9, N=1,295) [Figure 2b]. Although not significantly different, children in RLS experienced a mean 12-month gain in HAZ of 0.23 (95% CI 0.14-0.33) compared to a gain of 0.12 (95% CI 0.03-0.21) in children in DS (P=0.34). This remained after adjusting for age.
Twenty-nine studies (RLS=21, N=8,068; and DS=8, N=788) reported HAZ at 24-months post-ART (Table 2). Change in 24-month HAZ ranged from −0.86 to 2.81 in RLS and from 0.11 to 0.90 in DS. Twenty-three studies (RLS=15, N=3,904; and DS=8, N=788) were included in the pooled analysis at 24-months (data not shown). There was no significant difference in gains in HAZ following 24-months of ART between RLS and DS (0.41, 95%CI 0.22-0.60; and 0.18, 95% CI 0.08-0.28; respectively, P=0.42).
Figure 3 illustrates HAZ over time in 29 RLS studies and 12 DS studies.
Change in WHZ at 6, 12 and 24 Months post-ART
Few studies reported post-ART WHZ outcomes. Eleven studies included data on WHZ at 6-months (RLS=9, N=3,361; and DS=2, N=81), 13 studies included data at 12-months (RLS=11, N=3,089; and DS=2, N=81), and 9 studies at 24-months (RLS=8, N=1,750; and DS=1, N=36). Change in WHZ during 24-month follow-up ranged from 0 to 1.71 in RLS and 0.07 to 0.50 in DS [Table 2]. Pooled summary statistics were not performed due to the small number of studies reporting mean WHZ post-ART.
Potential Cofactors for Growth Reconstitution
Age at ART Initiation
Eleven studies reported data regarding associations between age at initiation and post-ART growth [1, 18-28]. Nine of these (RLS=7 and DS=2) reported an association between younger age at ART initiation and greater improvements in WAZ [1, 18-20, 22, 24-27] and HAZ [19, 20, 22, 25-28]. The remaining two studies (Thailand [21] and Malawi [23]) found no difference in growth and age at ART initiation. Adjusting for setting, older median cohort age was significantly associated with lower rate of change in weight (−0.07 SD per 1 year increase in age; 95% CI −0.12, −0.01; P=0.02) 12-months post-ART. Further, cohorts with median age <4 years at ART had greater rate of change in yearly WAZ as compared to cohorts aged >6 years at ART (0.52 SD; 95% CI 0.05-0.99; P=0.03). Similarly, younger cohorts (<4 years) had higher 6-month HAZ increase compared to older cohorts (>6 years) (0.15 SD, 95% CI 0.01-0.30; P=0.04), after adjusting for setting. The relationship between younger cohort age and greater improvements in HAZ did not persist beyond 6-months post-ART.
Nutrition Supplementation and ART
Information on nutrition supplementation was reported in fourteen studies, all in RLS. Types of supplementation included: rice/corn and vegetable oil (Haiti [29]), high-energy protein (Zambia [24]), Plumpy’nut (Malawi [23]), other ready-to-use therapeutic food (Malawi [30]), nutritional porridge (Kenya [18, 31]), corn-soy blend ready-to-eat meal supplement (South Africa [32]), fortified amylase-enriched maize product (South Africa [28]), food supplementation or support (Malawi/Mozambique/Guinea [10], Ethiopia [8], Cambodia [33]), and multivitamins (Kenya [18], South Africa [32], Uganda [34], Uganda/Zimbabwe [35], and India [36]). One (Kenya [18]) study evaluated receipt and duration of nutritional supplementation in growth analyses, while a second (Malawi [30]) evaluated 6-month nutritional recovery in malnourished children initiating ART with ready-to-use therapeutic food. A third (South Africa [32]) evaluated dietary iron intake and changes in hemoglobin 18-months post-ART among children receiving iron-free multivitamins. Specific eligibility criteria for nutritional support were generally not defined. Two studies (US [37] and Kenya [5]) explicitly stated that children received no supplements. After adjusting for cohort age and setting, receipt of nutrition supplements was significantly associated with greater height gains at 12- and 24-months post-ART (0.38, 95% CI, 0.02, 0.74, P=0.04; and 0.60, 95% CI, 0.20, 1.01, P=0.007; respectively); this difference did not remain significant after adjusting for baseline cohort length (0.19, 95% CI −0.19-0.57, P=0.31; and 0.36, 95% CI −0.12-0.83, P=0.13; respectively). Similarly, there was better weight gain at 24-months in studies with supplements than without supplements (0.55, 95% CI 0.06-1.03, P=0.03), however, this relationship was not as strong after adjusting for baseline cohort weight (0.45, 95% CI −0.03-0.93, P=0.06).
