Abstract
Background
Both transforming growth factor (TGF)-β1 and activin-A have been implicated in airway remodelling in asthma but the modulation of their specific signalling pathways after disease activation remains undefined.
Objective
To define the expression kinetics of TGF-β1, activin-A ligands and follistatin (a natural activin inhibitor), their Type I and Type II receptors (ALK-1, ALK-5 and ALK-4 and TβRII and ActRIIA/RIIB) and activation of signalling (via pSmad2), in the asthmatic airway following allergen challenge.
Methods
Immunohistochemistry was performed on bronchial biopsies from 15 mild atopic asthmatics (median age 25 years, median FEV1% predicted 97%) at baseline and 24 hours after allergen inhalation. Functional effects of activin-A were evaluated using cultured normal human bronchial epithelial (NHBE) cells.
Results
pSmad2+ epithelial cells increased at 24 hours (p=0.03) and pSmad2 was detected in submucosal cells. No modulation of activin-A, follistatin or TGF-β1 expression was demonstrated. Activin receptor+ cells increased after allergen challenge.: ALK-4 in epithelium (p=0.04) and submucosa (p=0.04), and ActRIIA in epithelium (p=0.01). The TGF-β receptor ALK-5 expression was minimal in the submucosa at baseline and after challenge and was down-regulated in the epithelium after challenge (p=0.02), whereas ALK-1 and TβRII expression in the submucosa increased after allergen challenge (p=0.03 and p=0.004 respectively). ALK-1 and ALK-4 expression by T cells was increased after allergen challenge. Activin-A induced NHBE cell proliferation, was produced by NHBE cells in response to TNF-α, and down-regulated TNF-α and IL-13-induced chemokine production by NHBE cells.
Conclusion
Both TGF-β and activin signalling pathways are activated upon allergen provocation in asthma. Activin-A may contribute to resolution of inflammation.
Keywords: Asthma, activin-A, TGF-β1
Introduction
Transforming growth factor (TGF)-β1 and activin-A are members of the TGF-β superfamily of growth factors. Generally TGF-β1 is a potent inhibitor of inflammation whilst an activator of tissue fibrosis 1. Whilst the exact function of activin-A remains uncertain, it is likely that activin-A also functions to modulate inflammatory responses 2whilst activating tissue repair programmes 3. Activin-A is rapidly induced in Th2 cells upon T cell activation suggesting activin-A may have functions also as a Th2 immunomodulatory cytokine 4;5.
TGF-β ligands are present in an inactive state bound to extracellular matrix and as intracellular stores 6,7, thus analysis of signalling pathway components is required to detect functional activity of these ligands. Activated ligand binds to and signals through a serine-threonine kinase specific Type II receptor. TGF-β1 signalling is via TβRII whilst activin signalling is predominantly via ActRIIA and ActRIIB. Ligand binding to the Type II receptor allows it to complex with and phosphorylate the Type I receptor (termed ALK: activin receptor-like kinase) leading to down stream signalling 8. The predominant Type I receptor for TGF-β1 is ALK-5 but this cytokine can also bind the more selectively expressed receptor ALK-1. Activins signal through ALK-4. Downstream signalling is via phosphorylation of receptor-regulated Smads (R-Smads) that translocate to the nucleus to initiate gene transcription. ALK-5 and ALK-4 signalling is via either phosphorylated (p) Smad2 or Smad3. ALK-1 signalling is via pSmad1/5. Strict regulation of signalling activity is achieved through the induction of inhibitory Smad7 which acts on the Type I receptor leading to receptor degradation. Activins are further regulated by a potent physiological inhibitor, follistatin 9.
Our group and others have previously shown rapid increases in pSmad2 together with eosinophil-derived TGF-β1 after allergen provocation in the asthmatic airway 10;11. We have also demonstrated rapid induction of airway remodelling and inflammation at 24 hours post-allergen challenge 10;12. However, a down-regulation of the key TGF-β1 Type I receptor ALK-5 in asthma compared to the normal airway has been previously detected 13. In addition, low levels of ALK5 expression are present in a murine model of allergen-induced airway injury 14 and lung injury-fibrosis 15. These data suggest that other TGF-β1 receptors and/or other cytokines may be involved in chronic allergic airway inflammation and remodelling in asthma. Activin-A has been implicated in airway inflammation in mouse models of allergen challenge 14, was elevated in serum from symptomatic asthmatics, and detected in peripheral blood Th2 cells from asthmatics 4. We therefore hypothesised that rapid expression of pSmad2 in the airways after allergen challenge in asthma may be related to activation of activin-A signalling. Here, we examined the time course of activation of TGF-β and activin-A signalling and receptor modulation at baseline and 24 hours after allergen challenge in mild asthma.
Methods
Volunteer Details and Study Design
The study was approved by the Royal Brompton and Harefield Hospital Ethics Committee and volunteers gave written informed consent. Fifteen volunteers with a history of atopic asthma together with either a 15% increase in FEV1 to β2 agonist, or methacholine PC20 ≤8 mg/ml were recruited. The median age was 25 (range 19-46) years with a FEV1 % predicted 97 (range 75.4-125.7) % at study entry with a methacholine PC20 of 2.1 (1.2-3.6) mg/ml (geometric mean ± 95% CI). All subjects demonstrated positive skin prick tests (wheal size ≥3mm) (SPT) to one or more of the aeroallergens house dust mite, cat dander or grass (ALK, Hørsholm, Denmark). Volunteers sensitive to pollens were studied outside of the season. Volunteers were controlled with only rescue β2 agonists at the time of study and had no clinical features of infection for at least 4 weeks before starting the study and none throughout the study period. The study design is previously described 12. Briefly, bronchial biopsies obtained at baseline and then 24 hours post allergen challenge were evaluated. All volunteers were non-smokers. All bronchoscopies were performed between 08.30-09.00am.
