Abstract
Aim:
This study was aimed at standardizing the “In-House fatty meal” methodology in cholescintigraphy and to determine gall bladder ejection fraction (GBEF) with this standardized meal.
Materials and Methods:
This is a prospective case–control study where 61 patients having right upper quadrant pain and postprandial bloating and 59 healthy volunteers were included. They underwent 99mTc-mebrofenin fatty meal cholescintigraphy following a standard protocol. Dynamic acquisitions over 120 min were done, with a fatty meal being given between 45- and 60-min. Gallbladder emptying kinetics was studied by assessing the time activity curves and calculation of GBEFs were made at 30-min, 45-min, and at 60-min and assessed.
Results:
The GBEF at 30-min was 74.42% ± 8.26% (mean ± standard deviation), at 45-min was 82.61% ± 6.5%, and at 60-min was 89.37% ± 4.48% in the volunteer group. The lower limit of GBEF in volunteers at 30-min was 58%, 45-min was 69%, and at 60-min was 81%. Receiver operating characteristic (ROC) analysis showed that 30-min GBEF provided the best separation between healthy and diseased subjects with an area under curve of 0.952 (95% confidence interval = 0.914–0.989). The lower limit of GBEF at 30-min was 58%.
Conclusions:
An in-House standard fatty meal could be a reproducible alternative to cholecystokinin as it is well-tolerated. Based on ROC curve analysis, we propose that 30-min GBEF provides good separation between healthy and diseased people with this in-House fatty meal. Hence, dynamic acquisitions beyond 30-min postingestion of the fatty meal may not be warranted.
Keywords: Dynamic cholescintigraphy, gallbladder ejection fraction, in-House fatty meal
INTRODUCTION
Gastrointestinal problems often present with postprandial bloating, epigastric burning, nausea, vomiting, indigestion, sour eructations, and early satiety. Biliary tract disorders are a common cause of symptomatic gastrointestinal disease, and it, therefore, becomes necessary to identify and characterize this segment.
Hepatobiliary imaging using 99mTc-mebrofenin (an iminodiacetic acid derivative) is a clinically relevant technique in this situation as it permits noninvasive assessment of the functional status of the biliary systems by tracer excretion dynamics. Being objective, it is reproducible at various time intervals and allows the simultaneous assessment of both morphological and physiological changes.[1]
Cholescintigraphy with sincalide (intravenous octapeptide of cholecystokinin [CCK]) is the gold standard to assess gallbladder function. Its nonavailability[2] and high cost warrants an alternative that can be easily administered with minimal side effects. A fatty meal fulfills these criteria besides being a physiological stimulus as it stimulates the release of endogenous CCK.
However, a great amount of variability in ejection fraction has been seen with various fatty meal assessments as it depends on the amount of fat present in it, and the methodology used.[3] Hence, there is a need to standardize fatty meal for normal subjects so as to define optimum time and gallbladder ejection fractions (GBEF) cut-off. This in turn would help in identifying the abnormal gallbladder function in these patients.
MATERIALS AND METHODS
This is a prospective case–control study. The study duration extended from June 2011 to December 2013. The study was approved by the Institutional Ethics Committee and written informed consent was obtained from all the subjects.
A detailed history was taken, and Rome III questionnaire for functional biliary disorders was used. All the subjects were prescreened with ultrasonography (USG) of the upper abdomen and upper gastrointestinal endoscopy to rule out organic causes of disease. Liver and pancreatic biochemistries were confirmed to be normal.
Selection of volunteers
Fifty-nine volunteers (mean age 42 years, 31 males, and 28 females) participated in the study. The volunteers had no symptoms (i.e., right upper quadrant/epigastric pain, nausea, vomiting), no history of gall bladder or hepatobiliary disease, diabetes mellitus, abdominal surgery or family history of hepatobiliary disease.
Selection of patients
The patients were referred to the Department of Nuclear Medicine for evaluation of recurrent right upper quadrant pain for the duration of 6 months by cholescintigraphy. The patients who fulfilled Rome III diagnostic criteria of functional gallbladder disorder were included in the study analysis. A total of 61 patients (mean age 42 years, 40 males, and 21 females) were included in the study. Diabetic patients were requested to avoid antidiabetic medications on the day of test to avoid hypoglycemia.