ART Regimen
Nine studies reported post-ART growth comparisons by regimen. Four of these (RLS=3 and DS=1) compared growth among children initiating protease inhibitor (PI) or non-nucleoside reverse-transcriptase inhibitor (NNRTI) based regimen. Two trials (Africa/India [38] and Europe/Americas [39]) reported significantly greater weight gains in nevirapine-naïve children randomized to NNRTI versus PI based regimens. Conversely, an observational study in Brazil [40] reported greater weight gains in children initiating PI-based ART. After adjusting for cohort age and setting, rate of weight gain did not differ between studies reporting NNRTI versus PI-containing regimens (6 mos: 0.13, 95% CI −0.48-0.73, P=0.67; 12 mos: −0.31, 95%CI, −0.98-0.36, P=0.35); similar associations remained after adjusting for baseline cohort weight. Similarly, there was no difference in height velocity by regimen in adjusted analyses (6 mos: 0.19, 95% CI −0.08-0.46, P=0.15; 12 mos: 0.11, 95% CI −0.19-0.40, P=0.44).
Discussion
In this systematic review and meta-analysis of 6, 12, and 24-month growth outcomes in HIV-infected children initiating ART, children in RLS had markedly lower weight and height at ART initiation compared to children in DS. Following ART, children in both settings experienced rapid improvements in weight and height. While the greatest gains in weight were observed during the first 6-months of therapy, gains in height were more modest and occurred later. Children in RLS had significant improvements in WAZ and HAZ at 6 and 12-months post-ART with higher rates of weight gain at 12 and 24-months post-ART compared to children in DS. Despite substantial growth reconstitution following ART, children in RLS continued to be an average of 1 SD below that of children in DS at 12-months post-ART due to baseline differences that never recovered.
Younger age at ART initiation was associated with greater gains in growth in nine studies, irrespective of setting. The most marked improvements in weight and height velocity were observed in children initiating ART prior to three years of age [1, 18-20, 22]. In the two studies reporting no difference in growth based on age at ART, the first consisted of few children less than 2 years of age [21], and the second reported high loss to follow-up rate and missing data [23]. While older children do experience post-ART catch-up growth, they do not reconstitute as rapidly as younger children and they may never reach population age-norms, particularly in height. These results emphasize the need for early identification and treatment of HIV in pediatric populations, as early ART initiation would be expected to avoid further growth compromise.
A few studies provided information on nutritional supplements, all from RLS [5, 8, 10, 18, 23, 24, 29-32, 34, 37]. While many ART programs in RLS provide nutritional supplements during ART, most studies did not clearly specify eligibility criteria or duration of supplementation, and only two evaluated the impact of supplements on growth. A Kenyan study reported greater post-ART weight gain in children receiving food supplements, and greater gains in height with multivitamins [18]. In a South African study of dietary iron intake, post-ART height gains were associated with higher hemoglobin levels [32]. In other studies mentioning nutritional support [23, 24, 29], the greatest gains in weight were among children with the lowest baseline WAZ at ART initiation. In the absence of HIV, catch-up growth to population norms following malnutrition depends on severity, duration, and age at onset [41]. International adoptees experience remarkable catch-up growth, with younger adoptees gaining as much as 2 SD in weight and height [42]. In Malawi, 80% of children recovered from moderate acute malnutrition after treatment with ready-to-use therapeutic supplementary food [43]. While food supplements as an adjunct to ART may accelerate weight recovery, it is unclear whether ART alone would produce the same rebound in a slightly longer timeframe or if underlying poverty contributes to the persistence of undernutrition. Our pooled analysis suggests greater 12 and 24-month height and 24-month weight gains in cohorts using supplements, however lack of direct comparison of growth outcomes by timing and supplement type make it difficult to interpret these results. Further research is needed to determine effectiveness and optimal supplement regimens as adjuncts to ART.