Immunohistochemistry (IHC)
Tissue processing and immunostaining was performed as previously described 16;17as was the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method 16 to identify specific binding of antibodies to cells. The APAAP reaction was visualised using appropriate Vectastain-ABC-AP kits and the Fast Red chromogen. A chicken polyclonal antibody against TGF-β1 and a polyclonal goat antibody against human activin-A were used (R & D Systems, Abingdon, UK). A polyclonal goat antibody against follistatin was used (R&D Systems). Inflammatory cell co-localisation of activin-A was performed using a standard double staining technique. Briefly, activin-A expression was localized using 3, 3′-diaminobenzidine (DAB) chromogen that produces a brown end product, whilst inflammatory cell markers were identified using Fast Red as previously described 12. The antibodies directed against the Type I and Type II receptors and Smads were a kind gift from Prof. P.Sideras, Biomedical Research Foundation of the Academy of Athens, Greece. Briefly, polyclonal antibodies were raised in rabbits against synthetic polypeptides and tested for specificity by immunoprecipitation and western blotting as previously described 14;18. These antibodies have been previously validated in human tissue 19. Double staining for ALK-1 (Autogen Bioclear, Wiltshire, UK), ALK-4 (GeneTex,Irvine,CA) and TBRII (R &D Systems) withCD3 (Dako, Cambridge, UK) was performed as previously described 16. Incubation of tissue sections with an irrelevant species IgG antibody served as a negative control. Cells counts were done in a blinded fashion by an independent observer using an Olympus BH-2 Microscope (Olympus Corp., Lake Success, NY, USA) as previously described 10.
Epithelial cell culture and stimulation
The primary cultured normal human bronchial epithelial cells (NHBE, Lonza Rockland Inc, Rockland, ME, USA) were seeded in 6-well plastic plates (Sigma-Aldrich, Gillingham, Dorset, UK) previously coated with 2.5 mg/ml collagen type I (Sigma-Aldrich) in 0.016 mM acetic acid. Cells were grown at 37°C in a humidified 5% CO2 atmosphere in bronchial epithelium growth medium (BEGM) supplemented with a bulletkit containing 0.5 ng/ml recombinant human epidermal growth factor (EGF), 500 ng/ml hydrocortisone, 0.005 mg/ml insulin, 0.035 mg/ml bovine pituitary extract, 500 nM ethanolamine, 500 nM phosphoethanolamine, 0.01 mg/ml transferrin, 6.5 ng/ml 3,3,5-triiodothyronine, 500 ng/ml adrenaline, and 0.1 ng/ml retinoic acid (Lonza). Once they reached 80% confluence, epithelial cells were used for experiments. Activin A, follistatin, IL-13 and TNF-α were all from R&D Systems.
The effect of activin A on NHBE cell proliferation was determined using the ViaLight Cell proliferation BioAssay Kit (Lonza) according to the manufacturer’s instructions after 24h stimulation. The concentrations of IL-6, CXCL8/IL-8, IL-13 and CCL5/RANTES were assessed by ELISA (Peprotech EC, London, UK) and activin-A was measured by activin-A Duoset ELISA (R&D Systems). A Human Chemokine Ten-Plex Antibody Bead Kit (Invitrogen, Carlsbad, California, USA) was used to detect the level of CCL11/eotaxin, CXCL1/GRO-alpha, CXCL10/IP-10, CXCL9/MIG, CCL2/MCP-1, CCL8/MCP-2, CCL7/MCP-3, CCL3/MIP-1alpha and CCL4/beta and CCL5/RANTES; the plate was analyzed with a Luminex 100TM instrument (Luminex xMAP Technology, Oosterhout, The Netherlands). ELISAs and the Luminex plate were all assessed on supernatants from the 24h stimulation time point.
Statistical analysis
Cell counts are presented as the median ± interquartile range. All paired within-subject data was analysed using the Wilcoxon signed rank test. For time course experiments comparability between the means was assessed by the Friedman test, and then Wilcoxon test as a post-test. Data was analysed using Graph Pad Prism Version 4 (Graph Pad Software Inc., San Diego, CA) or StatView (San Francisco, CA). Significance was accepted as p<0.05.
Results
Activation of pSmad2 signalling is seen 24 hours after allergen challenge
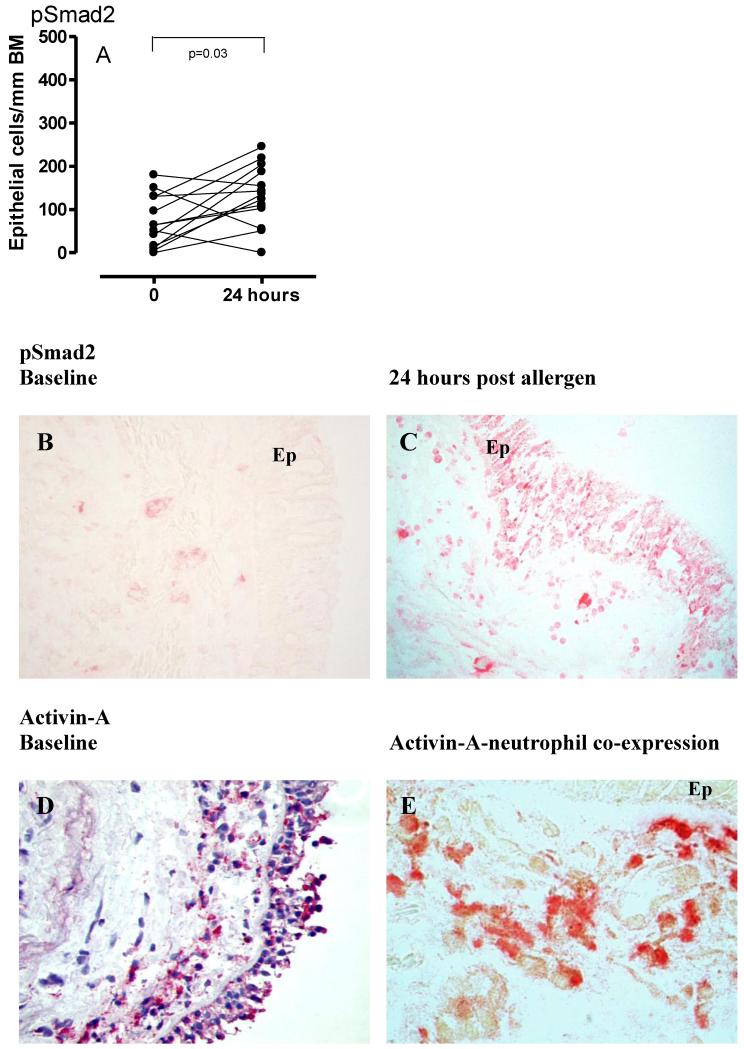
Allergen challenge was associated with significant increases in the number of pSmad2 positive epithelial cells at 24 hours post allergen challenge (p=0.03, n=13 volunteers) (Figure 1A-C), suggesting rapid activation of TGF-β and/or activin signalling in response to allergen. Submucosal cells also stained positive for pSmad2 after allergen challenge, although this increase was not significant (Table 1).
