Abstract
Rice is an important staple food for more than half of the world's population. Especially in Asian countries, rice is a major contributor to dietary glycaemic load (GL). Sustained consumption of higher-GL diets has been implicated in the development of chronic diseases such as type 2 diabetes mellitus. Given that a reduction in postprandial glycaemic and insulinaemic responses is generally seen as a beneficial dietary change, it is useful to determine the variation in the range of postprandial glucose (PPG) and insulin (PPI) responses to rice and the primary intrinsic and processing factors known to affect such responses. Therefore, we identified relevant original research articles on glycaemic response to rice through a systematic search of the literature in Scopus, Medline and SciFinder databases up to July 2014. Based on a glucose reference value of 100, the observed glycaemic index values for rice varieties ranged from 48 to 93, while the insulinaemic index ranged from 39 to 95. There are three main factors that appear to explain most of the variation in glycaemic and insulinaemic responses to rice: (1) inherent starch characteristics (amylose:amylopectin ratio and rice cultivar); (2) post-harvest processing (particularly parboiling); (3) consumer processing (cooking, storage and reheating). The milling process shows a clear effect when compared at identical cooking times, with brown rice always producing a lower PPG and PPI response than white rice. However, at longer cooking times normally used for the preparation of brown rice, smaller and inconsistent differences are observed between brown and white rice.
Keywords: Rice, Blood glucose, Insulin, Glycaemic index, Starch, Processing
Rice is a daily dietary staple food for more than half of the world's population, and the major single food source of carbohydrate and energy in China and many other Asian countries( 1 ). In South India, for example, nearly half of daily energy intake come from refined grains, and white polished rice constitutes >75 % of refined grain intake( 2 ). In China, brown rice is rarely consumed( 3 ). As a result, in Asian populations, white rice makes large contributions to dietary glycaemic load, an index reflecting the acute blood glucose-raising potential of foods or diets( 4 ). Higher levels of postprandial glycaemic exposure have been implicated in the development of chronic metabolic diseases, particularly type 2 diabetes mellitus and CVD( 5 ). A recent systematic review and meta-analysis has shown a clear relationship between white rice intake and the risk of type 2 diabetes mellitus, with higher levels of rice intake being more strongly associated with the risk in Asian than in Western populations( 6 , 7 ).
There are many varieties of rice grain in the world, which vary considerably in the postprandial blood glucose (PPG) response they produce( 8 ). The results of glycaemic index (GI) studies around the world( 9 ) report values ranging from 64 to 93. Moreover, the post-harvest treatment of rice and the method of consumer preparation can also play a significant role in this variation. Starch comprises two glucose polymers: amylose and amylopectin. Amylose is a linear and relatively short polymer of glucose units linked by α(1 → 4) bonds. Amylopectin is a branched and longer polymer where glucose units are arranged linearly through α(1 → 4), with branches emerging via α(1 → 6) bonds occurring every twenty-four to thirty glucose units( 10 ). It is well known that starches with a higher amount of amylose are more resistant to digestion( 11 ).
In addition to the variation in amylose content, cooking (and cooling) processes can influence starch digestibility via the degree of gelatinisation and retrogradation of rice starch. Gelatinisation is the collapse (disruption) of molecular order (breaking of H bonds) within the starch granule, manifested in irreversible changes in properties such as granular swelling, native crystallite melting, loss of birefringence and starch solubilisation during hydrothermal treatment( 12 ). This leads to the dissociation of crystalline regions in starch with associated hydration and swelling of starch granules, leading to higher starch availability to human digestive enzymes( 13 ). Retrogradation is the recrystallisation of amorphous phases created by gelatinisation( 14 ) and, in the case of amylose, results in the formation of type 3 resistant starch (RS3)( 15 ). RS3 is resistant to digestion, because it is heat stable and melts above 120°C( 16 ). In contrast, retrograded amylopectin is thought to melt upon reheating (cooking) due to the low melting point (46–65°C) of these crystallites, and therefore it is digestible upon cooking.
Post-harvest processing includes milling, parboiling and quick-cooking. The rice milling process starts with the husking stage to remove the husk from paddy rice, followed by the whitening–polishing stage to transform brown rice into polished white rice, and finally the grading and blending stage to obtain head rice with predefined amounts of broken rice. However, while this may affect the overall nutritional value, the effects on digestibility and PPG are less clear( 17 ). Other post-harvest treatments such as parboiling can also play a role in digestibility. Parboiling is a hydrothermal treatment that includes soaking in water, heating, drying and milling of paddy rice. During the parboiling process, the crystalline structure of the starch present in rice is transformed into an amorphous form. Pressure parboiling is accomplished by soaking paddy rice in warm water (65–68°C) for 4–5 h followed by steaming under pressure and drying( 18 ). Other post-harvest processes are used to produce quick-cooking rice. The latter is a precooked rice where the starch has been partially gelatinised by soaking in water and heating( 19 ). For consumer consumption, additional processes include cooking, storage and reheating. There are different ways of rice cooking depending on the ratios between rice and water, equipment (pressure cooking and steaming), and consumer preference (sticky rice, aromatic basmati, etc.). Cooking of polished white rice strongly affects gelatinisation. Retrogradation is affected by cooling and storage conditions (see also Fig. 3).
Fig. 3.
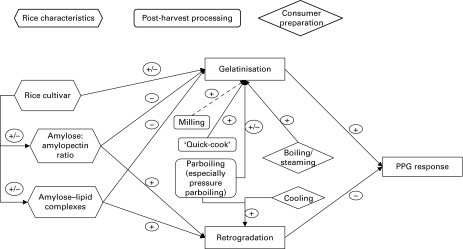
Relationship between rice characteristics, processing factors, physico-chemical processes and glycaemic response (+ indicates increased effect; − indicates decreased effect). This is a general figure, depending on specific processes, e.g. conditions of parboiling; the effects may differ. PPG, postprandial glucose response.
Given that reductions in PPG responses are generally seen as a beneficial dietary change( 5 ), it is useful to objectively establish the variation in the range of PPG responses to rice and the primary intrinsic and processing factors known to affect such responses. Therefore, we performed a systematic search of the literature characterising the range of PPG and PPI responses to different rice types, and considered this alongside available data on rice grain and processing characteristics. The main emphasis is on in vivo studies conducted in human subjects, supplemented in places by the in vitro literature related to specific mechanisms that may be relevant (e.g. influence of microstructure on rice).
Methods
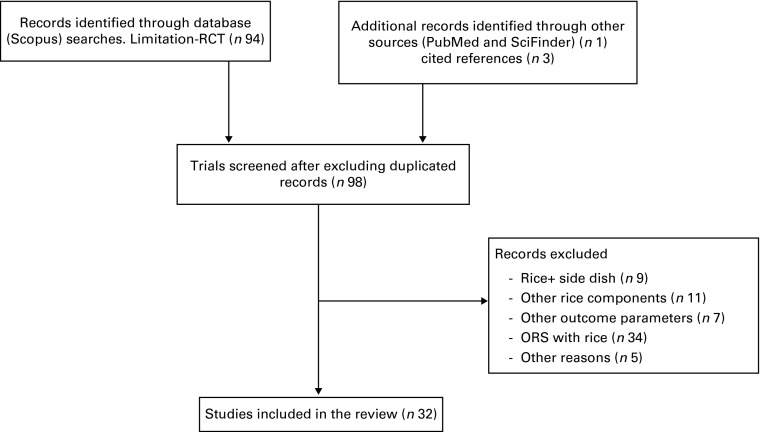
The literature database ‘Scopus’ was searched for the following combinations of keywords (without language or time restrictions): rice* AND glycaem* or glycem* or digestib* or glucose* or insulin* or hyperglycaem* or hyperglycem* or hypoglycaem* or hypoglycem* or normoglycaem* or normoglycem* AND combined with the title from 1980 through July 2014, resulting in ninety-four records. In addition, the PubMed and SciFinder databases were also searched using the same search terms, resulting in one additional article. A further three ‘missed’ articles were identified from the cited references in the articles identified in the formal searches, resulting in ninety-eight articles. From manual inspection of the ninety-eight abstracts, we identified twenty-eight original articles describing the results of thirty-two randomised clinical trials with rice as the test food and a measure of PPG (and in some cases also PPI) as an outcome measure (for a detailed flow chart, see Fig. 1).
