Summary
Fas-mediated apoptosis underlies a plethora of liver pathologies and toxicities. As a consequence, this process is a major research topic in the field of experimental and clinical hepatology. The present chapter describes the set-up of an in vitro model of hepatocellular apoptotic cell death. In essence, this system consists of freshly isolated hepatocytes cultured in a monolayer configuration, which are exposed to a combination of Fas ligand and cycloheximide. This in vitro model has been characterized by using a set of well-acknowledged cell death markers. This experimental system allows the study of the entire course of Fas-mediated hepatocellular cell death, going from early apoptosis to secondary necrosis, and hence can serve a broad range of in vitro pharmaco-toxicological purposes.
Keywords: apoptosis, secondary necrosis, in vitro model, Fas ligand, primary hepatocyte
1. Introduction
Historically, the liver has gained particular attention of toxicologists, as it represents a key target organ of chemical-induced cell injury. Indeed, hepatocytes drive the majority of the xenobiotic biotransformation machinery in the organism and are therefore frequently involved in toxicity (1-3). For many years, it was thought that cell death elicited by toxicants primarily occurred through necrosis. It has now become clear that an alternative cell death mode, namely apoptosis, predominates in toxicant-induced cytolethality (3-6). Apoptosis is a well-orchestrated process, relying on the proteolytic activity of an evolutionarily conserved family of cysteine proteases, called caspases. In fact, 2 major apoptotic pathways have been described, the extrinsic signalling cascade and the intrinsic pathway (3, 4, 6, 7). The former is initiated by the binding of a specific subset of ligands, including the Fas ligand, to their corresponding receptors at the cell membrane surface (6, 7). Hepatocytes highly express the Fas receptor (8) and its ligand binding results in the proteolytic cleavage and auto-activation of procaspase 8. Activated caspase 8 induces caspase 3, which subsequently cleaves a broad spectrum of cellular proteins. In the intrinsic apoptotic pathway, cytochrome C is released from mitochondria, a process that is mediated by members of the B-cell lymphoma-2 protein family. Activation of this pathway occurs upon DNA damage. Cytochrome C triggers caspase 9 activation, which in turn also induces caspase 3 (6, 7).
A number of protocols have been described for studying hepatocellular apoptosis in vivo, including the direct administration of cell death-evoking toxicants to animals (9), the application of genetically-modified subjects (10) and the use of partially hepatectomized rodents (11). The intraperitoneal injection of anti-Fas antibodies in mice, known to activate the Fas receptor, causes fulminant hepatic failure and severe hepatocyte injury, which eventually burgeons into animal death (12). Such experiments not only raise serious ethical questions, but are also of limited scientific value. Indeed, apoptotic cells are barely detectable in vivo, as they are rapidly engulfed by neigbouring phagocytes. During in vitro experimentation, where phagocytosis does not take place, the full course of apoptosis can be monitored, whereby the late apoptotic phase is typically followed by secondary necrosis (4-7). Cell lines are frequently used experimental tools in in vitro apoptosis research. However, these cells, such as HepG2 cells, Huh7 cells and Hep3B cells, are often derived from tumours and have typically acquired high resistance against apoptosis (13-15). Primary cells may offer a better alternative, as they display in vivo-like sensitivity to apoptosis, at least during short-term culture (14). Among the numerous experimental strategies that have been followed to provoke apoptotic cell death in primary hepatocyte cultures, the use of Fas receptor stimuli is a most reasonable approach, as it directly affects the physiological pathway (16). In vitro models of Fas-mediated hepatocellular cell death have yet been exploited for a wide range of biomedical applications (17-20). In the majority of such studies, apoptosis is induced without any further investigation of the cell death response as such. However, insight into the underlying mechanisms of cytolethality is of crucial importance, since they may have a direct impact on the outcome of the study. Therefore, the establishment of an in vitro model of Fas-mediated hepatocyte cell death along with its characterization are described in the current chapter.
2. Materials
2.1. Establishment of the in vitro model of Fas-mediated hepatocellular cell death
Hepatocyte seeding medium. William’s medium E containing 7 ng/mL glucagon, 292 mg/mL L-glutamine, 7.33 IE/mL sodium benzyl penicillin, 50 μg/mL kanamycin monosulfate, 10 μg/mL sodium ampicillin, 50 μg/mL streptomycin sulfate and 10% fetal bovine serum (see Note 1). Prepare in a laminar air flow cabinet and store for maximum 7 days at 4°C. Prior to use, the hepatocyte seeding medium should be placed for 30 min in a thermostated bath at 37°C (see Note 2).
