Abstract
Background
For many women with Rheumatoid Arthritis (RA) motherhood decisions are complicated by their condition and complex pharmacological treatments. Decisions about having children or expanding their family require relevant knowledge and consultation with their family and physician as conception and pregnancy has to be managed within the RA context. Relevant information is not readily available to women with RA. Therefore a randomized controlled study was conducted to evaluate the effectiveness of a new motherhood decision aid (DA) developed specifically for women with RA.
Methods
One hundred and forty-four women were randomly allocated to either an intervention or control group. All women completed a battery of questionnaires at pre-intervention, including, the Pregnancy in Rheumatoid Arthritis Questionnaire (PiRAQ), the Decisional Conflict Scale (DCS), the Hospital Anxiety and Depression Scale (HADS), and the Arthritis Self-Efficacy Scale (ASES), and provided basic demographic information. Women in the DA group were sent an electronic version of the DA, and completed the battery of questionnaires for a second time post-intervention.
Results
Women who received the DA had a 13 % increase in relevant knowledge (PiRAQ) scores and a 15 % decrease in scores on the decisional conflict (DCS), compared to the control group (1 %, 2 % respectively). No adverse psychological effects were detected as evident in unchanged levels of depression and anxiety symptoms.
Conclusions
The findings of this study suggest that this DA may be an effective tool in assisting women with RA when contemplating having children or more children.
Trial registration
Australian New Zealand Clinical Trials Registry, http://www.anzctr.org.au/, ACTRN12615000523505.
Background
The decision to have children is influenced by a number of factors including relationship quality and readiness, education level, financial considerations, and the availability of social support [1–3]. Women with chronic medical conditions may experience additional challenges associated with their health care needs, social stigma pertaining to their parenting capacity, and the impact of their condition on pregnancy and motherhood [4–7].
While parenting is generally accepted as a positive experience, for many women with chronic conditions such as Rheumatoid Arthritis (RA), the physical symptoms can significantly impair their role and performance as mothers [8–10]. Some women experience feelings of guilt and shame over the impact of their illness on parenting and have to adjust their expectations of themselves as mothers [11, 12].
RA is a chronic, autoimmune disease that occurs three times more frequently in women than men and may affect women during their childbearing and childrearing years [13, 14]. Given the early onset of RA and the high prevalence rates in women, reproductive health is a relevant concern for patients and health professionals [15]. Furthermore, living with RA in the first few years after diagnosis can be challenging, [16] and may coincide with, and have important implications for, family planning [11].
There is a longstanding link between RA and infertility with the incidence of nulliparity in women with RA greater than those without the disease [17]. However, lower birth rates may be due to: (i) reduced sexual function [18], (ii) deciding not to have a family, or to have smaller families due to concerns about the impact of RA or its treatment on the foetus [16, 17], or the perception of pregnancy as being risky [19], (iii) fear of transmitting the disease to the child [20]; and (iv) perceived lack of support from their health professionals [12].
It is well established that pregnancy and childbirth can affect the disease activity of RA [21]. During pregnancy a decision to limit the use of RA medication may be made because of the risks that pharmacological treatments can pose to the foetus. However, reduced uptake of disease modifying drugs may lead to increased disease activity in the mother [22]. The development of various biological pharmacotherapies for RA has, for women with RA, supported increased physical capability to manage pregnancy and overcome challenges of raising children [23]. However these therapies may concern women and compound the complexity of their motherhood decision [11].
For women with RA there are some risks associated with pregnancy. While remission can spontaneously occur during pregnancy [24–26] RA symptoms may exacerbate following delivery [27]. Furthermore, there is a link between RA, premature birth, and low birth weight- factors that could have long-term implications for the child’s health [28]. Previous research has identified some of the parenting challenges women with chronic conditions experience, however, little is known about how women with RA make motherhood decisions [11]. It is therefore important that the clinicians discuss with women fertility, pregnancy, and lactations in the context of RA and its treatment, and convey risks and benefits of different options. One approach to facilitating such communication is to provide support for both clinicians and patients, via accessible and targeted educational material in a form of a decision aid tool.
Decision aids (DAs) are based on a well-developed, evidenced-based approach to assisting individuals with making health treatment and screening decisions [29, 30]. The development of DAs is commonly guided by the International Patient Decision Aid Standards (IPDAS) [31]. DAs support active participation in decision making by weighing up the benefits and harms of treatment options based on comprehensive, specific and relevant information [29, 32] from unbiased, nondirective, current research evidence [33].
Results of a comprehensive systematic review based on 55 randomized controlled trial and over 500 DAs conducted by O’Connor and colleagues [34] have found support for the effectiveness of those aids. Stacey et al [32] conducted a recent update of this seminal work to include 115 randomized controlled trials (n = 34,444) of DAs. Some of the reviewed DAs refer to decisions regarding pregnancy and birthing options [33]; childbirth options [35, 36], infertility issues [37], miscarriage [38], pregnancy termination [39], and prenatal testing [40]. Another recent systematic review also found positive effects of DAs on informed decision making in pregnancy care, including increased knowledge, and decreased decisional conflict and anxiety [33]. Currently, there is only one published study of a DA on motherhood choices for women with a chronic condition (Multiple Sclerosis) [41].