Prior studies have yielded inconclusive data on the influence of ART regimen on growth reconstitution. The IMPAACT P1060 trial, undertaken in sub-Saharan Africa and India, reported better weight gains in the NVP group compared to LPV/r group [38]. However, this difference did not remain after 1 year [44]. Conversely, the PENPACT trial in Europe and North and South America showed greater weight gain in the PI group [45]. Similarly, a large observational study reported better weight and height growth among children initiating PI- versus NNRTI-based regimens [46]. In practice, younger children are preferentially provided LPV/r-based regimens to reduce the likelihood of NVP resistance among those exposed to PMTCT, which may confound ability to determine role of regimen in growth reconstitution. Despite concerns regarding LPV/r’s poor palatability which could compromise adherence [27, 28], a recent Cochrane review [47] concluded that LPV/r was a more efficacious first-line regimen than NVP among younger children. The relationship between post-ART growth gains and immune and viral response is not well understood. Post-ART gains in weight have been shown to correlate with improvements in CD4 and viral load [48, 49]; conversely, other studies have shown no association between post-ART virologic response and growth recovery suggesting other factors are required to promote growth [22, 50, 51]. We found no difference in growth response between studies reporting PI- and NNRTI-based regimens, and many studies lacked statistical power to compare growth outcomes by regimen.
This study has limitations inherent to systematic reviews and meta-analyses. Retention varied across studies. Children no longer in care may have been more likely to have died or worse growth outcomes than those in care. This would lead to overestimating growth reconstitution following ART. The studies included older children (mean age 7 years) and could be affected by survivor bias. Studies of younger children may have greater post-ART growth recovery compared to older cohorts. However, there was no difference in mean age at baseline between RLS and DS. This analysis excluded some studies reporting growth in weight and length rather than Z-scores. However, most studies included Z-scores and we believe exclusion of studies without Z-scores does not introduce substantial bias into our estimates of pooled changes in growth. Lastly, a few studies included children with prior mono/dual therapy. Compared to treatment-naïve children initiating ART, children with prior treatment may have a slower rate of post-ART growth.
This is the first systematic review of post-ART growth outcomes in children in RLS and DS. Previous reviews of pediatric post-ART outcomes have included only baseline growth measures [14] or provided limited data on follow-up growth in sub-Saharan Africa or RLS [3, 12, 13]. We expanded upon this by systematically screening, evaluating, and selecting studies including post-ART growth outcomes irrespective of study setting. Meta-analysis techniques were then used to provide standardized summary statistics of change in growth at 6, 12, and 24-months post-ART; affording a unique opportunity to compare the rate of change in weight and height in children receiving ART across studies in RLS and DS.
While post-ART rates of growth reconstitution in RLS and DS were comparable or higher in RLS, children in RLS had continued marked lower growth at 12- and 24-months post-ART. Projecting from 2-year growth reconstitution rates, if rates persisted at similar or lower reconstitution, it may take 4-5 years to reach population norms for WAZ while HAZ is likely to never fully recover. Few studies reported data on nutritional supplementation, making it difficult to assess the potential benefits of supplementation. Current programs often provide empiric nutritional supplementation to children starting ART, but it is not clear if this is beneficial because ART alone results in substantial growth reconstitution. Despite empiric nutritional supplementation in pediatric ART programs evidence of the effectiveness of nutritional therapy on growth and morbidity in children on ART is lacking. Improvements in programmatic data regarding nutritional supplements can help inform policies to optimize nutritional therapy for growth reconstitution in children on ART. Our study demonstrates marked persistent growth compromise in ART-treated children. Earlier diagnosis and treatment of children and further data regarding the role of supplementation will be important to enhance growth in these children.