Figure 1. pSmad2 expression baseline and post allergen.
pSmad2 (1A) expression in airway epithelium in response to allergen challenge at the 24 hour time point is summarised. Representative photomicrographs from a paired volunteer at baseline and 24 hours post-allergen are given (1B-C ×20 magnification). Representative photomicropgraph of activin-A expression in the airway (1D ×40) and co-localisation (brown via DAB) to elastase+ positive neutrophils (Fast Red) (1E ×100) is given.
Table 1. Summary of ligand and signalling component expression.
Epithelial expression (median ± interquartile range) is given as the number of cells per unit length of BM (cells/mm BM). Expression in the submucosa is expressed as cells/mm2.
| Epithelium | Submucosa | |||
|---|---|---|---|---|
|
| ||||
| Protein | Baseline | 24 hours | Baseline | 24 hours |
| TGF-β1 | 197.3 (67-236) | 240 (174.4-272) | 40 (26.7-60) | 44.80 (21.35-72) |
| Activin-A | 132 (80-170.7) | 114 (99.20-153) | 60 (32-112) | 83 (32-147.2) |
| Follistatin | 108 (0-156) | 121.6 (48-192) | Absent or minimal | Absent or minimal |
| ALK-5 | 225.6 (132.2-344.9) | 154.7* (64.70-210.2) | Absent or minimal | Absent or minimal |
| ALK-1 | 116.4 (77.85-177.5) | 169 (64.35-356) | 0 (0-9.35) | 18.30* (0-65.35) |
| ALK-4 | 178.7 (152-240) | 236* (224-310) | 10.70 (1.35-36.80) | 59.50* (6.4-146) |
| TβRII | 352 (304-400) | 360 (320-440) | 115.2 (32-148) | 213.3* (144-260) |
| ActRIIA | 0 (0-67.2) | 99* (0-169.6) | 4 (0-24) | 12 (0-39.2) |
| ActRIIB | 131.2 (48-166.4) | 144 (96-196) | Absent or minimal | Absent or minimal |
| pSmad2 | 64 (14-130.5) | 136* (78.65-196.2) | 4 (0-9.6) | 17.6 (2.3-69.3) |
p<0/05.
TGF-β1 and activin-A were expressed in the airway of mild asthmatics at baseline. There was no modulation of numbers of cells positive for TGF-β1, activin-A or follistatin post-allergen challenge in either epithelium or submucosa (Table 1). Of the activin-A positive submucosal cells, 51.1 % (24.9-59.1) were neutrophils. In addition, at 24 hours 32.5% (11.7-46.2) of the infiltrating neutrophil population stained for activin-A (n=6). Mast cells (tryptase+), CD4+ T cells and macrophages (CD68+) were also identified as sources of activin-A (data not shown). Representative photomicrographs of mucosal activin-A expression and co-localisation to neutrophils are shown (Figure 1D and E).
Allergen inhalation challenge modulates Type I and Type II receptor expression for TGF-β and activin-A
Since both TGF-β1 and activin-A signal via pSmad2, and both ligands are expressed in asthma, we examined the effect of allergen challenge on Type I and Type II receptor expression both for TGF-β1 (ALK-1, ALK-5 and TβRII) and activin-A (ALK4 and ActRIIA/ActRIIB).