Hepatocyte culture medium. Serum-free hepatocyte seeding medium supplemented with 25 μg/mL hydrocortisone sodium hemisuccinate and 0.5 μg/mL insulin (see Note 1). Prepare in a laminar air flow cabinet and store for maximum 7 days at 4°C. Prior to use, the hepatocyte culture medium should be placed for 30 min in a thermostated bath at 37°C (see Note 2).
Hepatocyte cell death medium. Hepatocyte culture medium supplemented with 200 ng/ml Fas ligand (Alexis, Switzerland) (see Note 3) and 2 μg/mL cycloheximide (Sigma-Aldrich, Belgium) (see Note 4). Prepare ex tempore in a laminar air flow cabinet. Prior to use, the hepatocyte cell death medium should be placed for 30 min in a thermostated bath at 37°C.
Incubator (37°C ± 1°C, 90% ± 5% humidity, 5% ± 1% CO2).
Laminar air flow cabinet.
3.5 cm diameter plastic cell culture dishes.
Thermostated bath (37°C).
2.2. Characterization of the in vitro model of Fas-mediated hepatocellular cell death
Phosphate-buffered saline (PBS). 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4.2H2O, 1.8 mM KH2PO4 in deionized water. Adjust to pH 7.4 and store for maximum 6 months at 4°C. Prior to use, PBS should be placed for 30 min at room temperature.
PBS supplemented with divalent cations (PBSD+). PBS containing 1.2 mM CaCl2 and 340 μM MgCl2.6H2O in deionized water. Adjust to pH 7.4 and store for maximum 6 months at 4°C. Prior to use, the PBSD+ solution should be placed for 30 min at room temperature.
Staining solution. 140 mM NaCl, 5 mM CaCl2, 10 mM N-[2-hydroxyethyl]piperazine-N’[2-ethaansulfonzuur], 2% annexin-V-fluos (Roche Diagnostics, Germany), 3 μg/mL Hoechst 33342 (Invitrogen, Belgium) and 1 μg/mL propidium iodide (Invitrogen, Belgium) in deionized water (see Note 5). Adjust to pH 7.4 and store for maximum 6 months at 4°C. Prior to use, the staining solution should be placed for 30 min at room temperature.
Fluorescence microscope, camera, computer and software.
3. Methods
3.1. Establishment of a monolayer culture of primary hepatocytes and induction of cell death
Use freshly isolated primary rat hepatocytes (21) (see Note 6).
Evenly plate the hepatocytes on 3.5 cm diameter plastic culture dishes at a density of 0.56 × 105 cells/cm2 in hepatocyte seeding medium (see Notes 7 and 9). Place the cell cultures in an incubator at 37°C and 5% CO2 for 4 h.
Remove the hepatocyte seeding medium and replace by identical volumes of hepatocyte culture medium. Place the cell cultures in an incubator at 37°C and 5% CO2 for 24 h.
Replace the hepatocyte culture medium. Place the cell cultures in an incubator at 37°C and 5% CO2 for 20 h.
Remove the hepatocyte culture medium and replace by identical volumes of hepatocyte cell death medium. Place the cell cultures in an incubator at 37°C and 5% CO2 (see Note 10).
Sample at the start of cell death induction and 2, 4 and 6 h thereafter (see Note 11).
3.2. In situ staining of cell death markers
Remove the hepatocyte cell death medium from the 3.5 cm diameter cell culture dishes and wash the cells twice with 2 mL of the PBSD+ solution.
Add 2 mL of the staining solution to each culture dish and leave on for 15 min at room temperature in the dark.
Remove the staining solution from the culture dishes and wash the cells 4 times with 2 mL of the PBSD+ solution.
Analyze the culture dishes by means of fluorescence microscopy at 100× magnification.