Supplementary Material
Fig 3. Change in weight-for-age (top) and height-for-age (bottom) following initiation of antiretroviral therapy.
Numbers correspond to articles as listed in the References. ART, antiretroviral therapy.
Acknowledgments
Sources of support: C.J.M. was supported by the University of Washington STD/AIDS Research Training Fellowship (NIH NRSA T32AI007140) and NIH research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program – BIRCWH) during manuscript preparation. Support for G.J.S. includes a NIH K24 grant (HD054314) and the University of Washington (UW) Global Center for Integrated Health of Women Adolescents and Children (Global WACh). Lastly, support by the NIH funded program, UW Center for AIDS Research (CFAR) (P30 AI027757).
Abbreviations
- WAZ
weight-for-age Z-score
- WHZ
weight-for-height Z-score
- HAZ
height-for-age Z-score
- HAART
highly active antiretroviral therapy
- ART
antiretroviral therapy
- DS
developed setting
- RLS
resource-limited setting
Footnotes
Conflicts of interest: There are no conflicts of interest.
Author’s Contributions: C.J.M., G.J.S., B.A.R. were involved in the inception of the research question, study design, and data analysis. C.J.M. and L.D. conducted the literature search and data abstraction. C.J.M., L.D., B.A.R., E.P.C., and G.J.S. were involved in data interpretation, editing, and revision of the paper. All authors have read and approved the text as submitted to AIDS.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent official views of the United States National Institutes of Health (NIH).
References
- 1.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 2.Fassinou P, Elenga N, Rouet F, Laguide R, Kouakoussui KA, Timite M, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d'Ivoire. AIDS. 2004;18:1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 3.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 4.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 5.Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45:311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS . Global report: UNAIDS report on the global AIDS epidemic 2012. In: UNAIDS, editor. JUNPoHA. Geneva, Switzerland: 2012. [Google Scholar]
- 7.Wagner A, Slyker J, Langat A, Inwani I, Adhiambo J, Benki-Nugent S, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:325. doi: 10.1186/s12887-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagstromer O, Lundstedt L, Balcha TT, Bjorkman P. Decentralised paediatric HIV care in Ethiopia: a comparison between outcomes of patients managed in health centres and in a hospital clinic. Glob Health Action. 2013;6:22274. doi: 10.3402/gha.v6i0.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Zhao D. Clinical, immunological, and virological outcomes of pediatric antiretroviral therapy in central China. BMC Res Notes. 2014;7:419. doi: 10.1186/1756-0500-7-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marazzi MC, De Luca S, Palombi L, Scarcella P, Ciccacci F, Ceffa S, et al. Predictors of adverse outcomes in HIV-1-infected children receiving combination antiretroviral treatment: results from a DREAM cohort in sub-Saharan Africa. Pediatr Infect Dis J. 2014;33:295–300. doi: 10.1097/INF.0b013e3182a0994b. [DOI] [PubMed] [Google Scholar]
- 11.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 12.Davies MA, Egger M, Keiser O, Boulle A. Paediatric antiretroviral treatment programmes in sub-Saharan Africa: a review of published clinical studies. Afr J AIDS Res. 2009;8:329–338. doi: 10.2989/AJAR.2009.8.3.9.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciaranello AL, Chang Y, Margulis AV, Bernstein A, Bassett IV, Losina E, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:e423–441. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Nations Statistics Division Composition of macro geographical regions, geographical sub-regions, and selected economic and other groupings. 2011 [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath CJ, Chung MH, Richardson BA, Benki-Nugent S, Warui D, John-Stewart GC. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–355. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, Krogstad PA, et al. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24:352–357. doi: 10.1097/01.inf.0000157095.75081.43. [DOI] [PubMed] [Google Scholar]
