Summary
The ubiquitous shortage of primary human hepatocytes has urged the scientific community to search for alternative cell sources, such as immortalized hepatic cell lines. Over the years, several human hepatic cell lines have been produced, whether or not using a combination of viral oncogenes and human telomerase reverse transcriptase protein. Conditional approaches for hepatocyte immortalization have also been established and allow generation of growth-controlled cell lines. A variety of immortalized human hepatocytes have already proven useful as tools for liver-based in vitro testing and fundamental research purposes. The present chapter describes currently applied immortalization strategies and provides an overview of the actually available immortalized human hepatic cell lines and their in vitro applications.
Keywords: cell line, liver, immortalization, human
1. Introduction
At present, primary human hepatocytes represent an important tool for research purposes, in particular in the field of in vitro pharmaco-toxicology (1). However, their use is largely impeded by inadequate supply, high cost, high variability and limited in vitro proliferation capacity. Alternative cell sources include hepatic cell lines and stem-cell derived hepatocytes (2, 3, 4). Several hepatic cell lines are nowadays available either directly derived from liver tumor tissue or generated from primary hepatocytes in vitro (5, 6). Although several hepatoma-derived cell lines, such as HepaRG cells, preserve important liver-specific functions, most of them do not exhibit sufficient in vivo-like functionality to be of pharmaco-toxicological relevance (7-12). Immortalized hepatocytes are usually derived from healthy primary hepatocytes by the use of a defined immortalization strategy. Several fetal and adult hepatic cell lines have already been established, whether or not using a combination of viral oncogenes and the human telomerase reverse transcriptase (hTERT) protein (13-19). In this chapter, a number of immortalization strategies are discussed and a state-of-the-art overview of the currently available immortalized human hepatic cell lines and their in vitro applications is provided.
2. Hepatocyte immortalization strategies
The most common methods for immortalization of primary hepatocytes are (i) overexpression of viral oncogenes, (ii) forced expression of hTERT or (iii) a combination of both (Table 1) (14, 20). A number of additional immortalization genes as well as conditional approaches for hepatocyte immortalization have also been described (Table 2).
Table 1. Overview of immortalization strategies, hepatic functionality and in vitro applications of human adult and fetal hepatic cell lines.
(A1AT, α1-antitrypsin; AFP, α-fetoprotein; AhR, aryl hydrocarbon receptor; ALB, albumin; A2M, α2-macroglobulin; APO, apolipoprotein; Arnt, AhR nuclear translocator; ASGP(R), asialoglycoprotein (receptor); BCRP, breast cancer resistance protein; BSEP, bile salt export pump; CAR, constitutive androstane receptor; C/EBP, Ccaat-enhancer-binding protein; ChREBP, carbohydrate-responsive element-binding protein; CK, cytokeratin; CLDN, claudin; CYP, cytochrome P450; EH, epoxide hydrolase; EMT, epithelial-mesenchymal transition; EPCAM, epithelial cell adhesion molecule; FXR, farnesoid X receptor; GGT, γ-glutamyltranspeptidase; G6P, glucose-6-phosphate; GPX, gluthatione peroxidase; GS, glutamine synthetase; GST, gluthatione S-transferase; HBCF, human blood coagulation factor; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HGFR, hepatocyte growth factor receptor; HNF, hepatocyte nuclear factor; HPV, human papillomavirus; hTERT, human telomerase reverse transcriptase; IL, interleukin; IFN, interferon; MDR, multidrug resistance protein; mRNA, messenger ribonucleic acid; MRP, multidrug resistance-associated protein; NADPH, nicotinamide adenine dinucleotide phosphate; NCAM, neural cell adhesion molecule; NTCP, sodium taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; PPAR, peroxisome proliferator-activated receptor; PXR, pregnane X receptor; Rb, retinoblastoma; RIPK4, receptor-interacting serine-threonine kinase 4; SOD, superoxide dismutase; SREBP, sterol regulatory element-binding protein; SV40 Tag, simian virus 40 large T antigen; TGF, transforming growth factor; TF, transferrin; (bil-)UGT, (bilirubin-)uridinediphosphate-glucuronosyltransferase).
| Adult hepatic cell line | ||||
|---|---|---|---|---|
| Cell line | Immortalization strategy | Hepatic functionality of immortalized cells | In vitro applications | Reference |
| Fa2N-4 | Transfection |
|
Routine screening system for PXR-mediated CYP3A4 induction. | (18,92,97) |
| SV40 Tag | ||||
| HepLi5 | Retroviral vector |
|
(21) | |
| SV40 Tag | ||||
| HepLL | Lipid mediated gene transfer (lipofectamine reagent) |
|
Testing of new drug carriers for anti-HBV drug delivery. | (22, 98) |
| SV40 Tag | ||||
| HepZ | Lipid mediated gene transfer (lipofectamine reagent) |
|
(47) | |
| Antisense constructions for Rb and p53 under control of ALB promoter + Cotransfection of E2F transcription factors and cyclin D1 | ||||
| HHE6E7T-1/2 | Small hepatocytes |
|
(16, 99) | |
| Lentiviral and retroviral vectors | ||||
| HPV16 E6/E7 + hTERT | ||||
| HHL(-5/-7/-16) | Retroviral vector |
|
Used as a cell model to investigate:
|
(15, 89-91, 100-102) |
| HPV16 E6/E7 | ||||
| IHH-A5 | Lipid mediated gene transfer (lipofectin reagent) |
|
Used a cell model to investigate:
|
(23, 85, 86, 103-105) |
| SV40 Tag | ||||
| PH5CH | Lipid mediated gene transfer (lipofectin reagent) |
|
Used a cell model to investigate:
|
(25, 74, 75, 81, 106-113) |
| SV40 Tag | ||||
| THLE | Retroviral vector |
|
THLE and THLE-CYP cells can be used to study cellular toxicity of compounds. Used a cell model to investigate:
|
(25, 93, 94, 111, 114-124) |
| SV40 Tag | ||||
| TPH1 | Strontium phosphate precipitation |
|
Used a cell model to investigate HCV infection and replication. Induces apoptosis of activated hepatic stellate cells. |
(45, 77, 78, 125-129) |
| HCV core gene | ||||
| Fetal and neonatal hepatic cell lines | ||||
|---|---|---|---|---|
| Cell line | Immortalization strategy | Hepatic functionality of immortalized cells | In vitro applications | Ref |
| FH-TERT | Retroviral vector |
|
Used as a stroma to induce human embryonic stem cellsdifferentiation into hematopoietic cells. Used as a cell model to investigate:
|
(34, 83, 84, 130) |
| hTERT | ||||
| Hc3716-hTERT | Retroviral vector |
|
Used as in vitro model for predicting the side-effects of telomere-targeting drugs. | (39) |
| hTERT | ||||
| NeHepLxHT | Retroviral vector |
|
Used as a cell model to investigate:
|
(14, 80, 131, 132) |
| hTERT | ||||
| OUMS-29 | Lipid mediated gene transfer (lipofectin reagent) |
|
Used as a cell model to investigate:
|
(17, 49, 62, 133-136) |
| SV40 Tag | ||||
Table 2. Overview of conditional immortalization strategies, hepatic functionality and in vitro applications of growth-controlled human adult and fetal hepatic cell lines.
