Abstract
FAM19A4 is an abbreviation for family with sequence similarity 19 (chemokine (C–C motif)-like) member A4, which is a secretory protein expressed in low levels in normal tissues. The biological functions of FAM19A4 remain to be determined, and its potential receptor(s) is unclarified. In this study, we demonstrated that FAM19A4 was a classical secretory protein and we verified for the first time that its mature protein is composed of 95 amino acids. We found that the expression of this novel cytokine was upregulated in lipopolysaccharide (LPS)-stimulated monocytes and macrophages and was typically in polarized M1. FAM19A4 shows chemotactic activities on macrophages and enhances the macrophage phagocytosis of zymosan both in vitro and in vivo with noticeable increases of the phosphorylation of protein kinase B (Akt). FAM19A4 can also increase the release of reactive oxygen species (ROS) upon zymosan stimulation. Furthermore, based on receptor internalization, radio ligand binding assays and receptor blockage, we demonstrated for the first time that FAM19A4 is a novel ligand of formyl peptide receptor 1 (FPR1). The above data indicate that upon inflammatory stimulation, monocyte/macrophage-derived FAM19A4 may play a crucial role in the migration and activation of macrophages during pathogenic infections.
Keywords: cytokine, FAM19A4, FPR1, macrophages, phagocytosis
Introduction
Cytokines are soluble mediators of cell communication that are critical in immune regulation.1 During innate immune responses, the principle sources of cytokines are macrophages, dendritic cells, natural killer cells and innate lymphoid cells. As a crucial component of innate immunity, macrophages play vital roles in phagocytosis to remove erythrocytes, apoptotic cells and cellular debris for protection from the invasion of pathogens.2 Meanwhile, macrophages produce various cytokines to protect the host and instruct the adaptive immune system to mount a response that is mutually regulated by cytokines.3,4 For instance, macrophages can express interferon γ (IFN-γ) after infection by pathogens,5,6 which can trigger a harsh proinflammatory response in turn on the macrophages that requires them to kill the intracellular pathogens. This pro-inflammatory response converts resting macrophages into potent cells with augmented phagocytosis and an increased generation of reactive oxygen species (ROS). Above all, cytokines and macrophages act and react upon one another using specific receptors.
In the recruitment and regulation of macrophages during inflammatory responses, G-protein coupled receptors are often involved. One such receptor, formyl peptide receptor 1 (FPR1), is highly expressed on macrophages and neutrophils; its functions include mediating cell migration, phagocytosis and the release of the superoxide ion.7,8,9 Microbe-derived peptides from bacteria and viruses, such as formyl-methionyl-leucyl phenylalanine (fMLF), N-formyl peptides from mitochondria and host-derived peptides such as the N-terminal peptides from Annexin-1, have been proved to be FPR1 ligands. More recently, synthetic low-molecular-weight ligands for FPR1 have emerged from a number of compound library screens.10 By binding to FPR1, they function as agonists of FPR1 to promote migration, phagocytosis and the release of the superoxide ion from macrophages and neutrophils,11 resulting in the systemic inflammatory response syndrome.12 However, these ligands are either from the infecting pathogens or serve as damage-associated molecular patterns, also called alarmins. Damage-associated molecular patterns are released upon mitochondrial damage and from apoptotic cells.13,14 They are passively released to activate the host immune responses associated with tissue and cell damage.
FAM19A4 (family with sequence similarity 19, member A4), a member of the TAFA family and also called TAFA4, is a secretory protein expressed in low levels in normal tissues as previously reported,15 but its bioactivities have not yet been studied. We identified FAM19A4 to be a novel cytokine matching criteria previously mentioned.16 In this study, we found that it was a classical secretory protein and verified for the first time that its mature protein consists of 95 amino acids. We proved that the expression of FAM19A4 was upregulated in LPS-stimulated monocytes and macrophages, especially in polarized M1. As a cytokine ligand of FPR1, FAM19A4 can chemoattract macrophages, promote phagocytosis against zymosan and increase ROS release. For its inducible expression pattern and the capacity to activate FPR1 and to promote macrophages, we expected that FAM19A4 might have essential roles in infectious diseases. As the first cytokine ligand of FPR1, FAM19A4 enhances communication among immune cells, which will be helpful in understanding infection and immunity.
Materials and methods
Mice
All mice used in this study were of a C57BL/6 background, were between 6–10 weeks old and were bred in the animal breeding facilities at the Peking University Health Science Center (Beijing, China) under specific pathogen-free conditions. The animal experimental procedures were approved by the ethics committee of the Peking University Health Science Center.