Fig. 1.
Flow chart of the systematic review article selection process. RCT, randomised controlled trial.
Results
Evidence base
Studies identified in the search and their key relevant results are presented in Table 1. In addition, specific comparisons of amylose content, parboiling and milling are presented in online Supplementary Tables S2, S3 and S4, respectively. The thirty-two randomised clinical trials on PPG responses to rice included different rice types (e.g. regional varieties) and different processes (milling, (par)boiling, ‘quick-cook’ and (pressure) cooking). Outcome measures for blood glucose included GI (twenty-seven studies) and/or the incremental area under the PPG response curve (iAUC, nineteen studies), or peak glucose values (eight studies). The iAUC is the actual blood glucose response to a given serving of rice, whereas the GI and the corresponding insulinaemic index (II) use a fixed available carbohydrate load (usually 50 g) and represent responses as a comparison with a reference (assigned a value of 100). Except where noted, the GI and II studies compared rice with glucose as the reference. A subset of studies reported the II (seven studies) or insulin AUC (eight studies). Furthermore, two studies took breath hydrogen into account as an indicator of carbohydrate malabsorption( 20 , 21 ).
Table 1.
Human in vivo studies on the postprandial glycaemic and insulinaemic effects of rice*
| Glycaemic response | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication+Expt | Participants | Food | Amylose (w/w%) | AUC | GI | Peak | Insulin response | ||||||||||||||||||||||
| Brand-Miller et al. (1992)( 9 ) | Healthy volunteers n 8, age 19–36 years, BMI 18–25 kg/m2 | Rice types grown in Australia | GI v. bread | II v. bread | |||||||||||||||||||||||||
| min = minutes boiled | |||||||||||||||||||||||||||||
| Doongara (white), 14 min | 28 | 64 | 40 | ||||||||||||||||||||||||||
| Doongara (brown), 30 min | 28 | 66 | 39 | ||||||||||||||||||||||||||
| Pelde (brown), 30 min | 20 | 76 | 55 | ||||||||||||||||||||||||||
| Sunbrown (quick), 16 min | NR | 80 | 54 | ||||||||||||||||||||||||||
| Calrose (white), 14 min | 20 | 83 | 67 | ||||||||||||||||||||||||||
| Calrose (brown), 35 min | 20 | 87 | 51 | ||||||||||||||||||||||||||
| Pelde (parboiled), 14 min | 20 | 87 | 57 | ||||||||||||||||||||||||||
| Waxy rice, 14 min | < 2 | 88 | 89 | ||||||||||||||||||||||||||
| Pelde white, 14 min | 20 | 93 | 67 | ||||||||||||||||||||||||||
| Ranawana et al. (2009)( 18 ) | Healthy subjects n 14, age 18–65 years, BMI < 30 kg/m2 | min = minutes boiled | |||||||||||||||||||||||||||
| Guilin rice noodles, 8 min | 76 | 37 | |||||||||||||||||||||||||||
| Jiangxi rice noodles, 8 min | 74 | 40 | |||||||||||||||||||||||||||
| Easy-cook long grain rice, 15 min | 76 | 47 | |||||||||||||||||||||||||||
| Long-grain rice (Indica type), 15 min | 91 | 47 | |||||||||||||||||||||||||||
| White basmati rice, 10 min | 20–25 | 94 | 50 | ||||||||||||||||||||||||||
| White (60 %) and brown (40 %) basmati rice, 25 min | 92 | 59 | |||||||||||||||||||||||||||
| Basmati+wild rice, 20 min | 20–25 | 96 | 63 | ||||||||||||||||||||||||||
| Brown basmati rice, 25 min | 20–25 | 116 | 75 | ||||||||||||||||||||||||||
| Thai red rice, 25 min | 111 | 76 | |||||||||||||||||||||||||||
| Easy-cook basmati rice, 15 min | 20–25 | 111 | 80 | ||||||||||||||||||||||||||
| Thai glutinous rice, 10 min | < 2 | 144 | 92 | ||||||||||||||||||||||||||
| Li et al. (2010)( 20 ) | Healthy subjects n 16 (n 9 male/n 7 female), age 23–26 years, BMI 18–24 kg/m2 | RS-enriched (RS 20 %) (high amylose) | |||||||||||||||||||||||||||
| Indica type (Oryza sativa L. cultivar Te-Qing) | GI | Peak | II | ||||||||||||||||||||||||||
| RS-enriched (RS 20 %, high amylose), produced with an antisense inhibition starch-branching enzyme | 48 | 6·8 | 34 | ||||||||||||||||||||||||||
| Wild type (RS 2%) | 77 | 7·2 | 54 | ||||||||||||||||||||||||||
| Casiraghi et al. (1993)( 21 ) | Healthy subjects n 9, mean age 26 years, BMI 22 kg/m2 | Italian Fino ribe rice, processed as: | GI v. bread | ||||||||||||||||||||||||||
| Parboiled (15 min boiling time) | 70 | ||||||||||||||||||||||||||||
| Quick-cooking parboiled (8 min) | 79 | ||||||||||||||||||||||||||||
| Conventionally polished (20 min) | 115 | ||||||||||||||||||||||||||||
| Al-Mssallem et al. (2011)( 22 ) | Healthy subjects n 13 (n 6 male/n 7 female), 25–42 years, BMI 25·6 (sem 1·0) kg/m2 | Long-grain rice variety ‘UBR’ and traditional Saudi Arabian rice ‘HR’ | |||||||||||||||||||||||||||
| UBR | 19 | 54 | 78 | ||||||||||||||||||||||||||
| HR | 26 | 59 | 56 | ||||||||||||||||||||||||||
| Juliano & Goddard (1986) Expt 1( 23 ) | n 16 | Rice cooked: same degree of doneness | (tAUC 0–180 min) | AUC (μU/ml) | |||||||||||||||||||||||||
| Labelle | 28 | 19·0† | 86 | ||||||||||||||||||||||||||
| Newrex | 24 | 19·3† | 64 | ||||||||||||||||||||||||||
| Juliano & Goddard (1986) Expt 2(23) | n 33 | Rice cooked: same degree of doneness | (tAUC 0–180 min) | AUC (μU/ml) | |||||||||||||||||||||||||
| Mochi Gome | 1 | 19·2† | 113 | ||||||||||||||||||||||||||
| Labelle | 24 | 19·3† | 95 | ||||||||||||||||||||||||||
| Pecos | 18 | 19·7† | 110 | ||||||||||||||||||||||||||
| Juliano et al. (1989)( 24 ) | T2DM subjects n 8 | Long-grain non-waxy (RD21 and RD23) and waxy rice | |||||||||||||||||||||||||||
| Non-waxy rice | 16 | 71 | |||||||||||||||||||||||||||
| Waxy rice | 2 | 75 | |||||||||||||||||||||||||||
| Panlasigui et al. (1991) Expt 1(25) | Healthy subjects n 11 (n 4 male/n 7 female), age 23–44 years, 90–110 % ideal body weight | Long-grain, non-waxy rice: IR62, IR36 and IR42; white rice: boiled for 22 min | mmol × min/l | AUC (pmol × min/l) | |||||||||||||||||||||||||