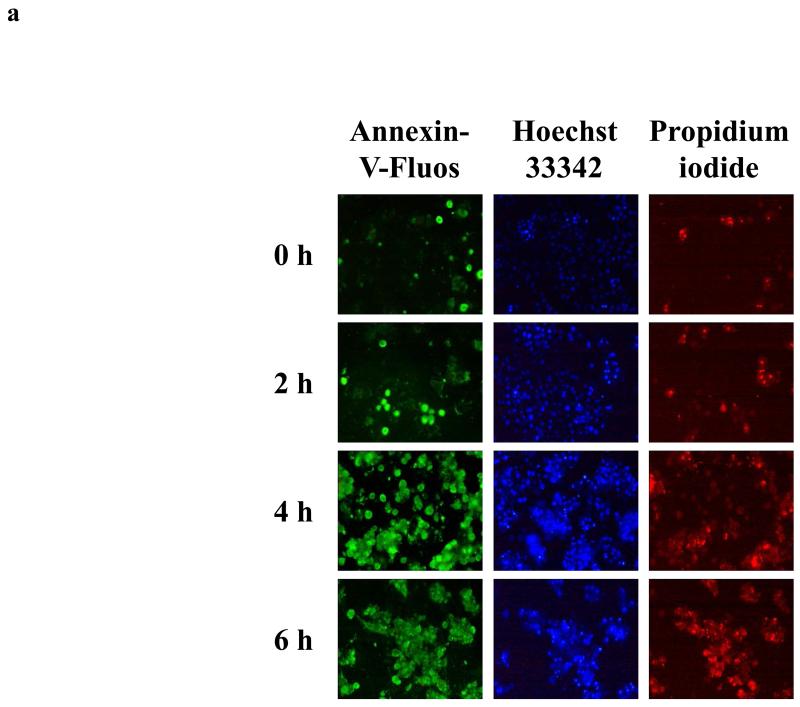
Take at least 5 images per culture dish by using appropriate filters (Fig. 1) (see Note 12).
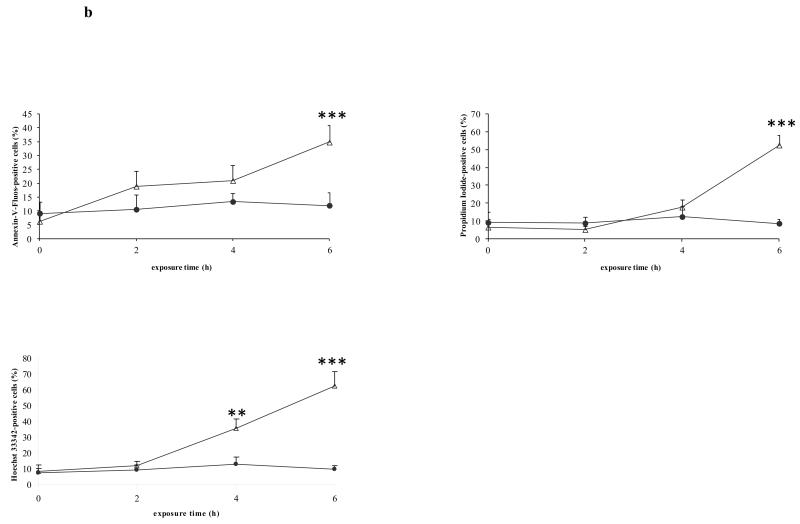
Count the number of cells positive for the marker concerned in each image and express relative to the total number of nuclei present (Fig. 1) (see Notes 13, 14, 15 and 16).
Figure 1. In situ staining of cell death markers.
Freshly isolated rat hepatocytes were cultivated in a monolayer configuration and were exposed to 200 ng/mL Fas ligand and 2 μg/mL cycloheximide, starting at 44 h postplating. a) Samples were taken at the start of the exposure (0 h), and after 2, 4 and 6 h, and were stained with annexin-V-fluos, Hoechst 33342 and propidium iodide. b) The number of cells, positive for the concerned marker, was counted in each image and expressed relative to the total number of nuclei present. Data are expressed as the mean ± standard deviation of 3 independent experiments. Results were evaluated by 1-way analysis of variance followed by post hoc Bonferroni tests. ● = control; Δ = Fas ligand/cycloheximide; **p < 0.01; ***p < 0.001. Reproduced in modified form with permission from (32).