Cell preparation
HEK293 (CRL-1573), RAW264.7 (TIB-71) and THP-1 (TIB-202) cells were purchased from ATCC (Manassas, VA, USA). The HEK293T cells were kindly provided by T. Matsuda, Japan. Human peripheral blood was obtained from the Beijing Red Cross Blood Center. The peripheral blood mononuclear cells (PBMCs) were separated by Polymorphprep (Axis-Shield, Oslo, Norway) at a 1.077 density following the manufacturer's instructions. The PBMCs were located at the interface between RPMI 1640 and Polymorphprep.
The HEK293T and RAW264.7 cells were cultured in complete Dulbecco's Modification of Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS) (inactivated FBS for the latter). The HEK293T cells were transfected with VigoFect (Vigorous, Beijing, China) and the RAW264.7 cells were transfected with Lipofectamin2000 (Invitrogen, Koghbatsi, Yerevan, USA), each according to the manufacturers' instructions. The HEK293 cells, the THP-1 cells, the PBMCs, the human macrophages and the mice peritoneal macrophages were maintained in RPMI 1640 with 10% FBS. Monocytes were obtained from the PBMCs after adhesion for 4 h.
Reagents and antibodies
The pCMV-FPR1 expression plasmid was kindly provided by Professor Philip M. Murphy from the Laboratory of Molecular Immunology, National Institutes of Health. The pEGFP-N1-FPR1 and pcDNA3.1-FAM19A4-myc-his plasmids were constructed in our laboratory. The eukaryotic expression and affinity-purification of the FAM19A4-myc-his protein were commissioned to be completed by Crown Bioscience, Inc. (Beijing, China). The polyclonal antibodies against FAM19A4 were generated by immunizing rabbits with the eukaryotic FAM19A4 protein (300 µg/rabbit). The antibody was purified from the rabbits' serum using Protein G agarose beads.
FBS was purchased from Biochrom AG (Berlin, Germany). Brefeldin A (BFA). PMA, LPS (Escherichia coli 055:B5), Boc-MLF and thioglycollate broth were all purchased from Sigma-Aldrich (St. Louis, MO, USA). The FITC-zymosan and reverse transcription kits were purchased from Invitrogen. The cDNA panels of multiple human tissues, the Human MTC Panels I and II and the Human Immune System MTC Panel were purchased from Clontech (Mountain View, CA, USA). The protease inhibitor cocktail and the PhosSTOP cocktail were obtained from Roche (Rotkreuz, Switzerland). Rabbit monoclonal antibodies (mAbs) of Akt (pan) and Phospho-Akt (Ser473) for the Western blot were obtained from Cell signaling Technology (Danvers, MA, USA). The PE-labeled anti-mouse F4/80 antibodies for flow cytometry (FACS) were purchased from BD Biosciences (San Jose, CA, USA).
Real-time PCR
The expression of FAM19A4 at the mRNA level was examined using real-time PCR with the UPL probes and GAPDH as the control. The following primers were used:
hFAM19A4-F: 5′-Tgctgccagattactcaggtt-3′
hFAM19A4-R: 5′-gcacacctctctcttcgctac-3′
GAPDH-F: 5′-tccactggcgtcttcacc-3′
GAPDH-R: 5′-ggcagagatgatgaccctttt-3′
TNF-F: 5′-cagcctcttctccttcctgat-3′
TNF-R: 5′-gccagagggctgattagaga-3′
The samples were run in triplicate with the following cycling conditions: 10 min denaturing at 95 °C, followed by 45 cycles of 15 s at 95 °C and 1 min at 60 °C.
Immunoprecipitation
The cell culture medium was collected and concentrated through ultrafiltration. The medium was then incubated with the Protein G agarose beads that were conjugated with rabbit anti-FAM19A4 or normal rabbit IgG at 4 °C for 2 h. Next, the beads were collected by centrifugation, washed five times in phosphate buffer saline (PBS), boiled at 99 °C for 10 min in loading buffer and analyzed by western blotting.