TGF-β receptors
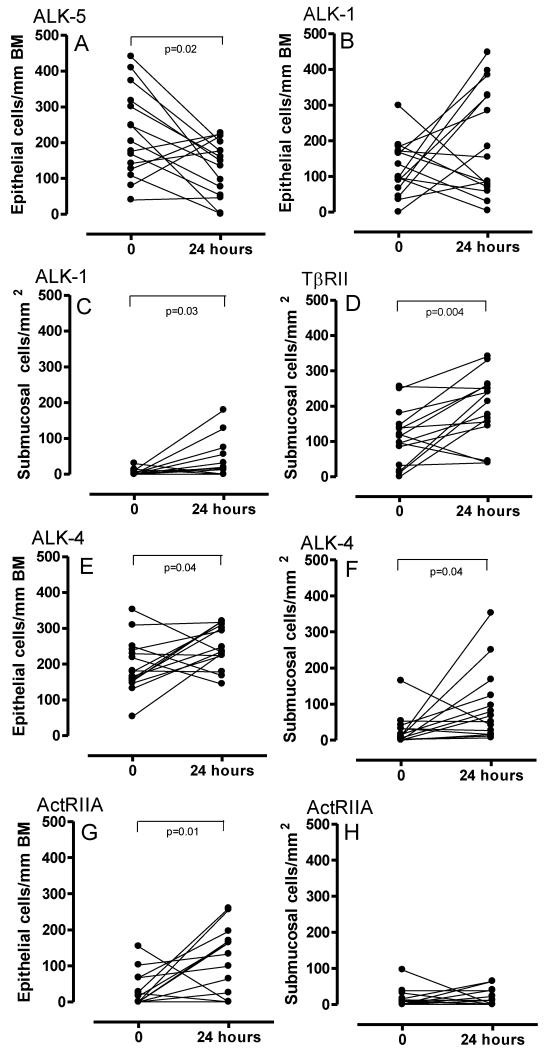
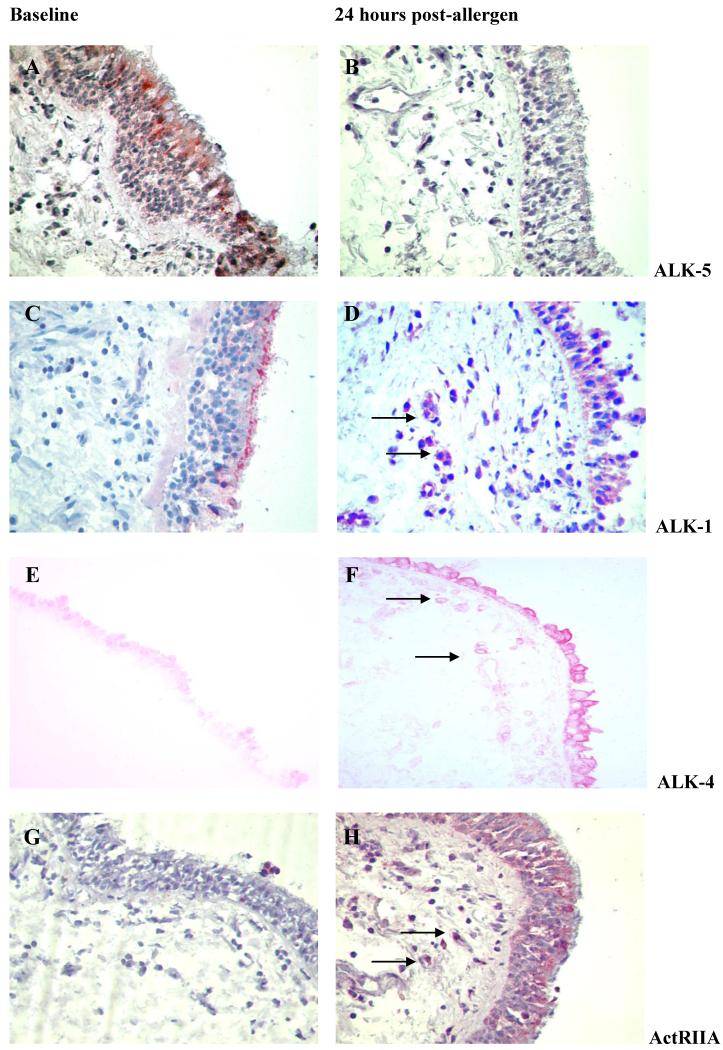
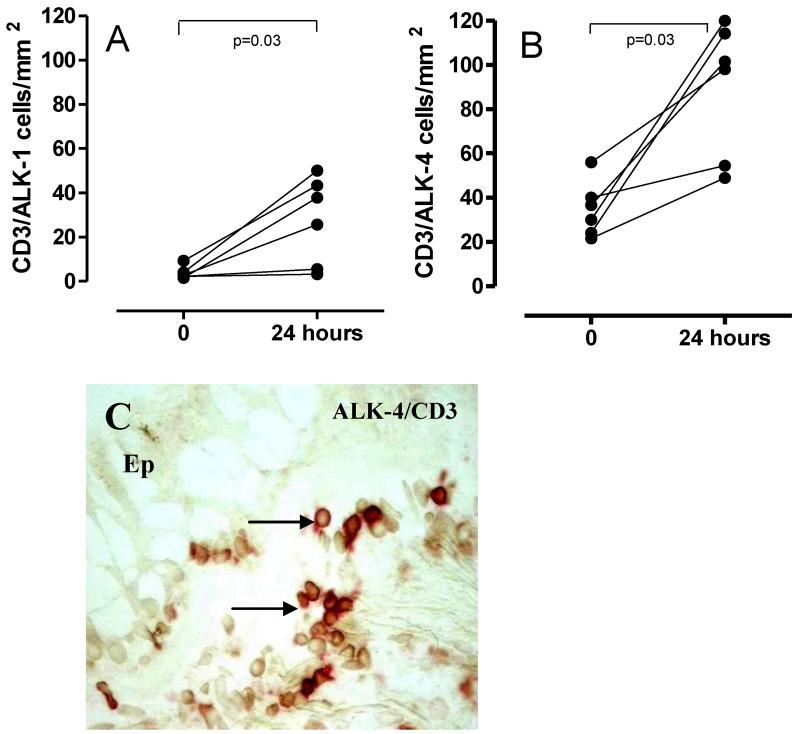
Allergen challenge was associated with a decrease in the number of epithelial cells expressing ALK-5 at 24 hours (p=0.02) (Figure 2A and 3A-B). Scattered submucosal inflammatory-like cells staining positive for ALK-5 were identified in low numbers only and not in all volunteers. Similarly, ALK-5 expression was not detected in either fibroblast-like cells or airway smooth muscle cells. However, there was increased expression of ALK-1 in epithelial cells from baseline to 24 hours post allergen challenge (Table 1) (Figure 2B and 3C-D). Furthermore, significantly increased numbers of submucosal cells expressed ALK-1 at 24 hours (p=0.03) (Figure 2C and 3C-D). No modulation of epithelial TβRII expression was found (Table 1). There were significantly increased numbers of submucosal cells expressing TβRII at the 24 hour time point following allergen challenge (p=0.004) (Figure 2D). ALK-1 was expressed on CD3+ T cells at baseline and expression was increased post-allergen challenge (p=0.03) (n=6) (Figure 4A). After allergen challenge 71.65% (median) of CD3+ T cells were ALK-1+ (58.55-84.70). Both before and after allergen challenge, all CD3+ T cells identified also stained for TBRII (data not shown).