Acknowledgements
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel - Belgium (Willy Gepts Fonds UZ-VUB), the University of Sao Paulo - Brazil (USP), the São Paulo Research Foundation - Brazil (FAPESP), the Fund for Scientific Research - Flanders (FWO-Vlaanderen), the European Research Council (project CONNECT), the European Union (FP7) and Cosmetics Europe (projects DETECTIVE and HeMiBio).
Footnotes
The composition of the cell culture medium is a major determinant of the response of primary hepatocytes to experimentally induced cell death. Several commonly used cell culture additives can counteract the occurrence of apoptosis in primary hepatocyte cultures, including glucocorticosteroids (22, 23), insulin (24), glucagon (18) and serum (25). The composition of the cell culture medium used in the current protocol has been optimized in such a way that a cell death response, going from early apoptosis to secondary necrosis, is generated and completed in a total time span of 6 h in cultures of primary rat hepatocytes. The composition of the cell culture medium might be subject to optimization when using hepatocytes from other species.
The sterility of the cell culture media can be checked by adding 1 mL of the medium to 25 mL sterile thioglycollate medium (Oxoid, Belgium) and placing for 2 days in an incubator.
It is strongly recommended to use the soluble Fas ligand and not anti-Fas antibodies to induce cell death in primary hepatocyte cultures. Although both trigger the same pathway, anti-Fas antibodies mediate different effects with different sensitivity in comparison with Fas ligand (26-28).
It is strongly recommended to boost the apoptotic effect of Fas ligand by combining with an inhibitor of protein translation, such as cycloheximide. The concentrations of both Fas ligand and cycloheximide have been optimized in such a way that a cell death response, going from early apoptosis to secondary necrosis, is generated and completed in a total time span of 6 h in cultures of primary rat hepatocytes. These concentrations might be subject to optimization when seeding the cells at cell density other than used in the current protocol or when using hepatocytes from other species.
The concentrations of annexin-V-fluos, Hoechst 33342 and propidium iodide as well as incubation time with these dyes have been optimized for primary rat hepatocyte cultures cultivated at the indicated cell density. These parameters might be subject to optimization when seeding the cells at another cell density or when using hepatocytes from other species.
It is of utmost importance to assess cell viability following hepatocyte isolation, as this procedure typically causes considerable harm to cells. This is routinely done by means of trypan blue exclusion. Cell viability before plating should be at least 85%.
It is strongly recommended to avoid the use of collagen-coated cell culture dishes, as the presence of an extracellular matrix scaffold counteracts the occurrence of cell death (29).
Cell density is a major determinant of the response of cultured hepatocytes to experimentally induced cell death. Hepatocytes cultured at low density (i.e. 0.35 × 105 cells/cm2) display less apoptotic activity in comparison with counterparts seeded at high density (i.e. 1.4 × 105 cells/cm2) (30). The cell density in the current protocol (i.e. 0.56 × 105 cells/cm2) has been optimized in such a way that a cell death response, going from early apoptosis to secondary necrosis, is generated and completed in a total time span of 6 h in cultures of primary rat hepatocytes. The cell density might be subject to optimization when using hepatocytes from other species.
The size of the culture dishes depends on the type of analysis that is intended. For in situ staining of cell death markers, 3.5 cm diameter cell culture dishes are sufficient.
The time of cell death induction is a major determinant of the response of cultured hepatocytes to experimentally induced cell death. Considerable spontaneous cell death is observed in the first 24 h following cell plating as a result of insults underwent during the hepatocyte isolation procedure (31). This might interfere with the experimentally induced cell death process. On the other hand, hepatocytes become resistant to cell death induction as a function of cultivation time, which reflects the progressive dedifferentiation process known to take place in primary hepatocyte cultures (2). Based on own experience, the best time to induce cell death is between 40 and 48 h postplating.
The total duration of sampling in the current protocol (i.e. 6 h) has been optimized in such a way that the entire cell death response, going from early apoptosis to secondary necrosis, can be studied in primary cultures of rat hepatocytes. This timing might be subject to optimization when using hepatocytes from other species.
Excitation wavelengths for Hoechst 33342, annexin-V-fluos and propidium iodide are 346, 488 and 535 nm, while emission wavelengths are 497, 518 and 617 nm, respectively.
Counting should be done in a double blinded fashion. Alternatively, yet technically more demanding, fluorescence-assisted cell sorting can be used for high-throughput counting.