Western blot
The cell culture supernatants were collected and centrifuged (1500 r.p.m. for 10 min at 4 °C) to eliminate the residual cells and debris. The cells were lysed in lysis buffer (20 mM HEPES (pH 7.4), 1% Triton X-100, 100 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM PMSF and 1% protease inhibitor cocktail). The protein concentration was measured using a BCA protein assay reagent (Pierce, Rockford, IL, USA) with bovine serum albumin as the standard. The cell culture medium, lysates and immunoprecipitation samples were separated on 12.5%–15% SDS–PAGE and were subsequently transferred to nitrocellulose membranes (Hybond, Escondido, CA, USA). After being blocked in 5% bovine serum albumin (BSA) in TBS-T (Tris-buffered saline containing 0.05% Tween 20) for 1 h at room temperature, the membranes were incubated with the appropriate primary antibodies overnight at 4 °C, extensively washed in TBS-T, and then incubated for 1 h at room temperature with the HRP-labeled goat anti-rabbit. Following three additional washes in TBS-T, the signals were finally visualized with ECL Substrate (Pierce, Rockford, IL, USA) and imaged by LAS500 (GE, New York City, NY, USA).
For detecting the phosphorylation of the signaling molecules, PhosSTOP was added to the lysis buffer. The resolved proteins were detected after being transferred with the indicated primary antibodies, followed by incubation in the specific secondary antibodies (Cell signaling Technology, Danvers, MA, USA).
Phagocytosis assay in vitro and in vivo
In vitro, the human macrophages derived from monocytes and RAW264.7 cells were pre-treated with FAM19A4 (10 ng/ml in D) for 2 h and then challenged with FITC-zymosan at a ratio of 10∶1 cells for 30 min in the absence of the serum. Phagocytosis was determined by confocal microscopy (Leica TCS SP8, Wetzlar, Germany) or on a FACSVerse (BD Biosciences, San Jose, CA, USA) using the BD FACSuite software. The calculation of the relative recognition index (RRI) was conducted using the equation RRI=percentage of zymosan-FITC+×MFI (mean fluorescence intensity) of zymosan-FITC+. The ratio of RRI compared to the control group represents the ability to conduct phagocytosis. Eight- to 10-week-old C57BL/6J mice were used to assess the effects of FAM19A4 on the in vivo uptake of zymosan by the macrophages. A total of 100 ng or 1 µg per mouse in 1 ml PBS of FAM19A4 (PBS as the control) was pre-injected into the peritoneum of the mice; 108 particles per mouse of FITC-zymosan was injected 2 h later. Thirty minutes later, the infiltrate in the peritoneal cavity was collected, and the fluorescence intensity was analyzed using flow cytometry. The scatter profile and the PE-F4/80 staining were used to gate the macrophages in the peritoneal washout.
Measurement of intracellular ROS
The mice peritoneal macrophages were recruited using thioglycollate broth (4%) stimulation for 2 days. ROS generation in the macrophages was monitored with the oxidation sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) obtained from the Beyotime Institute of Biotechnology (Shanghai, China). In brief, the macrophages were incubated for 30 min at 37 °C with 10 µM H2DCF-DA in serum-free medium. Then, the cells were washed and pre-treated with or without FAM19A4 for 2 h before being stimulated with zymosan (100 µg/ml). The fluorescence of DCF was determined by FACS.
Chemotaxis assay
The pCMV-FPR1 (kindly provided by Professor Philip M Murphy from the Laboratory of Molecular Immunology, National Institutes of Health) or the pEGFP-N1-FPR1-EGFP plasmid was transiently transfected into the HEK293 cells by electroporation at 120 V for 20 ms using an electric pulse generator (Electro Square Porator ECM 830; BTX, San Diego, CA, USA), and a chemotaxis assay was performed 48 h later. The chemotaxis assays were performed in 48-well microchemotaxis chambers (Neutroprobe, Bethesda, MD, USA) as described previously. In brief, FAM19A4 or fMLF (28 µl/well) diluted in HEPES-buffered RPMI 1640 medium supplemented with 0.1% BSA was placed in the lower chambers. The cells were resuspended in the same medium at a density of 106 cells/ml and were added to the upper chambers (50 µl/well). The upper and lower chambers were separated by a Rat Tail Collagen Type 1 (Biomedical Technologies, Stoughton, MA, USA) coated polyvinylpyrrolidone-free polycarbonate filter (Neutroprobe, Bethesda, MD, USA) with 12-µm (for HEK293) or 5-µm pores (for macrophages). The chambers were incubated for 6 h at 37 °C in an atmosphere of 5% CO2. Afterward, the filters were removed from the chambers, washed, fixed and stained with the Three Step Stain Set (Richard-Allan Scientific, Kalamazoo, MI, USA). The cells that migrated into each filter were counted in five randomly selected high-power fields (×400) per well.17 Significant chemotaxis was compared with the medium and was defined as a chemotactic index >2.0. In the cross-desensitization chemotaxis assay, fMLF and FAM19A4 were tested for their abilities to chemoattract the HEK293-FPR1 cells with or without being pre-treated with fMLF or FAM19A4 for 1 h and the chemotaxis index was calculated.
