Abstract
To identify native wildlife species possibly susceptible to infection with Schmallenberg virus (SBV), a midge-transmitted orthobunyavirus that predominantly infects domestic ruminants, samples from various free-living ruminants, but also carnivores, small mammals and wild boar were analyzed serologically. Before 2011, no SBV-specific antibodies were detectable in any of the tested species, thereafter, a large proportion of the ruminant population became seropositive, while every sample taken from carnivores or small mammals tested negative. Surprisingly, SBV-specific-antibodies were also present in a large number of blood samples from wild boar during the 2011/2012 and 2012/2013 hunting seasons. Hence, free-ranging artiodactyls may play a role as wildlife host.
Introduction, methods and results
Schmallenberg virus, a midge-transmitted orthobunyavirus, was initially detected in domestic ruminants near the German/Dutch border in late 2011 [1]. Since then, the virus spread very rapidly among European livestock. After the first vector season a very high seroprevalence of approximately 70% to nearly 100% was observed in domestic ruminants in the centre of the epidemic in North-Western Germany, the Netherlands and Belgium [2-5]. In the following vector season, SBV still circulated in that area, but at a much lower level [6], and in 2013, cases of viral genome detection were reported only sporadically to the German Animal Disease Reporting System (TSN). However, in summer and autumn 2014, SBV reappeared to a greater extent [7] and the reasons for that observation are not completely elucidated until now. One possible explanation could be the existence of transient reservoir hosts for the virus apart from the major target species. Until now, viral genome or specific antibodies were detected predominantly in domestic and wild ruminants, such as cattle, sheep, goats, mouflon, bison, moose, alpacas, buffalos, bison, and deer [8-12]. However, antibodies were also found in a dog in Sweden [13], and type I interferon receptor knock-out mice are susceptible to an experimental SBV-infection [14]. To examine whether free-living carnivores or small mammals, i.e. rodents and shrews, may be infected by SBV, 339 blood samples from a variety of carnivores (red fox - Vulpes vulpes, raccoon dog - Nyctereutes procyonoides, raccoon - Procyon lotor, marten - Martes spp.) as well as 195 samples from small mammals (members of the families Muridae, Cricetidae and Soricidae; approved by the competent authority, LANUV NRW, ref. 8.87-51.05.20.09.210) were collected between 2011 and 2012 and tested for the presence of SBV-specific antibodies. Though the detection of specific antibodies does not inevitably reflect a productive infection, the short viraemia of only a few days [1,15] makes the detection of anti-SBV antibodies to a much more promising diagnostic test system than the detection of the virus itself, especially for epidemiological investigations.
Wild boar (Sus scrofa), considered as a reservoir for several viruses of livestock and humans, is the second most abundant ungulate in Europe. Based on official hunting statistics Germany is one of the countries with the highest population densities of wild boar in Europe [16]. In previous investigations neutralizing antibodies against Akabane virus, a member of the Simbu sero-group of the genus Orthobunyavirus, were detected in warthogs and bush pigs in Africa [17,18] and in pigs in Taiwan [19].
To investigate whether wild boar are susceptible to an SBV-infection and may serve as a reservoir, a total of 2077 blood samples taken post mortem in 2006 and between August 2010 and December 2013 was analyzed for the presence of SBV-specific antibodies. 1646 of the 2077 samples were collected in North Rhine-Westphalia, the German federal state where the first case of SBV-infection was detected [1]. In the 2013/2014 hunting seasons, predominantly young animals (<1 year) were sampled. In addition, samples from European mouflon (Ovis orientalis musimon), as a wild sheep the only free-living wild form of susceptible domestic animals in Germany, and further free-living ruminants such as roe deer (Capreolus capreolus), fallow deer (Dama dama), red deer (Cervus elaphus), and sika deer (Cervus nippon) were analyzed (Table 1). Blood samples from deer and mouflon as well as wild boar and carnivores were collected in cooperation with local hunters according to the appropriate German legislation. No ethical/welfare authority approval was required as samples were collected post-mortem by the hunters. All blood samples were examined with an indirect or a competitive commercially available SBV-antibody ELISA (ID Screen® Schmallenberg virus Indirect or ID Screen® Schmallenberg virus Competition, both IDvet, Grabels, France) according to the manufacturer’s recommendations. In the indirect ELISA kit an Anti-multi-species IgG-HRP conjugate is included. Samples with a doubtful ELISA result as well as a representative number of samples from each species with positive and negative ELISA results were retested by a standard micro-neutralization assay as described previously [15].
