Abstract
Regulatory T (Treg) cells are necessary for immune system homeostasis and the prevention of autoimmune diseases. Foxp3 is specifically expressed in Treg cells and plays a key role in their differentiation and function. Foxp3+ Treg cells are consisted of naturally occurring, thymus-derived Treg (nTreg) and peripheral-induced Treg (iTreg) cells that may have different functional characteristics or synergistic roles. All-trans retinoic acid (atRA), a vitamin A metabolite, regulates a wide range of biological processes, including cell differentiation and proliferation. Recent studies demonstrated that atRA also regulates the differentiation of T helper (Th) cells and Treg cells. Moreover, atRA also sustains nTreg stability under inflammatory conditions. In this review, we summarize the significant progress of our understanding of the role(s) and mechanisms of atRA in Treg biology.
Keywords: atRA, autoimmune diseases, Foxp3, Treg cells
Introduction
Autoimmunity is a heterogeneous disorder, which includes at least 80 diseases and is controlled by complex genetic and environmental factors. The pathogenesis of autoimmunity is hypothesized to result from a breakdown of immune tolerance, including central and/or peripheral mechanisms, and this loss of control ultimately culminates in autoimmune diseases.1 Whereas the immune system plays an important role in the prevention of autoimmune diseases through self-tolerance mechanisms, it must be efficient in protecting the host from insult by exogenous pathogens. In this regard, the CD4+ T-cell represents the chief protagonist, and plays a critical role in controlling the adaptive immune system.2 A current paradigm in immunology is that autoimmunity is elicited by an imbalance between pathogenic T cells and Foxp3+ regulatory T (Treg) cells.3 These Treg cells prevent autoimmune and inflammatory diseases by suppressing the activities of deleterious effector T helper (Th) cells.4
CD4+CD25+Foxp3+ Treg cells are a specialized CD4+ T-cell lineage that plays a central role in maintaining self-tolerance, and the dysfunction of these cells is implicated in the development of various autoimmune diseases.5,6,7 CD4+CD25+Foxp3+ Treg cells comprise at least two distinct subsets in the periphery, natural Treg cells (nTreg cells) produced by the thymus after recognition of high-affinity self-antigen and then move to the periphery, and induced Treg cells (iTreg cells) that are converted from conventional non-Treg cells as a consequence of peripheral exposure to antigens in the presence of transforming growth factor-beta (TGF-β) signaling.8 The comparison of the similarities and differences between nTreg and iTreg cells has been previously reviewed.3,9,10
Foxp3+ iTreg cells can be induced ex vivo by TGF-β or IL-10.11,12 Although many factors may promote the differentiation and development of iTreg cells, TGF-β and its receptor signaling pathway is critical because Foxp3+ iTreg cells cannot be induced without a TGF-β signal.13,14 IL-2 is also important for the development and maintenance of iTreg cells.15 All-trans retinoic acid (atRA), a vitamin A metabolite, regulates a wide range of biological processes, including cell differentiation and proliferation. Recent studies revealed that atRA regulates the differentiation of Th cells and Foxp3+ Treg cells.16,17 Additionally, atRA promotes the development and function of CD4+ iTreg cells, although its effect on CD8+ iTreg cells is minimal.18,19,20,21 Moreover, atRA also helps preserve nTreg cell stability under inflammatory conditions.22,23 In this review, we summarize our understanding of the role of atRA in Treg cell biology, its related molecular mechanisms and potential clinical application for patients with autoimmune diseases and who need organ transplantation.