Receptor internalization assay
FPR1 was expressed in the HEK293 cells as described above. The HEK293-FPR1 cells were stimulated with fMLF (0.1 µM), FAM19A4 (0.1 µM) or as negative controls in suspension for 1 h. The cells were then stained with anti-FPR1 or mouse IgG primary antibodies followed by goat anti-mouse-PE IgG as the detection antibody. The FPR1 expression was then analyzed on a FACSVerse flow cytometer. The internalization rate of FPR1 and the significant differences compared to the control were calculated.
In the receptor internalization assay that was conducted by confocal microscopy, the pEGFP-N1-FPR1 plasmid was transiently expressed in the HEK293 cells as described above. The transfected cells were stimulated with fMLF, FAM19A4 or the negative controls for 1 h in the supernatant, and the subcellular localization of FPR1-EGFP was observed using a confocal microscope. The scale bars are 25 µm.
Radio ligand binding assay
The FPR1-EGFP-expressing HEK293 cells were prepared. FAM19A4 was labeled with 125I. 125INa was purchased from DuPont, Inc (Wilmington, DE, USA). The 125I-FAM19A4 in the binding buffer was concentrated at 13050CPM/100 µl. For the saturation experiment, equivalent quantities of 125I-FAM19A4 were incubated with varying quantities of the FPR1 cell membrane extract in binding buffer. For the competition binding assays, equivalent quantities of the cell membrane extract and 125I-FAM19A4 were incubated with varying quantities of unlabeled FAM19A4, fMLF or the myc-his peptide. All the reactions were incubated for binding, and radioactivity was evaluated as described previously.17
Statistical analysis
The data were expressed as the mean±s.e.m. The statistical analyses were performed using two-tailed Student's t-tests (unpaired) in Prism 5.0 (GraphPad Software, San Diego, CA, USA). Significant differences between groups are represented by *P<0.05, **P<0.01 and ***P<0.001.
Results
FAM19A4 is a classical secretory protein.
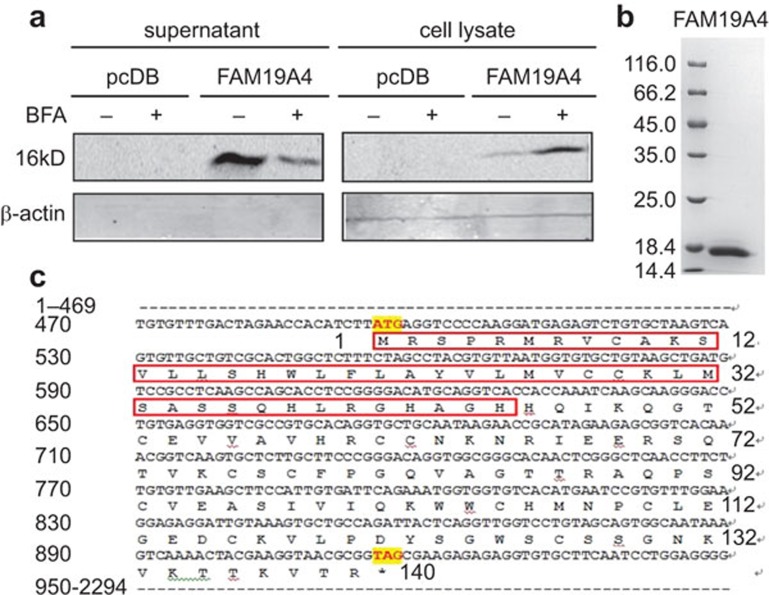
FAM19A4 belongs to a novel secretory protein family called TAFA, which has five homological members, TAFA1-5. FAM19A4 has been reported to be secreted when overexpressed in CHO cells.15 To further verify its secretory pattern, we carried out the BFA inhibition test. As shown in Figure 1a, FAM19A4 was secreted into the culture supernatant of the HEK293T cells and the secretion could be inhibited by the Golgi blocker BFA. This suggests that FAM19A4 is a classical secretory protein. The protein was then affinity-purified from the supernatant with a high purity (over 95%, Figure 1b) and low endotoxin levels (less than 0.11 EU/mg).
Figure 1.
FAM19A4 is a classical secretory protein. (a) The HEK293T cells were transfected with pcDNA3.1-FAM19A4-myc-his using VigoFect. BFA (10 µg/ml) was added to the cell culture medium 24 h prior to the harvest. (b) The eukaryotic FAM19A4-myc-his protein was >95% pure and had less than 0.11 EU/mg of endotoxins. (c) The signal peptide (marked with the red box) and the sequence of the FAM19A4 protein. BFA, brefeldin A.