This paper reports on the randomized controlled study that was conducted to evaluate the effectiveness of a DA developed for women with RA [42]. It was hypothesised that, based on previous DA studies, this DA would (1) increase relevant knowledge and decrease decisional conflict (primary outcomes), and (2) not effect motherhood decision, symptoms of depression and anxiety, or arthritis self-efficacy (secondary outcomes).
Method
Ethics
This study was approved (H6884) by the Western Sydney University Human Ethics Committee and conducted according to the Helsinki ethical principles of research. All participants provided written informed consent and were not compensated for their participation.
Patients and methods
The reporting of this randomized controlled study was informed by the CONSORT guidelines [43]. Eligible women were those (i) aged within their child bearing and rearing years that (ii) had been clinically diagnosed with RA and currently under the care of a rheumatologist, and (iii) contemplating having children or more children. No other inclusion or exclusion criteria were applied. One hundred and eighty-eight women enrolled in the study and were randomly allocated to the intervention (DA) or no intervention (control) group (Fig. 1). Of those, 167 women (89 %) completed the pre-intervention questionnaire and 144 women (86 %) the post-intervention questionnaire. Overall, 76 % of women initially enrolled in the study completed both pre- and post-intervention questionnaires.
Fig. 1.
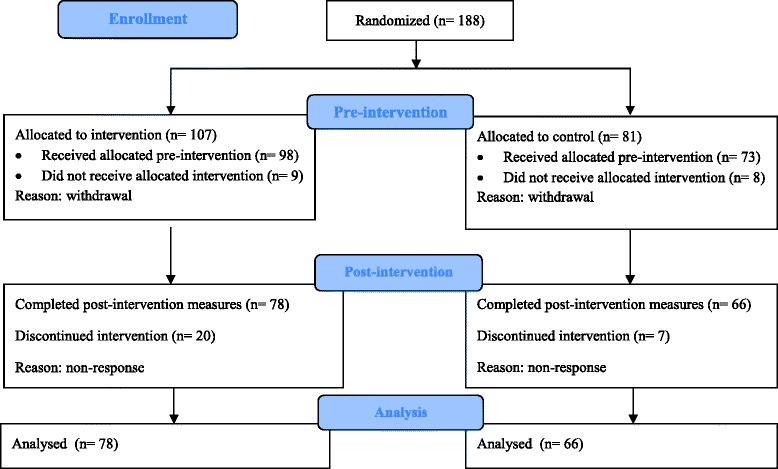
CONSORT flow-chart
Recruitment took place over a 12-month period via online advertising including a Google ad campaign, social media, media releases, website content and relevant Arthritis websites, and print advertising including general practitioner newsletters, posters, and flyers distributed to rheumatology clinics. As women provided consent, a member of the research team randomly allocated them to either the DA or control group, using the Bernoulli function in Excel. As a consequence of the random allocation, the control and intervention groups were not balanced for parity or gravity. The pre- and post-intervention questionnaires were completed online via SurveyMonkey.
Once women in the intervention (DA) group had completed the baseline questionnaire, they were sent a link to the electronic version of the DA via email or SurveyMonkey, and were asked to read it within two weeks. All women in the sample possessed an active email account and preferred the DA to be emailed rather than mailed to them. Women in both groups were followed up via phone or emailed approximately two to four weeks after the baseline questionnaire. All women in the intervention group were asked if they had read and understood the DA, and had any questions or feedback about the DA or the study topic. Women in the control group were contacted and asked if they had any questions or feedback about the questionnaire or study topic. Once follow-up contact had been made, women in both groups were sent a link to the post-intervention questionnaire via SurveyMonkey. The questionnaire was identical to the one completed in the pre-intervention phase except for the exclusion of demographic questions. Non-respondents were followed up via phone or email. The process of completing pre and post surveys ranged between four to 12 weeks.
Measures
The demographic questions included: age, education, marital status, country of birth, RA duration, number of children, and pregnancy status. Participants were also asked whether they wanted children prior to the RA diagnosis, if the diagnosis of RA complicated their decision to have children (Yes, No, or Unsure), and how certain they were in wanting children in the future (decision certainty) (-5, Definitely would not have children, to +5, Definitely would have children and a score of 0 indicating unsure).
Primary outcome measures
The Pregnancy in Rheumatoid Arthritis Questionnaire (PiRAQ) was developed to assess the RA, pregnancy, and parenting knowledge. Its items correspond directly to the DA content including questions relating to general knowledge about RA, the physical and psychosocial effects of RA, effects of RA on fertility and pregnancy, the effects of RA medicines during conception, pregnancy, and breastfeeding, postnatal considerations, and parenting with DA. Scale development experts were consulted in the development of items, and the final selection was based on pilot results. The PiRAQ was piloted with a consumer sample (women with RA and partners) (n = 17). Based on the pilot results the number of items was reduced from 50 to 39 as some lacked discriminatory power. The scores for PiRAQ are calculated by summating the number of correct responses, and range between 0 and 39 with higher scores indicating greater knowledge. In this study, the PiRAQ’s internal consistency coefficient was 0.83.