Figure 2. TGF-β and activin receptor modulation following allergen provocation in asthma.
Epithelial ALK-5 (2A) and ALK-1 (2B) (2C-submucosa) expression before and post-allergen challenge is summarised. Submucosal cell expression of TβRII is also presented (2D). ALK-4 and ActRIIA expression is summarised in Figure 2E-F and 2G-H respectively. Epithelial cells are expressed as cells/ mm BM and submucosal cells as cells per mm2.
Figure 3. Receptor modulation in response to allergen challenge.
Representative photomicrographs of ALK-5 expression (3A-B), ALK-1 (3C-D), ALK-4 (3E-F) and ActRIIA (3G-H) at baseline and post-allergen are presented (×40 magnification). Submucosal cells expressing ALK-1, ALK-4 and ActRIIA are arrowed.
Figure 4. ALK-1 and ALK-4 modulation of expression CD3+ T cells post-allergen.
Numbers of T cells expressing receptors for TGFβ (ALK-1 Figure 4A) and activin (ALK-4, Figure 4B) were enumerated before and after allergen challenge by double staining for CD3 and ALK-1 or ALK-4. Counts are expressed as cells per mm2 of biopsy. A representative photomicrograph for double staining for CD3 (stained red) and ALK-4 (DAB) is shown in Figure 4C. Double stained cells are seen as a darker red-brown colour. The epithelium is indicated as Ep to orientate the section. N=6.
Activin receptors
At 24 hours after allergen challenge there were increased numbers of epithelial cells (p=0.04) (Figure 2E) and submucosal inflammatory-like cells staining for ALK4 (p=0.04) (Figure 2F). ALK-4 expression was evident in fibroblast-like cells post-allergen (3.2 cells/mm2 ± 0-35.2 range versus 11.40 cells/mm2 ± 4-190 range). Increased numbers of epithelial cells stained for ActRIIA at 24 hours after allergen challenge (p=0.01) (Figure 2G). Representative photomicrographs are given in Figure 3E-F and 3G-H. There was a non significant trend for increased numbers of submucosal cells staining for ActRIIA post-allergen (Figure 2H). No modulation of ActRIIB was demonstrated in either tissue compartment (Table 1). ALK-4 expression was expressed on CD3+ T cells at baseline with rapid modulation of expression post-allergen (p=0.03)(n=6) (Figure 4B and 4C). After allergen challenge 96.5% (median) of CD3+ T cells were ALK-4+ (91.89-100).
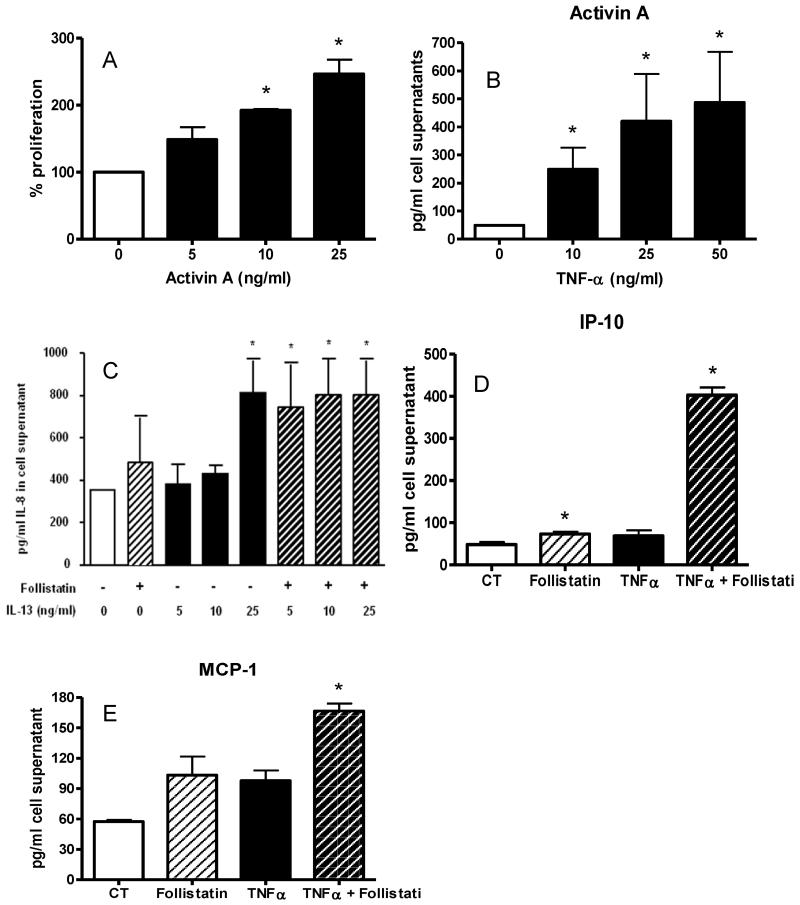
Activin-A induces proliferation of and inhibits cytokine-induced chemokine production by primary human bronchial epithelial cells
NHBE cells were stimulated with increasing doses of activin-A for 6, 24 and 48 hours. A dose dependent increase in NHBE cell proliferation was observed at each time point, reaching significance at 10 and 25 ng/ml (Figure 5A). Activin did not induce release of IL-6, CXCL8/IL-8, IL-13or CCL11/eotaxin, CXCL1/GRO-alpha, CXCL10/IP-10, CXCL9/MIG, CCL2/MCP-1, CCL8/MCP-2, CCL7/MCP-3, CCL/3MIP-1alpha and CCL4/beta and CCL5/RANTES from NHBE (data not shown). TNF-α increased the release of activin-A by NHBE cells, which also released activin-A without stimulation (Figure 5B). Furthermore, the activin inhibitor follistatin augmented IL-13 induction of CXCL8/IL-8 by NHBE (Figure 5C). In addition, although at the concentrations tested TNF-α and IL-13 did not induce release of CXCL10/IP-10 or CCL2/MCP-1 from NHBE, blockade of activin by follistatin induced significant production of both chemokines by IL-13 or TNF-α-stimulated NHBE (Figure 5D and 5E and data not shown) suggesting that activin acts to inhibit cytokine-induced chemokine production by bronchial epithelial cells.