(A1AT, α1-antitrypsin; AFP, α-fetoprotein; ALB, albumin; ASGP(R), asialoglycoprotein (receptor); Bmi-1, B lymphoma Mo-MLV insertion region 1 homolog; C/EBP, Ccaat-enhancer-binding protein; CD, cluster of differentiation; CK, cytokeratin; CYP, cytochrome P450; GS, glutamine synthetase; GST, gluthatione S-transferase; HBCF, human blood coagulation factor; HNF, hepatocyte nuclear factor; hTERT, human telomerase reverse transcriptase; mRNA, messenger ribonucleic acid; PT, prothrombin; SV40 Tag, simian virus 40 large T antigen; TF, transferrin; (bil-)UGT, (bilirubin-)uridinediphosphate-glucuronosyltransferase).
| Adult hepatic cell line | ||||
|---|---|---|---|---|
| Cell line | Immortalization strategy | Hepatic functionality of conditional immortalized cells | In vitro applications | Reference |
| 16T-3 | Retroviral vector | Reverted 16T-3 cells:
|
(43) | |
| hTERT | ||||
| Tamoxifen-mediated self-excision (Cre-LoxP) | ||||
| HepLi-4 | Retroviral vector | Reverted HepLi-4 cells:
|
(26) | |
| SV40 Tag | ||||
| Tamoxifen-mediated self-excision (Cre-LoxP) | ||||
| HLTC-7/ -11/ -15/ -17/ -19 | Retroviral vector |
|
(28) | |
| SV40 Tag | ||||
| Temperature-based regulation | ||||
| IHH10(.3)/12 | Lentiviral vector |
|
A novel in vitro model to investigate the mechanisms and consequences of lipid accumulation in hepatocytes, independently of insulin resistance. | (19, 87) |
| SV40 Tag + hTERT (IHH10) or SV40 Tag + hTERT + Bmi-1 (IHH12) | ||||
| Recombinase- based control (Cre-LoxP) | ||||
| NKNT-3 | Retroviral vector |
|
Used in combination with HCV like particles as a model system for studying viral binding and entry. | (6, 29,52, 71, 79) |
| SV40 Tag | ||||
| Recombinase- based control (Cre-loxP) | ||||
| YOCK-13 | Retroviral vector |
|
(42) | |
| hTERT | ||||
| Tamoxifen-mediated self-excision (Cre-LoxP) | ||||
| Fetal hepatic cell lines | ||||
|---|---|---|---|---|
| Cell line | Immortalization strategy | Hepatic functionality of conditional immortalized cells | In vitro applications | Reference |
| cBAL111 | Lentiviral vector |
|
(13) | |
| hTERT | ||||
| Transcriptional regulation (Tet-on approach) | ||||
| HepCL | Retroviral vector |
|
(27) | |
| SV40 Tag | ||||
| Temperature-based regulation | ||||
2.1. Immortalization genes
2.1.1. Viral oncogenes
Several human hepatic cell lines have been generated using viral oncogenes, such as the simian virus 40 large T antigen (SV40 Tag) and the human papillomavirus 16 (HPV16) E6/E7 genes, suggesting that overexpression of these viral oncogenes could be sufficient to surmount the premature in vitro growth arrest of cultured adult hepatocytes (15, 17, 18, 20-29). Indeed, in contrast to fetal hepatocytes, human adult hepatocytes do not undergo spontaneous cell growth in vitro and possess very limited proliferation capacity, even when cultivated in growth-promoting culture systems (6, 20, 30, 31). It has been proposed that this in vitro precocious growth arrest could be the result of a telomere-independent senescence mechanism, which possibly involves tumor suppressor proteins and cyclin-dependent kinase inhibitors (32). Viral oncogenes typically promote cell cycling by inhibiting the p16/retinoblastoma protein (pRB) and p53 pathways (20, 33). However, while the use of viral oncogenes, such as SV40 Tag, is sufficient to produce immortalized rodent cells, overexpression of these oncogenes probably only extends lifespan in human cells. Human hepatocytes, like other somatic cells, do not possess telomerase activity and are subject to replicative senescence (20, 32, 34, 35). Consequently, immortalization per se requires telomerase reactivation either through mutations or by involving a second immortalizing gene. In particular, hTERT has been used for this purpose (16, 19, 20, 34, 36-38).
2.1.2. Human telomerase reverse transcriptase
The single use of hTERT for immortalization is limited to a number of human cell types, including fetal and neonatal hepatocytes (6, 13, 14, 34, 36, 39, 40). These immature cells are still able to proliferate in vitro and hence do not need cell cycle stimulation for immortalization (6, 13, 14, 34, 40, 41). However, fetal and neonatal human hepatocytes do not display indefinite growth potential due to telomere-dependent senescence. As a result, overexpression of hTERT is needed to immortalize these hepatocytes (13, 14, 34, 40). Contradicting results have been obtained when merely hTERT is used to immortalize mature hepatocytes (16, 42, 43). Since telomerase activity most likely does not allow adult hepatocytes to overcome the suggested telomere-independent growth arrest, overexpression of hTERT may not be adequate to stimulate the progression of adult hepatocytes through the cell cycle (5, 13, 20, 44).
2.1.3. Miscellaneous immortalization genes
The hepatitis C core protein has been described as a specific immortalization agent for mature human hepatocytes (36, 45, 46). A particular cell line was developed by co-transfecting human adult hepatocytes with p53 and pRB antisense constructs and plasmids that include E2F and cyclin D1 genes (47). Furthermore, specific combinations of immortalization genes, such as SV40 Tag with hTERT and B lymphoma Moloney Murine Leukemia virus (Mo-MLV) insertion region 1 homolog (Bmi-1), have also proven useful for the immortalization of mature human hepatocytes. Bmi-1 has a similar function as the HPV16E7 oncogene and its expression inactivates the p16/pRB pathway. However, the simultaneous transduction with Bmi-1 and hTERT, like for the combined HPV16E7/hTERT approach, appears to be insufficient to immortalize non-proliferating adult hepatocytes (16, 19).
2.2. Gene transfer
Appropriate gene transfer is of utmost importance for hepatocyte immortalization (37). In this regard, both viral and non-viral transfer methods have been used to generate immortalized hepatocyte-derived cell lines.