- 20.Kekitiinwa A, Lee KJ, Walker AS, Maganda A, Doerholt K, Kitaka SB, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–392. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 21.Aurpibul L, Puthanakit T, Taecharoenkul S, Sirisanthana T, Sirisanthana V. Reversal of growth failure in HIV-infected Thai children treated with non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. AIDS Patient Care STDS. 2009;23:1067–1071. doi: 10.1089/apc.2009.0093. [DOI] [PubMed] [Google Scholar]
- 22.Musoke PM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D, Mubiru MM, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. doi: 10.1186/1471-2431-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigel R, Phiri S, Chiputula F, Gumulira J, Brinkhof M, Gsponer T, et al. Growth response to antiretroviral treatment in HIV-infected children: a cohort study from Lilongwe, Malawi. Trop Med Int Health. 2010;15:934–944. doi: 10.1111/j.1365-3156.2010.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutcliffe CG, van Dijk JH, Munsanje B, Hamangaba F, Sinywimaanzi P, Thuma PE, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. doi: 10.1186/1471-2334-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwiru RS, Spiegelman D, Duggan C, Seage GR, 3rd, Semu H, Chalamilla G, et al. Growth among HIV-infected children receiving antiretroviral therapy in Dar es Salaam, Tanzania. J Trop Pediatr. 2014;60:179–188. doi: 10.1093/tropej/fmt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GC, Yu T, Min XH, Zhao LN, Qing Q, Yuan YH, et al. Prognosis of 153 patients with decompensated hepatitis B virus-related cirrhosis is improved after 3-year continuous lamivudine treatment. Chin Med J (Engl) 2013;126:1538–1543. [PubMed] [Google Scholar]
- 27.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J. 2011;30:974–979. doi: 10.1097/INF.0b013e31822539f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhagan MK, Kauchali S, Van den Broeck J. Clinical and contextual determinants of anthropometric failure at baseline and longitudinal improvements after starting antiretroviral treatment among South African children. Trop Med Int Health. 2012;17:1092–1099. doi: 10.1111/j.1365-3156.2012.03026.x. [DOI] [PubMed] [Google Scholar]
- 29.George E, Noel F, Bois G, Cassagnol R, Estavien L, De Rouzier PM, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. Journal of Infectious Diseases. 2007;195:1411–1418. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 30.Kim MH, Cox C, Dave A, Draper HR, Kabue M, Schutze GE, et al. Prompt initiation of ART With therapeutic food is associated with improved outcomes in HIV-infected Malawian children with malnutrition. J Acquir Immune Defic Syndr. 2012;59:173–176. doi: 10.1097/QAI.0b013e3182405f8f. [DOI] [PubMed] [Google Scholar]
- 31.Song R, Jelagat J, Dzombo D, Mwalimu M, Mandaliya K, Shikely K, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics. 2007;120:e856–861. doi: 10.1542/peds.2006-1122. [DOI] [PubMed] [Google Scholar]
- 32.Kruger HS, Balk LJ, Viljoen M, Meyers TM. Positive association between dietary iron intake and iron status in HIV-infected children in Johannesburg, South Africa. Nutr Res. 2013;33:50–58. doi: 10.1016/j.nutres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Isaakidis P, Raguenaud ME, Te V, Tray CS, Akao K, Kumar V, et al. High survival and treatment success sustained after two and three years of first-line ART for children in Cambodia. J Int AIDS Soc. 2010;13:11. doi: 10.1186/1758-2652-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlow-Mosha LN, Bagenda DS, Mudiope PK, Mubiru MC, Butler LM, Fowler MG, et al. The long-term effectiveness of generic adult fixed-dose combination antiretroviral therapy for HIV-infected Ugandan children. Afr Health Sci. 2012;12:249–258. doi: 10.4314/ahs.v12i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendergast A, Bwakura-Dangarembizi M, Cook A, Bakeera-Kitaka S, Natukunda E, Nahirya P, et al. Hospitalisation for severe malnutrition amongst HIV-infected children starting antiretroviral therapy in the ARROW trial. AIDS. 2011 doi: 10.1097/QAD.0b013e328345e56b. [DOI] [PubMed] [Google Scholar]