| IR42 | 26·7 | 55 | 61 | 9240 | |||||||||||||||||||||||||
| IR62 | 27 | 65 | 72 | 7131 | |||||||||||||||||||||||||
| IR36 | 26·7 | 81 | 91 | 9415 | |||||||||||||||||||||||||
| Panlasigue et al. (1991) Expt 2( 25 ) | Healthy subjects n 11 (n 3 male/n 8 female), age 23–50 years, 90–110 % ideal body weight | Long-grain, non-waxy rice: IR62, IR36 and IR42; white rice: 50 g | mmol × min/l | ||||||||||||||||||||||||||
| Expt 2: boiled for minimum cooking | |||||||||||||||||||||||||||||
| IR42, boiled for 14 min | 26·7 | 26·7 | 81 | ||||||||||||||||||||||||||
| IR62, boiled for 20 min | 27 | 27 | 75 | ||||||||||||||||||||||||||
| IR36, boiled for 19 min | 26·7 | 26·7 | 78 | ||||||||||||||||||||||||||
| Panlasigui & Thompson (2006) Expt 1(26) | Healthy subjects n 10 (n 3 male/n 7 female), age 24–50 years, 90–110 % ideal body weight | mmol × min/l | GI v. bread | ||||||||||||||||||||||||||
| IR42 rice, brown rice | 26·7 | 107 | 83 | ||||||||||||||||||||||||||
| IR42 rice, white rice | 26·7 | 134 | 94 | ||||||||||||||||||||||||||
| Panlasigui & Thompson (2006) Expt 2( 26 ) | T2DM patients n 9 (n 5 male/n 4 female), age 45–64 years | mmol × min/l | GI v. bread | ||||||||||||||||||||||||||
| IR42 rice, brown rice | 26·7 | 406 | 56 | ||||||||||||||||||||||||||
| IR42 rice, white rice | 26·7 | 626 | 87 | ||||||||||||||||||||||||||
| Kim et al. (2004)(27) | T2DM patients n 10 (n 4 male/n 6 female), mean age 57 years, BMI 24 kg/m2 | Korean rice products: | mmol/l per 4 h | mg/dl per 4 h | |||||||||||||||||||||||||
| Garaeduk: 16 mm stick of steamed, extruded rice flour | 730 | 1742 | |||||||||||||||||||||||||||
| Cooked rice: gelatinised grains, boiled polished rice | 914 | 2571 | |||||||||||||||||||||||||||
| Bagsulgi (rice cake): large block of steamed rice flour | 1070 | 3266 | |||||||||||||||||||||||||||
| Larsen et al. (2000)(28) | T2DM patients n 9, age 60 years, BMI 26·6 kg/m2 | Indica rice variety BR16, high amylose, long grain | iAUC (mmol/l per 3 h) | GI v. bread | iAUC (pmol/l per 3 h) | ||||||||||||||||||||||||
| Pressure parboiled rice | 27 | 231 | 39 | 10·5 | 7590 | ||||||||||||||||||||||||
| Traditional mild parboiled rice | 27 | 274 | 46 | 11 | 7719 | ||||||||||||||||||||||||
| Non-parboiled rice | 27 | 335 | 55 | 10·9 | 7595 | ||||||||||||||||||||||||
| White bread | 626 | 100 | 14 | 1652 | |||||||||||||||||||||||||
| Kataoka et al. (2012)(29) | Healthy Chinese n 32, age 33 years, BMI 22·9 kg/m2 and Healthy European subjects n 31, age 34 years, BMI 25·8 kg/m2 | Rice types: jasmine rice; basmati; brown rice; Doongara; parboiled rice (Uncle Ben's) | iAUC European/Chinese (mmol × min/l) | GI European/Chinese | |||||||||||||||||||||||||
| Doongara | 30( 9 ) | 109/179 | 55/67 | ||||||||||||||||||||||||||
| Parboiled | 112/194 | 57/72 | |||||||||||||||||||||||||||
| Basmati | 20–25( 9 ) | 116/184 | 57/67 | ||||||||||||||||||||||||||
| Brown | 129/210 | 65/78 | |||||||||||||||||||||||||||
| Jasmine | Low( 32 ) | 140/225 | 68/80 | ||||||||||||||||||||||||||
| Trinidad et al. (2013)( 30 ) | Healthy volunteers n 9–10, age 27–55 years | Cooked milled and brown rice | mmol × min/l | ||||||||||||||||||||||||||
| Milled rice | |||||||||||||||||||||||||||||
| PSB rc10 | 27 | 188 | 50 | ||||||||||||||||||||||||||
| IR64 | 22·9 | 212 | 57 | ||||||||||||||||||||||||||
| PSB Rc18 | 18 | 221 | 59 | ||||||||||||||||||||||||||
| IMS2 | 0·6 | 233 | 63 | ||||||||||||||||||||||||||
| PSB Rc12 | 21 | 236 | 63 | ||||||||||||||||||||||||||
| NSIC RC160 | 15·3 | 259 | 70 | ||||||||||||||||||||||||||
| Sinandomeng | 12·6 | 280 | 75 | ||||||||||||||||||||||||||
| Brown rice | |||||||||||||||||||||||||||||
| IR64 | 22 | 189 | 51 | ||||||||||||||||||||||||||
| Sinandomeng | 12·1 | 204 | 55 | ||||||||||||||||||||||||||
| Zarrati et al. (2008)( 31 ) | Healthy subjects n 30 (n 13 male/n 17 female), age 35 years, BMI 23 kg/m2 | One Iranian rice type: Kazemi and imported rices | Maximum changes | II | |||||||||||||||||||||||||
| Sorna pearl | 32 | 52 | 1·2 | 47 | |||||||||||||||||||||||||
| Basmati | 31 | 61 | 1·7 | 52 | |||||||||||||||||||||||||
| Kazemi | 27 | 68 | 1·5 | 62 | |||||||||||||||||||||||||
| Larsen et al. (1996)( 32 ) | T2DM patients n 12 (n 7 male/n 5 female), mean age 58 years, BMI 30 kg/m2 | Dehulled, milled rices: | iAUC (mmol/l per 3 h) | GI v. bread | mmol/l | iAUC (pmol/l per 3 h) | |||||||||||||||||||||||
| BR2 = low amylose variety | |||||||||||||||||||||||||||||
| BR4 = low gelatinisation temperature and gel consistency v. BG16 | |||||||||||||||||||||||||||||
| BR4-PB | 27 | 361 | 47 | 14·5 | 12 964 | ||||||||||||||||||||||||
| BR16-PB | 28 | 391 | 50 | 14·7 | 12 821 | ||||||||||||||||||||||||
| BR16-NP | 28 | 411 | 53 | 14·8 | 11 087 | ||||||||||||||||||||||||
| BR2-PB | 12 | 566 | 73 | 15·9 | 16 215 | ||||||||||||||||||||||||
| White bread | 756 | 100 | 17·3 | 20 183 | |||||||||||||||||||||||||
| Goddard et al. (1984)( 33 ) | n 33 (n 16 male/n 17 female), age 27–81 years, within 20 % desirable body weight | Long-grain rice: Labelle | 23–25 | 19·4† | 6·3 | 100 μU/ml | |||||||||||||||||||||||
| Medium-grain rice: Pecos | 14–17 | 20·0† | 6·6 | 105 | |||||||||||||||||||||||||
| Sweet rice: Mochi Gome | < 2 | 19·4† | 6·8 | 110 | |||||||||||||||||||||||||
| Hettiarachchi et al. (2001)( 34 ) | Healthy subjects n 22, age 25–50 years | Shri Lankan rice varieties (red v. white and parboiled v. raw rice) | GI v. bread | ||||||||||||||||||||||||||
| Rice breeding Institute: | |||||||||||||||||||||||||||||
| Bg 350, raw, red | 55 | ||||||||||||||||||||||||||||
| Bw 351, parboiled, red | 56 | ||||||||||||||||||||||||||||
| Bw 2726-B, parboiled, red | 58 | ||||||||||||||||||||||||||||
| Bg 94-1, parboiled, white | 62 | ||||||||||||||||||||||||||||
| BW 302, raw, white | 64 | ||||||||||||||||||||||||||||