2.2.1. Plasmid transfection
Since the immortalization process will select cells that stably express the immortalization genes, simple transfection methods can be used (48). At present, various approaches are available for transfecting plasmids into primary hepatocytes (37, 48). The strontium phosphate precipitation method has been used to immortalize human hepatocytes (45). Although this method is cheap and allows robust transfection of primary hepatocytes with low toxicity, it is generally accompanied by limited gene transfer efficiency (37). Liposomes have also been explored as gene carriers for hepatocyte immortalization (17, 22, 23, 25, 47, 49). When properly optimized, lipid-mediated gene transfer strategies can achieve high gene transfer efficiencies compared to the previously mentioned phosphate-precipitation-based transfection approach (37). Furthermore, combination with hepatocyte-specific ligands allows more hepatocyte-specific transfections (48).
2.2.2. Viral transduction
Transduction with viral vectors is a frequently used strategy for gene transfer. Among the available viral vectors, retroviral and lentiviral vectors enable stable integration of the immortalization gene and thus ensure persistent transgene expression in the progeny (48, 50). Furthermore, these vectors do not induce harmful immune responses and are able to integrate large genes (51). Retroviral vectors have often been used to produce human hepatic cell lines (14-16, 21, 24, 26-28, 34, 39, 42, 43, 52). An important drawback of these vectors, however, is their inability to transduce non-dividing cells, which hampers their use for non-proliferating cells, including hepatocytes (51, 53). Although transduction efficiencies generally remain limited even when growth factors are used, it has been reported that the addition of hepatocyte growth factor to the cell culture medium increases the transduction efficiency in primary human hepatocyte cultures (48, 51, 53-55). Lentiviral vectors derived from the human immunodeficiency virus can overcome these flaws and effectively transduce both dividing and non-dividing cells when applied at a relatively high titer (51, 53, 54, 56). Several studies are based upon lentiviral gene transfer for immortalization of human adult and fetal hepatocytes (13, 16, 19). It has been demonstrated that the lentiviral transduction procedure as such does not interfere with the differentiated hepatic phenotype of primary human hepatocytes (57). Moreover, the addition of growth factors to the cell culture medium can markedly enhance the expression of lentiviral genes in both human adult and fetal hepatocytes when low vector titers are used. This transduction approach therefore offers the opportunity to lower the viral load, which in turn reduces cost and cellular toxicity (56). Also, the antioxidant vitamin E is known to promote lentiviral transduction rates of human adult hepatocytes (53).
2.2.3. Human artificial chromosomes
The successful immortalization of rat hepatocytes and human fibroblasts using human artificial chromosomes vectors expressing SV40 Tag or hTERT, respectively, opens new perspectives for human hepatocyte immortalization (58-60). Although the transfer efficiency is generally lower compared to viral vectors, human artificial chromosomes have many characteristics of an ideal gene delivery vector. Indeed, they are able to incorporate complete genomic loci and maintain a mitotically stable episomal expression throughout many cell divisions. Furthermore, due to their episomal nature, integration-related complications, such as oncogenesis, can be avoided (50).
2.3. Hepatic functionality of the immortalized human hepatocytes
In general, most human hepatic cell lines possess reduced or only limited liver-specific functionality. Strategies that are typically used to counteract the loss of functionality in primary hepatocyte cultures, including the establishment of co-culture systems or the overexpression of liver-enriched transcription factors, have also proven beneficial for immortalized human hepatocytes (61, 62). In this context, overexpression of specific cytochrome P450 (CYP) enzymes lies at the basis of the development of the THLE-CYP sublines (63). On the other hand, as differentiation and proliferation are mutually exclusive in vitro, overexpression of the cyclin-dependent kinase inhibitor p21 and the use of conditional immortalization strategies were reported to boost to some extent the differentiated phenotype of hepatocytes (19, 29, 43, 64, 65).
2.4. Conditional immortalization strategies
Conditional immortalization supports controlled expansion of cells. At present, 3 different strategies are applied for human hepatocytes, namely (i) temperature-based regulation, (ii) recombinase-based regulation and (iii) transcriptional regulation (36) (Table 2 and 3).
Table 3. Conditional approaches for human hepatocyte immortalization (36).
(rtTA, reverse tetracycline transactivator; SV40 Tag, simian virus 40 large T antigen; TRE, tetracycline responsive element; tTA, tetracycline transactivator).
| Conditional approach | Immortalization construct | Growth curve |
|---|---|---|
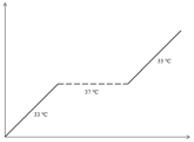
| Temperature-based regulation | Thermolabile SV40 Tag mutant |
|
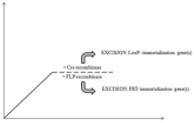
| Recombinase-based control | LoxP – immortalization gene(s) – LoxP FRT – immortalization gene(s) – FRT |
|
| Transcriptional regulation | TRE- immortalization gene(s) |

|
2.4.1. Temperature-based regulation
This method is based upon the application of a temperature-unstable SV40 Tag mutant. At permissive temperature (i.e. 33°C), the immortalizing gene is fully active and stimulates hepatocyte proliferation. However, at higher temperatures (i.e. 37 to 39°C), the immortalization gene is typically degraded and cell cycle progression is no longer supported (36). Since no other temperature-sensitive immortalizing genes have yet been identified, this method is restricted to the use of SV40 Tag (36). Importantly, the temperature shift related to this methodology might induce variations in cellular properties, which can complicate the interpretation of the study outcome (36, 66, 67). More sophisticated systems based upon recombinase or transcriptional regulation are believed to offer a better solution (68).
2.4.2. Recombinase-based control
The site-specific recombinase strategy results in an irreversible reversion of immortalization, due to permanent removal of immortalization genes (36, 69). The Cre-LoxP site-specific recombination system has often been used to establish reversible immortalization (69, 70). In this method, the immortalization genes are flanked by 2 identical DNA sequences, called LoxP sites, and their excision is regulated by Cre recombinase (20, 70). Hence, proper reversion relies on the effective transfer of the recombinase gene (36). A new method based on tamoxifen-mediated self-excision has been introduced and renders secondary virus-mediated transfer of the recombinase gene superfluous (26, 42, 43). Additionally, the incorporation of a negative selection marker, such as the suicide gene herpes simplex virus thymidine kinase, allows the removal of cells that underwent improper recombination by exposure to ganciclovir (19, 70). Reversible immortalization of numerous human hepatocyte-derived cell lines, including NKNT-3, IHH and 16T-3 cells, depends on this recombinase-based control approach (19, 42, 43, 71).