Decisional conflict scale (DCS) [44]
The DCS is a 16-item self-report questionnaire which measures personal perceptions of: (1) uncertainty in health-related decision making; (2) factors contributing to the uncertainty and; (3) the perceived effectiveness of decision making. It consists of five subscales: informed, values clarity, uncertainty, support, and effective decision. The scale includes five response criteria ranging from Strongly Agree (0) to Strongly Disagree (4) with scores ranging from 0 (no decisional conflict) to 100 (extremely high decisional conflict) [44]. The scale has good test-retest reliability (0.81) and internal consistency coefficients range from 0.78-0.89 [44] and in this study was 0.95.
Secondary outcome measures
Arthritis self-efficacy scale (ASES) [45]
The ASES provides a measure of the patient’s perceived ability to control and manage a number of aspects of their arthritis [45]. A short form of the original ASES was used in this study [46]. It contains eight items from the original scale and has been validated [47]. Scores range from 1-10 with higher scores indicating higher levels of self-efficacy. Internal consistency for the short form of the ASES has been reported above 0.90 [46, 47] and in this study was 0.91.
The hospital anxiety and depression scale (HADS) [48]
The HADS consists of 14 items related to feelings of anxiety (7 items) and depression (7 items). Responses are scored from 0-3, with 3 indicating higher symptom frequencies [49]. Scores may be calculated for each subscale (anxiety and depression) and range from 0-21, with clinical cut off for possible anxiety or depression being a score of 8-10 and probable indication being a score of 11 and over. The scale’s internal consistency coefficients exceed 0.80 [48] and in this study were 0.85. HADS is reported to be suitable for the use with RA populations [50].
Decision aid
Motherhood choices: decision Aid for women with rheumatoid arthritis
Development of the DA was conducted based on the Ottawa Decision Support Framework and following the guidelines of the IPDAS Collaboration [31]. Initial content for the DA was devised based on areas of need identified in the literature. It was then further informed with input from consumer (i.e. women with RA) and expert (i.e. rheumatologists, researchers, health care professionals) panels (n = 33). The DA was delivered to the panels via an online survey, with questions pertaining to: content, clarity/readability, balance of information, structure/presentation, and the option to provide qualitative feedback. Panel members also met face-to-face within an advisory committee as part of the feedback gathering process. Statements that were agreed upon by 80 % of the panels’ members were retained, and those with lower agreement rate were revised. Consumer panel members and participants in the pilot study contributed their experiences (‘women’s stories’) to the DA. Pilot testing of the DA, as with the PiRAQ, was conducted with a sample of 17 consumers. In addition to participants completing a battery of questionnaires, they also received a draft version of the DA to comment on its content, clarity, balance of information, and structure. A revised version of the DA was then reviewed by another panel of experts (n = 5). Lastly, the Flesch-Kinkaaid readability test was used to ensure that the DA text met the general population’s reading age.
The final DA is a 45-page resource available in electronic or paper versions, and contains three distinct sections: 1) Information on RA, conception, pregnancy, and parenting; 2) decision making activities; and 3) resources. It provides information about RA and its effects, the impact of RA and medications on conception, pregnancy and motherhood, the impact of pregnancy on RA, and ‘women’s stories’. The DA also contains worksheets to assist women in the decision making process (a decision tree, pros/cons scales, support networks, a knowledge checklist, and note taking). The DA is available online on the Arthritis NSW website, http://arthritisnsw.org.au/arthritis/research/ra-and-motherhood/ and is listed on the Ottawa Hospital Research Institute’s Patient Decision Aids website: http://decisionaid.ohri.ca/AZsumm.php?ID=1787. The DA is currently being reviewed based on recent relevant literature.
Statistical analysis
Analyses were conducted using IMB SPSS Statistics (21.0), with statistical significance set at p < .05 (two-tailed). Independent t-tests and ANOVAs were used to assess differences between groups and across time. DAs are reported to increase knowledge by 19 points, and reduce decisional conflict by nine points [51]. A sample size of 130 subjects is sufficient to detect moderate effects (0.13) [52] on each of these differences with 95 % power at a 0.05 level of significance [53]. According to Cohen’s specifications for ANOVA analyses small, medium, and large effect sizes are classified as 0.02, 0.13, and 0.26 respectively [52].
Results
A total of 188 women consented to participate in the study. Forty-four (28 DA; 16 Control) participants did not complete pre or post questionnaire and after a number of efforts to contact them, were assumed to have withdrawn from the study. The final sample of 144 participants consisted of 78 women (mean age 31.26, SD =4.26) in the DA group, and 66 women (mean age 30.67, SD = 5.38) in the control group. There were no significant differences on demographic data between women in the DA group, and women in the control group. Demographic data is presented in Table 1.
Table 1.