Figure 5. Effect of activin on human bronchial epithelial cells.
Activin caused dose-dependent proliferation of NHBE cells in culture (Figure 5A). Data are for 24 hours culture and are means and SE for 5 separate experiments. Figure 5 B: TNF-α induced-dose dependent release of activin A from NHBE cells (24 hour time point, means and SE, n=5). Figure 5C: 10 ng/ml IL-13 induced dose-dependent release of IL-8 from NHBE and this was significantly increased in the presence of 100 ng follistatin (24 hour time point, n=5). Figure 5 D and E. TNF-α alone did not induce release of CXCL10/IP-10 or CCL2/MCP-1 from NHBE cells but this was significantly induced in the presence of the activin-inhibitor follistatin at 100 ng/ml (n=5). In all figures * indicates p<0.05 for comparison with medium alone values.
Discussion
This study suggests that rapid activation of pSmad2 in response to allergen challenge in asthma may result from signalling by both activins and TGF-β. We report rapid modulation of selected ligand-specific receptor expression. In particular ALK-5, the Type I receptor implicated to date in TGF-β1 signalling, was down-regulated in airway epithelium with absent or decreased expression in the submucosa, whereas we detected ALK-1 expression by airway epithelium and submucosal cells with increases after allergen challenge, raising the possibility that TGF-β may also signal via ALK-1 in the asthmatic airway. ALK-4, the only activin Type I receptor, was expressed at baseline and further up-regulated in response to allergen challenge, suggesting that activin-mediated signalling pathways have important roles in the airway response to allergen induced airway inflammation and remodelling events in asthma. Activin induced proliferation of bronchial epithelial cells in culture and inhibited cytokine-induced chemokine release by these cells.
Neither TGF-β1 or activin-A ligand expression was modulated in response to disease activation, in our study. Torrego et al. have previously reported an increase in TGF-β2 (but not TGF-β1 or TGF-β3 expression in the airway after allergen challenge)11, whereas Rosendahl and colleagues reported an increase in mRNA for TGF-β3 and activin-A in lungs from mice sensitized and challenged with ovalbumin, but no changes in mRNA for TGF-β1 or TGF-β2 14. However since both TGF-β1 and activin-A are stored in tissues in inactive forms and immunohistochemistry and in situ hybridization cannot distinguish inactive forms from activated ligands, we suggest that detection of pSmad2 is a better indicator of activation of both TGF-β and activin pathays afer allergen challenge in asthma
Here we identified neutrophils as a source of activin-A in the asthmatic airway after allergen challenge. Neutrophils have already been identified as an important source of TGF-β1 in asthma 20 and thus may have a role in tissue remodelling. The exact contribution of neutrophil derived activin-A to asthma pathogenesis will need further focus.
The rapid and consistent down-regulation in the expression pattern of epithelial ALK-5 24 hours after allergen exposure raises the possibility that there may be a regulatory mechanism in place to attenuate the cellular response to TGF-β1. This observation is in keeping with data from an allergen-induced mouse model of airway injury 14and a rat model of bleomycin-induced lung fibrosis 15, both of which demonstrated reduced expression of ALK-5 with activation of fibrosis. ALK-5 expression was not detected on submucosal inflammatory-like cells at any time point in the mild asthmatics studied here. Decreased ALK-5 expression has, however, been documented in the asthmatic airway in more symptomatic subjects previously 13. Inflammatory cell expression of ALK-5 is related to the state of cell differentiation and activation as has been demonstrated in inactive monocytes which express a relatively high proportion of ALK-5 early on but with cell activation there is down-regulation of ALK-5 with concomitant loss of functional responses to TGF-β ligand 21.
ALK-1 expression was increased after allergen challenge in epithelium and particularly submucosal cells. Unidentified stromal cells of nasal tissue have been shown to express ALK-1 22 and a mouse model of allergen-induced airway injury demonstrates ALK-1 expression in submucosal infiltrating cells, fibroblasts, epithelium and vascular structures 14.
The functional consequence of ALK-1 expression in the context of airway inflammation and remodelling in asthma remains to be determined. In endothelial cells at least ALK-1 activation leads to cell proliferation and migration whilst ALK-5 signalling antagonises such responses 23;24. Whilst we appreciate that our data is derived from an immunohistochemical approach which is semi-quantitative at best and that several pathways will interact with the TGF-β signalling cascade, it is still important to consider the possibility that the trend towards increased ALK-1 expression alongside decreased or absent ALK-5 expression observed here may reflect down-regulation of ALK-5 mediated signalling programmes whilst antagonistic ALK-1 mediated signalling programmes are activated. ALK-1 signals through the Smad1/5 pathway and our recent work demonstrating increased allergen-induced signalling of pSmad1/5 expression would thus also support ALK-1 mediated signalling 25. Several bone morphogenetic proteins (BMPs) such as BMP-7 are anti-fibrotic, and it is therefore possible that ALK-1 induction is an attempt at regulating the airway-injury response 26. It will be important to understand how TGF-β1 and the BMP activated ALK-1 interacts to determine functional cellular outcomes.