Foxp3 and Treg cell subsets
Foxp3, an X chromosome linked factor that controls Treg cell development and function, is the major transcription factor for determining the fate and identity of Treg cells and is specifically expressed in Treg cells.24,25 Foxp3 is generally postulated to positively control Treg cell function in a binary fashion, because its expression in conventional T cells is sufficient to specify immune-suppressive activities.7 Foxp3 is critically involved in the development and function of Treg cells, its expression appears to play a necessary role in governing Treg cell action. Treg cells also prevent autoimmune and inflammatory diseases by suppressing the potentially deleterious activities of Th cells.4 In contrast, the downregulation of Foxp3 or Foxp3 deficiency results in multiorgan autoimmune diseases. For example, downregulation of Foxp3 in antigen-experienced Treg cells coincides with the onset of pro-inflammatory and immunoregulatory cytokine secretion, such as IL-2, IFN-γ and IL-10, in these cells.26 Recent data indicate that mature Foxp3+ Treg cells express the highest levels of neuropilin-1 (Nrp-1), which is usually expressed on thymus-derived natural regulatory T cells. This suggests that the overwhelming majority of thymus-derived, natural Treg cells express Nrp-1.27 Similarly, Helios provides an additional marker for the discrimination of nTreg cells from iTreg cells, although its specificity remains a concern.28,29 Nrp-1 also identifies Foxp3+ cell stability because Nrp-1+ nTreg cells are more stable compared with Nrp-1− nTreg cells. Nrp-1+ nTreg cells have lower methylation levels in the Treg cell-specific demethylated region.30 The Treg cell-specific demethylated region colocalizes with conserved non-coding sequence-2 of Foxp3, a region involved in the maintenance of Foxp3 expression.31
One paradigm of immunology is that autoimmunity is elicited by an imbalance between pathogenic T and Foxp3+ Treg cells. The pathophysiology driven by autoimmune diseases can alter the phenotypic and functional activity of Treg cells. Foxp3 expression in Treg cells is closely associated with their functional activities. The plasticity of Foxp3 expression by nTreg cells under inflammatory conditions may also play an important role in infectious diseases, in which early inflammatory cytokines induced by the innate immune response may not only downregulate Treg cell function, but may also change Treg cells into T effector cells locally in the infected tissues, thereby enhancing immunity.1 The adoptive transfer of nTreg cells prevents the initiation and development of autoimmune diseases in many animal models; however, the therapeutic effect of nTreg cells on autoimmune diseases remains unsatisfactory. The key reason is that inflammatory cytokines, such as IL-6, TNF-α and IL-1, may decrease Foxp3 expression and subsequently reduce the functional activity of nTreg cells.22,23,32,33,34,35,36
The stability of Treg cell subsets
Recent studies demonstrated that nTreg cells from both mouse and human are instable and dysfunctional under inflammatory conditions.7,32,34,35,37,38 These cells not only lose their suppressive ability after encountering inflammatory environments, but they can convert into pathogenic cells that may actually accelerate the inflammatory process.1 In addition, the repeat expansion of nTreg cells, even in the absence of pro-inflammatory cytokines, can also result in the loss of Foxp3 expression. This finding has very important implications for clinical utility because nTreg cells initially exist as a very small cell population.26 It is therefore critical to identify approaches that maintain Foxp3 expression and Treg cell function during expansion, particularly under inflammatory conditions.
Rapamycin (RAPA) may be an ideal candidate for promoting nTreg cell stability. RAPA, an mTOR kinase inhibitor, is an immunosuppressive drug that inhibits effector T-cell proliferation, migration and cytokine production,39 and can selectively promote the expansion of suppressive human CD4+CD25hiFoxp3+ T cells isolated from healthy donors and patients with diabetes.40,41 It remains unclear whether RAPA selectively suppresses the expansion of non-Treg cells, thereby indirectly promoting the expansion of Foxp3+ Treg cells.16 Although a comparison study has shown that both RAPA and atRA had similar effects on promoting and stabilizing Treg cells during their expansion,42 a more recent study demonstrated that atRA exhibits superior efficacy relative to RAPA for stabilizing nTreg cells under inflammatory conditions.23 The mechanism by which atRA stabilizes nTreg cells is discussed below.
iTreg cells exhibit several characteristic differences in stability and functionality relative to nTreg cells. Whereas IL-6 can convert nTreg cells into Th17 and Th1 cells, it does not have this effect on iTreg cells.32 Conversely, iTreg cells are stable and function effectively in an inflammatory environment.32,43 It is likely that TGF-β treatment reduces IL-6 receptor expression and thereby suppresses its signaling pathway.32 Therefore, iTreg cells may play a complementary role to nTreg cells, particularly in response to self-antigens, which are not expressed in the thymus. It is possible that under inflammatory conditions, the induction of iTreg cells is suppressed and self-reactive cells develop directly into effector-memory T cells and promote autoimmune disease. Whereas TGF-β is crucial for promoting the development of iTreg cells, the presence of IL-6 interferes with the ability of TGF-β to promote this differentiation.44 However, because iTreg cells are stable and functional under inflammatory conditions, after they have been induced, they can expand ex vivo following adoptive transfer for cell-based therapeutic treatment of patients with autoimmune inflammatory diseases.3,45
atRA and Treg cell function
atRA, the primary biologically active metabolite of vitamin A, plays vital roles in embryonic development, vision, skin homeostasis and reproduction, and it also crucial for maintenance of the immune system.46 atRA produced by dendritic cells facilitates the de novo generation of Foxp3+ Treg cells from naive CD4+CD25− T cell populations in mice,47,48 but also suppress the de novo differentiation of naive CD4+ cells into Th17 cells.22 The effect of atRA on Treg and Th17 cells is dependent upon the RA receptor/retinoid X receptor heterodimer.49,50 Because the pathogenesis and development of many autoimmune diseases is affected by the imbalance between Treg and Th17 cells, the role of atRA in regulating this balance may greatly affect the progress of autoimmune diseases.