The results of the N-terminal sequencing verified that the mature protein started from the amino acid Histidine at position 46 (Figure 1c). Therefore, the signal peptide contained the first 45 N-terminal amino acids, and the secreted FAM19A4 was composed of 95 amino acids (Figure 1c), which was not consistent with our prediction (http://www.cbs.dtu.dk/services/SignalP/).
FAM19A4 is upregulated upon inflammatory stimuli in monocytes and macrophages.
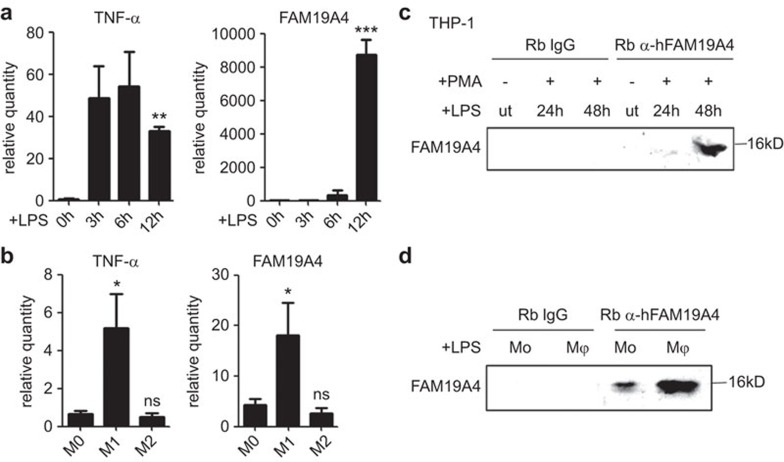
The expression pattern of a cytokine is useful for determining its physiological or pathological function. FAM19A4 was reported at constitutively low levels in most normal tissues, with relatively higher expression levels in the brain;15 this was consistent with our results (Supplementary Figure 1). However, the GEO Profiles database suggests that it is upregulated in monocytes and macrophages with LPS stimulation (GDS2856 record, GPL570, 242348_at; GDS2430 record, GPL97, 242348_at). To verify this concept, the PMA-pre-treated THP-1 cells were stimulated with LPS and the kinetic expression of FAM19A4 during the activation was examined. We found that it was barely detectable at the mRNA level in the resting THP-1 cells and increased upon LPS stimulation in a time-dependent pattern that was most obvious at 12 h at the mRNA level (Figure 2a).
Figure 2.
The expression of FAM19A4 was inducible in the monocytes and macrophages. (a, c) The THP-1 cells were pre-treated with PMA (10 ng/ml) for 24 h and then stimulated with LPS (1 µg/ml). (a) FAM19A4 increased in the stimulated THP-1 cells at the mRNA level. The relative quantity is the ratio of 2−ΔΔCt compared to the control. (c) FAM19A4 increased in the cell culture medium of the stimulated THP-1 cells at the protein level. (b) FAM19A4 increased in the macrophages during their differentiation to M1 at the mRNA level. (d) FAM19A4 increased in the cell culture medium of the macrophages after LPS stimuli. The medium was first ultrafiltered and then immunoprecipitated using anti-FAM19A4 or normal rabbit IgG as the control (ut: untreated). The representative result of three independent experiments is shown.
LPS stimulation represents a pro-inflammatory trigger that can induce differentiation of the macrophages toward the M1 subset.18 Considering that FAM19A4 is upregulated in LPS stimulated THP-1 cells, we wondered whether the M1 subset of macrophages had a higher expression level of FAM19A4 than the M0 or M2 subsets. Therefore, we isolated human peripheral blood monocytes and induced them into macrophages with M-CSF stimulation in vitro. With LPS combined with IFN-γ or IL-4 stimulation for 24 h, the macrophages differentiated into the M1 or M2 subsets, respectively. FAM19A4 was induced by the M1 polarized activation but not by M2 at the mRNA level (Figure 2b). These observations were consistent with the GEO data. TNF-α was used as the positive control to confirm the activation of the THP-1 cells and to identify the subset of M1 but not M2.
Furthermore, we found that FAM19A4 increased in the THP-1 cells upon LPS stimulation at 48 h in the culture supernatant at the protein level (Figure 2c and Supplementary Figure 2). The monocytes could also secrete FAM19A4 after 48 h of LPS stimulation but at a reduced amount compared to the M1 macrophages (Figure 2d).