- 36.Devi NP, Chandrasekaran K, Bhavani PK, Thiruvalluvan C, Swaminathan S. Persistence of stunting after highly active antiretroviral therapy in HIV-infected children in South India. Indian Pediatr. 48:333–334. [PubMed] [Google Scholar]
- 37.Miller TL, Mawn BE, Orav EJ, Wilk D, Weinberg GA, Nicchitta J, et al. The effect of protease inhibitor therapy on growth and body composition in human immunodeficiency virus type 1-infected children. Pediatrics. 2001;107:E77. doi: 10.1542/peds.107.5.e77. [DOI] [PubMed] [Google Scholar]
- 38.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PENPACT-1 First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diniz LM, Maia MM, Camargos LS, Amaral LC, Goulart EM, Pinto JA. Impact of HAART on growth and hospitalization rates among HIV-infected children. J Pediatr (Rio J) 2011;87:131–137. doi: 10.2223/JPED.2064. [DOI] [PubMed] [Google Scholar]
- 41.Boersma B, Wit JM. Catch-up growth. Endocr Rev. 1997;18:646–661. doi: 10.1210/edrv.18.5.0313. [DOI] [PubMed] [Google Scholar]
- 42.Van Ijzendoorn MH, Bakermans-Kranenburg MJ, Juffer F. Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. J Dev Behav Pediatr. 2007;28:334–343. doi: 10.1097/DBP.0b013e31811320aa. [DOI] [PubMed] [Google Scholar]
- 43.Lagrone L, Cole S, Schondelmeyer A, Maleta K, Manary MJ. Locally produced ready-to-use supplementary food is an effective treatment of moderate acute malnutrition in an operational setting. Ann Trop Paediatr. 2010;30:103–108. doi: 10.1179/146532810X12703901870651. [DOI] [PubMed] [Google Scholar]
- 44.Barlow-Mosha L, Angelidou K, Lindsey JC, Mofenson L, Palumbo P, Jean-Philippe P, et al. Conference on Retroviruses and Opportunistic Infections (CROI) Seattle, Washington, USA: 2015. Long-term outcomes of HIV-infected children initiating NVP vs LPV/r-based treatment. [Google Scholar]
- 45.Team P-S, Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11:273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gsponer T, Weigel R, Davies MA, Bolton C, Moultrie H, Vaz P, et al. Variability of growth in children starting antiretroviral treatment in southern Africa. Pediatrics. 2012;130:e966–977. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penazzato M, Prendergast AJ, Muhe LM, Tindyebwa D, Abrams EJ. Optimization of antiretroviral therapy in HIV-infected children under 3 years of age: a systematic review. AIDS. 2014;28(Suppl 2):S137–146. doi: 10.1097/QAD.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 48.Verweel G, van Rossum AM, Hartwig NG, Wolfs TF, Scherpbier HJ, de Groot R. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109:E25. doi: 10.1542/peds.109.2.e25. [DOI] [PubMed] [Google Scholar]
- 49.Guillen S, Ramos JT, Resino R, Bellon JM, Munoz MA. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- 50.De Beaudrap P, Rouet F, Fassinou P, Kouakoussui A, Mercier S, Ecochard R, et al. CD4 cell response before and after HAART initiation according to viral load and growth indicators in HIV-1-infected children in Abidjan, Cote d'Ivoire. J Acquir Immune Defic Syndr. 2008;49:70–76. doi: 10.1097/QAI.0b013e3181831847. [DOI] [PubMed] [Google Scholar]
- 51.Van Rossum AM, Gaakeer MI, Verweel S, Hartwig NG, Wolfs TF, Geelen SP, et al. Endocrinologic and immunologic factors associated with recovery of growth in children with human immunodeficiency virus type 1 infection treated with protease inhibitors. Pediatr Infect Dis J. 2003;22:70–76. doi: 10.1097/00006454-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Nacro B, Zoure E, Hien H, Tamboura H, Rouet F, Ouiminga A, et al. Pharmacology and immuno-virologic efficacy of once-a-day HAART in African HIV-infected children: ANRS 12103 phase II trial. Bull World Health Organ. 2011;89:451–458. doi: 10.2471/BLT.10.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48:1–10. [PubMed] [Google Scholar]