atRA appears to promote gut homing of CD4+ T cells by inducing CCR9 and α4β7 expression, and the expression of these molecules also indicates that a given population of T cells respond to atRA.51 An initial study showed that atRA suppresses Th1 but promotes Th2 cells.52 Vitamin A deficiency results in immune dysfunction via excessive IFN-γ production and impaired antibody responses. A recent study reported that atRA inhibits Th17 cell differentiation but promotes Foxp3+ Treg cells,17,18,53 although the role of atRA in CD4+ and CD8+ iTreg cell differentiation may be different.21 The orphan nuclear receptor, RORγt, has been implicated in the gene transcription of Th17 cells. TGF-β induces high levels of RORγt and further promotes Th17 cell development in the presence of IL-6. However, the addition of atRA to cultures containing TGF-β and IL-6 greatly reduces RORγt expression and Th17 cell differentiation.54
The key role played by atRA in immune tolerance is via the induction of iTreg cells. atRA plays a crucial role in maintaining gut mucosa tolerance to commensal bacteria and food antigens through the induction of both Foxp3+ Treg cells and IL-10-producing Treg cells.55,56 atRA is primarily produced by CD103+ dendritic cells in the intestine. These CD103+ dendritic cells originate in the lamina propria, but migrate to the mesenteric lymph nodes where they drive the differentiation of gut-homing Foxp3+ Treg cells through the production of retinoic acid from dietary vitamin A.57 Whereas TGF-β alone is not sufficient to drive the development of human iTreg cells, the addition of atRA provides the necessary stimulus for human iTreg cell induction, demonstrating its value in clinical translation.19 The study of molecular mechanisms demonstrated that although atRA does not significantly affect the phosphorylation levels of Smad2/3, it promotes iTreg cell induction in CD4+ cells isolated from Smad3 knockout and Smad2 conditional knockout mice. By contrast, atRA markedly increases the activation of the ERK1/2 signaling pathway, and the resultant signaling promotes Foxp3 expression.20 Although DNA methylation at the Foxp3 gene locus affects Foxp3 expression and maintenance by Treg cells,58 atRA enhances the differentiation and stability of iTreg cells in the absence of any alteration of DNA methylation. Instead, atRA acts via increased histone methylation and acetylation within the promoter and conserved non-coding DNA sequence elements at the Foxp3 gene locus;20 however, atRA can inhibit the methylation of the Foxp3 gene of nTreg in the presence of inflammatory cytokines.23
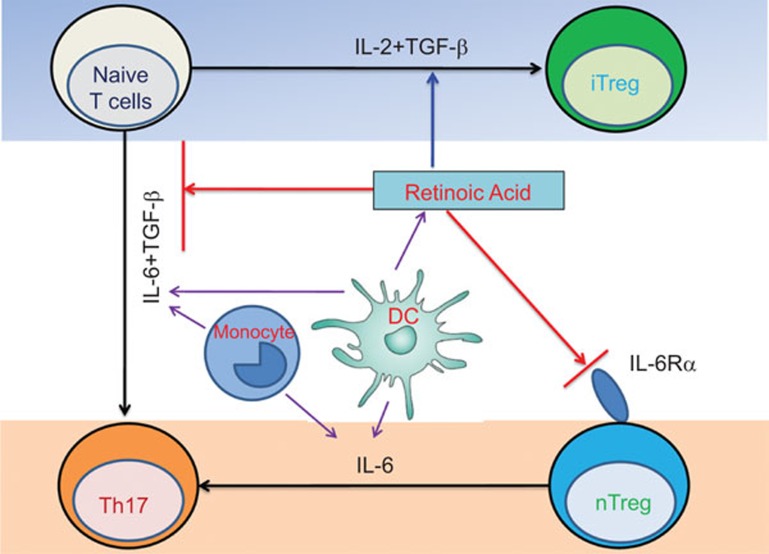