Exclusion criteria
Patients with gallstones on USG or peptic ulcer disease on endoscopy
Patients with previous history of gallbladder surgery
Patients with history of lactose intolerance
Patients fasting for less than 4 h or longer than 24 h or are on total parenteral nutrition
Pregnant or lactating women
Patients on medications affecting gall bladder contraction (prokinetics like cisapride or itopride, cholinomimetics such as bethanechol, prostigmine, and erythromycin, inhibitory hormones such as somatostatin, nitric acid releaser such as arginine, calcium channel antagonist nifedipine, progesterone, trimebutine, loperamide, ondansetron).
Instruments used and imaging protocol
A minimum of 4 h fasting was ascertained for all subjects, and a written informed consent was obtained. In the pretest “4 h fasting,” drinking of water was not allowed and no specific dietary precautions were followed. The patient was made to lie supine underneath a large-field-of-view gamma camera (Siemens Symbia T6) fitted with a low-energy, high-resolution collimator. Patient was positioned under the gamma camera centering the right upper quadrant of the abdomen. After an intravenous injection of 3 mCi of 99mTc-mebrofenin, sequential images of 1 min/frame in anterior projection were acquired, on a 64 × 64 computer matrix, for 120 min. An “in-House fatty meal” was given at 45-min in the supine position with the help of a straw, to provoke gallbladder contraction.
In-House fatty meal
An in-House fatty meal prepared by the dietary department of our institute was used for the study.
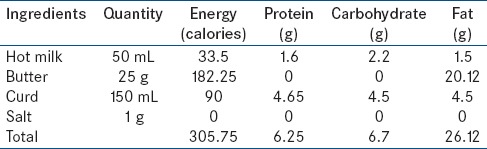
Ingredients: Milk, butter, yogurt, salt
Preparation: Add 25 g of butter in 50 mL of hot milk and mix it properly. Then add 150 mL of curd and 1 g of salt into the mixture and blend it in the mixer grinder to make it a homogenous drink. Serve 200 mL of a fatty meal. The composition of the meal is depicted in Table 1
Consumption: 200 mL of the fatty meal is consumed as a single meal. It should be used within 6 h of its preparation
Intake method: A minimum of 4 h of fasting is required. After 45-min of study acquisition, it is to be consumed orally immediately
Storage: Keep in cool and dry place since its preparation
Overdose: It does not have any side effects. No fat toxicity was reported.
Table 1.
Composition of in-House fatty meal
Radiopharmaceutical
99mTc-mebrofenin was used in our study of the gall bladder emptying kinetics. It is supplied by “Board of Radiation and Isotope Technology,” Mumbai, India as “TCK-39.” Reconstitution with 99mTc-pertechnetate is done under strict aseptic precautions at room temperature.
Image interpretation
Studies were analyzed for hepatic uptake and excretion of tracer into the biliary system, time to visualization of activity in the small bowel, time to visualization of the gall bladder. 99mTc-mebrofenin is rapidly cleared from the bloodstream by the hepatocytes after an intravenous injection. The extrahepatic bile ducts and gallbladder are visualized normally at 15–30 min and the small intestine at 30–60 min.
A tight region of interest (ROI) was drawn over the gallbladder and on hepatic parenchyma for background correction in a composite image. Decay-corrected counts from the gallbladder ROI's were used to calculate the GBEF with the help of formula as follows:

Premeal counts in GB were obtained from the 45th frame. GBEF was calculated at 30-min, 45-min, and 60-min using the above-mentioned formula. Time activity curves were generated and analyzed for various patterns of gallbladder emptying [Figure 1].
Figure 1.