Demographic data for 144 participants
| Measures | DA group | Control group |
|---|---|---|
| 54 % (n = 78) | 46 % (n = 66) | |
| Age (Yrs) | 31.26 (4.26) | 30.43 (5.07) |
| Education | ||
| Secondary Education (12 years) | 8 % (n = 6) | 10.8 % (n = 7) |
| Tertiary Education | 92 % (n = 72) | 88 % (n = 57) |
| Marital status | ||
| Single | 10 % (n = 8) | 15 % (n = 10) |
| Married | 87 % (n = 68) | 83 % (n = 54) |
| Divorced/separated | 3 % (n = 2) | 2 % (n = 1) |
| RA Duration in years | 8.43 (6.97) | 7.71 (7.19) |
| No. of children | ||
| 0 | 65 % (n = 50) | 69 % (n = 45) |
| 1 | 26 % (n = 20) | 23 % (n = 15) |
| 2 | 9 % (n = 7) | 6 % (n = 4) |
| Pregnant at time of survey | 4 % (n = 3) | 6 % (n = 4) |
| Wanted children before RA diagnosis | ||
| Yes | 79 % (n = 61) | 71 % (n = 46) |
| No | 3 % (n = 2) | 11 % (n = 7) |
| Unsure | 18 % (n = 14) | 18 % (n = 12) |
| Want to have (more) children in the future | ||
| Definitely not | 0 % (n = 0) | 0 % (n = 0) |
| Unsure | 51 % (n = 40) | 38 % (n = 25) |
| Definitely yes | 49 % (n = 38) | 60 % (n = 39) |
No significant differences were found on demographic data between women in the DA and control groups
Independent samples t-tests revealed no significant differences between the DA and control groups on the PiRAQ, t(142) = 1.642, p = 0.103, DCS t(142) = 1.614, p = 0.109, HADS-A t(142) = 1.338, p = 0.183, HADS-D t(142) = 0.764, p = 0.446,, or on RA-Complicated Decision, t(142) = 1.156, p = 0.250 at baseline.
The 2 x 2 mixed between-within subjects ANOVA was conducted to assess the impact of the DA on women’s scores on the PiRAQ, DCS, ASES, HADS-A, and HADS-D, and questions measuring RA-Complicated Decision and Decisional Certainty at follow up (post intervention) (Table 2). Participants in the DA and control groups both had moderate knowledge at pre-intervention, with control group scores remaining unchanged, and the DA group increasing to moderate to high knowledge at post-intervention. Both groups reported relatively low levels of decisional conflict at pre-intervention; however the DA group reported significantly lower decisional conflict at post-intervention. Participants in both groups reported moderate self-efficacy, high levels of anxiety symptoms and low levels of depression symptoms.
Table 2.
Means, standard deviations, confidence intervals, and effect sizes for each outcome measure at pre and post intervention
| DA group | Control group | ƞp2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Mean (95 % CI) | SD | Mean (95 % CI) | SD | Mean (95 % CI) | SD | Mean (95 % CI) | SD | ||
| PiRAQ | 26.70 | 5.94 | 31.92 | 4.66 | 25.32 | 4.60 | 26.09 | 5.34 | 0.214** |
| (25.49-27.91) | (30.80-33.05) | (24.01-26.62) | (24.88-27.31) | ||||||
| DCS | 43.34 | 23.92 | 28.14 | 18.06 | 36.96 | 23.32 | 34.80 | 21.86 | 0.117** |
| (38.05-48.63) | (23.69-32.59) | (31.20-42.71) | (29.96-39.64) | ||||||
| Informed | 39.42 | 26.92 | 24.57 | 17.81 | 44.95 | 28.34 | 39.27 | 27.53 | 0.036* |
| (33.25-45.60) | (19.47-29.67) | (38.24-51.66) | (33.72-44.81) | ||||||
| Values Clarity | 36.54 | 25.56 | 25.32 | 19.62 | 32.07 | 27.44 | 31.06 | 26.13 | 0.051* |
| (30.62-42.46) | (20.21-30.43) | (25.64-38.50) | (25.51-36.62) | ||||||
| Support | 33.65 | 23.12 | 26.82 | 21.47 | 31.31 | 25.19 | 29.29 | 20.94 | 0.014* |
| (28.26-39.05) | (22.06-31.57) | (25.45-37.18) | (24.13-34.46) | ||||||
| Uncertainty | 49.89 | 27.87 | 40.06 | 28.52 | 43.81 | 28.92 | 44.82 | 30.79 | 0.049* |
| (43.55-56.24) | (33.44-46.69) | (36.91-50.72) | (37.63-52.02) | ||||||
| Effective Decision | 32.05 | 21.75 | 27.40 | 21.52 | 31.06 | 25.55 | 30.87 | 21.28 | 0.009 |
| (26.78-37.33) | (22.61-32.20) | (25.33-36.80) | (25.66-36.08) | ||||||
| Decision Certainty | 3.72 | 1.66 | 3.51 | 1.87 | 3.91 | 1.72 | 3.74 | 1.76 | 0.014 |
| (3.34-4.10) | (3.11-3.92) | (3.50-4.32) | (3.30-4.19) | ||||||
| Complicated Decision | 0.91 | 0.49 | 0.91 | 0.50 | 0.82 | 0.46 | 0.96 | 0.539 | 0.012 |
| (0.80-1.02) | (0.79-1.03) | (0.70-0.93) | (0.83-1.08) | ||||||
| ASES | 5.43 | 1.87 | 5.81 | 1.92 | 5.74 | 2.00 | 5.56 | 2.03 | 0.030* |
| (5.00-5.86) | (5.37-6.25) | (5.27-6.21) | (5.08-6.04) | ||||||
| HADS-A | 8.87 | 3.73 | 8.71 | 3.77 | 8.00 | 3.66 | 8.14 | 4.13 | 0.004 |
| (8.04-9.70) | (7.82-9.59) | (7.09-8.91) | (7.17-9.10) | ||||||
| HADS-D | 5.42 | 3.41 | 5.40 | 3.68 | 4.94 | 3.46 | 5.20 | 3.92 | 0.004 |
| (4.65-6.19) | (4.55-6.25) | (4.10-5.78) | (4.27-6.13) | ||||||
*p-value < .05
**p-value < .001
CI Confidence Interval, PiRAQ: Pregnancy in Rheumatoid Arthritis Questionnaire, DCS Decisional Conflict Scale, ASES Arthritis Self-Efficacy Scale, HADS-A Hospital Anxiety and Depression Scale- Anxiety, HADS-D Hospital Anxiety and Depression Scale- Depression