2.4.3. Transcriptional regulation
In this method, the reversibility of immortalization does not rely on recombinase activity, but is achieved through transcriptional control of immortalization gene expression. Consequently, repetitive cycles of hepatocyte proliferation and growth arrest are possible and the risk of chromosomal rearrangement is prevented (36, 68, 72). The transcriptional control of immortalizing genes can be accomplished by the use of an artificial promoter/transactivator system, such as the well-known tetracycline system (36). As such, 2 approaches are currently available, namely the tet-off and the tet-on system, which comprise a tetracycline-regulated promoter and a tetracycline transactivator (tTA) or reverse tetracycline transactivator (rtTA), respectively. In the tet-on system, binding of doxycycline to the transactivator will induce the expression of the regulated gene. This is not the case for the tet-off system, since only unbound tTA is able to interact with the gene promoter (72, 73). The tet-on approach has been used for the development of the human fetal liver cell line cBAL111 (13).
3. In vitro applications of immortalized human hepatocytes
In recent years, both adult and fetal human hepatic cell lines have been explored for research purposes (Table 1 and 2). Several immortalized human hepatocytes, including PH5CH, TPH1, NKNT-3 and NeHepLxHT cells, have indeed been successfully used as tools in research focused on hepatitis C virus or hepatitis B virus (HBV) (74-81). A murine model of HBV viremia based upon a human hepatocyte-derived cell line transfected with HBV DNA has been described and offers the opportunity for in vivo HBV research (82). Human hepatic cell lines have also been applied as cellular models to investigate the processes of hepatocarcinogenesis and steatosis (83-88). Moreover, the HHL cell line proved useful during the development of adeno-associated viral vectors for liver-directed gene therapy (89-91).
Besides their application in fundamental research, different hepatic cell lines are equally addressed as suitable in vitro tools for screening and safety testing of drug candidates. In this regard, Hc3716-hTERT immortalized hepatocytes constitute an appropriate in vitro model for predicting the side-effects of telomere-targeting drugs (39). Furthermore, Fa2N4 cells may be used as a routine screening system for pregnane X receptor-mediated CYP3A4 induction (92). Similarly, the hepatic THLE cell line and THLE-CYP sublines have been reported as promising models for investigation of CYP-mediated drug metabolism and liver toxicity (63, 93-95). However, NKNT-3 cells appeared to be less suitable than the hepatoma cell line HCC1.2 for the development of improved in vitro genotoxicity test systems (96).
4. Conclusion
In vitro expansion of human hepatocytes has gained considerable attention over the years, as it may serve a plethora of fundamental and applied research and screening purposes. Freshly isolated mature hepatocytes inherently have poor growth potential, a finding that has prompted the search for strategies to immortalize these particular human cells, while maintaining their liver-specific functions. The available methods thus far include transfection or transduction with prototypical immortalization genes and conditional immortalization by temperature-based regulation, recombinase-based control and transcriptional regulation. Although hepatocyte immortalization has been explored for decades, cell lines with in vivo-like hepatic functionality are largely lacking. In the upcoming years, more attention should be paid to the search for culture systems that support the differentiation status of immortalized human hepatocytes.
Acknowledgements
This work was financially supported by the grants from the University Hospital of Vrije Universiteit Brussel (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research Flanders (FWO-Vlaanderen), the European Union (FP7/Cosmetics Europe projects HeMiBio and DETECTIVE) and the European Research Council (ERC Starting Grant project CONNECT).
References
- 1.Hewitt NJ, Lechon MJ, Houston JB, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- 2.Sinz M, Kim S. Stem cells, immortalized cells and primary cells in ADMET assays. Drug Discov Today Technol. 2006;3:79–85. doi: 10.1016/j.ddtec.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Soldatow VY, Lecluyse EL, Griffith LG, et al. In vitro models for liver toxicity testing. Toxicol Res. 2013;2:23–39. doi: 10.1039/C2TX20051A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues RM, De Kock J, Branson S, et al. Human skin-derived stem cells as a novel cell source for in vitro hepatotoxicity screening of pharmaceuticals. Stem Cells Dev. 2014;23:44–55. doi: 10.1089/scd.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen JW, Bhatia SN. Improving the next generation of bioartificial liver devices. Semin Cell Dev Biol. 2002;13:447–454. doi: 10.1016/s1084952102001337. [DOI] [PubMed] [Google Scholar]
- 6.Chamuleau RA, Deurholt T, Hoekstra R. Which are the right cells to be used in a bioartificial liver? Metab Brain Dis. 2005;20:327–335. doi: 10.1007/s11011-005-7914-4. [DOI] [PubMed] [Google Scholar]
- 7.Brandon EF, Raap CD, Meijerman I, et al. An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol Appl Pharmacol. 2003;189:233–246. doi: 10.1016/s0041-008x(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 8.Choi S, Sainz B, Corcoran P, et al. Characterization of increased drug metabolism activity in dimethyl sulfoxide (DMSO)-treated Huh7 hepatoma cells. Xenobiotica. 2009;39:205–217. doi: 10.1080/00498250802613620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim PL, Tan W, Latchoumycandane C, et al. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro. 2007;21:1390–1401. doi: 10.1016/j.tiv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Szabo M, Veres Z, Baranyai Z, et al. Comparison of human hepatoma HepaRG cells with human and rat hepatocytes in uptake transport assays in order to predict a risk of drug induced hepatotoxicity. PLoS One. 2013;8:e59432. doi: 10.1371/journal.pone.0059432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aninat C, Piton A, Glaise D, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- 12.Guillouzo A, Corlu A, Aninat C, et al. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Deurholt T, van Til NP, Chhatta AA, et al. Novel immortalized human fetal liver cell line, cBAL111, has the potential to differentiate into functional hepatocytes. BMC Biotechnol. 2009;9:89. doi: 10.1186/1472-6750-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid Y, Gaddipati JP, Yadav D, et al. Establishment of a human neonatal hepatocyte cell line. In Vitro Cell Dev Biol Anim. 2009;45:535–542. doi: 10.1007/s11626-009-9219-0. [DOI] [PubMed] [Google Scholar]