FAM19A4 shows chemotactic activity on macrophages.
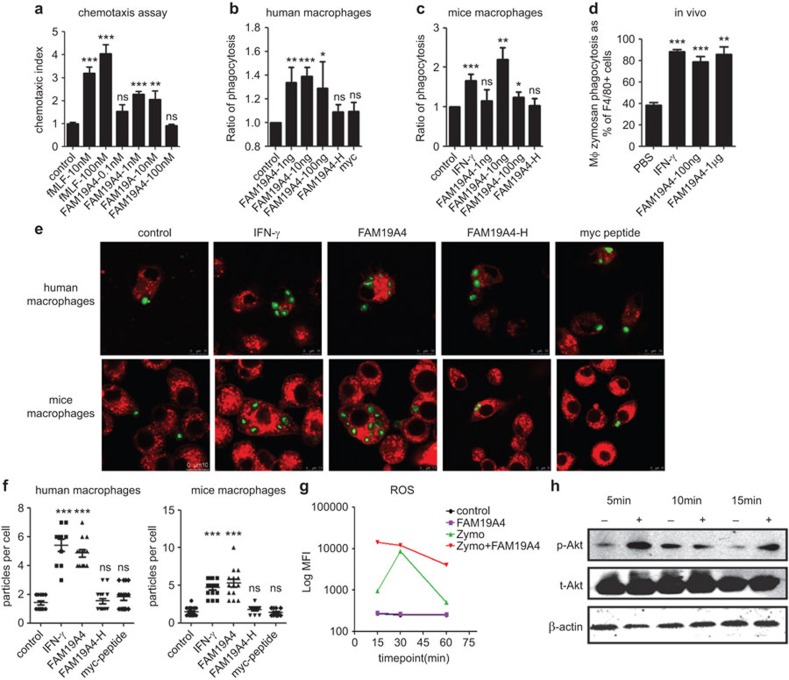
Because FAM19A4 was secreted by the monocytes and macrophages upon stimulation, we analyzed whether it had functions toward the macrophages. Based on the CC and CXC motifs of FAM19A4A, we first hypothesized about its chemotactic activities on macrophages. In the dose-response chemotaxis assays, FAM19A4 induced a typical bell-shaped curve that delineated its chemotactic activity on macrophages; the optimal dose was approximately 1 nM (Figure 3a).
Figure 3.
FAM19A4 chemo-attracts macrophages and promotes the phagocytosis of macrophages both in vitro and in vivo. (a) The chemotactic activity of FAM19A4 on human macrophages. FAM19A4 promotes the phagocytosis of human macrophages derived from monocytes (b, e) and RAW264.7 cells (c, e); this was analyzed using flow cytometry or confocal microscopy. (d) FAM19A4 increases the phagocytosis of mouse peritoneal resident macrophages in vivo; this was also analyzed by flow cytometry. There were at least five mice in each group. (f) The calculation of the number of FITC-zymosan particles that were swallowed by a single macrophage; this was imaged by confocal microscopy (n>10). (g) FAM19A4 promotes ROS release upon zymosan stimulation in mice peritoneal macrophages. The relative fluorescence intensity is taken as the average of the values from four repeated experiments. (h) The RAW264.7 cells were pre-treated for 2 h in the presence or absence of FAM19A4 and were then challenged with zymosan (100 µg/ml) at different time points. The expression of total protein and the phosphorylated forms of Akt were determined by western blotting. The result of one experiment, representative of four total experiments, is presented.
FAM19A4 promotes phagocytosis of macrophages involving Akt activation both in vitro and in vivo
Macrophages are phagocytic cells that play important roles in the activation of the innate immune responses. They can engulf and destroy pathogens through phagocytosis.3,19 They can also produce cytokines in response to external stimuli, and some of the cytokines affect their function in turn. By testing human macrophages derived from peripheral blood monocytes by flow cytometry, we found that FAM19A4 could enhance the uptake of zymosan by the macrophages in a dose-dependent manner. This worked at a range of low concentrations on the bell-shaped curve; the optimal dose was approximately 10 ng/ml (Figure 3b).