Interestingly, atRA also helps maintain Foxp3 expression during the nTreg cell expansion process.22 This has recently been extended to human nTreg cells.23 Compared with RAPA, the effect of atRA on stabilizing Foxp3 expression and Treg cell function is superior under inflammatory conditions.23 atRA promotes the expression of cytotoxic T lymphocyte antigen 4(CTLA-4), a cell-surface receptor typically expressed by Treg cells on the majority of TGF-β-generated Foxp3+ cells. The B7/CTLA-4 signal is crucial for the development and function of iTreg cells.59 atRA also enhances the expression of surface TGF-β on nTreg cells,23 which is another possible mechanism for nTreg cell stabilization. In addition, atRA also downregulates IL-6R expression and IL-6R signaling on nTreg cells, rendering nTreg cells resistant to the pro-inflammatory cytokine, IL-6, which is usually elevated in autoimmune diseases.60 The key role atRA plays in promoting iTreg cells development and nTreg cells stabilization is summarized in Figure 1. These characteristics highly suggest that atRA-treated nTreg cells from patients with rheumatoid arthritis and other autoimmune diseases could potentially be used to control disease development and even cure patients. The combination of atRA and TGF-β also provides another approach to develop Treg cell therapy for patients with autoimmune diseases and the prevention of allograft reject in patients with organ transplantation.
Figure 1.
Immunomodulatory effects of atRA on CD4+ T-cell subsets. atRA maintains immune homeostasis by working with TGF-β to promote Treg cell induction from naive T cells, while inhibiting Th17 cell induction in the presence of inflammatory cytokines such as IL-6. In addition, atRA inhibits Th17 differentiation from nTreg cells by reducing their expression of IL-6Rα. atRA, all-trans retinoic acid; TGF, transforming growth factor; Treg, regulatory T.
Conclusions
Treg cells are a distinct lineage of CD4+ T cells that are essential for maintaining immune system homeostasis by promoting self-tolerance and restraining excessive immune responses. Many mechanisms are involved in Treg cell development and function. Foxp3 is the most specific hallmark of Treg cell subsets. Foxp3 expression and stability are closely related to the functionality of Treg cells. Foxp3 expression on nTreg cells is unstable in the presence of IL-6 and other pro-inflammatory cytokines. RAPA and/or atRA can stabilize nTreg cells, but atRA has superior effects on nTreg cell stabilization under inflammatory conditions. atRA also promotes the differentiation of TGF-β-induced iTreg cells and inhibits Th1 and Th17 cell differentiation. These results highlight the role of atRA in promoting the development of iTreg cells and stabilizing the phenotype and function of nTreg cells, indicating that approaches with atRA-primed Treg cells have potential therapeutic value for patients with autoimmune diseases and those undergoing organ transplantation.
Acknowledgments
This work was supported in part by grants from the NIH AR059103 and AI084359 (to SGZ), from the National Natural Science Foundation of China 81274161 and 81001307, the Zhejiang Provincial Natural Science Foundation of China Y2090918, the Health Bureau of Zhejiang Province 2012RCA046 (to JM) and the National Natural Science Foundation of China 81370433, 81170084 (to ZL).