Primary outcomes
Post-intervention scores on the PiRAQ, revealed a significant time by group interaction, F(1, 141) = 38.474, p < 0.001, ƞp2 = 0.214 (moderate to large effect size), indicating larger improvement in knowledge in the DA group compared to the control group. There was also a significant main effect for group, F(1, 141) = 20.787, p < 0.001, ƞp2 = 0.128 (moderate effect size). Therefore knowledge improved across both groups, but was significantly greater in the DA group.
Decisional conflict (DCS) also reduced significantly more in the DA group than the control group, as indicated by a time by group interaction, F(1, 142) = 18.794, p < 0.001, ƞp2 = 0.117 (moderate effect size). Significant interaction effects were found for three of the four DCS subscales: ‘informed’, ‘values clarity’, and ‘uncertainty’ with all interaction effects being Fs ≥ 5.28, ps ≤ 0.023, ƞp2 ≥ 0.036. There was no main effect for group F(1, 141) = 0.002, p = 0.967, ƞp2 = 0.000 but there was a significant main effect for time on the ‘support’ subscale, F(1, 142) = 6.719, p = 0.011, ƞp2 = 0.045. While the effect size was small, this indicates that both the DA and control groups felt more supported in their decision making from pre- to post-intervention.
Secondary outcomes
Anxiety did not change over time (HADS-A), F(1, 141) = 0.004, p = 0.947, ƞp2 = 0.000, nor differ between groups at post-intervention, F(1, 141) = 1.411, p = 0.237, ƞp2 = 0.010 and there was no interaction effect, F(1, 141) = 0.520, p = 0.472, ƞp2 = 0.004. Depression (HADS-D), also did not significantly change over time F(1, 141) = 0.335, p = 0.552, ƞp2 = 0.003, or between groups, F(1, 141) = 0.337, p = 0.562, ƞp2 = 0.002 with no significant interaction effect, F(1, 141) = 0.500, p = 0.481, ƞp2 = 0.004.
Similarly, there was no significance difference between RA-Complicated Decision from pre to post-intervention, F(1, 142) = 1.415, p = 0.236, ƞp2 = 0.010 across time or groups. Furthermore, the DA did not impact on women’s decision certainty with no significant interaction or main effects (all Fs ≤ 2.471, ps ≥ .152, ƞp2 ≤ .014) detected.
For arthritis self-efficacy (ASES), the main effects for time F(1, 142) = .550, p = 0.460, ƞp2 = 0.004) and group (F(1, 142) = 0.011, p = 0.917, ƞp2 < 0.001) were not significant. However, there was a significant interaction effect, F(1, 142) = 4.401, p = 0.038, ƞp2 = 0.030, indicating a post-intervention increase for participants in the DA group, although the effect size was small.
Discussion
The aim of this randomized controlled study was to evaluate the effectiveness of a DA resource tool developed for women with RA when considering motherhood. In the DA group knowledge increased by approximately 13 % and decisional conflict decreased by 15 % compared to less than 1 % and 2 % respectively in the control group. Similar changes in knowledge and decisional conflict were reported in Prunty et al’s [41] study. While the knowledge increased was lower than the average of 19 % across DA studies [51] this may be due to the already high knowledge at the pre-DA stage. The decisional conflict decline was, however, considerably higher than the average improvement of nine percent [32]. Importantly, the DA did not lead to any increases in psychological distress (anxiety and depression). This confirms that the DA is not associated with negative, unintended consequences as also noted in Prunty et al’s [41] study.
Despite careful attention to the methodology, a few limitations remain, which should be taken into consideration when interpreting the findings. Firstly, reliance on self-selection, and drop out (which in this study was 44/188 participants) effects may have resulted in a sample that is not representative of the broader population of women with RA; particularly in relation to their high rates of tertiary-education, relatively high level of knowledge about RA and pregnancy, and low levels of decisional conflict. Secondly, the evaluation of the knowledge pre and post DA relied on a scale that was specifically developed for this study, as there is no existing RA knowledge scale that addresses pregnancy/parenthood related content, and therefore had limited psychometric evaluation. Thirdly, the DA was self-administered and relied upon self-report data. Fourthly, the short timeframe of the present study means that the usefulness of the DA in actual decision making was not established. Finally, whilst the findings suggest this DA is a useful resource, its effectiveness in face-to-face clinician-patient settings is yet to be evaluated.