Table 1.
Serological results of German wildlife screening for Schmallenberg virus infection
| Species | Hunting season or time period | Samples | Positive (%) | Negative (%) |
|---|---|---|---|---|
| Mouflon | 2011/2012 | 4 | 4 (100) | 0 |
| 2012/2013 | 31 | 26 (83.87) | 5 (16.13) | |
| 2013/2014 | 9 | 3 (33.33) | 6 (66.67) | |
| Deera | 2000/2001 | 134 | 0 | 134 (100) |
| 2011/2012 | 136 | 41 (30.15) | 95 (69.85) | |
| 2012/2013 | 760 | 278 (36.58) | 482 (63.42) | |
| 2013/2014 | 324 | 65 (20.06) | 259 (79.94) | |
| 2014/2015 | 4 | 2 (50) | 2 (50) | |
| Carnivoresb | 2011/2012 | 281 | 0 | 281 (100) |
| 2012/2013 | 58 | 0 | 58 (100) | |
| Small mammalsc | 2011-2012 | 195 | 0 | 195 (100) |
The results are divided by species and hunting seasons (huntable animals) resp. time period (small mammals). A hunting season takes from 1st April to 31st March next year.
aRoe deer, red deer, sika deer, and fallow deer.
bRed fox, marten, badger, raccoon dog, and raccoon.
cRodents, and shrews.
No antibodies against SBV could be detected in samples of the 339 wild carnivores collected from 2011 to 2013, and in samples of the 195 small mammals collected in 2011 and 2012.
In contrast, within this time frame (2011–2012) about 30% of the deer and all 4 tested mouflons were SBV-antibody-positive (Table 1). Furthermore, in the hunting season 2012/2013 antibodies against SBV were detectable in approximately 84% of the mouflons and 37% of the samples from deer. In the following season the seroprevalence declined to about 33% and 20%, respectively (Table 1).
In addition to the samples taken after the presumed date of SBV-introduction into Europe, historical samples collected from wild ruminants (roe deer, red deer, and fallow deer) in Germany before 2011 were analyzed. All 134 samples tested negative in an SBV-specific antibody-ELISA (Table 1). The same holds true for wild boar, every sample taken before autumn 2011 tested negative.
However, from October 2011 onwards, SBV-specific antibodies were frequently also detected in wild boar (Figure 1). In the hunting season 2011/2012, 105 out of 316 samples tested positive (33%), in the following season SBV-specific antibodies were detectable in 11% of the samples (119 out of 1114), while in 2013/2014 all of the analyzed 32 samples scored negative (Figure 1).
Figure 1.
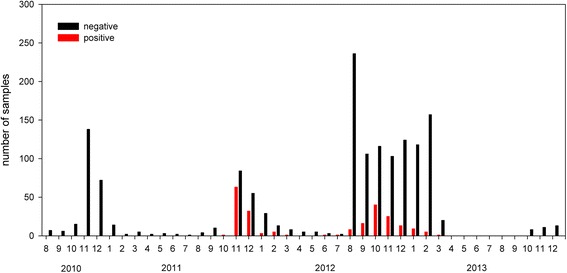
Frequency of Schmallenberg virus-specific antibodies in wild boar. Samples were collected between August 2010 and December 2013 and analyzed by a commercially available ELISA resp. serum neutralization test. The number of negative results per month is shown as a black bar and the number of positive results is displayed as a red bar. Only samples with information about months of culling were depicted.