- 54.Benki-Nugent S, Eshelman C, Wamalwa D, Langat A, Tapia K, Okinyi HM, et al. Correlates of age at attainment of developmental milestones in HIV-infected infants receiving early antiretroviral therapy. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coovadia A, Abrams EJ, Stehlau R, Meyers T, Martens L, Sherman G, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies MA, Keiser O, Technau K, Eley B, Rabie H, van Cutsem G, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 58.Eley B, Davies MA, Apolles P, Cowburn C, Buys H, Zampoli M, et al. Antiretroviral treatment for children. S Afr Med J. 2006;96:988–993. [PubMed] [Google Scholar]
- 59.Jaspan HB, Berrisford AE, Boulle AM. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008;27:993–998. doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- 60.Purchase SE, Van der Linden DJ, McKerrow NH. Feasibility and effectiveness of early initiation of combination antiretroviral therapy in HIV-infected infants in a government clinic of Kwazulu-Natal, South Africa. J Trop Pediatr. 2012;58:114–119. doi: 10.1093/tropej/fmr053. [DOI] [PubMed] [Google Scholar]
- 61.Reddi A, Leeper SC, Grobler AC, Geddes R, France KH, Dorse GL, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reitz C, Coovadia A, Ko S, Meyers T, Strehlau R, Sherman G, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–251. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 64.Musiime V, Kayiwa J, Kiconco M, Tamale W, Alima H, Mugerwa H, et al. Response to Antiretroviral Therapy of HIV Type 1-Infected Children in Urban and Rural Settings of Uganda. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0313. [DOI] [PubMed] [Google Scholar]
- 65.van Dijk JH, Sutcliffe CG, Hamangaba F, Bositis C, Watson DC, Moss WJ. Effectiveness of efavirenz-based regimens in young HIV-infected children treated for tuberculosis: a treatment option for resource-limited settings. PLoS One. 2013;8:e55111. doi: 10.1371/journal.pone.0055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devi NP, Chandrasekaran K, Bhavani PK, Thiruvalluvan C, Swaminathan S. Persistence of stunting after highly active antiretroviral therapy in HIV-infected children in South India. Indian Pediatr. 2011;48:333–334. [PubMed] [Google Scholar]
- 67.Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Moothi SN, Solomon S. Safety, tolerability and effectiveness of generic HAART in HIV-Infected children in South India. Journal of Tropical Pediatrics. 2009;55:155–159. doi: 10.1093/tropej/fmn080. [DOI] [PubMed] [Google Scholar]
- 68.Lodha R, Upadhyay A, Kabra SK. Antiretroviral therapy in HIV-1 infected children. Indian Pediatr. 2005;42:789–796. [PubMed] [Google Scholar]
- 69.Parakh A, Dubey AP, Kumar A, Maheshwari A, Saxena R. Efficacy of first-line, WHO recommended generic HAART regimens in Indian children. Kathmandu Univ Med J (KUMJ) 2009;7:220–225. doi: 10.3126/kumj.v7i3.2727. [DOI] [PubMed] [Google Scholar]
- 70.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janssens B, Raleigh B, Soeung S, Akao K, Te V, Gupta J, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–1140. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 72.Sophan S, Meng CY, Pean P, Harwell J, Hutton E, Trzmielina S, et al. Virologic and immunologic outcomes in HIV-infected Cambodian children after 18 months of highly active antiretroviral therapy (HAART) Southeast Asian J Trop Med Public Health. 2010;41:126–137. [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang F, Haberer JE, Zhao Y, Dou Z, Zhao H, He Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007;46:594–598. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 74.Bunupuradah T, Puthanakit T, Kosalaraksa P, Kerr S, Boonrak P, Prasitsuebsai W, et al. Immunologic and virologic failure after first-line NNRTI-based antiretroviral therapy in Thai HIV-infected children. AIDS Res Ther. 2011;8:40. doi: 10.1186/1742-6405-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansudewechakul R, Naiwatanakul T, Katana A, Faikratok W, Lolekha R, Thainuea V, et al. Successful clinical outcomes following decentralization of tertiary paediatric HIV care to a community-based paediatric antiretroviral treatment network, Chiangrai, Thailand, 2002 to 2008. J Int AIDS Soc. 2012;15:17358. doi: 10.7448/IAS.15.2.