| Bg 300, parboiled, white | 66 | ||||||||||||||||||||||||||||
| Bw 400, raw, red | 66 | ||||||||||||||||||||||||||||
| Bg 450, raw, white | 67 | ||||||||||||||||||||||||||||
| Bg 94-1, raw, white | 68 | ||||||||||||||||||||||||||||
| Bw 2726-B, raw, red | 68 | ||||||||||||||||||||||||||||
| Bw 351, raw, red | 73 | ||||||||||||||||||||||||||||
| Srinivasa et al. (2013)( 35 ) | Healthy volunteers n 83 (n 64 male/n 19 female), age 18–37 years, body weight 44–74 kg | Thermally treated Indian basmati rice | mmol × min/l | mg/l | |||||||||||||||||||||||||
| 182 | 55 | 76 | |||||||||||||||||||||||||||
| Henry et al. (2005)( 36 ) | n 8, mean age 37 years, BMI 23 kg/m2 | Basmati rice, Indian, boiled 8 min | 69 | ||||||||||||||||||||||||||
| Basmati rice, Indian, easy-cook, boiled 9 min | 67 | ||||||||||||||||||||||||||||
| Basmati rice, boiled 12 min | 52 | ||||||||||||||||||||||||||||
| Basmati rice, organic, boiled 9 min | 57 | ||||||||||||||||||||||||||||
| Karupaiah et al. (2011)( 37 ) | Healthy subjects n 9 (n 6 male/n 4 female), age < 30 years, BMI 23 kg/m2 | Transgressive brown rice, cross between wild rice O. rufipogon Griff. and O. sativa L. subsp. indica cultivar MR219, polished version and white rice (Cap Rambutan) | mmol × min/l | II | |||||||||||||||||||||||||
| Brown rice | 13 | 84 | 51 | 39 | |||||||||||||||||||||||||
| Polished rice | 15 | 130 | 79 | 63 | |||||||||||||||||||||||||
| White rice | 18 | 141 | 86 | 68 | |||||||||||||||||||||||||
| Chiu & Stewart (2013)( 38 ) | Healthy subjects n 21 (n 12 male/n 9 female), age 18–65 years, BMI 18·5–30·1 kg/m2 | Refrigerated long-grain rice prepared with rice cooker (2·55 g RS/100 g as consumed) high RS | |||||||||||||||||||||||||||
| Refrigerated short-grain rice prepared with pressure cooker (0·20 g RS/100 g) low RS | |||||||||||||||||||||||||||||
| High-RS rice | 211 | 84 | |||||||||||||||||||||||||||
| Low-RS rice | 181 | 78 | |||||||||||||||||||||||||||
| Wolever et al. (1986) Expt 1( 39 ) | Diabetics n 18, of which NIDDM n 13 (n 6 female/n 7 male), age 67 years, 124 % ideal body weight and IDDM n 5 (n 4 female/n 1 male), age 54 years, 104 % ideal weight | NIDDM/IDDM (mmol × min/l) | NIDDM/IDDM (GI v. bread) | NIDDM/IDDM (mmol/l) | |||||||||||||||||||||||||
| White bread | 951/1220 | 100/100 | 7·7/9·7 | ||||||||||||||||||||||||||
| White bread+tomato | 1003/1208 | 107/95 | 8·2/9·6 | ||||||||||||||||||||||||||
| 15 min regular rice | 23 | 816/1019 | 86/77 | 6·4/7·8 | |||||||||||||||||||||||||
| 15 min parboiled rice | 23 | 614/710 | 68/64 | 4·7/5·9 | |||||||||||||||||||||||||
| Wolever et al. (1986) Expt 2( 39 ) | Diabetics n 18, of which NIDDM n 13 (n 6 female/n 7 male), age 67 years; 124 % ideal body weight and IDDM n 5 (n 4 female/n 1 male), age 54 years, 104 % ideal body weight | GI v. bread | |||||||||||||||||||||||||||
| White bread+tomato | 103 | ||||||||||||||||||||||||||||
| 5 min regular rice | 58 | ||||||||||||||||||||||||||||
| 15 min regular rice | 83 | ||||||||||||||||||||||||||||
| Instant rice | 65 | ||||||||||||||||||||||||||||
| 5 min parboiled rice | 54 | ||||||||||||||||||||||||||||
| 15 min parboiled rice | 67 | ||||||||||||||||||||||||||||
| 25 min parboiled rice | 66 | ||||||||||||||||||||||||||||
| Jung et al. (2009)( 40 ) | Healthy females n 12, mean age 22 years, BMI 21 kg/m2 | Korean (Pungtak region) rice, processed as: | II | ||||||||||||||||||||||||||
| Uncooked rice powder | 50 | 74 | 74 | ||||||||||||||||||||||||||
| Freeze-dried uncooked rice powder | 59 | 68 | 68 | ||||||||||||||||||||||||||
| Cooked rice (boiled 15 min) | 72 | 95 | 95 | ||||||||||||||||||||||||||
| Parastouei et al. (2011)( 51 ) | Healthy young adults n 10, mean age 20 years, BMI 20 kg/m2 | ‘Iranian’ white rice (no further details on type): | |||||||||||||||||||||||||||
| Fluffy (soaked 35 min → boiled 10 min → drained and simmered 20–30 min) | 55 | ||||||||||||||||||||||||||||
| Steamed (boiled 5–8 min → simmered 30 min) | 66 | ||||||||||||||||||||||||||||
| Truong et al. (2014)( 57 ) | Healthy volunteers n 12 (n 9 female/n 3 male), age 18–65 years, BMI 23 kg/m2 | Four brands of Jasmine rice: | |||||||||||||||||||||||||||
| Della (USA) | Low | 96 | |||||||||||||||||||||||||||
| Jazzmen (USA) | Low | 106 | |||||||||||||||||||||||||||
| Reindeer (Thailand) | Low | 115 | |||||||||||||||||||||||||||
| Mahatma (Thailand) | Low | 116 | |||||||||||||||||||||||||||
| Gatti et al. (1987)( 59 ) | Healthy subjects n 14 (n 9 male/n 5 female), age 21–32 years, body weight 88–115 kg | Rice was cooked in two different ways: | 60 min | AUC (U/ml) | |||||||||||||||||||||||||
| Boiled in salt water | 61 | 2536 | |||||||||||||||||||||||||||
| Baked for 10 min at 160°C after boiling | 43 | 2676 | |||||||||||||||||||||||||||
| Matsuo et al. (1999) Expt 1( 60 ) | Healthy adults n 8 (n 3 male/n 5 female), mean age 25 years, BMI 20 kg/m2 | Short-grain Koshihikari rice | 48 | II = 65 | |||||||||||||||||||||||||
| 3 h GI and II v. glucose reference | |||||||||||||||||||||||||||||
| Shobana et al. (2012)( 61 ) | Healthy volunteers n 23, age 18–45 years, BMI < 23·0 kg/m2 | Indian rice varieties (Sona Masuri, Ponni and Surti Kolam) | mmol × min/l | ||||||||||||||||||||||||||
| Ponni | 175 | 70 | |||||||||||||||||||||||||||
| Sona Masuri | 172 | 72 | |||||||||||||||||||||||||||
| Surti Kolam | 185 | 77 | |||||||||||||||||||||||||||
GI, glycaemic index; II, insulinaemic index; NR, not reported; RS, resistant starch; UBR, Uncle Ben's rice; HR, Hassawi rice; tAUC, total AUC; T2DM, type 2 diabetes mellitus; iAUC, incremental AUC; PB, parboiled; NP, not parboiled; Bg, Bathalagaoda; Bw, Bombuwala; NIDDM, non-insulin-dependent diabetes mellitus; IDDM, insulin-dependent diabetes mellitus.