- 15.Clayton RF, Rinaldi A, Kandyba EE, et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005;25:389–402. doi: 10.1111/j.1478-3231.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsuruga Y, Kiyono T, Matsushita M, et al. Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant. 2008;17:1083–1094. doi: 10.3727/096368908786991542. [DOI] [PubMed] [Google Scholar]
- 17.Fukaya K, Asahi S, Nagamori S, et al. Establishment of a human hepatocyte line (OUMS-29) having CYP 1A1 and 1A2 activities from fetal liver tissue by transfection of SV40 LT. In Vitro Cell Dev Biol Anim. 2001;37:266–269. doi: 10.1007/BF02577541. [DOI] [PubMed] [Google Scholar]
- 18.Hariparsad N, Carr BA, Evers R, et al. Comparison of immortalized Fa2N-4 cells and human hepatocytes as in vitro models for cytochrome P450 induction. Drug Metab Dispos. 2008;36:1046–1055. doi: 10.1124/dmd.108.020677. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TH, Mai G, Villiger P, et al. Treatment of acetaminophen-induced acute liver failure in the mouse with conditionally immortalized human hepatocytes. J Hepatol. 2005;43:1031–1037. doi: 10.1016/j.jhep.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Cascio SM. Novel strategies for immortalization of human hepatocytes. Artif Organs. 2001;25:529–538. doi: 10.1046/j.1525-1594.2001.025007529.x. [DOI] [PubMed] [Google Scholar]
- 21.Pan X, Li J, Du W, et al. Establishment and characterization of immortalized human hepatocyte cell line for applications in bioartificial livers. Biotechnol Lett. 2012;34:2183–2190. doi: 10.1007/s10529-012-1025-1. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Li LJ, Cao HC, et al. Establishment of highly differentiated immortalized human hepatocyte line with simian virus 40 large tumor antigen for liver based cell therapy. ASAIO J. 2005;51:262–268. doi: 10.1097/01.mat.0000161045.16805.8b. [DOI] [PubMed] [Google Scholar]
- 23.Schippers IJ, Moshage H, Roelofsen H, et al. Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol Toxicol. 1997;13:375–386. doi: 10.1023/a:1007404028681. [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer AM, Cole KE, Smoot DT, et al. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci USA. 1993;90:5123–5127. doi: 10.1073/pnas.90.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi M, Hirohashi S. Cell lines from non-neoplastic liver and hepatocellular carcinoma tissue from a single patient. In Vitro Cell Dev Biol Anim. 1996;32:135–137. doi: 10.1007/BF02723678. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Li J, Lv G, et al. Evaluation of a reversibly immortalized human hepatocyte line in bioartificial liver in pigs. Afr J Biotechnol. 2012;11:4116–4126. [Google Scholar]
- 27.Chen Y, Li J, Liu X, et al. Transplantation of immortalized human fetal hepatocytes prevents acute liver failure in 90% hepatectomized mice. Transplant Proc. 2010;42:1907–1914. doi: 10.1016/j.transproceed.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 28.Smalley M, Leiper K, Tootle R, et al. Immortalization of human hepatocytes by temperature-sensitive SV40 large-T antigen. In Vitro Cell Dev Biol Anim. 2001;37:166–168. doi: 10.1290/1071-2690(2001)037<0166:IOHHBT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N, Fujiwara T, Westerman KA, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287:1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- 30.Ilyin G, Rescan C, Rialland M, et al. Growth control and cell cycle progression in cultured hepatocytes. In: Berry M, Edwards A, editors. The Hepatocyte Review. Kluwer Academic Publishers; The Netherlands: 2000. pp. 263–280. [Google Scholar]
- 31.Runge DM, Runge D, Dorko K, et al. Epidermal growth factor- and hepatocyte growth factor-receptor activity in serum-free cultures of human hepatocytes. J Hepatol. 1999;30:265–274. doi: 10.1016/s0168-8278(99)80073-7. [DOI] [PubMed] [Google Scholar]
- 32.Ozturk M, Arslan-Ergul A, Bagislar S, et al. Senescence and immortality in hepatocellular carcinoma. Cancer Lett. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 34.Wege H, Le HT, Chui MS, et al. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology. 2003;124:432–444. doi: 10.1053/gast.2003.50064. [DOI] [PubMed] [Google Scholar]
- 35.Chiu CP, Harley CB. Replicative senescence and cell immortality: the role of telomeres and telomerase. Proc Soc Exp Biol Med. 1997;214:99–106. doi: 10.3181/00379727-214-44075. [DOI] [PubMed] [Google Scholar]
- 36.Lipps C, May T, Hauser H, et al. Eternity and functionality: rational access to physiologically relevant cell lines. Biol Chem. 2013;394:1637–1648. doi: 10.1515/hsz-2013-0158. [DOI] [PubMed] [Google Scholar]
- 37.McLean J. Immortalization Strategies for Mammalian Cells. In: Jenkins N, editor. Animal Cell Biotechnology: Methods and Protocols. Humana Press; United States of America: 1999. pp. 61–72. [Google Scholar]
- 38.Noguchi H, Kobayashi N. Controlled expansion of mammalian cell populations by reversible immortalization. J Biotechnol Biomater. 2013;3:158. [Google Scholar]
- 39.Waki K, Anno K, Ono T, et al. Establishment of functional telomerase immortalized human hepatocytes and a hepatic stellate cell line for telomere-targeting anticancer drug development. Cancer Sci. 2010;101:1678–1685. doi: 10.1111/j.1349-7006.2010.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wege H, Chui MS, Le HT, et al. In vitro expansion of human hepatocytes is restricted by telomere-dependent replicative aging. Cell Transplant. 2003;12:897–906. doi: 10.3727/000000003771000138. [DOI] [PubMed] [Google Scholar]
- 41.Curran TR, Bahner RI, Oh W, et al. Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Exp Cell Res. 1993;209:53–57. doi: 10.1006/excr.1993.1284. [DOI] [PubMed] [Google Scholar]
- 42.Okitsu T, Kobayashi N, Jun HS, et al. Transplantation of reversibly immortalized insulin-secreting human hepatocytes controls diabetes in pancreatectomized pigs. Diabetes. 2004;53:105–112. doi: 10.2337/diabetes.53.1.105. [DOI] [PubMed] [Google Scholar]
- 43.Totsugawa T, Yong C, Rivas-Carrillo JD, et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74–82. doi: 10.1016/j.jhep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology. 2004;45:33–38. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- 46.Basu A, Meyer K, Ray RB, et al. Hepatitis C virus core protein is necessary for the maintenance of immortalized human hepatocytes. Virology. 2002;298:53–62. doi: 10.1006/viro.2002.1460. [DOI] [PubMed] [Google Scholar]