A number of samples with positive ELISA results from each species were also confirmed by a highly specific serum neutralization test, and the resulting titers ranged from 1:5 up to 1:30 (mouflon), 1:640 (roe deer), 1:15 (fallow deer), 1:60 (red deer), 1:60 (sika deer), or 1:80 (wild boar).
Nevertheless, during the entire period from 2011 to the 2014–2015 hunting season, malformations were not reported by hunters, neither in wild ruminants, nor in wild boar.
Discussion
As shown for alpine ungulates or deer hunted in countries that border Germany, SBV is capable to infect several ruminant species [9,11,12]. In the present study, however, SBV-specific antibodies were not only detected in a wide range of ruminants, but also in wild boar which belong to the closely related Suidae family within the order Artiodactyla.
After experimental SBV-infection of domestic pigs, in only a small proportion of animals a temporary seroconversion was observed; neutralization titers that barely reached the limit of detection were measured in a few animals for a short time, while the SBV-specific ELISA scored negative in every case [20]. As opposed to experimentally inoculated domestic pigs, neutralizing anti-SBV antibodies could not be detected in field-collected sera [20]. On the contrary, a large proportion of wild boar tested positive by ELISA in our study, and neutralization titers even exceeded those measured in wild ruminants, i.e. mouflon, fallow deer, red deer, or sika deer. Therefore, and because of the positive results in two independent test systems, and the absence of measurable SBV-specific antibodies before 2011, the year of presumed virus introduction into Europe, unspecific reactions are very unlikely. Especially as the insect vectors responsible for SBV-transmission, such as Culicoides midges of the Obsoletus group [21], evidently also feed on members of the Suidae family [22]. The reasons for the obvious differences in the susceptibility of domestic pigs and wild boar to an SBV-infection, however, need to be evaluated in future studies. In this context the possibility has to be considered that midges might feed repeatedly on an individual animal which could induce a measurable immune response also in pigs resp. wild boar. Furthermore, it might be possible that the pathogen is only mechanically transmitted by the vector [18].
Though the applied ELISA tests might cross-react with antibodies against viruses closely related to SBV and the serum neutralization test is in general considered as the most sensitive and specific system for the detection of SBV-specific antibodies [23], only a subset of samples could be tested in this assay. Since the samples were taken from hunted animals under non-sterile conditions in the present study, the quality (bacterial contamination, cytotoxicity) hampered the cell culture-based neutralization assay. However, a good correlation between ELISA results and neutralization titers was observed in every tested sample (data not shown), and the commercially available SBV-ELISAs have been previously successfully applied not only for sera from cattle, sheep or goats, for which they have been originally produced, but also for further species such as wild ruminants, domestic pigs or mice [20,24,25]. Here, the applicability of this test system was demonstrated for wild boar as well.
In keeping with domestic ruminants, SBV was not present in German wildlife until late 2011. Thereafter, a large proportion of seropositive animals was found. The lower seroprevalence in the wild boar population after the 2012/2013 hunting season corresponds to that observed in domestic ruminants such as cattle [6] and further free-living ruminants (Table 1). Most likely caused by a high seroprevalence in the population of susceptible animals after the first vector season, the virus circulated only on a limited scale in the following years resulting in a missing infection of the SBV-naïve young stock which in turn has led to a decline in herd seroprevalence. Apart from the supply with seronegative offspring over the time, a gradual reduction of SBV-specific antibodies in individual animals could be an explanation for the declining herd seroprevalence. However, in other animal species, such as cattle, the titers of anti-SBV antibodies are mostly stable for at least two years [26], and in the present study, predominantly young animals were tested after the 2011/2012 hunting season. SBV-specific antibodies were detectable in a number of those animals in the last years which is not only explainable by maternal antibodies (e.g. for animals older than 6 months), but also by new infections caused by a low level of virus circulation. The annual testing of subadults could show whether this low level of infections will persist in the next years and, if so, it will lead to a renewed virus circulation on a larger scale which might be expected as soon as the level of the specific immunity within the complete population will further decline.