The spots in the cell culture dishes that are used for counting are selected at random. However, since the cells are generally less homogeneously seeded at the borders of the cell culture dish, it is not recommended to consider spots for counting in these areas.
At least 6 spots per cell culture dish should be counted. From a statistical point of view, it is strongly recommended to perform the experiments on hepatocytes isolated from the liver of 3 different human donors or animals. Results can be processed and evaluated by analysis of variance followed by post hoc Bonferroni tests.
Small groups of apoptotic bodies must be considered as remnants from a single cell.
References
- 1.Vanhaecke T, Rogiers V. Hepatocyte cultures in drug metabolism and toxicological research and testing. Methods Mol Biol. 2006;320:209–227. doi: 10.1385/1-59259-998-2:209. [DOI] [PubMed] [Google Scholar]
- 2.Vinken M, Papeleu P, Snykers S, et al. Involvement of cell junctions in hepatocyte culture functionality. Crit Rev Toxicol. 2006;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Gores GJ, Cederbau AI, et al. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 4.Raffray M, Cohen GM. Apoptosis and necrosis in toxicology: a continuum or distinct modes of cell death? Pharmacol Ther. 1997;75:153–177. doi: 10.1016/s0163-7258(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Lechon MJ, O’Connor E, Castell JV, et al. Sensitive markers used to identify compounds that trigger apoptosis in cultured hepatocytes. Toxicol Sci. 2002;65:299–308. doi: 10.1093/toxsci/65.2.299. [DOI] [PubMed] [Google Scholar]
- 6.Gill GH, Dive D. Apoptosis: basic mechanisms and relevance to toxicology. In: Roberts R, editor. Apoptosis in toxicology. Taylor & Francis; London: 2000. pp. 1–20. [Google Scholar]
- 7.Yin X-M, Dong Z. Essentials of apoptosis: a guide for basic and clinical research. Humana Press; New Jersey: 2003. [Google Scholar]
- 8.Feldmann G. Liver apoptosis. J Hepatol. 1997;26:1–11. doi: 10.1016/s0168-8278(97)80491-6. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa S, Usuda K, Fujieda Y, et al. Apoptosis and cell proliferation in rat hepatocytes induced by barbiturates. J Vet Med Sci. 2000;62:23–28. doi: 10.1292/jvms.62.23. [DOI] [PubMed] [Google Scholar]
- 10.Guicciardi ME, Bronk SF, Werneburg NW, et al. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Baier PK, Baumgartner U, Wolff-Vorbeck G, et al. Hepatocyte proliferation and apoptosis in rat liver after liver injury. Hepatogastroenterology. 2006;53:747–752. [PubMed] [Google Scholar]
- 12.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 13.Valavanis C, Hu Y, Yang Y, et al. Model cell lines for the study of apoptosis in vitro. In: Schwartz LM, Ashwell JD, editors. Apoptosis: methods in cell biology. Academic Press; San Diego: 2001. pp. 417–436. [DOI] [PubMed] [Google Scholar]
- 14.Schulze-Bergkamen H, Untergasser A, Dax A, et al. Primary human hepatocytes: a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol. 2003;38:736–744. doi: 10.1016/s0168-8278(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 15.Lei XY, Zhong M, Feng LF, et al. siRNA-mediated Bcl-2 and Bcl-xl gene silencing sensitizes human hepatoblastoma cells to chemotherapeutic drugs. Clin Exp Pharmacol Physiol. 2007;34:450–456. doi: 10.1111/j.1440-1681.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- 16.Maeda S. Mechanisms of active cell death in isolated hepatocytes. In: Berry MN, Edwards AM, editors. The hepatocyte review. Kluwer Academic Publishers; London: 2000. pp. 281–300. [Google Scholar]
- 17.Azzaroli F, Mehal W, Soroka CJ, et al. Ursodeoxycholic acid diminishes Fas-ligand-induced apoptosis in mouse hepatocytes. Hepatology. 2002;36:49–54. doi: 10.1053/jhep.2002.34511. [DOI] [PubMed] [Google Scholar]