FAM19A4 has been conserved during evolution, and human FAM19A4 and its murine homologue have 93% identities in their whole amino-acid sequences. Therefore, we examined the function of the recombinant human FAM19A4 protein on murine cells in vitro and in vivo. Consistent with the effects on human macrophages, FAM19A4 promoted the phagocytosis of the murine macrophage cell line RAW264.7 in vitro (Figure 3c) and of the mice resident peritoneal macrophages in vivo (Figure 3d). Using confocal microscopy, we found that FAM19A4 could markedly increase the number of zymosan particles swallowed intracellularly by a single cell at a concentration of 10 ng/ml (Figure 3e), which exerted similar effects as IFN-γ (Figure 3f). The spinning confocal microscopy further demonstrated that FAM19A4 promoted the motoricity of the macrophages toward the zymosan particles and the ability to swallow them (Supplementary Movies 1 and 2). As negative controls of the system, the heated FAM19A4 (FAM19A4-H) or the myc-his tag peptide (myc peptide) had no effect, which ruled out the unspecific effect from endotoxins left in the recombinant protein (FAM19A4-H) and the myc-his tag (myc peptide). These results confirmed the promoting effect of FAM19A4 on phagocytosis of both human and mice macrophages.
As a part of the respiratory burst triggered by zymosan phagocytosis,20 ROS production in mice macrophages was subsequently detected with H2DCF-DA as probes. FAM19A4 could significantly increase the release of intracellular ROS that were aroused by zymosan, but it could not lead to an increase of ROS by itself (Figure 3g).
In macrophages, phagocytosis is regulated by phospholipid-modifying enzymes such as PI3K.20 Upon activation of PI3K, Akt is recruited to the plasma membrane and is further activated through phosphorylation on Ser473.21 We detected the total Akt levels and its phosphorylated form on Ser473 in the mice macrophages 5–15 min after the zymosan challenge. Pre-treatment of the cells with FAM19A4 for 2 h markedly increased the phosphorylation of Akt (Figure 3h). Therefore, the PI3K pathway could be one of the targets of FAM19A4 that leads to the enhancement of phagocytosis.
These results clarified the function of FAM19A4 in the phagocytosis of macrophages, and they suggest that there might a functional receptor of FAM19A4 on the stimulated macrophages that is involved in phagocytosis and ROS release.
FPR1 is a functional receptor of FAM19A4
According to the function of FAM19A4 toward macrophages, we hypothesized that its receptor might be a chemotactic receptor that is highly expressed on macrophages and mediates phagocytosis.
When searching for such a chemotactic receptor that can promote phagocytosis as well, we focused on FPR1, which has been reported to be a non-classical chemokine receptor highly expressed on stimulated macrophages that also regulates phagocytosis.22,23 To test our hypothesis, we overexpressed FPR1 on the HEK293 cells and observed the effects of FAM19A4 on the cells. The chemotaxis assays showed that FAM19A4 was able to recruit FPR1-expressing HEK293 cells in a dose-dependent manner (Figure 4a). Furthermore, it could mutually antagonize the chemotactic activity of fMLF, the classical ligand of FPR1 (Figure 4b).
Figure 4.
FPR1 is a functional receptor of FAM19A4. (a) FAM19A4 was tested for its chemotactic abilities on the HEK293-FPR1 cells, and significant differences compared with the medium were calculated. (b) In the cross-desensitization chemotaxis assay, fMLF and FAM19A4 were tested for their ability to chemoattract HEK293-FPR1 cells with or without pre-treatment with fMLF or FAM19A4 for 1 h and the chemotaxis index was calculated. (c) fMLF (0.1 µM) or FAM19A4 (0.1 µM) were used to treat the HEK293 cells expressing FPR1-EGFP for 1 h; these cells were then analyzed by confocal microscopy. The scale bars represent 25 µm. (d) After HEK293-FPR1 was stimulated with fMLF, FAM19A4 or the negative controls in suspension for 1 h, the quantity of FPR1 on the cell surface was evaluated using flow cytometry. The internalization rate of FPR1 and significant differences compared to the control were calculated. (e) In the saturation experiment, equivalent quantities of 125I-FAM19A4 were incubated with varying quantities of the FPR1-transfected cell membrane extract in binding buffer. The y-axis represents the radioactivity of the binding, and the x-axis represents the concentration of FPR1. (f) In the competitive binding assays, equivalent quantities of the cell membrane extract and 125I-FAM19A4 were incubated with varying quantities of unlabeled ligands. The y-axis represents the percentage of the max radioactivity of the specific binding complexes, and the x-axis represents the logarithmic form of the concentration of the competitors. (h, i) Boc-MLF (1 µM) was used to pre-treat the human (g) or mice (h) macrophages for 10 min followed by incubation with FAM19A4 for 30 min; the phagocytosis assay (g) or the ROS detection (h) were then performed as described before. fMLF, formyl-methionyl-leucyl phenylalanine; FPR1, formyl peptide receptor 1.