For the GI and II values, 50 g of available carbohydrates were used, with glucose as the reference (except where noted) being assigned the value of 100.
The AUC was not calculated by the trapezoidal method but by the following formula: (time 1)/4+(time 2)/2+¾ time 3+time 4+time 5.
Characterisation of rice and processing
In most studies, rice was well characterised with respect to the percentage of amylose (nine studies), dietary fibre (four studies), RS (two studies) and available starch (sixteen studies). In some studies, gelatinisation or amylograph measurements of milled rice flour were taken into account( 22 – 26 ), while in others, in vitro glucose release assays were included( 21 , 24 , 27 ). A few studies reported grain size, rheology or retrogradation determined by differential scanning calorimetry (a thermo-analytical technique to identify phase transition)( 28 ). The processes explored in the studies involved post-harvest treatments such as parboiling and milling (Fig. 2) .
Fig. 2.
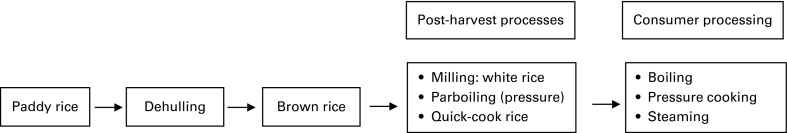
Rice processing steps.
Variation observed in the glycaemic index and insulinaemic index and its causes
The observed GI values ranged from 48 to 93, while the II values (0–120 min) ranged from 39 to 95 (Table 1).
In the studies that specifically tested or varied the amylose content and its quantitative relationship with glycaemic and insulinaemic responses( 9 , 18 , 20 , 22 , 23 , 29 – 33 ), the latter measures were significantly inversely associated with the amylose content( 9 , 18 , 20 , 29 – 32 ) (see also online Supplementary Table S2). However, some studies did not find this inverse relationship for all glycaemic parameters( 22 , 23 , 33 ). Large differences in amylose content (2 % v. approximately 30 % amylose) were often associated with relatively large glycaemic and insulinaemic effects (approximately 300 % decrease in PPG; approximately 55 % decrease in PPI)( 9 , 18 , 29 ). However, there were also studies in which this effect was inconsistent( 30 ) or not observed( 23 (Expt 2) , 33 ).
Rice that received post-harvest treatments such as parboiling( 21 , 29 , 34 ) and quick-cooking( 18 , 21 ) generally gave a lower GI compared with white rice not subjected to these post-harvest treatments (see also online Supplementary Table S3). Larsen et al. ( 28 ) reported that an increased severity of parboiling conditions leads to significant decreases in PPG responses due to the formation of RS. In that study, mild traditional parboiling had no effect on the GI, whereas severely pressure parboiling reduced the GI by almost 30 % compared with non-parboiled rice. However, one study did not show an effect of parboiling( 32 ), and the reported GI of a thermally treated Indian basmati rice variety (thermal treatment not specified) was 55( 35 ), which was in the range between 52 and 59 reported for non-thermally treated Indian basmati rice by Henry et al. ( 36 ). The influence of another post-harvest treatment, milling, by which brown rice is transformed into white rice, was considered in several studies( 9 , 18 , 26 , 30 , 37 ) (see online supplementary Table S4). In those studies where cooking times were identical( 26 , 30 , 37 ), brown rice always produced lower PPG and PPI responses. However, when realistic (longer) cooking times were applied to brown rice( 9 , 18 ), the difference between brown and white rice was smaller and inconsistent.
Consumer processing can also make a large contribution to the formation of RS in rice. Chiu & Stewart( 38 ) quantified RS content in four white rice varieties (jasmine, long grain, medium grain and short grain) cooked in three different ways (oven-baked, conventional rice cooker and pressure cooker), and analysed the RS content immediately after preparation or after 3 d of refrigeration at 4°C. Refrigerated long-grain rice cooked in a conventional rice cooker had the highest RS content, while the refrigerated short-grain rice cooked in a pressure cooker had the lowest RS content. However, in this case, the GI values did not differ significantly between the higher-RS and lower-RS rice varieties. Consumer processing can also have a large effect on gelatinisation. Wolever et al. ( 39 ) showed that the GI generally increased with cooking time for rice, while Jung et al. ( 40 ) showed a marked increase in gelatinisation upon cooking rice and a somewhat higher GI and II.
Discussion
The literature reveals considerable variation in the glycaemic or insulin response to rice. This is largely attributable to (1) starch characteristics, (2) post-harvest processing (particularly parboiling and to a much lesser extent dehulling and milling) and (3) consumer processing (cooking, storage and reheating). The relationships among rice characteristics and processing factors, and their physico-chemical effects and impact on glycaemic responses are qualitatively shown in Fig. 3.
Influence of the composition and processing of rice
The most consistently important source of variation in PPG responses to rice is amylose content. The amylose content of rice varies between 0 % (waxy rice) and 30 % (Doongara)( 9 ), with basmati having an intermediate value (20–25 % amylose( 41 )). One of the reasons for the lower PPG responses to high amylose varieties is incomplete gelatinisation of amylose under normal cooking conditions, while amylopectin is fully gelatinised under these conditions( 42 ). Gelatinisation temperature is known to be positively correlated with amylose content( 43 ), implying that rice with a higher amylose content requires a higher gelatinisation temperature due to restrained swelling by amylose, resulting in a longer required cooking time( 44 ). The formation of complexes between amylose and lipids upon heating further contributes to reduced access to starch by gut enzymes( 33 ). These complexes with lipids are only found in association with amylose; therefore, rice with the highest amylose content would have more lipid–amylose complexes( 33 ). In addition, a higher amylose content (after cooking and cooling) leads to a greater degree of retrogradation( 18 ). A recent study found the major gene associated with the variation in the GI was the waxy gene( 44 ), which codes for different structures of amylose within the grain and leads to different retrogradation rates( 45 ).