- 18.Fladmark KE, Gjertsen BT, Doskeland SO, et al. Fas/APO-1(CD95)-induced apoptosis of primary hepatocytes is inhibited by cAMP. Biochem Biophys Res Commun. 1997;232:20–25. doi: 10.1006/bbrc.1997.6214. [DOI] [PubMed] [Google Scholar]
- 19.Fu T, Blei AT, Takamura N, et al. Hypothermia inhibits Fas-mediated apoptosis of primary mouse hepatocytes in culture. Cell Transplant. 2004;13:667–676. doi: 10.3727/000000004783983495. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, He W, Liu F, et al. Inhibition of mouse hepatocyte apoptosis via anti-Fas ribozyme. World J Gastroenterol. 2004;10:2567–2570. doi: 10.3748/wjg.v10.i17.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papeleu P, Vanhaecke T, Henkens T, et al. Isolation of rat hepatocytes. Methods Mol Biol. 2006;320:229–237. doi: 10.1385/1-59259-998-2:229. [DOI] [PubMed] [Google Scholar]
- 22.Bailly-Maitre B, de Sousa G, Boulukos K, et al. Dexamethasone inhibits spontaneous apoptosis in primary cultures of human and rat hepatocytes via Bcl-2 and Bcl-xL induction. Cell Death Differ. 2001;8:279–288. doi: 10.1038/sj.cdd.4400815. [DOI] [PubMed] [Google Scholar]
- 23.Bailly-Maitre B, de Sousa G, Zucchini N, et al. Spontaneous apoptosis in primary cultures of human and rat hepatocytes: molecular mechanisms and regulation by dexamethasone. Cell Death Differ. 2002;9:945–955. doi: 10.1038/sj.cdd.4401043. [DOI] [PubMed] [Google Scholar]
- 24.Bresgen N, Ohlenschlager I, Wacht N, et al. Ferritin and FasL (CD95L) mediate density dependent apoptosis in primary rat hepatocytes. J Cell Physiol. 2008;217:800–808. doi: 10.1002/jcp.21555. [DOI] [PubMed] [Google Scholar]
- 25.Ethier C, Raymond VA, Musallam L, et al. Antiapoptotic effect of EGF on mouse hepatocytes associated with downregulation of proapoptotic Bid protein. Am J Physiol Gastrointest Liver Physiol. 2003;285:G298–G308. doi: 10.1152/ajpgi.00040.2003. [DOI] [PubMed] [Google Scholar]
- 26.Fadeel B, Thorpe CJ, Yonehara S, et al. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201–209. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- 27.Legembre P, Beneteau M, Daburon S, et al. Cutting edge: SDS-stable Fas microaggregates: an early event of Fas activation occurring with agonistic anti-Fas antibody but not with Fas ligand. J Immunol. 2003;171:5659–5662. doi: 10.4049/jimmunol.171.11.5659. [DOI] [PubMed] [Google Scholar]
- 28.Thilenius AR, Braun K, Russell JH. Agonist antibody and Fas ligand mediate different sensitivity to death in the signaling pathways of Fas and cytoplasmic mutants. Eur J Immunol. 1997;27:1108–1114. doi: 10.1002/eji.1830270510. [DOI] [PubMed] [Google Scholar]
- 29.Vanhaecke T, Henkens T, Kass GE, et al. Effect of the histone deacetylase inhibitor trichostatin A on spontaneous apoptosis in various types of adult rat hepatocyte cultures. Biochem Pharmacol. 2004;68:753–760. doi: 10.1016/j.bcp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Qiao L, Farrell GC. The effects of cell density, attachment substratum and dexamethasone on spontaneous apoptosis of rat hepatocytes in primary culture. In Vitro Cell Dev Biol Anim. 1999;35:417–424. doi: 10.1007/s11626-999-0117-2. [DOI] [PubMed] [Google Scholar]
- 31.Vinken M, Decrock E, Doktorova T, et al. Characterization of spontaneous cell death in monolayer cultures of primary hepatocytes. Arch Toxicol. 2011;85:1589–1596. doi: 10.1007/s00204-011-0703-4. [DOI] [PubMed] [Google Scholar]
- 32.Vinken M, Decrock E, De Vuyst E, et al. Biochemical characterization of an in vitro model of hepatocellular apoptotic cell death. Altern Lab Anim. 2009;37:209–218. doi: 10.1177/026119290903700210. [DOI] [PubMed] [Google Scholar]