- 47.Werner A, Duvar S, Müthing J, et al. Cultivation and characterization of a new immortalized human hepatocyte cell line, HepZ, for use in an artificial liver support system. Ann NY Acad Sci. 1999;875:364–368. doi: 10.1111/j.1749-6632.1999.tb08518.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Mani P, Sarkar DP, et al. Ex vivo gene transfer into hepatocytes. Methods Mol Biol. 2009;481:117–140. doi: 10.1007/978-1-59745-201-4_11. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi N, Noguchi H, Watanabe T, et al. Role of immortalized hepatocyte transplantation in acute liver failure. Transplant Proc. 2001;33:645–646. doi: 10.1016/s0041-1345(00)02182-5. [DOI] [PubMed] [Google Scholar]
- 50.Kazuki Y, Oshimura M. Human artificial chromosomes for gene delivery and the development of animal models. Mol Ther. 2011;19:1591–1601. doi: 10.1038/mt.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahler MH, Irani A, Malhi H, et al. The application of a lentiviral vector for gene transfer in fetal human hepatocytes. J Gene Med. 2000;2:186–193. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<186::AID-JGM100>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi N, Noguchi H, Fujiwara T, et al. Establishment of a highly differentiated immortalized adult human hepatocyte cell line by retroviral gene transfer. Transplant Proc. 2000;32:2368–2369. doi: 10.1016/s0041-1345(00)01702-4. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen TH, Oberholzer J, Birraux J, et al. Highly efficient lentiviral vector-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Mol Ther. 2002;6:199–209. doi: 10.1006/mthe.2002.0653. [DOI] [PubMed] [Google Scholar]
- 54.Ohashi K, Park F, Kay MA. Hepatocyte transplantation: clinical and experimental application. J Mol Med. 2001;79:617–630. doi: 10.1007/s001090100260. [DOI] [PubMed] [Google Scholar]
- 55.Pages JC, Andreoletti M, Bennoun M, et al. Efficient retroviral-mediated gene transfer into primary culture of murine and human hepatocytes: expression of the LDL receptor. Hum Gene Ther. 1995;6:21–30. doi: 10.1089/hum.1995.6.1-21. [DOI] [PubMed] [Google Scholar]
- 56.Selden C, Mellor N, Rees M, et al. Growth factors improve gene expression after lentiviral transduction in human adult and fetal hepatocytes. J Gene Med. 2007;9:67–76. doi: 10.1002/jgm.1000. [DOI] [PubMed] [Google Scholar]
- 57.Zamule SM, Strom SC, Omiecinski CJ. Preservation of hepatic phenotype in lentiviral-transduced primary human hepatocytes. Chem Biol Interact. 2008;173:179–186. doi: 10.1016/j.cbi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito M, Ito R, Yoshihara D, et al. Immortalized hepatocytes using human artificial chromosome. Cell Transplant. 2008;17:165–171. doi: 10.3727/000000008783906883. [DOI] [PubMed] [Google Scholar]
- 59.Ito M, Ikeno M, Nagata H, et al. Treatment of nonalbumin rats by transplantation of immortalized hepatocytes using artificial human chromosome. Transplant Proc. 2009;41:422–424. doi: 10.1016/j.transproceed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 60.Shitara S, Kakeda M, Nagata K, et al. Telomerase-mediated life-span extension of human primary fibroblasts by human artificial chromosome (HAC) vector. Biochem Biophys Res Commun. 2008;369:807–811. doi: 10.1016/j.bbrc.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe T, Shibata N, Westerman KA, et al. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75:1873–1880. doi: 10.1097/01.TP.0000064621.50907.A6. [DOI] [PubMed] [Google Scholar]
- 62.Inoue Y, Miyazaki M, Tsuji T, et al. Reactivation of liver-specific gene expression in an immortalized human hepatocyte cell line by introduction of the human HNF4alpha2 gene. Int J Mol Med. 2001;8:481–487. doi: 10.3892/ijmm.8.5.481. [DOI] [PubMed] [Google Scholar]
- 63.Soltanpour Y, Hilgendorf C, Ahlström MM, et al. Characterization of THLE-cytochrome P450 (P450) cell lines: gene expression background and relationship to P450-enzyme activity. Drug Metab Dispos. 2012;40:2054–2058. doi: 10.1124/dmd.112.045815. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi N, Kunieda T, Sakaguchi M, et al. Active expression of p21 facilitates differentiation of immortalized human hepatocytes. Transplant Proc. 2003;35:433–434. doi: 10.1016/s0041-1345(02)03784-3. [DOI] [PubMed] [Google Scholar]
- 65.Kunieda T, Kobayashi N, Sakaguchi M, et al. Transduction of immortalized human hepatocytes with p21 to enhance differentiated phenotypes. Cell Transplant. 2002;11:421–428. [PubMed] [Google Scholar]
- 66.Allen KJ, Reyes R, Demmler K, et al. Conditionally immortalized mouse hepatocytes for use in liver gene therapy. J Gastroenterol Hepatol. 2000;15:1325–1332. [PubMed] [Google Scholar]
- 67.Fox IJ, Chowdhury NR, Gupta S, et al. Conditional immortalization of Gunn rat hepatocytes: an ex vivo model for evaluating methods for bilirubin-UDP-glucuronosyltransferase gene transfer. Hepatology. 1995;21:837–846. [PubMed] [Google Scholar]
- 68.May T, Hauser H, Wirth D. Transcriptional control of SV40 T-antigen expression allows a complete reversion of immortalization. Nucleic Acids Res. 2004;32:5529–5538. doi: 10.1093/nar/gkh887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerman KA, Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci USA. 1996;93:8971–8976. doi: 10.1073/pnas.93.17.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paillard F. Reversible cell immortalization with the Cre-lox system. Hum Gene Ther. 1999;10:1597–1598. doi: 10.1089/10430349950017590. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi N, Noguchi H, Fujiwara T, et al. Establishment of a reversibly immortalized human hepatocyte cell line by using Cre/loxP site-specific recombination. Transplant Proc. 2000;32:1121–1122. doi: 10.1016/s0041-1345(00)01154-4. [DOI] [PubMed] [Google Scholar]
- 72.Anastassiadis K, Rostovskaya M, Lubitz S, et al. Precise conditional immortalization of mouse cells using tetracycline-regulated SV40 large T-antigen. Genesis. 2010;48:220–232. doi: 10.1002/dvg.20605. [DOI] [PubMed] [Google Scholar]
- 73.Jazwa A, Florczyk U, Jozkowicz A, et al. Gene therapy on demand: site-specific regulation of gene therapy. Gene. 2013;525:229–238. doi: 10.1016/j.gene.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 74.Ikeda M, Kato N, Mizutani T, et al. Analysis of the cell tropism of HCV by using in vitro HCV-infected human lymphocytes and hepatocytes. J Hepatol. 1997;27:445–454. doi: 10.1016/s0168-8278(97)80347-9. [DOI] [PubMed] [Google Scholar]
- 75.Kato N, Ikeda M, Mizutani T, et al. Replication of hepatitis C virus in cultured non-neoplastic human hepatocytes. Jpn J Cancer Res. 1996;87:787–792. doi: 10.1111/j.1349-7006.1996.tb02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikeda F, Dansako H, Nishimura G, et al. Amino acid substitutions of hepatitis C virus core protein are not associated with intracellular antiviral response to interferon-α in vitro. Liver Int. 2010;30:1324–1331. doi: 10.1111/j.1478-3231.2010.02299.x. [DOI] [PubMed] [Google Scholar]