The in vitro literature showed that the rice cultivar, clustered as Indica, Japonica and Hybrid rice type, plays a pivotal role in the rate and degree of starch digestion: low-amylose Indica showed a faster and higher degree of digestion than low-amylose Japonica, while a high-amylose Japonica was faster and more completely digested (reflected by a higher content of rapidly digestible starch and a lower content of slowly digestible starch and RS) than high-amylose Indica( 11 ). In addition, Benmoussa et al. ( 46 ) showed that amylopectin fine structure in rice cultivars affects starch digestion properties in vitro: cultivars with the highest amount of slowly digestible starch contained mainly long-chain amylopectin.
Post-harvest treatments such as parboiling( 21 , 29 , 34 ) and quick-cooking( 18 , 21 ) also have a large influence on the GI (see online Supplementary Table S3). Gelatinisation and re-crystallisation are the major changes that occur in rice starch during parboiling( 47 ). The parboiling process increases the gelatinisation temperature of rice that is proportional to the severity of the heat treatment( 48 ). This is probably the reason why pressure parboiling lowers the GI to such a large extent, especially of high-amylose starches( 49 ). The pressure parboiling process increases gelatinisation temperature due to the formation of retrograded amylose and amylopectin. Wet heating and subsequent drying during these processes result in the gelatinisation of starch, followed by retrogradation of amylose and amylopectin( 18 ) leading to higher levels of RS. It is possible that amylopectin crystallites (part of RS) retain some of the associating forces during reheating, and are partly responsible for the low glucose response observed during pressure parboiling. The amylose–lipid complexes have a melting temperature above 100°C and are not melted during the cooking process, resulting in higher levels of RS( 28 ).
Another way of achieving a high RS content is to apply multiple heating/cooling cycles( 50 ). After three heating/cooling cycles, the RS content of legumes, cereals and tubers increased from 4·18, 1·86 and 1·51 % to 8·16, 3·25 and 2·51 %, respectively, on a DM basis. However, a ten times greater RS content in rice varieties had no effect on the GI( 38 ). It is possible that the tested range of difference in RS content in that study was not sufficient to observe a change in the GI( 38 ), which is confirmed by the fact that only large differences in amylose content (leading to high RS content after cooking and cooling) lead to relatively large effects on the GI( 9 ).
Another final process shown to have a major influence on the PPG response is the gelatinisation process during cooking, which needs moisture and a high temperature (above gelatinisation temperature) for a particular period of time. Using different rice types with the same high amylose content, Panlasigui et al. ( 25 ) reported that PPG responses differed between rice types when a fixed cooking time was used; however, these differences disappeared when the minimum cooking time for each particular rice type was used. This is likely attributed to other physico-chemical properties of rice types. Physico-chemical parameters that predict lower blood glucose responses are high gelatinisation temperature, high minimum cooking time, lower viscosity measured by amylograph consistency (amylograph is an instrument for measuring gelatinisation temperature and viscosity of flour and starch pastes), and low volume expansion upon cooking, all parameters relating to lower gelatinisation( 25 ). Steaming also gave a larger PPG response than boiling and simmering( 51 ), which may reflect greater gelatinisation by steaming.
A factor that has a relatively less impact on PPG responses is physical size and form of the whole kernel rice, probably due to the fact that size is minimised by chewing( 52 ). Particle size only plays a major role when the rice is milled to rice flour, resulting in the higher surface area:starch ratio that leads to an increased rate of digestion( 53 ). In addition, the effect of brown rice v. white rice on glycaemic and insulinaemic responses shows a clear difference( 26 , 30 , 37 ) when compared at identical cooking times: for instance, brown rice always gives a lower PPG and PPI response (see online Supplementary Table S4). However, in reality, consumers cook brown rice longer than white rice, resulting in a mixed outcome: in some cases, white rice was found to have a higher glycaemic response( 9 ) (for Pelde), or a neutral effect( 9 ) (for Doongara and Calrose) or even a lower response than brown rice( 18 ). In most of these studies( 9 , 18 , 30 ) commercially available white rice was taken at random and not milled from the same batch of brown rice. Therefore, the variety and physico-chemical properties of rice samples may have differed( 53 ). Only two studies( 26 , 37 ) used white and brown rice from the same batch. However, a recent longer-term study showed that the iAUC over 5 d consumption was 19·8 % lower for a group eating brown v. white rice, as measured with a continuous glucose monitoring device( 54 ). However, it is not clear whether brown rice and white rice were of the same rice variety. Therefore, the results cannot clearly be attributed to the milling process alone. It is possible that the dietary fibre-rich bran fraction in brown rice can continue to serve as a barrier to digestive enzymes( 53 ), but several other modes of action are also possible. The magnitude of the effect of milling and polishing could also be somewhat dependent on the rice strain and cooking conditions( 18 ). White rice has a shorter minimum cooking time and higher volume expansion than brown rice, indicating that white rice is more easily hydrated and gelatinised compared with brown rice, and therefore more readily digested resulting in a higher PPG response( 53 ) when cooked under the same conditions.
In addition to the rice source and processing, there is an inter-individual variation observed in PPG (iAUC and peak blood glucose) responses to carbohydrate-rich foods. This was reported to account for at least 20 % of the total variation in PPG responses( 55 ). One of the factors that could be responsible for the inter-individual variation in PPG responses to rice could be ethnicity. The PPG (+iAUC) response was 60 % greater for five rice varieties and 39 % greater for glucose among the Chinese population compared with Europeans( 29 ) (Table 1). The most likely explanation for these ethnic differences is that the Chinese population are more likely to become insulin resistant than Europeans of the same or higher relative body weight and waist circumference( 56 ). Truong et al. ( 57 ) also observed that Asian Americans on average exhibited higher levels of blood glucose than Caucasians after consumption of a control food with 50 g carbohydrates. Therefore, when comparing the results across studies, ethnicity of the subjects should be taken into account: i.e. Asian people typically have a higher PPG response than Caucasians, which may also increase the apparent magnitude of differences between rice types and characteristics.
A final factor contributing towards the inter-individual variation in PPG responses is the degree of habitual mastication( 52 ). The latter may be a considerable contributor, especially to foods consisting of intact grains (such as rice) that rely on mechanical breakdown for carbohydrate release. Indeed, a recent study( 58 ) showed that rice chewed fifteen times produced a PPG, peak PPG and GI response significantly lower than that when chewed thirty times.