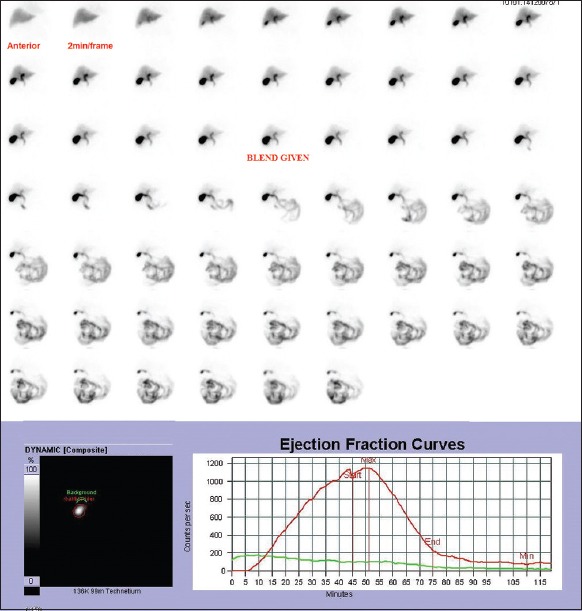
Dynamic images of normal gallbladder after injection of 2 mCi of 99mTc-mebrofenin with the fatty meal being given at 45th min. It also shows ejection fraction curve, from which gall bladder ejection fractions is calculated. Gallbladder ejection fractions at 30-min – 77%, at 45-min – 91%, and at 60-min 92%
Statistical analysis
Data entry and validation was done by MS-EXCEL spreadsheet (Microsoft office Excel 2007) SPSS software (version 11.0, Chicago: SPSS Inc). All the continuous variables were assessed for the normality using Shapiro–Wilk test and Kolmogorov–Smirnov test. As the sample size was small, Shapiro–Wilk test was used. Continuous variables which followed Gaussian distribution were expressed as mean ± standard deviation (SD), otherwise median (75th percentile). All the categorical variables were expressed as percentages. The statistical difference between the two groups which was normally distributed was derived by independent sample t-test and by ANOVA test if more than two groups along with Tukey's honestly significant difference (HSD)test as post-hoc analysis. Receiver operating characteristic (ROC) curve was used to assess the diagnostic accuracy of GBEF calculated at 30-min, 45-min, and 60-min and area under curve (AUC) was obtained. All the P < 0.05 was considered as statistically significant.
RESULTS
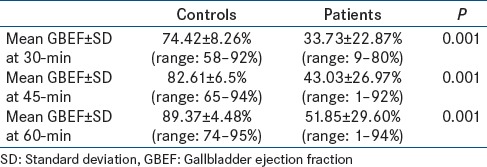
In volunteers, the GBEF at 30-min after fatty meal ingestion ranged from 58% to 92% (mean ± SD: 74.42% ± 8.26%). GBEF at 45-min ranged from 65% to 94% (mean ± SD: 82.61% ± 6.5%) and at 60-min ranged from 74% to 95% (mean ± SD: 89.37% ± 4.48%). A comparison of GBEF between volunteers and patients at various time points (30-, 45-, 60-min) in Table 2.
Table 2.
Comparison of GBEF in volunteers and patients at various time points
Using an independent sample t-test, we compared the GBEF at various time intervals (30-, 45-, and 60-min) in controls and cases. We found that GBEF measurements at each time point (30-, 45-, and 60-min) provided reasonably good separation between healthy controls and cases P < 0.05. ROC curve analysis was used to assess the diagnostic accuracy of GBEF measured at 3-time points [Figure 2]. Area under ROC curve was largest for 30-min GBEF (AUC 0.952; 95% confidence interval [CI] = 0.914–0.989), followed by 45-min (AUC 0.939; 95% CI = 0.892–0.986), and 60-min (AUC 0.064, 95% CI = 0.0184–0.110). Furthermore, all the 3 measures were statistically significant (P < 0.005).
Figure 2.