- 77.Raychoudhuri A, Shrivastava S, Steele R, et al. Hepatitis C virus infection impairs IRF-7 translocation and alpha interferon synthesis in immortalized human hepatocytes. J Virol. 2010;84:10991–10998. doi: 10.1128/JVI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raychoudhuri A, Shrivastava S, Steele R, et al. ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J Virol. 2011;85:12881–12889. doi: 10.1128/JVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Triyatni M, Saunier B, Maruvada P, et al. Interaction of hepatitis C virus-like particles and cells: a model system for studying viral binding and entry. J Virol. 2002;76:9335–9344. doi: 10.1128/JVI.76.18.9335-9344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu GY, He G, Li CY, et al. Hepatic expression of HCV RNA-dependent RNA polymerase triggers innate immune signaling and cytokine production. Mol Cell. 2012;48:313–321. doi: 10.1016/j.molcel.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu S, Chen J, Wu M, et al. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol. 2010;91:2080–2090. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 82.Brown JJ, Parashar B, Moshage H, et al. A long-term hepatitis B viremia model generated by transplanting non-tumorigenic immortalized human hepatocytes in Rag-2-deficient mice. Hepatology. 2000;31:173–181. doi: 10.1002/hep.510310126. [DOI] [PubMed] [Google Scholar]
- 83.Heim D, Cornils K, Schulze K, et al. Retroviral insertional mutagenesis in telomerase-immortalized hepatocytes identifies RIPK4 as novel tumor suppressor in human hepatocarcinogenesis. Oncogene. 2014 doi: 10.1038/onc.2013.551. in press. [DOI] [PubMed] [Google Scholar]
- 84.Wege H, Heim D, Lütgehetmann M, et al. Forced activation of β-catenin signaling supports the transformation of hTERT-immortalized human fetal hepatocytes. Mol Cancer Res. 2011;9:1222–1231. doi: 10.1158/1541-7786.MCR-10-0474. [DOI] [PubMed] [Google Scholar]
- 85.Guller MC, Andre J, Legrand A, et al. c-Fos accelerates hepatocyte conversion to a fibroblastoid phenotype through ERK-mediated upregulation of paxillin-Serine178 phosphorylation. Mol Carcinog. 2009;48:532–544. doi: 10.1002/mc.20492. [DOI] [PubMed] [Google Scholar]
- 86.Guller M, Toualbi-Abed K, Legrand A, et al. c-fos overexpression increases the proliferation of human hepatocytes by stabilizing nuclear cyclin D1. World J Gastroenterol. 2008;14:6339–6346. doi: 10.3748/wjg.14.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Gottardi A, Vinciguerra M, Sgroi A, et al. Microarray analyses and molecular profiling of steatosis induction in immortalized human hepatocytes. Lab Invest. 2007;87:792–806. doi: 10.1038/labinvest.3700590. [DOI] [PubMed] [Google Scholar]
- 88.Martel C, Allouche M, Esposti DD, et al. Glycogen synthase kinase 3-mediated voltage-dependent anion channel phosphorylation controls outer mitochondrial membrane permeability during lipid accumulation. Hepatology. 2013;57:93–102. doi: 10.1002/hep.25967. [DOI] [PubMed] [Google Scholar]
- 89.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finn JD, Hui D, Downey HD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18:135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121:2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LeCluyse E, Sinz M, Hewitt N, et al. Cytochrome P450 Induction. In: Lu C, Li A, editors. Enzyme Inhibition in Drug Discovery and Development: The Good and the Bad. John Wiley & Sons, Inc.; United States of America: 2010. pp. 265–314. [Google Scholar]
- 93.Rand AA, Rooney JP, Butt CM, et al. Cellular toxicity associated with exposure to perfluorinated carboxylates (PFCAs) and their metabolic precursors. Chem Res Toxicol. 2014;27:42–50. doi: 10.1021/tx400317p. [DOI] [PubMed] [Google Scholar]
- 94.Thompson RA, Isin EM, Li Y, et al. In vitro approach to assess the potential for risk of idiosyncratic adverse reactions caused by candidate drugs. Chem Res Toxicol. 2012;25:1616–1632. doi: 10.1021/tx300091x. [DOI] [PubMed] [Google Scholar]
- 95.Dambach DM, Andrews BA, Moulin F. New technologies and screening strategies for hepatotoxicity: use of in vitro models. Toxicol Pathol. 2005;33:17–26. doi: 10.1080/01926230590522284. [DOI] [PubMed] [Google Scholar]
- 96.Winter HK, Ehrlich VA, Grusch M, et al. Use of four new human-derived liver-cell lines for the detection of genotoxic compounds in the single-cell gel electrophoresis (SCGE) assay. Mutat Res. 2008;657:133–139. doi: 10.1016/j.mrgentox.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Mills JB, Rose KA, Sadagopan N, et al. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J Pharmacol Exp Ther. 2004;309:303–309. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- 98.Wang B, Chen G, Mao Z, et al. Preparation and cellular uptake of PLGA particles loaded with lamivudine. Chin Sci Bull. 2012;57:3985–3993. [Google Scholar]
- 99.Tsuruga Y, Kiyono T, Matsushita M, et al. Effect of intrasplenic transplantation of immortalized human hepatocytes in the treatment of acetaminophen-induced acute liver failure SCID mice. Transplant Proc. 2008;40:617–619. doi: 10.1016/j.transproceed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Willberg CB, Ward SM, Clayton RF, et al. Protection of hepatocytes from cytotoxic T cell mediated killing by interferon-alpha. PLoS One. 2007;2:e791. doi: 10.1371/journal.pone.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho L, Titus AS, Banerjee KK, et al. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging. 2013;5:835–849. doi: 10.18632/aging.100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wargent ET, Zaibi MS, Silvestri C, et al. The cannabinoid Δ(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes. 2013;3:e68. doi: 10.1038/nutd.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caron S, Huaman Samanez C, Dehondt H, et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol Cell Biol. 2013;33:2202–2211. doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lauressergues E, Bert E, Duriez P, et al. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology. 2012;62:784–796. doi: 10.1016/j.neuropharm.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 105.Lasfer M, Vadrot N, Aoudjehane L, et al. Cadmium induces mitochondria-dependent apoptosis of normal human hepatocytes. Cell Biol Toxicol. 2008;24:55–62. doi: 10.1007/s10565-007-9015-0. [DOI] [PubMed] [Google Scholar]
- 106.Kato N, Ikeda M, Sugiyama K, et al. Hepatitis C virus population dynamics in human lymphocytes and hepatocytes infected in vitro. J Gen Virol. 1998;79:1859–1869. doi: 10.1099/0022-1317-79-8-1859. [DOI] [PubMed] [Google Scholar]