Conclusions
While rice as a total category may be a major global contributor to dietary glycaemic load, there is a wide variation in glycaemic and insulinaemic responses to rice as consumed. This can be largely attributed to the inherent starch characteristics of specific cultivars; however, within a given rice type, the mode of post-harvesting processing and ‘at-home’ preparation can also have a large influence. A reduced glycaemic impact is mediated mainly by the relative content of amylose (v. amylopectin), reduction in gelatinisation, or the facilitation of retrogradation. Perhaps, surprisingly, milling and polishing (thus white v. brown rice) has been found to have inconsistent impacts on acute glycaemic responses when compared at realistic cooking times that are longer for brown rice. The glycaemic response to rice can be further influenced by individual characteristics of the consumer, such as chewing habit and ethnicity. In order to interpret and compare the reported PPG responses between different studies in rice, the rice cultivar, amylose:amylopectin ratio, post-harvest processing parameters and cooking conditions should be considered. In addition, a lower PPG response to rice can be achieved by choosing right conditions, for example high amylose content, minimised cooking times (or pressure parboiled) and cooled before consumption. The opposite effect (a higher PPG response) can be achieved by selecting for low-amylose (waxy) white rice, with a long cooking time, and consuming directly after cooking.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0007114515001841.
click here to view supplementary material
Acknowledgements
The present study was not supported by any external funding.
The authors' contributions are as follows: H. M. B. carried out the systematic review; H. M. B. and D. J. M. extracted the data from the articles; H. M. B. wrote the manuscript with significant contributions from D. J. M. and J. S. t. H.
H. M. B., D. J. M. and J. S. t. H. are employees of Unilever. Unilever manufactures and markets consumer food products, including products used for the preparation of rice-based dishes.
Abbreviations: GI, glycaemic index; iAUC, incremental AUC; II, insulinaemic index; PPG, postprandial glucose response; PPI, postprandial insulin response; RS, resistant starch
References
- 1. Kennedy G, Burlingame B & Nguyen VN (2003) Nutritional contribution of rice and impact of biotechnology and biodiversity in rice-consuming countries. In Proceedings of the 20th Session of the International Rice Commission, Bangkok, Thailand. Rome: FAO. [Google Scholar]
- 2. Kumar S, Mohanraj E, Sudha V, et al. (2011) Perceptions about varieties of brown rice: a qualitative study from Southern India. J Am Diet Assoc 111, 1517–1522. [DOI] [PubMed] [Google Scholar]
- 3. Zhang G, Malik VS, Pan A, et al. (2010) Substituting brown rice for white rice to lower diabetes risk: a focus-group study in Chinese adults. J Am Diet Assoc 110, 1216–1221. [DOI] [PubMed] [Google Scholar]
- 4. Mohan V, Radhika G, Vijayalakshmi P, et al. (2010) Editorial: can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess grain (rice) intake? Ind J Med Res 131, 369–372. [PubMed] [Google Scholar]
- 5. Blaak EE, Antoine JM, Benton D, et al. (2012) Impact of postprandial glycaemia on health and prevention of disease. Obes Rev 13, 923–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu EA, Pan A, Malik V, et al. (2012) White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. Br Med J 344, e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neal B (2012) White rice and risk of type 2 diabetes. Br Med J 344, e2021. [DOI] [PubMed] [Google Scholar]
- 8. Foster-Powell K, Holt SHA & Brand-Miller JC (2002) International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76, 5–56. [DOI] [PubMed] [Google Scholar]
- 9. Brand Miller J, Pang E & Bramall L (1992) Rice: a high or low glycemic index food? Am J Clin Nutr 56, 1034–1036. [DOI] [PubMed] [Google Scholar]
- 10. Sajilata MG, Singhal RS & Kulkarni PR (2006) Resistant starch – a review. Compr Rev Food Sci Food Safety 5, 1–17. [DOI] [PubMed] [Google Scholar]
- 11. Hu P, Zhao H, Duan Z, et al. (2004) Starch digestibility and the estimated glycemic score of different types of rice differing in amylose content. J Cereal Sci 40, 231–237. [Google Scholar]
- 12. Atwell WA, Hood LF, Lineback DR, et al. (1988) The terminology and methodology associated with basic starch phenomena. Cereal Foods World 33, 306–311. [Google Scholar]
- 13. Tester RF & Sommerville MD (2003) The effects of non-starch polysaccharides on the extent of gelatinization, swelling and alpha-amylase hydrolysis of maize and wheat starches. Food Hydrocolloids 17, 41–54. [Google Scholar]
- 14. Faraj A, Vasanthan T & Hoover R (2004) The effect of extrusion cooking on resistant starch formation in waxy and regular barley flours. Food Res Int 37, 517–525. [Google Scholar]
- 15. Mitra A, Bhattacharya D & Roy S (2007) Role of resistant starches particularly rice containing resistant starches in type 2 diabetes. J Hum Ecol 21, 47–51. [Google Scholar]
- 16. Sievert D & Pomeranz Y (1989) Enzyme-resistant starch. I. Characterization and evaluation by enzymatic, thermoanalytical and microscopic methods. Cereal Chem 66, 342–347. [Google Scholar]
- 17. Dipti SS, Bergman C, Indrasari SD, et al. (2012) The potential of rice to offer solutions for malnutrition and chronic diseases. Rice 5, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ranawana DV, Henry CJK, Lightowler HJ, et al. (2009) Glycaemic index of some commercially available rice and rice products in Great Britain. Int J Food Sci Nutr 60, 99–110. [DOI] [PubMed] [Google Scholar]
- 19. Owens G (editor) (2001) Cereals Processing Technology. Cambridge: Woodhead Publishing Limited. [Google Scholar]
- 20. Li M, Piao J-H, Tian Y, et al. (2010) Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H2 in healthy Chinese adults. Br J Nutr 103, 1029–1034. [DOI] [PubMed] [Google Scholar]
- 21. Casiraghi MC, Brighenti F, Pellegrini N, et al. (1993) Effect of processing on rice starch digestibility evaluated by in vivo and in vitro methods. J Cereal Sci 17, 147–156. [Google Scholar]
- 22. Al-Mssallem MQ, Hampton SM, Frost GS, et al. (2011) A study of Hassawi rice (Oryza sativa L.) in terms of its carbohydrate hydrolysis (in vitro) and glycaemic and insulinaemic indices (in vivo). Eur J Clin Nutr 65, 627–634. [DOI] [PubMed] [Google Scholar]
- 23. Juliano BO & Goddard MS (1986) Cause of varietal difference in insulin and glucose responses to ingested rice. Qual Plant Plant Foods Hum Nutr 36, 35–41. [Google Scholar]
- 24. Juliano BO, Perez CM, Komindr S, et al. (1989) Properties of Thai cooked rice and noodles differing in glycemic index in non-insulin-dependent diabetics. Plant Foods Hum Nutr 39, 369–374. [DOI] [PubMed] [Google Scholar]
- 25. Panlasigui L, Thompson LU, Juliano BO, et al. (1991) Rice varieties with similar amylose content differ in starch digestibility and glycemic response in humans. Am J Clin Nutr 54, 871–877. [DOI] [PubMed] [Google Scholar]