These limitations notwithstanding, there are a number of strengths of this study. Firstly, the development of the DA was guided by, and aligned with, the IPDAS criteria [31]. The DA was systematically developed in consultation with both experts and consumers, which informed relevance, clarity, balance, and readability of the DA. It was then piloted and further refined before examining its effectiveness via a randomized controlled study. Lastly, the effectiveness of the DA is consistent with findings of other DAs across a range of health decisions [33, 35]. Given the comparable findings of Prunty and colleagues [41] and the current study it is likely that similar DAs could benefit women with other chronic conditions.
Two issues, however, should be acknowledged. Firstly it is important to note that as there are common concerns and challenges across chronic conditions [11, 54], other DAs may model the core features included in this DA, while generic features, such as value clarifying exercises, may be used in counselling settings [41]. Secondly, despite some similarities, there are considerable differences across chronic conditions (i.e. symptoms, subtypes, treatment, progression, and prognosis). These factors may have unique implications for fertility, pregnancy, and outcomes. Therefore, the existing DAs should not be generalised to motherhood decision making in other chronic conditions.
Given that having children is a decision that may be revisited at different times, a longitudinal evaluation of the impact of DAs is recommended [41] as well as further evaluation of the DA’s usefulness, in terms of its components (particularly in relation to treatment options), across different populations and formats (i.e. briefer, interactive, translated in other languages), and across settings (i.e. clinical, counselling, etc.). Finally, the findings in this study from the DCS’s subscales may be indicative of areas of the DA that could be further strengthened when updating or revising this resource.
Conclusions
In summary, a DA has been developed to support decision-making for women with RA considering having children or more children. The DA is consistent with the IPDAS criteria, the gold standard for the development of decision aids. This initial evaluation suggests that it is effective in improving relevant knowledge and reducing decisional conflict without influencing women’s decisions or causing distress. This DA therefore has a direct application to patient care in that it may facilitate communication and shared decision making with family and health professionals.
Availability of supporting data
For access to study data please contact the corresponding author.
Acknowledgments
We thank Associate Professor Julie Pallant and Professor Allan Tennant for advice and assistance with the PiRAQ development and revision; expert and consumer panels for contribution to the DA development; Lisa Hallab for research assistance work, and Arthritis NSW and partner investigator, Mrs Diana Aspenell, for contribution to the study’s development and delivery. This work was supported by the Australian Research Council, [grant number LP0989906] titled ‘Motherhood choices: a decision aid for women with Rheumatoid Arthritis’ in partnership with Arthritis NSW. The Australian Research Council had no role in the design, collection, analysis, and interpretation of data; in the writing of the manuscript; or the decision to submit the manuscript for publication.
Abbreviations
- ASES
Arthritis Self-Efficacy Scale
- DA
Decision Aid
- DCS
Decisional Conflict Scale
- HADS
Hospital Anxiety and Depression Scale
- PiRAQ
Pregnancy in Rheumatoid Arthritis Questionnaire
- RA
Rheumatoid Arthritis
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TM contributed to the design, collection, data analyses and interpretation and writing of the manuscript. ED conducted data analyses and interpretation and contributed to writing of the manuscript. NM contributed to the design, data interpretation and writing of the manuscript. LS contributed to the design, data interpretation and writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
T. Meade, Email: t.meade@westernsydney.edu.au
E. Dowswell, Email: e.dowswell@westernsydney.edu.au
N. Manolios, Email: nicholas.manolios@sydney.edu.au
L. Sharpe, Email: louise.sharpe@sydney.edu.au
References
- 1.Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34:80–93. doi: 10.1016/S1701-2163(16)35138-6. [DOI] [PubMed] [Google Scholar]
- 2.Kariman N, Simbar M, Ahmadi F, Vedadhir A. Concerns about one’s own future or securing child’s future: paradox of childbearing decision making. Health. 2014 [Google Scholar]
- 3.Tough S, Tofflemire K, Benzies K, Fraser-Lee N, Newburn-Cook C. Factors influencing childbearing decisions and knowledge of perinatal risks among canadian men and women. Matern Child Health J. 2013 doi: 10.1007/s10995-006-0156-1. [DOI] [PubMed] [Google Scholar]
- 4.Chuang CH, Velott DL, Weisman CS. Exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions. Matern Child Health J. 2010 doi: 10.1007/s10995-009-0518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iezzoni LI, Yu J, Wint AJ, Smeltzer SC, Ecker JL. Prevalence of current pregnancy among US women with and without chronic physical disabilities. Med Care. 2013 doi: 10.1097/MLR.0b013e318290218d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne D, McPherson KM. Becoming mothers. Multiple sclerosis and motherhood: a qualitative study. Disabil Rehabil. 2010; doi:10.3109/09638280903204708 [DOI] [PubMed]
- 7.Redshaw M, Malouf R, Gao H, Gray R. Women with disability: the experience of maternity care during pregnancy, labour and birth and the postnatal period. BMC Pregnancy Childbirth. 2013; doi:10.1186/1471-2393-13-174. [DOI] [PMC free article] [PubMed]
- 8.Del Fabro Smith L, Suto M, Chalmers A, Backman CL. Belief in doing and knowledge in being mothers with arthritis. OTJR. 2011; doi:10.3928/15394492-20100222-01.