- 107.Ikeda M, Nozaki A, Sugiyama K, et al. Characterization of antiviral activity of lactoferrin against hepatitis C virus infection in human cultured cells. Virus Res. 2000;66:51–63. doi: 10.1016/s0168-1702(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki K, Aoki K, Ohnami S, et al. Adenovirus-mediated gene transfer of interferon alpha inhibits hepatitis C virus replication in hepatocytes. Biochem Biophys Res Commun. 2003;307:814–819. doi: 10.1016/s0006-291x(03)01255-5. [DOI] [PubMed] [Google Scholar]
- 109.Ikeda M, Sugiyama K, Mizutani T, et al. Human hepatocyte clonal cell lines that support persistent replication of hepatitis C virus. Virus Res. 1998;56:157–167. doi: 10.1016/s0168-1702(98)00063-x. [DOI] [PubMed] [Google Scholar]
- 110.Tsuchihara K, Tanaka T, Hijikata M, et al. Specific interaction of polypyrimidine tract-binding protein with the extreme 3′-terminal structure of the hepatitis C virus genome, the 3′X. J Virol. 1997;71:6720–6726. doi: 10.1128/jvi.71.9.6720-6726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cuconati A, Mills C, Goddard C, et al. Suppression of AKT anti-apoptotic signaling by a novel drug candidate results in growth arrest and apoptosis of hepatocellular carcinoma cells. PLoS One. 2013;8:e54595. doi: 10.1371/journal.pone.0054595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brockhausen J, Tay SS, Grzelak CA, et al. miR-181a mediates TGF-β-induced hepatocyte EMT and is dysregulated in cirrhosis and hepatocellular cancer. Liver Int. 2014 doi: 10.1111/liv.12517. in press. [DOI] [PubMed] [Google Scholar]
- 113.Wilson R, Warner N, Ryan K, et al. The hepatitis B e antigen suppresses IL-1β-mediated NF-κB activation in hepatocytes. J Viral Hepat. 2011;18:e499–e507. doi: 10.1111/j.1365-2893.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 114.Shah F, Louise-May S, Greene N. Chemotypes sensitivity and predictivity of in vivo outcomes for cytotoxic assays in THLE and HepG2 cell lines. Bioorg Med Chem Lett. 2014;24:2753–2757. doi: 10.1016/j.bmcl.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 115.Gustafsson F, Foster AJ, Sarda S, et al. A correlation between the in vitro drug toxicity of drugs to cell lines that express human P450s and their propensity to cause liver injury in humans. Toxicol Sci. 2014;137:189–211. doi: 10.1093/toxsci/kft223. [DOI] [PubMed] [Google Scholar]
- 116.Rodrigues AV, Rollison HE, Martin S, et al. In vitro exploration of potential mechanisms of toxicity of the human hepatotoxic drug fenclozic acid. Arch Toxicol. 2013;87:1569–1579. doi: 10.1007/s00204-013-1056-y. [DOI] [PubMed] [Google Scholar]
- 117.Foster AJ, Prime LH, Gustafsson F, et al. Bioactivation of the cannabinoid receptor antagonist rimonabant to a cytotoxic iminium ion metabolite. Chem Res Toxicol. 2013;26:124–135. doi: 10.1021/tx300418w. [DOI] [PubMed] [Google Scholar]
- 118.Antolino-Lobo I, Meulenbelt J, Nijmeijer SM, et al. Differential roles of phase I and phase II enzymes in 3,4-methylendioxymethamphetamine-induced cytotoxicity. Drug Metab Dispos. 2010;38:1105–1112. doi: 10.1124/dmd.110.032359. [DOI] [PubMed] [Google Scholar]
- 119.Saha S, New LS, Ho HK, et al. Direct toxicity effects of sulfo-conjugated troglitazone on human hepatocytes. Toxicol Lett. 2010;195:135–141. doi: 10.1016/j.toxlet.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Saha S, New LS, Ho HK, et al. Investigation of the role of the thiazolidinedione ring of troglitazone in inducing hepatotoxicity. Toxicol Lett. 2010;192:141–149. doi: 10.1016/j.toxlet.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 121.Ip BC, Hu KQ, Liu C, et al. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res. 2013;6:1304–1316. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krajka-Kuzniak V, Paluszczak J, Oszmianski J, et al. Hawthorn (Crataegus oxyacantha L.) bark extract regulates antioxidant response element (ARE)-mediated enzyme expression via Nrf2 pathway activation in normal hepatocyte cell line. Phytother Res. 2014;28:593–602. doi: 10.1002/ptr.5035. [DOI] [PubMed] [Google Scholar]
- 123.Krajka-Kuzniak V, Paluszczak J, Celewicz L, et al. Phloretamide, an apple phenolic compound, activates the Nrf2/ARE pathway in human hepatocytes. Food Chem Toxicol. 2013;51:202–209. doi: 10.1016/j.fct.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 124.Krajka-Kuzniak V, Paluszczak J, Baer-Dubowska W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol In Vitro. 2013;27:149–156. doi: 10.1016/j.tiv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 125.Ait-Goughoulte M, Kanda T, Meyer K, et al. Hepatitis C virus genotype 1a growth and induction of autophagy. J Virol. 2008;82:2241–2249. doi: 10.1128/JVI.02093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kanda T, Basu A, Steele R, et al. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J Virol. 2006;80:4633–4639. doi: 10.1128/JVI.80.9.4633-4639.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanda T, Steele R, Ray R, et al. Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J Virol. 2007;81:12375–12381. doi: 10.1128/JVI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Basu A, Saito K, Meyer K, et al. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis. 2006;11:1391–1400. doi: 10.1007/s10495-006-8312-z. [DOI] [PubMed] [Google Scholar]
- 129.Mazumdar B, Meyer K, Ray R. N-terminal region of gelsolin induces apoptosis of activated hepatic stellate cells by a caspase-dependent mechanism. PLoS One. 2012;7:e44461. doi: 10.1371/journal.pone.0044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Gao Y, Theng SS, Zhuo J, et al. FAT10, an ubiquitin-like protein, confers malignant properties in non-tumorigenic and tumorigenic cells. Carcinogenesis. 2014;35:923–934. doi: 10.1093/carcin/bgt407. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Toh HC, Chow P, et al. MicroRNA-224 is up-regulated in hepatocellular carcinoma through epigenetic mechanisms. FASEB J. 2012;26:3032–3041. doi: 10.1096/fj.11-201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomimaru Y, Xu CQ, Nambotin SB, et al. Loss of exon 4 in a human T-cell factor-4 isoform promotes hepatic tumourigenicity. Liver Int. 2013;33:1536–1548. doi: 10.1111/liv.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nonaka M, Tazuma S, Hyogo H, et al. Cytoprotective effect of tauroursodeoxycholate on hepatocyte apoptosis induced by peroxisome proliferator-activated receptor gamma ligand. J Gastroenterol Hepatol. 2008;23:e198–e206. doi: 10.1111/j.1440-1746.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- 135.Nakamura K, Yamagishi S, Yoshida T, et al. Hydrogen peroxide stimulates pigment epithelium-derived factor gene and protein expression in the human hepatocyte cell line OUMS-29. J Int Med Res. 2007;35:427–432. doi: 10.1177/147323000703500319. [DOI] [PubMed] [Google Scholar]
- 136.Shishido S, Koga H, Harada M, et al. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003;37:136–147. doi: 10.1053/jhep.2003.50014. [DOI] [PubMed] [Google Scholar]