- 26. Panlasigui LN & Thompson LU (2006) Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int J Food Sci Nutr 57, 151–158. [DOI] [PubMed] [Google Scholar]
- 27. Kim JC, Kim J-I, Kong B-W, et al. (2004) Influence of the physical form of processed rice products on the enzymatic hydrolysis of rice starch in vitro and on the postprandial glucose and insulin responses in patients with type 2 diabetes mellitus. Biosci Biotechnol Biochem 68, 1831–1836. [DOI] [PubMed] [Google Scholar]
- 28. Larsen HN, Rasmussen OW, Rasmussen PH, et al. (2000) Glycaemic index of parboiled rice depends on the severity of processing: study in type 2 diabetic subjects. Eur J Clin Nutr 54, 380–385. [DOI] [PubMed] [Google Scholar]
- 29. Kataoka M, Venn BJ, Williams SM, et al. (2013) Glycaemic responses to glucose and rice in people of Chinese and European ethnicity. Diabet Med 30, 101–107. [DOI] [PubMed] [Google Scholar]
- 30. Trinidad TP, Mallillin AC, Encabo RR, et al. (2013) The effect of apparent amylose content and dietary fibre on the glycemic response of different varieties of cooked milled and brown rice. Int J Food Sci Nutr 64, 89–93. [DOI] [PubMed] [Google Scholar]
- 31. Zarrati M, Pirali M, Mirmiran P, et al. (2008) Glycemic index of various brands of rice in healthy individuals. Int J Endocrinol Metab 4, 200–204. [Google Scholar]
- 32. Larsen HN, Christensen C, Rasmussen OW, et al. (1996) Influence of parboiling and physico-chemical characteristics of rice on the glycaemic index in non-insulin-dependent diabetic subjects. Eur J Clin Nutr 50, 22–27. [PubMed] [Google Scholar]
- 33. Goddard MS, Young G & Marcus R (1984) The effect of amylose content on insulin and glucose responses to ingested rice. Am J Clin Nutr 39, 388–392. [DOI] [PubMed] [Google Scholar]
- 34. Hettiarachchi P, Jiffry MTM, Jansz ER, et al. (2001) Glycaemic indices of different varieties of rice grown in Sri Lanka. Ceylon Med J 46, 11–14. [DOI] [PubMed] [Google Scholar]
- 35. Srinivasa D, Raman A, Meena P, et al. (2013) Glycaemic index (GI) of an Indian branded thermally treated Basmati rice variety: a multi centric study. J Assoc Phys India 61, 716–720. [PubMed] [Google Scholar]
- 36. Henry CJK, Lightowler HJ, Strik CM, et al. (2005) Glycaemic index and glycaemic load values of commercially available products in the UK. Br J Nutr 94, 922–930. [DOI] [PubMed] [Google Scholar]
- 37. Karupaiah T, Aik CK, Heen TC, et al. (2011) A transgressive brown rice mediates favourable glycaemic and insulin responses. J Sci Food Agric 91, 1951–1956. [DOI] [PubMed] [Google Scholar]
- 38. Chiu Y-T & Stewart ML (2013) Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac J Nutr 22, 372–379. [DOI] [PubMed] [Google Scholar]
- 39. Wolever TMS, Jenkins DJA, Kalmusky J, et al. (1986) Comparison of regular and parboiled rices: explanation of discrepancies between reported glycemic responses to rice. Nutr Res 6, 349–357. [Google Scholar]
- 40. Jung EY, Suh HJ, Hong WS, et al. (2009) Uncooked rice of relatively low gelatinization degree resulted in lower metabolic glucose and insulin responses compared with cooked rice in female college students. Nutr Res 29, 457–461. [DOI] [PubMed] [Google Scholar]
- 41. Bhattacharjee P, Singhal RS & Kulkarni PR (2002) Basmati rice: a review. Int J Food Sci Techn 37, 12. [Google Scholar]
- 42. Björck I, Granfeldt Y, Liljeberg H, et al. (1994) Food properties affecting the digestion and absorption of carbohydrates. Am J Clin Nutr 59, Suppl. 3, 699S–705S. [DOI] [PubMed] [Google Scholar]
- 43. Fredriksson H, Silverio J, Andersson R, et al. (1998) The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr Polym 35, 119–134. [Google Scholar]
- 44. Fitzgerald MA, Rahman S, Resurreccion AP, et al. (2011) Identification of a major genetic determinant of glycaemic index in rice. Rice 4, 66–74. [Google Scholar]
- 45. Tran NAV, Daygon DA, Resurreccion R, et al. (2011) A single nucleotide polymorphism on the Waxy gene explains gel consistency. Theor Appl Genet 123, 519–525. [DOI] [PubMed] [Google Scholar]
- 46. Benmoussa M, Moldenhauer KAK & Hamaker BR (2007) Rice amylopectin fine structure variability affects starch digestion properties. J Agric Food Chem 55, 1475–1479. [DOI] [PubMed] [Google Scholar]
- 47. Oli P, Ward R, Adhikari B, et al. (2014) Parboiled rice: understanding from a materials science approach. J Food Eng 124, 173–183. [Google Scholar]
- 48. Islam MR, Shimizu N & Kimura T (2002) Effect of processing conditions on thermal properties of parboiled rice. Food Sci Technol Res 8, 131–136. [Google Scholar]
- 49. Zavareze EdR, Storck CR, de Castro LAS, et al. (2010) Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem 121, 358–365. [Google Scholar]
- 50. Yadav BS, Sharma A & Yadav RB (2009) Studies on effect of multiple heating/cooling cycles on the resistant starch formation in cereals, legumes and tubers. Int J Food Sci Nutr 60, 258–272. [DOI] [PubMed] [Google Scholar]
- 51. Parastouei K, Shahaboddin ME, Motalebi M, et al. (2011) Glycemic index of Iranian rice. Sci Res Essays 6, 5302–5307. [Google Scholar]
- 52. Ranawana V, Henry JK & Pratt M (2010) Degree of habitual mastication seems to contribute to interindividual variations in the glycemic response to rice but not to spaghetti. Nutr Res 30, 382–391. [DOI] [PubMed] [Google Scholar]
- 53. Chang UJ, Hong YH, Jung EY, et al. (2014) Rice and the glycemic index: benefits, risks and mechanisms of whole grains in health promotion. In Wheat and Rice in Disease Prevention and Health, pp. 357–363 [Watson RR, Preedy V and Zibadi S, editors]. London: Elsevier, Inc. [Google Scholar]
- 54. Mohan V, Spiegelman D, Sudha V, et al. (2014) Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: randomized trial. Diabetes Technol Ther 16, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vega-Lopez S, Ausman LM, Griffith JL, et al. (2007) Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 30, 1412–1417. [DOI] [PubMed] [Google Scholar]
- 56. Dickinson S, Colagiuri S, Faramus E, et al. (2002) Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr 132, 2574–2579. [DOI] [PubMed] [Google Scholar]
- 57. Truong TH, Yuet WC & Hall MD (2014) Glycemic index of American-grown jasmine rice classified as high. Int J Food Sci Nutr 65, 436–439. [DOI] [PubMed] [Google Scholar]
- 58. Ranawana V, Leow MK-S & Henry CJK (2014) Mastication effects of the glycaemic index: impact on variability and practical implications. Eur J Clin Nutr 68, 137–139. [DOI] [PubMed] [Google Scholar]
- 59. Gatti E, Testolin G, Noè D, et al. (1987) Plasma glucose and insulin responses to carbohydrate food (rice) with different thermal processing. Ann Nutr Metab 31, 296–303. [DOI] [PubMed] [Google Scholar]
- 60. Matsuo T, Mizushima Y, Komuro M, et al. (1999) Estimation of glycemic and insulinemic responses to short-grain rice (Japonica) and a short-grain rice-mixed meal in healthy young subjects. Asia Pac J Clin Nutr 8, 190–194. [DOI] [PubMed] [Google Scholar]
- 61. Shobana S, Kokila A, Lakshmipriya N, et al. (2012) Glycaemic index of three Indian rice varieties. Int J Food Sci Nutr 63, 178–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0007114515001841.
click here to view supplementary material