In domestic ruminants, the most important effect of SBV is stillbirth, premature birth and the induction of severe congenital malformations when dams are infected during a critical period of pregnancy [4,27]. Despite the high rate of SBV-infections in the first two years (autumn 2011 and 2012), no aborted, stillborn and/or malformed fawns or boar piglets were reported, neither from German hunters or forest rangers, nor from further European countries such as Belgium or the UK [12,28]. This may be due to the fact, that embryos can only be infected in a critical time of pregnancy i.e. after establishment of the first placentome and before the fetus is immunologically competent. Until now, only one case of a cervine fetus with SBV-typical malformation was found in utero; however, further abnormalities were also visible and an SBV-specific RT-PCR tested negative [29]. Consequently, it remains unclear whether SBV may cause transplacental infection in wild animals with the effects seen in cattle or sheep. This question is difficult to be answered for wildlife because aborted fetuses or unviable newborn malformed animals might be quickly eaten by scavengers, and therefore are extremely difficult to collect. All carnivores, which might be in contact to the virus by eating aborted fetuses and stillborn newborns, tested in contrast to ruminants or wild boar negative for SBV-specific antibodies. In addition to carnivores, free-living shrews and rodents are most likely also not a reservoir for SBV; specific antibodies were not detected in the tested species which display an intact interferon system in contrast to the SBV susceptible type I interferon receptor deficient mice [14,24].
In conclusion, SBV-specific antibodies were detectable in all free-ranging cervids and wild sheep (mouflons) present in Germany, but also in wild boar, indicating that not only ruminants but further members of the artiodactyls are susceptible to an SBV-infection. Hence, free-ranging artiodactyls but not small mammals or wild carnivores may play a role as an additional host in the epidemiology of SBV.
Acknowledgements
The authors especially thank the hunters for providing the wildlife samples, Michael Schürmann, Ulrich Kros for coordination of animal sampling in North Rhine-Westphalia, Sabrina Schmidt, Mathias Schlegel, Christian Kretzschmar, Konrad Wanka, Dörte Kaufmann, Ulrike M. Rosenfeld, Hanan Sheikh Ali, Kathrin Baumann, Nastasja Kratzmann, Theres Wollny, Angele Breithaupt, Stephan Drewes, Samuel Petri and Paul Dremsek for dissection of the small mammals as well as Deborah Basso, and Claudia Bunzenthal for storage and shipment of wild boar samples. Small mammal samples from North Rhine-Westphalia were kindly provided by Jens Jacob, Daniela Reil, Christian Imholt, Daniela Imholt and Mechthild Budde from UFOPLAN projects 3709 41 401 and 3713 48 401. Martin Eiden provided blood samples from wild ruminants especially collected before 2011. We are grateful to Anne Leske and Bianka Hillman for excellent technical assistance. This work was financially supported by the Germany Federal Ministry of Food, Agriculture and consumer protection and the European Union as outlined in Council Decision 2012/349/EU concerning a financial contribution by the Union for studies on Schmallenberg virus.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SM designed the study, performed and analyzed the serological assays. KW analyzed the data and drafted the manuscript. BH participated in the design of the study and in sample acquisition. RGU, WL, KB, and UW collected, stored and shipped the samples. MB conceived the study, and participated in its design. All authors read and approved the final manuscript.
Contributor Information
Susan Mouchantat, Email: susan.mouchantat@fli.bund.de.
Kerstin Wernike, Email: kerstin.wernike@fli.bund.de.
Walburga Lutz, Email: walburga.lutz@lanuv.nrw.de.
Bernd Hoffmann, Email: bernd.hoffmann@fli.bund.de.
Rainer G. Ulrich, Email: rainer.ulrich@fli.bund.de
Konstantin Börner, Email: konstantin-b@web.de.
Ulrich Wittstatt, Email: ulrich.wittstatt@landeslabor-bbb.de.
Martin Beer, Email: martin.beer@fli.bund.de.