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phongsamart W, Hansudewechakul R, Bunupuradah T, Klinbuayaem V, Teeraananchai S, Prasithsirikul W, et al. Long-term outcomes of HIV-infected children in Thailand: the Thailand Pediatric HIV Observational Database. Int J Infect Dis. 2014;22:19–24. doi: 10.1016/j.ijid.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Efficacy of non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy in Thai HIV-infected children aged two years or less. Pediatr Infect Dis J. 2009;28:246–248. doi: 10.1097/INF.0b013e31818dd72b. [DOI] [PubMed] [Google Scholar]
- 78.Puthanakit T, Saphonn V, Ananworanich J, Kosalaraksa P, Hansudewechakul R, Vibol U, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis. 2012;12:933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansudewechakul R, Sirisanthana V, Kurniati N, Puthanakit T, Lumbiganon P, Saphonn V, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr. 2010;55:503–509. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierre RB, Steel-Duncan JC, Evans-Gilbert T, Rodriguez B, Moore J, Palmer P, et al. Effectiveness of antiretroviral therapy in treating paediatric HIV/AIDS in Jamaica. West Indian Med J. 2008;57:223–230. [PubMed] [Google Scholar]
- 81.Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, Gibb DM, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11:273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aboulker JP, Babiker A, Chaix ML, Compagnucci A, Darbyshire J, Debre M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18:237–245. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 83.Buchacz K, Cervia JS, Lindsey JC, Hughes MD, Seage GR, 3rd, Dankner WM, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108:E72. doi: 10.1542/peds.108.4.e72. [DOI] [PubMed] [Google Scholar]
- 84.Chadwick EG, Yogev R, Alvero CG, Hughes MD, Hazra R, Pinto JA, et al. Long-term outcomes for HIV-infected infants less than 6 months of age at initiation of lopinavir/ritonavir combination antiretroviral therapy. AIDS. 2011;25:643–649. doi: 10.1097/QAD.0b013e32834403f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dreimane D, Nielsen K, Deveikis A, Bryson YJ, Geffner ME. Effect of protease inhibitors combined with standard antiretroviral therapy on linear growth and weight gain in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2001;20:315–316. doi: 10.1097/00006454-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 86.Faye A, Bertone C, Teglas JP, Chaix ML, Douard D, Firtion G, et al. Early multitherapy including a protease inhibitor for human immunodeficiency virus type 1-infected infants. Pediatr Infect Dis J. 2002;21:518–525. doi: 10.1097/00006454-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Thuret I, Michel G, Chambost H, Tamalet C, Giraud P, Brunet C, et al. Combination antiretroviral therapy including ritonavir in children infected with human immunodeficiency. AIDS. 1999;13:81–87. doi: 10.1097/00002030-199901140-00011. [DOI] [PubMed] [Google Scholar]
- 88.Scherpbier HJ, Bekker V, Pajkrt D, Jurriaans S, Lange JM, Kuijpers TW. Once-daily highly active antiretroviral therapy for HIV-infected children: safety and efficacy of an efavirenz-containing regimen. Pediatrics. 2007;119:e705–715. doi: 10.1542/peds.2006-1367. [DOI] [PubMed] [Google Scholar]
- 89.Kim RJ, Rutstein RM. Impact of antiretroviral therapy on growth, body composition and metabolism in pediatric HIV patients. Paediatr Drugs. 2010;12:187–199. doi: 10.2165/11532520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 90.Puthanakit T, Bunupuradah T. Early versus deferred antiretroviral therapy in children in low-income and middle-income countries. Curr Opin HIV AIDS. 2010;5:12–17. doi: 10.1097/COH.0b013e3283339b27. z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.