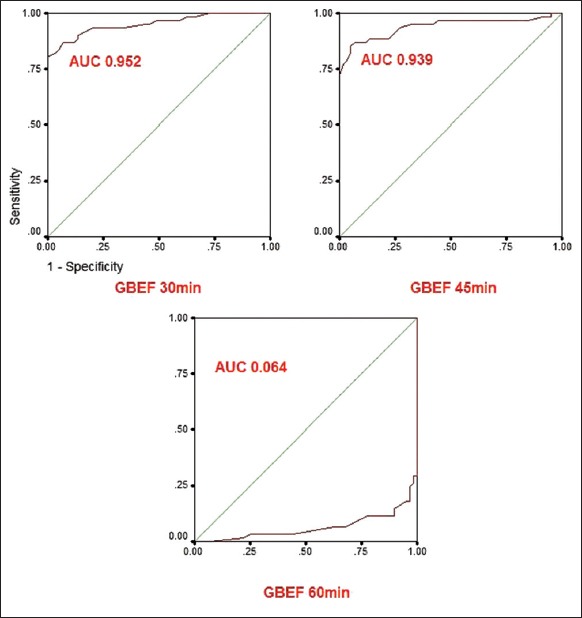
Receiver operating characteristic curves for gall bladder ejection fractions measurements at 30-min, 45-min, and 60-min. Area under receiver operating characteristic curve was largest for 30-min (area under curve 0.952; 95% confidence interval = 0.914–0.989) followed by 45-min (area under curve 0.939; 95% confidence interval = 0.892–0.986)
Since the AUC was maximum for 30-min GBEF, we considered 30-min as the appropriate time point to assess GBEF. And also the majority of the volunteers had 74% of gall bladder emptying at 30-min itself though gall bladder continued to contract slowly later [Figure 3]. As the data of GBEF at 30-min followed Gaussian distribution, the lower limit of GBEF was calculated using mean −2SD, which was 58%. Those patients with GBEF at 30-min <58% were considered as having hypokinetic gall bladder [Figure 4].
Figure 3.
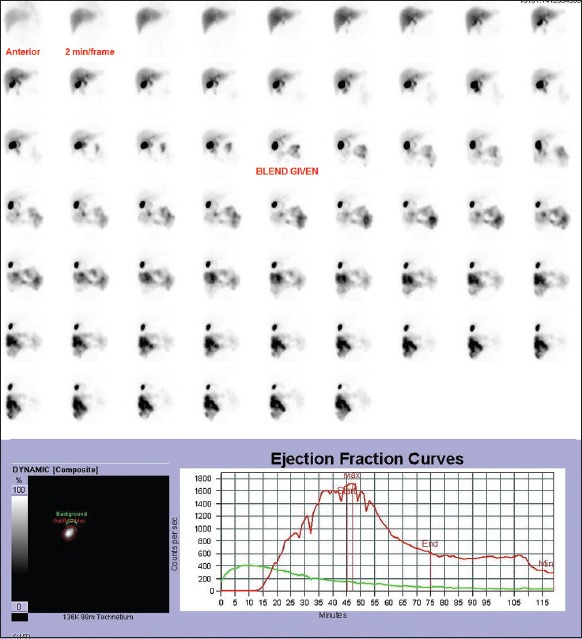
Dynamic images of normal gallbladder after injection of 2 mCi of 99mTc-mebrofenin. Ejection fraction curve shows that gallbladder continues to contract slowly beyond 30-min. Gallbladder ejection fraction at 30-min – 64%
Figure 4.
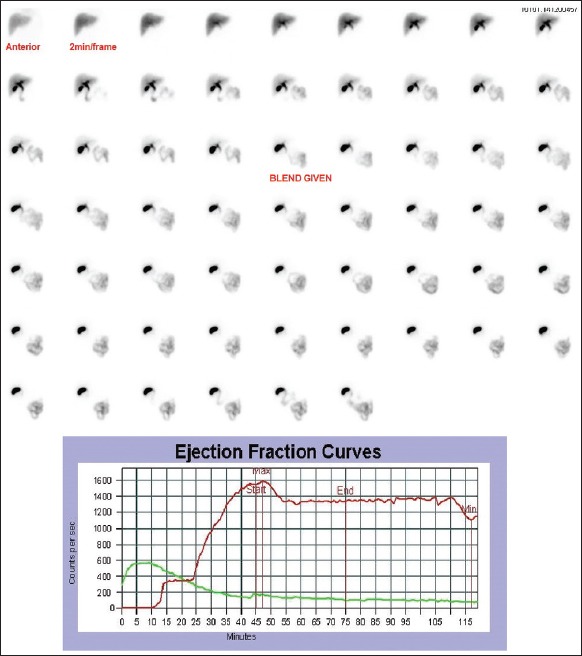
Dynamic images of hypokinetic gall bladder after injection of 2 mCi of 99mTc-mebrofenin. Ejection fraction curve shows a hypokinetic gallbladder pattern. Gallbladder ejection fraction at 30-min – 14%
Treatment and follow-up
All the patients with GBEF <58% were treated with ursodeoxycholic acid in a dose of 300 mg/day for 6 months. Patients reported to follow-up after 6 months and clinical assessment was done in terms of symptomatic relief and development of new symptoms. They reported significant improvement in their symptoms and quality of life.