References
- 1.Hoffmann B, Scheuch M, Höper D, Jungblut R, Holsteg M, Schirrmeier H, Eschbaumer M, Goller KV, Wernike K, Fischer M, Breithaupt A, Mettenleiter TC, Beer M. Novel orthobunyavirus in Cattle, Europe, 2011. Emerg Infect Dis. 2012;18:469–472. doi: 10.3201/eid1803.111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elbers AR, Loeffen WL, Quak S, de Boer-Luijtze E, van der Spek AN, Bouwstra R, Maas R, Spierenburg MA, de Kluijver EP, van Schaik G, van der Poel WH. Seroprevalence of Schmallenberg virus antibodies among dairy cattle, the Netherlands, winter 2011–2012. Emerg Infect Dis. 2012;18:1065–1071. doi: 10.3201/eid1807.120323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garigliany MM, Bayrou C, Kleijnen D, Cassart D, Desmecht D. Schmallenberg virus in domestic cattle, Belgium, 2012. Emerg Infect Dis. 2012;18:1512–1514. doi: 10.3201/eid1809.120716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernike K, Conraths F, Zanella G, Granzow H, Gache K, Schirrmeier H, Valas S, Staubach C, Marianneau P, Kraatz F, Höreth-Böntgen D, Reimann I, Zientara S, Beer M. Schmallenberg virus-two years of experiences. Prev Vet Med. 2014;116:423–434. doi: 10.1016/j.prevetmed.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Wernike K, Silaghi C, Nieder M, Pfeffer M, Beer M. Dynamics of Schmallenberg virus infection within a cattle herd in Germany, 2011. Epidemiol Infect. 2014;142:1501–1504. doi: 10.1017/S0950268813002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meroc E, Poskin A, Van Loo H, Van Driessche E, Czaplicki G, Quinet C, Riocreux F, De Regge N, Caij B, van den Berg T, Hooyberghs J, Van der Stede Y (2015) Follow-up of the Schmallenberg virus seroprevalence in Belgian cattle. Transbound Emerg Dis 62:e80-84 [DOI] [PubMed]
- 7.Wernike K, Hoffmann B, Conraths FJ, Beer M. Schmallenberg virus reoccurrence, Germany, 2014. Emerg Infect Dis. 2015;21:1202–1204. doi: 10.3201/eid2107.150180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anonymous . Technical and scientific studies on Schmallenberg virus - final report about “Commission Implementing Decision of 27 June 2012 supporting studies on Schmallenberg virus by the five-country consortium of veterinary research institutes”. 2014. [Google Scholar]
- 9.Chiari M, Sozzi E, Zanoni M, Alborali LG, Lavazza A, Cordioli P. Serosurvey for Schmallenberg virus in alpine wild ungulates. Transbound Emerg Dis. 2014;61:1–3. doi: 10.1111/tbed.12158. [DOI] [PubMed] [Google Scholar]
- 10.EFSA (2013) “Schmallenberg” virus: analysis of the epidemiological data (May 2013). EFSA Supporting Publications 2013. EN-3429. http://www.efsa.europa.eu/de/supporting/doc/429e.pdf; accessed 15/07/2013
- 11.Larska M, Krzysiak MK, Kęsik-Maliszewska J, Rola J. Cross-sectional study of Schmallenberg virus seroprevalence in wild ruminants in Poland at the end of the vector season of 2013. BMC Vet Res. 2014;10:967. doi: 10.1186/s12917-014-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linden A, Desmecht D, Volpe R, Wirtgen M, Gregoire F, Pirson J, Paternostre J, Kleijnen D, Schirrmeier H, Beer M, Garigliany MM. Epizootic Spread of Schmallenberg Virus among Wild Cervids, Belgium, Fall 2011. Emerg Infect Dis. 2012;18:2006–2008. doi: 10.3201/eid1812.121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wensman JJ, Blomqvist G, Hjort M, Holst BS. Presence of antibodies to Schmallenberg virus in a dog in Sweden. J Clin Microbiol. 2013;51:2802–2803. doi: 10.1128/JCM.00877-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wernike K, Breithaupt A, Keller M, Hoffmann B, Beer M, Eschbaumer M. Schmallenberg virus infection of adult type I interferon receptor knock-out mice. PLoS One. 2012;7:e40380. doi: 10.1371/journal.pone.0040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernike K, Eschbaumer M, Schirrmeier H, Blohm U, Breithaupt A, Hoffmann B, Beer M. Oral exposure, reinfection and cellular immunity to Schmallenberg virus in cattle. Vet Microbiol. 2013;165:155–159. doi: 10.1016/j.vetmic.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011;156:1691–1705. doi: 10.1007/s00705-011-1080-2. [DOI] [PubMed] [Google Scholar]