In contrast to ALK-5, ALK-4 expression increased throughout the epithelium and submucosal cells following allergen challenge. Furthermore, rapid upregulation of ActRIIA was detected in the epithelium following challenge with increased numbers of submucosal cells also expressing ActRIIA. Given the absence of ALK-5 expression in the airway submucosa in our study and others 13 these findings may suggest that activin-A may be an important contributor to airway responses to allergen challenge. To support this in animal models of lung fibrosis the activin antagonist follistatin abolishes fibrosis even in the presence of TGF-β1 27 and fibroblasts rapidly up-regulate ALK-4 expression 14. Here, we detected ALK-4 expression by fibroblast-like cells but did not see any up-regulation of follistatin after allergen challenge of asthmatics, suggesting that activin-A may act unopposed to activate airway fibroblasts. These findings support and extend those of Karagiannidis et al who showed increased activin-A in serum from symptomatic asthmatics and activation of airway fibroblasts in-vitro by activin-A 4.
The observation of increased ALK-4 expression and pSmad2 activation in airway epithelium after allergen challenge in asthma lead us to examine the effects of activin A on primary human airway epithelial cells in culture. Activin-A induced proliferation but not cytokine or chemokine release by NHBE. Furthermore, our data using the natural activin inhbitor, follistatin, raise the possibility that activin-A may act as an inhibitor of cytokine-induced pro-inflammatory chemokine release from the airway epithelium. These findings lead us to postulate a role for activin signalling in repair and resolution of inflammation after allergen challenge in asthma. Interestingly, rhinovirus infection also induces activin release from bronchial epithelial cells, and it will be of interest to determine whether this cytokine has a role in resolution of virus-induced airway inflammation 28. TGF-β1 is also reported to inhibit cytokine-induced chemokine production from epithelial cells 29and increases mucin production30.
Our demonstration of the expression of ALK-1 and ALK-4 on CD3+ T cells and modulation of expression in response allergen-provocation of asthma, suggests that both TGF-β1 and activin-A may act in resolution of T cell-mediated airway inflammation, since both cytokines can suppress effector T cell function 31;32. Activin-A has recently been reported to synergize with TGF-β1 for expansion of regulatory T cells 33. In addition TGF-β1 together with IL-6, acts in the differentiation of Th17 T cells, and this may be relevant to a possible role for such cells in chronic asthma. 34 Further studies will be required to explore these areas.
In conclusion, allergen provocation of asthma leads to rapid activation of TGF-b and activin signaling pathways, whereas receptor expression and our studies of airway epithelial cell function suggest a role for activin-A in resolution of inflammation and initiation of airway remodeling after allergen challenge. Alternate TGF-b1 pathways via ALK-1 rather than ALK-5 may also be operative. Further interventional approaches will be required to dissect these pathways in vivo, but it is clear that targetingTGF-b superfamily signaling in asthma will be ineffective unless the integrated and interactive signaling pathways that are in operation are considered as a whole.
Keymessages.
Activin receptor up-regulation alongside down-regulation of expression of TGF-β1 receptor ALK-5 after allergen challenge, together with induction of bronchial epithelial cell proliferation suggests that activin may have a role in allergen-induced remodelling and resolution of inflammation in asthma.
Acknowledgments
The authors thank Prof. Paschalis Sideras, Biomedical Research Foundation of the Academy of Athens, for the gift of TGF-β family signalling antibodies used in this study.
Funding; Imperial College Trust Fund
DSR was supported by a Wellcome Trust leave award for clinical academics. CML is a Wellcome Trust Senior Fellow.