DISCUSSION
Cholescintigraphy is a reproducible, nonobserver dependent, and noninvasive method to assess gallbladder function. GBEF is considered a reliable indicator of gall bladder function. It has a high positive predictive value in diagnosing chronic acalculous cholecystitis.
Intravenous CCK assessment of gallbladder ejection is the gold standard. Nonavailability[2] of CCK warrants use of a physiological stimulus and fatty meal is used as an alternative. A variety of fatty meals such as lactose-free fatty meal,[2] a corned beef, and cheese sandwich with milk,[3] dried egg yolk,[4] lipomul,[5] corn oil emulsion,[6] chocolate,[7] yoghurt, and chocolate,[8] Humana,[9] whipped cream,[10] half and half milk,[11,12] and whole milk[13,14] have been tried. Fatty meal, however, has been institution-specific usually and probably lacked standardization. As the precise timing of cessation of GB emptying is debated, fatty meal ejection fraction values do not have established normal values. Hence, standardization of times for calculating GBEF is to be evolved.
A fatty meal stimulates the release of endogenous CCK from the endocrine cells lining the mucosa of the duodenum, jejunum, and ileum. It may take as much as 6–26 min after a meal for endogenous CCK level to rise above the threshold to initiate gall bladder contraction and emptying. Once the gall bladder contraction begins, it continues for 1–3 h postmeal.
Our data in 59 controls showed that the mean GBEF at 30-min after fatty meal ingestion was 74.4% ± 8.27%, at 45-min was 83% ± 6.6%, and at 60-min was 89.37% ± 4.48%. These values were significantly different from each other with a P < 0.05. Different methods have been used to establish normal ranges and identify individuals requiring treatment. When the frequency distribution of population data resembles a Gaussian curve, it is traditional to use mean −2SD or 95% confidence limits to set the lower limit of normal GBEF values. GBEF at 30- and 45-min in our controls showed Gaussian distribution. The lower limit of GBEF at 30- and 45-min was 58% and 69%, respectively. GBEF at 60-min did not follow Gaussian distribution, hence we considered 5th percentile, which was 81% as the lower limit.
Kakhki et al.[9] (n = 36) in their study had mean GBEF of 69.54% ± 21.04% at 30-min and 84.26% ± 11.41% at 60-min. They used 120 mL of the fatty meal (10 g fat) using a commercially available formula (Humana) as a cholecystagogue. The lower limit of normal GBEF values were 27.46% at 30-min and 61.44% at 60-min. In our study, the mean GBEF at 30-min was 74.4% ± 8.27% and at 60-min was 89.37% ± 4.48%. The lower limit of GBEF at 30- and 60-min is 58% and 81%, respectively. Both the mean GBEF as well as the lower limit of GBEF were high in our study. Shafer et al.[5] studied 7 healthy volunteers and found mean GBEF ± SD to be 31% ± 11% with the use of Lipomul. The lower limit of normal GBEF at 60-min was 16%.
Ziessman et al.[2] studied 17 healthy volunteers and found that the lower limit of normal for GBEF at 60-min is 33% using a lactose-free food supplement (240-mL supplement containing 11.4 g of fat, 355 kcal, 50 g of carbohydrate). Krishnamurthy and Brown studied 13 healthy subjects using half and half milk (240 mL/70 kg containing 24 g fat, 8 g carbohydrate, 8 g protein, 320 calories) as a cholecystagogue. They reported that mean ± SD of GBEF at 60-min was 53.6% ± 20.2%. The number of subjects in their study was too small, and the GBEFs did not have a Gaussian distribution. The GBEF at 60-min did not follow Gaussian distribution in our study also. The mean ± SD of GBEF at 60-min was 89.37% ± 4.48%, which is more in comparison to the above study.