- 17.Al-Busaidy S, Hamblin C, Taylor WP. Neutralising antibodies to Akabane virus in free-living wild animals in Africa. Trop Anim Health Prod. 1987;19:197–202. doi: 10.1007/BF02242116. [DOI] [PubMed] [Google Scholar]
- 18.Hamblin C, Anderson EC, Jago M, Mlengeya T, Hipji K. Antibodies to some pathogenic agents in free-living wild species in Tanzania. Epidemiol Infect. 1990;105:585–594. doi: 10.1017/S0950268800048226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CC, Huang TS, Deng MC, Jong MH, Lin SY. Natural infections of pigs with akabane virus. Vet Microbiol. 2003;94:1–11. doi: 10.1016/S0378-1135(03)00062-2. [DOI] [PubMed] [Google Scholar]
- 20.Poskin A, Van Campe W, Mostin L, Cay B, De Regge N. Experimental Schmallenberg virus infection of pigs. Vet Microbiol. 2014;170:398–402. doi: 10.1016/j.vetmic.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen LD, Kristensen B, Kirkeby C, Rasmussen TB, Belsham GJ, Bodker R, Bøtner A. Culicoids as vectors of Schmallenberg virus. Emerg Infect Dis. 2012;18:1204–1206. doi: 10.3201/eid1807.120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartsch S, Bauer B, Wiemann A, Clausen PH, Steuber S. Feeding patterns of biting midges of the Culicoides obsoletus and Culicoides pulicaris groups on selected farms in Brandenburg, Germany. Parasitol Res. 2009;105:373–380. doi: 10.1007/s00436-009-1408-y. [DOI] [PubMed] [Google Scholar]
- 23.van der Poel WH, Cay B, Zientara S, Steinbach F, Valarcher JF, Bøtner A, Mars MH, Hakze-van der Honing R, Schirrmeier H, Beer M. Limited interlaboratory comparison of Schmallenberg virus antibody detection in serum samples. Vet Rec. 2014;174:380. doi: 10.1136/vr.102180. [DOI] [PubMed] [Google Scholar]
- 24.Kraatz F, Wernike K, Hechinger S, König P, Granzow H, Reimann I, Beer M. Deletion mutants of Schmallenberg virus are avirulent and protect from virus challenge. J Virol. 2015;89:1825–1837. doi: 10.1128/JVI.02729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laloy E, Breard E, Sailleau C, Viarouge C, Desprat A, Zientara S, Klein F, Hars J, Rossi S. Schmallenberg virus infection among red deer, France, 2010–2012. Emerg Infect Dis. 2014;20:131–134. doi: 10.3201/eid2001.130411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbers AR, Stockhofe-Zurwieden N, van der Poel WH. Schmallenberg virus antibodies in adult cows and maternal antibodies in calves. Emerg Infect Dis. 2014;20:901–902. doi: 10.3201/eid2005.130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conraths FJ, Peters M, Beer M. Schmallenberg virus, a novel orthobunyavirus infection in ruminants in Europe: potential global impact and preventive measures. N Z Vet J. 2013;61:63–67. doi: 10.1080/00480169.2012.738403. [DOI] [PubMed] [Google Scholar]
- 28.Barlow A, Green P, Banham T, Healy N. Serological confirmation of SBV infection in wild British deer. Vet Rec. 2013;172:429. doi: 10.1136/vr.f2438. [DOI] [PubMed] [Google Scholar]
- 29.Decors A, Moinet M, Décôté Y, Pozet F, Viry A, Faure E, Rossi S. Chevrette suspecte de maladie de Schmallenberg. 2014. [Google Scholar]