References
- 1Zhou X, Bailey-Bucktrout SL, Jeker LT, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009; 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Sawa S, Kamimura D, Jin GH, Morikawa H, Kamon H, Nishihara M et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med 2006; 203: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3+ regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol 2012; 4: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H, Zheng SG. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol 2010; 2: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Evridiki Sgouroudis E et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008; 28: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol 2008; 28: 677–684. [DOI] [PubMed] [Google Scholar]
- 7Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 2007; 445: 766–770. [DOI] [PubMed] [Google Scholar]
- 8Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003; 3: 253–257. [DOI] [PubMed] [Google Scholar]
- 9Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 1772–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol 2008; 29: 429–435. [DOI] [PubMed] [Google Scholar]
- 11Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol 2002; 169: 4183–4189. [DOI] [PubMed] [Google Scholar]
- 12Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389: 737–742. [DOI] [PubMed] [Google Scholar]
- 13Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol 2010; 184: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Lu L, Ma J, Wang X, Wang JL, Zhang F, Yu JN et al. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur J Immunol 2010; 40: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007; 178: 2018–2027. [DOI] [PubMed] [Google Scholar]
- 16Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity 2008; 29: 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3+ Treg cell conversion of naive T cells. Immunity 2009; 30: 471–472; author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 2008; 111: 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One 2010; 5: e15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y et al. All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One 2011; 6: e24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Ma J, Liu Y, Li Y, Gu J, Liu J, Tang JY et al. Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J Leuk Biol 2014; 95: 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol 2010; 185: 2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA 2014; 111: E3432–E3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 2005; 201: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005; 22: 329–341. [DOI] [PubMed] [Google Scholar]
- 26Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 2009; 39: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 27Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 2012; 209: 1713–1722, S1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184: 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One 2011; 6: e20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Lin X, Chen M, Liu Y, Guo Z, He X, Brand D et al. Advances in distinguishing natural from induced Foxp3+ regulatory T cells. Int J Clin Exp Pathol 2013; 6: 116–123. [PMC free article] [PubMed] [Google Scholar]
- 31Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010; 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 2008; 180: 7112–7116. [DOI] [PubMed] [Google Scholar]
- 33Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20: 62–68. [DOI] [PubMed] [Google Scholar]
- 34Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008; 112: 2340–2352. [DOI] [PubMed] [Google Scholar]
- 35Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 2009; 113: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 2013; 39: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol 2007; 178: 6725–6729. [DOI] [PubMed] [Google Scholar]
- 38Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 2009; 323: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 39Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol 2010; 22: 655–661. [DOI] [PubMed] [Google Scholar]
- 40Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 2006; 177: 8338–8347. [DOI] [PubMed] [Google Scholar]
- 41Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 2009; 58: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Scotta C, Esposito M, Fazekasova H, Fanelli G, Edozie FC, Ali N et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4+CD25+FOXP3+ T regulatory cell subpopulations. Haematologica 2013; 98: 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43O'Connor RA, Leech MD, Suffner J, Hämmerling GJ, Anderton SM. Myelin-reactive, TGF-beta-induced regulatory T cells can be programmed to develop Th1-like effector function but remain less proinflammatory than myelin-reactive Th1 effectors and can suppress pathogenic T cell clonal expansion in vivo. J Immunol 2010; 185: 7235–7243. [DOI] [PubMed] [Google Scholar]
- 44Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 45Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3+ regulatory T cells. J Mol Cell Biol 2012; 4: 29–37. [DOI] [PubMed] [Google Scholar]
- 46Kropotova ES, Zinov'eva OL, Zyrianova AF, Choı ˘nzonov EL, Afanas'ev SG, Cherdyntseva NV et al. Expression of genes involved in retinoic acid biosynthesis in human gastric cancer. Mol Biol (Mosk) 2013; 47: 317–330. Russian. [DOI] [PubMed] [Google Scholar]
- 47Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007; 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007; 204: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol 2007; 37: 2396–2399. [DOI] [PubMed] [Google Scholar]
- 50Takeuchi H, Yokota-Nakatsuma A, Ohoka Y, Kagechika H, Kato C, Song SY et al. Retinoid X receptor agonists modulate Foxp3+ regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J Immunol 2013; 191: 3725–3733. [DOI] [PubMed] [Google Scholar]
- 51Evans TI, Reeves RK. All-trans-retinoic acid imprints expression of the gut-homing marker alpha4beta7 while suppressing lymph node homing of dendritic cells. Clin Vaccine Immunol 2013; 20: 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 2003; 15: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 53Shi Q, Cao H, Liu J, Zhou X, Lan Q, Zheng S et al. CD4+ Foxp3+ regulatory T cells induced by TGF-beta, IL-2 and all-trans retinoic acid attenuate obliterative bronchiolitis in rat trachea transplantation. Int Immunopharmacol 2011; 11: 1887–1894. [DOI] [PubMed] [Google Scholar]
- 54Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol 2007; 19: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Kim CH. Regulation of FoxP3 regulatory T cells and Th17 cells by retinoids. Clin Dev Immunol 2008; 2008: 416910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol 2014; in press. [DOI] [PubMed]
- 57Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol 2011; 32: 412–419. [DOI] [PubMed] [Google Scholar]
- 58Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol 2006; 176: 3321–3329. [DOI] [PubMed] [Google Scholar]
- 60Houssiau FA, Devogelaer JP, van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum 1988; 31: 784–788. [DOI] [PubMed] [Google Scholar]