The lower limit of GBEF in our study was high in comparison to other studies. It is probably reflective of the high fat (26.12 g) content in the meal. Stone et al.[15] has suggested that 4 g of fat does not result in gallbladder contraction, and at least 10 g of fat is required to produce GB emptying. Fat intake in Indian population is probably income dependent and, therefore, is highly skewed, the intake being low among the rural and urban low-income groups. However, the minimal intakes of visible fat in Indian adults ranges between 20–40 g/p/d.[16] The fat stimulus in this study is based on an Indian Council of Medical Research study group report[16] and was standardized at 26.12 g. This is a 200 mL liquid meal, which was prepared by mixing curd, milk, and butter. Normal values of fatty meal cholescintigraphy are also likely dependent on the content of the meal, the amount of fat in the meal, and the methodology used.[15] Gastric emptying rate may also affect GBEF values. Delayed gastric emptying may delay endogenous production of CCK from the proximal small bowel.[14] Postural variation like upright position[17] may stimulate earlier production of endogenous CCK and result in greater gallbladder emptying during acquisition time.[13,14] The longer latent period using fatty meals as compared to the CCK-8 is probably due to the time taken for the release of endogenous CCK. As it can take about 10–20 min after a meal for the serum CCK level to rise above a threshold and induce contraction and emptying of the gallbladder. The mean latent period (time from fatty meal ingestion to the beginning of gallbladder emptying) was 15.5 min. Postprandial serum CCK levels stay above the threshold for 2–3 h postmeal[18,19] and the gallbladder emptying is maintained as long as the serum CCK levels remain above the threshold for contraction. Variability of results in various other studies may be due to the small sample size, and different methodologies.
Bartel et al.[6] using ROC analysis found that 60-min GBEF provided the best separation between normal and diseased GB. The area under ROC curve was maximum for 60-min (0.963% at 95% CI). Using corn oil emulsion (170 calories per 30-mL patient dose) as a fatty meal, in 23 healthy subjects, they found GBEF at 30-, 60-, and 90-min were 30% ± 22% (range, 0–85%), 57% ± 24% (range, 5–99%), and 72% ± 23% (range, 25–100%), respectively. On the contrary, in our study, we found that the area under ROC curve was largest for 30-min GBEF (0.954). For our study, GBEF at 30-min provided the best separation between healthy and diseased GB. However, it is interesting to note that the gallbladder was still emptying even at 75-min after ingestion of fatty meal in few patients. This may be due to continuous gastric emptying of fatty meal into the duodenum, which would further stimulate the release of endogenous CCK followed by subsequent contraction of the gallbladder.
CONCLUSIONS
In-House fatty meal could be a cheaper reproducible noninvasive equivalent to CCK in the assessment of gall bladder kinetics. The fat causes release of endogenous CCK and its ease of preparation, administration, and lack of side effects makes it an economical alternative. GBEF at 30-min allows identification of gall bladder dysfunction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cerçi SS, Ozbek FM, Cerçi C, Baykal B, Eroglu HE, Baykal Z, et al. Gallbladder function and dynamics of bile flow in asymptomatic gallstone disease. World J Gastroenterol. 2009;15:2763–7. doi: 10.3748/wjg.15.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziessman HA, Jones DA, Muenz LR, Agarval AK. Cholecystokinin cholescintigraphy: Methodology and normal values using a lactose-free fatty-meal food supplement. J Nucl Med. 2003;44:1263–6. [PubMed] [Google Scholar]