- 9.Clowse MEB, Chakravarty E, Costenbader KH, Chambers C, Michaud K. Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. 2012; doi:10.1002/acr.21593. [DOI] [PubMed]
- 10.Townsend A, Backman CL, Adam P, Li LC. Women’s accounts of help-seeking in early rheumatoid arthritis from symptom onset to diagnosis. Chronic Illn. 2014; doi:10.1177/1742395314520769 [DOI] [PMC free article] [PubMed]
- 11.Meade T, Sharpe L, Hallab L, Aspanell D, Manolios N. Navigating motherhood choices in the context of rheumatoid arthritis: women’s stories. Musculoskeletal Care. 2013; doi:10.1002/msc.1031 [DOI] [PubMed]
- 12.Vallido T, Wilkes L, Carter B, Jackson D. Mothering disrupted by illness: a narrative synthesis of qualitative research. J Adv Nurs. 2010; doi:10.1111/j.1365-2648.2010.05350.x [DOI] [PubMed]
- 13.Jørgensen KT, Harpsøe MC, Jacobsen S, Jess T, Frisch M. Increased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth Cohort. Rheumatology. 2014; doi:10.1093/rheumatology/keu150. [DOI] [PubMed]
- 14.Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF. Rheumatoid arthritis and outcomes in first and subsequent births based on data from a national birth registry. Acta Obstet Gynecol Scand. 2014; doi:10.1111/aogs.12324. [DOI] [PubMed]
- 15.Østensen M. Contraception and pregnancy counselling in rheumatoid arthritis. Curr Opin Rheumatol. 2014; doi:10.1097/BOR.0000000000000044. [DOI] [PubMed]
- 16.Kristiansen TM, Primdahl J, Antoft R, Horslev-Petersen K. Everyday life with rheumatoid arthritis and implications for patient education and clinical practice: a focus group study. Musculoskeletal Care. 2012; doi:10.1002/msc.224. [DOI] [PubMed]
- 17.Provost M, Eaton JL, Clowse MEB. Fertility and infertility in rheumatoid arthritis. Curr Opin Rheumatol. 2014; doi:10.1097/BOR.0000000000000058. [DOI] [PubMed]
- 18.El Miedany Y, El Gaafary M, El Aroussy N, Youssef S, Ahmed I. Sexual dysfunction in rheumatoid arthritis patients: arthritis and beyond. Clin Rheumatol. 2012; doi:10.1007/s10067-011-1891-2. [DOI] [PubMed]
- 19.Lin HC, Chen SF, Lin HC, Chen YH: Increased risk of adverse pregnancy outcomes in women with rheumatoid arthritis: a nationwide population-based study. Ann Rheum Dis. 2010; doi:10.1136/ard.2008.105262. [DOI] [PubMed]
- 20.Østensen M, Brucato A, Carp H, Chambers C, Dolhain RJEM, Doria A, et al. Pregnancy and reproduction in autoimmune rheumatic diseases. Rheumatology. 2011; doi:10.1093/rheumatology/keq350. [DOI] [PubMed]
- 21.Hazes JMW, Coulie PG, Geenen V, Vermeire S, Carbonnel F, Louis E, Masson P, De Keyser F. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology. 2011; doi:10.1093/rheumatology/ker302. [DOI] [PMC free article] [PubMed]
- 22.De Steenwinkel FDO, Hokken-Koelega ACS, de Ridder MAJ, Hazes JMW, Dolhain RJEM. Rheumatoid arthritis during pregnancy and postnatal catch-up growth in the offspring. Arthritis Rheum. 2014; doi:10.1002/art.38519. [DOI] [PubMed]
- 23.Williams M, Chakravarty EF. Rheumatoid arthritis and pregnancy: impediments to optimal management of both biologic use before, during and after pregnancy. Curr Opin Rheumatol. 2014; doi:10.1097/BOR.0000000000000046. [DOI] [PubMed]
- 24.Förger F, Vallbracht I, Helmke K, Villiger PM, Østensen M. Pregnancy mediated improvement of rheumatoid arthritis: effect of therapy on disease activity and autoantibodies. Swiss Med Wkly. 2012; doi:10.4414/smw.2012.13644. [DOI] [PubMed]
- 25.Makol A, Wright K, Amin S. Rheumatoid arthritis and pregnancy: safety considerations in pharmacological management. Drugs. 2011; doi:10.2165/11596240-000000000-00000. [DOI] [PubMed]
- 26.Østensen M, Villiger PM, Förger F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun Rev. 2012; doi:10.1016/j.autrev.2011.11.013 [DOI] [PubMed]
- 27.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008; doi:10.1002/art.24003. [DOI] [PubMed]
- 28.Chakravarty EF. Rheumatoid arthritis and pregnancy: beyond smaller and preterm babies. Arthritis Rheum. 2011; doi:10.1002/art.30206. [DOI] [PubMed]
- 29.Lowden D, Lee V, Ritchie JA. Redefining self: patients' decision making about treatment for multiple sclerosis. J Neurosci Nurs. 2014; doi:10.1097/jnn.0000000000000064 [DOI] [PubMed]