Abbreviations
- ALK
Activin-like kinase
- APAAP
Alkaline phosphatase anti-alkaline phosphatase
- BMP
Bone morphogenetic protein
- FEV1
Forced expiratory volume 1 second
- MBP
Major basic protein
- NHBE
Normal human bronchial epithelial cells
- PC20
Provocative concentration 20
- SPT
Skin prick test
- TGF-β
Transforming growth factor
Reference List
- 1.Ling E, Robinson DS. Transforming growth factor-beta1: its antinflammatory and pro-fibrotic effects. Clin.Exp.Allergy. 2002;32:175–178. doi: 10.1046/j.1365-2222.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 2.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc.Natl.Acad.Sci.U.S.A. 2007;104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulyok S, Wankell M, Alzheimer C, Werner S. Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol.Cell Endocrinol. 2004;225:127–132. doi: 10.1016/j.mce.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, Menz G, Uhlig S, Blaser K, Schmidt-Weber CB. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J.Allergy Clin.Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J.Immunol. 2006;177:6787–6794. doi: 10.4049/jimmunol.177.10.6787. [DOI] [PubMed] [Google Scholar]
- 6.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J.Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 7.Jones KL, de Kretser DM, Phillips DJ. Effect of heparin administration to sheep on the release profiles of circulating activin A and follistatin. J.Endocrinol. 2004;181:307–314. doi: 10.1677/joe.0.1810307. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto O, Nakamura T, Shoji H, Shimasaki S, Hayashi Y, Sugino H. A novel role of follistatin, an activin-binding protein, in the inhibition of activin action in rat pituitary cells. Endocytotic degradation of activin and its acceleration by follistatin associated with cell-surface heparan sulfate. J.Biol.Chem. 1997;272:13835–13842. doi: 10.1074/jbc.272.21.13835. [DOI] [PubMed] [Google Scholar]
- 10.Phipps S, Benyahia F, Ou TT, Barkans J, Robinson DS, Kay AB. Acute allergen-induced airway remodeling in atopic asthma. Am.J.Respir.Cell Mol.Biol. 2004;31:626–632. doi: 10.1165/rcmb.2004-0193OC. [DOI] [PubMed] [Google Scholar]
- 11.Torrego A, Hew M, Oates T, Sukkar M, Fan CK. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–313. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariyawasam HH, Aizen M, Barkans J, Robinson DS, Kay AB. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am.J.Respir.Crit Care Med. 2007;175:896–904. doi: 10.1164/rccm.200609-1260OC. [DOI] [PubMed] [Google Scholar]
- 13.Balzar S, Chu HW, Silkoff P, Cundall M, Trudeau JB, Strand M, Wenzel S. Increased TGF-beta2 in severe asthma with eosinophilia. J.Allergy Clin.Immunol. 2005;115:110–117. doi: 10.1016/j.jaci.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-beta/activin-Smad2 pathway during allergic airway inflammation. Am.J.Respir.Cell Mol.Biol. 2001;25:60–68. doi: 10.1165/ajrcmb.25.1.4396. [DOI] [PubMed] [Google Scholar]
- 15.Khalil N, Parekh TV, O’Connor RN, Gold LI. Differential expression of transforming growth factor-beta type I and II receptors by pulmonary cells in bleomycin-induced lung injury: correlation with repair and fibrosis. Exp.Lung Res. 2002;28:233–250. doi: 10.1080/019021402753570527. [DOI] [PubMed] [Google Scholar]
- 16.Hamid Q, Azzawi M, Ying S, Moqbel R, Wardlaw AJ, Corrigan CJ, Bradley B, Durham SR, Collins JV, Jeffery PK. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J.Clin.Invest. 1991;87:1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J.Allergy Clin.Immunol. 1993;92:313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 18.Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A, Sagara H, Setoguchi Y, Okada T, Okumura K, Ogawa H, Fukuda T. Expression of Smad7 in bronchial epithelial cells is inversely correlated to basement membrane thickness and airway hyperresponsiveness in patients with asthma. J.Allergy Clin.Immunol. 2002;110:873–878. doi: 10.1067/mai.2002.129236. [DOI] [PubMed] [Google Scholar]
- 20.Chu HW, Trudeau JB, Balzar S, Wenzel SE. Peripheral blood and airway tissue expression of transforming growth factor beta by neutrophils in asthmatic subjects and normal control subjects. J.Allergy Clin.Immunol. 2000;106:1115–1123. doi: 10.1067/mai.2000.110556. [DOI] [PubMed] [Google Scholar]
- 21.Brandes ME, Mai UE, Ohura K, Wahl SM. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J.Immunol. 1991;147:1600–1606. [PubMed] [Google Scholar]
- 22.Sadick H, Riedel F, Naim R, Goessler U, Hormann K, Hafner M, Lux A. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica. 2005;90:818–828. [PubMed] [Google Scholar]
- 23.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol.Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 25.Kariyawasam HH, Xanthou G, Barkans J, Aizen M, Kay AB, Robinson DS. Basal expression of bone morphogenetic protein receptor is reduced in mild asthma. Am.J.Respir.Crit Care Med. 2008;177:1074–1081. doi: 10.1164/rccm.200709-1376OC. [DOI] [PubMed] [Google Scholar]
- 26.Scherner O, Meurer SK, Tihaa L, Gressner AM, Weiskirchen R. Endoglin differentially modulates antagonistic transforming growth factor-beta1 and BMP-7 signaling. J.Biol.Chem. 2007;282:13934–13943. doi: 10.1074/jbc.M611062200. [DOI] [PubMed] [Google Scholar]
- 27.Aoki F, Kurabayashi M, Hasegawa Y, Kojima I. Attenuation of bleomycin-induced pulmonary fibrosis by follistatin. Am.J.Respir.Crit Care Med. 2005;172:713–720. doi: 10.1164/rccm.200412-1620OC. [DOI] [PubMed] [Google Scholar]
- 28.Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, Proud D. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J.Allergy Clin.Immunol. 2008;121:1238–1245. doi: 10.1016/j.jaci.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Adachi Y, Mio T, Takigawa K, Striz I, Romberger DJ, Robbins RA, Spurzem JR, Heires P, Rennard SI. Mutual inhibition by TGF-beta and IL-4 in cultured human bronchial epithelial cells. Am.J.Physiol. 1997;273:L701–L708. doi: 10.1152/ajplung.1997.273.3.L701. [DOI] [PubMed] [Google Scholar]
- 30.Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, Wenzel SE. Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. Am.J.Pathol. 2004;165:1097–1106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson NC, Phillips DJ, McAlpine T, Shin A, Svobodova S, Toy T, Pillay V, Kirkpatrick N, Zanker D, Wilson K, Helling I, Wei H, Chen W, Cebon J, Maraskovsky E. Activin-A: a novel dendritic cell-derived cytokine that potently attenuates CD40 ligand-specific cytokine and chemokine production. Blood. 2008;111:2733–2743. doi: 10.1182/blood-2007-03-080994. [DOI] [PubMed] [Google Scholar]
- 33.Huber S, Stahl FR, Schrader J, Luth S, Presser K, Carambia A, Flavell RA, Werner S, Blessing M, Herkel J, Schramm C. Activin a promotes the TGF-beta-induced conversion of CD4+ J.Immunol. 2009;182:4633–4640. doi: 10.4049/jimmunol.0803143. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J.Allergy Clin.Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]