- 3.Patankar R, Ozmen MM, Aldous A, Khader Z, Fleming JS, Johnson CD. Standardization of a technique for BrIDA cholescintigraphy. Nucl Med Commun. 1996;17:724–8. doi: 10.1097/00006231-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Sacchetti G, Mandelli V, Roncoroni L, Montanari C. Influence of age and sex on gallbladder emptying induced by a fatty meal in normal subjects. Am J Roentgenol Radium Ther Nucl Med. 1973;119:40–5. doi: 10.2214/ajr.119.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Shafer RB, Marlette JM, Morley JE. The effects of Lipomul, CCK, and TRH on gallbladder emptying. Clin Nucl Med. 1983;8:66–9. doi: 10.1097/00003072-198302000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bartel TB, Juweid ME, Ponto JA, Graham MM. Corn oil emulsion: A simple cholecystagogue for diagnosis of chronic acalculous cholecystitis. J Nucl Med. 2005;46:67–74. [PubMed] [Google Scholar]
- 7.Cay A, Imamoglu M, Sarihan H, Ahmetoglu A. Ultrasonographic evaluation of fatty meal stimulated gallbladder contraction in the diagnosis of biliary dyskinesia in children. Acta Paediatr. 2006;95:838–42. doi: 10.1080/08035250500459733. [DOI] [PubMed] [Google Scholar]
- 8.Al-Muqbel K, Bani Hani M, Daradkeh M, Rashdan A. Usefulness of fatty meal-stimulated cholescintigraphy in the diagnosis and treatment of chronic acalculous cholecystitis. Ann Nucl Med. 2009;23:137–42. doi: 10.1007/s12149-008-0221-5. [DOI] [PubMed] [Google Scholar]
- 9.Kakhki VR, Zakavi SR, Davoudi Y. Normal values of gallbladder ejection fraction using 99mTc-sestamibi scintigraphy after a fatty meal formula. J Gastrointestin Liver Dis. 2007;16:157–61. [PubMed] [Google Scholar]
- 10.Jacobs MP, Peterson CD. An alternative to kinevac. J Nucl Med. 2002;43:1727. [PubMed] [Google Scholar]
- 11.Krishnamurthy GT, Brown PH. Comparison of fatty meal and intravenous cholecystokinin infusion for gallbladder ejection fraction. J Nucl Med. 2002;43:1603–10. [PubMed] [Google Scholar]
- 12.Bobba VR, Krishnamurthy GT, Kingston E, Turner FE, Brown PH, Langrell K. Gallbladder dynamics induced by a fatty meal in normal subjects and patients with gallstones: Concise communication. J Nucl Med. 1984;25:21–4. [PubMed] [Google Scholar]
- 13.Mackie CR, Baxter JN, Grime JS, Hulks G, Cuschieri A. Gall bladder emptying in normal subjects – A data base for clinical cholescintigraphy. Gut. 1987;28:137–41. doi: 10.1136/gut.28.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xynos E, Pechlivanides G, Zoras OJ, Chrysos E, Tzovaras G, Fountos A, et al. Reproducibility of gallbladder emptying scintigraphic studies. J Nucl Med. 1994;35:835–9. [PubMed] [Google Scholar]
- 15.Stone BG, Ansel HJ, Peterson FJ, Gebhard RL. Gallbladder emptying stimuli in obese and normal-weight subjects. Hepatology. 1992;15:795–8. doi: 10.1002/hep.1840150508. [DOI] [PubMed] [Google Scholar]
- 16.Nutrient Requirements and Recommended Dietary Allowances for Indians: A Report of the Expert Group of the Indian Council of Medical Research (ICMR) – National Institute of Nutrition, ICMR, Hyderabad. 2009. [Last accessed on 2015 Apr 15]. Available from: http://www.icmr.nic.in/final/RDA-2010.pdf .
- 17.Ziessman HA. Keep it simple-it's only gastric emptying. In: Freeman LM, editor. Nuclear Medicine Annual. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. pp. 233–60. [Google Scholar]
- 18.Upp JR, Jr, Nealon WH, Singh P, Fagan CJ, Jonas AS, Greeley GH, Jr, et al. Correlation of cholecystokinin receptors with gallbladder contractility in patients with gallstones. Ann Surg. 1987;205:641–8. doi: 10.1097/00000658-198706000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vezina WC, McAlister VC, Wall WJ, Engel CJ, Grant DR, Ghent CN, et al. Normal fasting volume and postprandial emptying of the denervated donor gallbladder in liver transplant recipients. Gastroenterology. 1994;107:847–53. doi: 10.1016/0016-5085(94)90135-x. [DOI] [PubMed] [Google Scholar]