- 30.Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Legare F. Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision AIDS. Med Decis Making. 2012; doi:10.1177/0272989x12443311 [DOI] [PubMed]
- 31.Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333. [DOI] [PMC free article] [PubMed]
- 32.Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014; doi:10.1136/bmj.38926.629329.AE. [DOI] [PubMed]
- 33.Vlemmix F, Warendorf JK, Rosman AN, Kok M, Mol BWJ, Morris JM, et al. Decision aids to improve informed decision-making in pregnancy care: a systematic review. BJOG. 2013; doi:10.1111/1471-0528.12060 [DOI] [PubMed]
- 34.O'Connor AM, Stacey D, Rovner D, Holmes-Rovner M, Tetroe J, Llewellyn-Thomas H, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Sys Rev. 2001; doi:10.1002/14651858.CD001431 [DOI] [PubMed]
- 35.Nassar N, Roberts CL, Raynes-Greenow CH, Barratt A, Peat B. Evaluation of a decision aid for women with breech presentation at term: a randomized controlled trial. BJOG. 2007; doi:10.1111/j.1471-0528.2006.01206.x [DOI] [PMC free article] [PubMed]
- 36.Roberts CL, Raynes-Greenow CH, Nassar N, Trevena L, McCaffery K. Protocol for a randomized controlled trial of a decision aid for the management of pain in labour and childbirth. BMC Pregnancy Childbirth. 2004; doi:10.1186/1471-2393-4-24 [DOI] [PMC free article] [PubMed]
- 37.Healthwise. Infertility: should I have treatment? 2011. https://decisionaid.ohri.ca/Azsumm.php?ID=1134.
- 38.Healthwise. Miscarriage: should I have treatment to complete a miscarriage? 2013. https://decisionaid.ohri.ca/Azsumm.php?ID=1180.
- 39.Wong SSM, Thornton JG, Gbolade B, Bekker HL. A randomized controlled trial of a decision-aid leaflet to facilitate women’s choice between pregnancy termination methods. BJOG. 2006; doi:10.1111/j.1471-0528.2006.00930.x [DOI] [PubMed]
- 40.Nagle C, Gunn J, Bell R, Lewis S, Meiser B, Metcalfe S, et al. Use of a decision aid for prenatal testing of fetal abnormalities to improve women’s informed decision making: a cluster randomized controlled trial. BJOG. 2008; doi:10.1111/j.1471-0528.2007.01576.x [DOI] [PubMed]
- 41.Prunty MC, Sharpe L, Butow P, Fulcher G. The motherhood choice: a decision aid for women with multiple sclerosis. Patient Educ Couns. 2008; doi:10.1016/j.pec.2007.10.021 [DOI] [PubMed]
- 42.Meade T, Sharpe L, Aspanell D, Manolios N. Rheumatoid arthritis and motherhood: development of a resource to support women and their partners when contemplating family. Arthritis Matters Magazine. 2012; Summer Edition:10.
- 43.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ. 2010; doi:10.1136/bmj.c869. [DOI] [PMC free article] [PubMed]
- 44.O'Connor AM. User Manual- Decisional Conflict Scale. 1993. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 45.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995;38:1429–46. doi: 10.1002/art.1780381010. [DOI] [PubMed] [Google Scholar]
- 47.Mueller A, Hartmann M, Mueller K, Eich W. Validation of the arthritis self-efficacy short-form scale in German fibromyalgia patients. Eur J Pain. 2003;7:163–71. doi: 10.1016/S1090-3801(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 48.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 49.Whelan-Goodinson R, Ponsford J, Schonberger M. Validity of the hospital anxiety and depression scale to assess depression and anxiety following traumatic brain injury as compared with the structured clinical interview for DSM-IV. J Affect Disord. 2009; doi:10.1016/j.jad.2008.06.007 [DOI] [PubMed]
- 50.Covic T, Pallant JF, Tennant A, Cox S, Emery P, Conaghan PG. Variability in depression prevalence in early rheumatoid arthritis: a comparison of the CES-D and HAD-D Scales. BMC Musculoskel. 2009; doi:10.1186/1471-2474-10-18. [DOI] [PMC free article] [PubMed]
- 51.O’Connor AM, Legare F, Stacey D. Risk communication in practice: the contribution of decision aids. Brit Med J. 2003; doi:10.1136/bmj.327.7417.736 [DOI] [PMC free article] [PubMed]
- 52.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Erlbaum; 1988
- 53.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; doi:10.3758/BF03193146 [DOI] [PubMed]
- 54.Prunty M, Sharpe L, Butow P, Fulcher G. The motherhood choice: themes arising in the decision-making process for women with Multiple Sclerosis. Mult Scler. 2008; doi:10.1016/j.pec.2007.10.021 [DOI] [PubMed]