However, FAM19A4 could induce FPR1 internalization if it was the agonist of the receptor. The surface expression of FPR1 on the transfected HEK293 cells was then examined by flow cytometry before and after treatment with FAM19A4. As shown in Figure 4c, FAM19A4 decreased the surface level of FPR1 (approximately 20%) as fMLF did, suggesting the internalization of the receptor. Confocal microscopy further visualized the internalized receptor by the FAM19A4 treatment using HEK293 cells that transiently overexpressed FPR1-EGFP (Figure 4d).
To further confirm that FPR1 is a specific receptor for FAM19A4, we performed radioactive binding assays. The recombinant FAM19A4 protein was iodinated with 125I and used as a tracer. The saturation binding assays indicated that FAM19A4 could saturate FPR1-transfected HEK293 cells with a Kd of 0.4447 nM (Figure 4e). The competition binding assays were performed with unlabeled FAM19A4, fMLF and the myc-his tag peptide. The unlabeled FAM19A4 displaced 125I-FAM19A4 from the FPR1-transfected cells with a Ki of 58.81 pM, indicating the specificity of the binding; fMLF could also compete for the binding of 125I-FAM19A4 on the target cells, verifying the specific binding to FPR1. As the control, the myc-his tag peptide was not competitive at all, ruling out the potential influence of this tag in the recombinant protein (Figure 4f).
Whether the functions of FAM19A4 on the macrophages were mediated by FPR1 was subsequently clarified using Boc-MLF, a specific antagonist of FPR1.24 The macrophages were pre-treated with Boc-MLF for 10 min, followed by incubation with FAM19A4 for 30 min and stimulation with zymosan for 15–60 min. We found that the Boc-MLF pre-treatment weakened the increased phagocytosis and the ROS releasing abilities caused by FAM19A4 (Figure 4g and h). This result further demonstrates that FPR1 is a functional receptor for FAM19A4.
Discussion
FAM19A4, also called TAFA4, was first reported, along with its family members TAFA1, 2, 3 and 5, to be secreted when overexpressed in CHO cells.15 In this study, we have shown that it is secreted via the Golgi-dependent classical pathway and that its signal peptide contains the first 45 amino acids from the N-terminal, which is inconsistent with our prediction. Additionally, we have for the first time identified that its mature protein is composed of 95 amino acids.
As expected, FAM19A4 enhances the motoricity of macrophages. It chemo-attracts macrophages and promotes the macrophages to move toward zymosan particles and swallow more of these particles intracellularly at a low concentration (Supplementary Movies 1 and 2). As part of the essential pathway in the phagocytosis of macrophages, Akt is recruited to the plasma membrane upon the activation of PI3K through the binding of its plekstrin homology domain to PIP3, which is catalyzed by PI3K. Further activation of Akt requires phosphorylation on Ser473 by the mTORC2 complex.21 FAM19A4 acts on a particular stage of the PI3K pathway to increase the phosphorylation of Akt on Ser473 (Figure 3h) and finally promote phagocytosis by the macrophages.
FPR1 is a G-protein coupled receptor that was originally identified to be highly expressed on macrophages. It plays roles in chemotaxis, the killing of microorganisms through phagocytosis, and the generation of ROS. In addition, FPR1 is thought to play a role in sensing the endogenous signals of dysfunctional cells, which should attract leukocytes to the sites of inflammation and tissue damage.11,22,23,25,26 As the ligand with the highest affinity for FPR1, fMLF has a Kd of 0.04 nM.27 According to the ratio of Ki for fMLF (Ki=0.08139 pM) and FAM19A4 (Ki=58.81 pM) from the competition binding analysis, the Kd of FAM19A4 is 722 times that of fMLF. Thus, the calculated Kd of FAM19A4 is approximately 30 nM, suggesting a higher affinity than some known ligands for FPR1, such as the endogenous peptides Ac2-26 cleaved from Annexin-1 (a Kd value of ∼0.9 µM).11,28 Therefore, FAM19A4 is an endogenous ligand with a high affinity.
FPR1 can be activated by peptides from bacteria and viruses as well as by the endogenous damage-associated molecular patterns from bone and liver mitochondria to induce the severe inflammatory response syndrome.29,30,31 As the endogenous ligand of FPR1, the N-terminal peptide Ac2-26 is cleaved from Annexin-1 during inflammation and epithelial wound healing.32 All the known ligands are passively released to activate the host immune responses when infections occur. FAM19A4 would be more important as a cytokine ligand for FPR1. It evokes active host defenses in inflammatory responses. As a cytokine, FAM19A4 promotes communication among the immune cells via FPR1 and provides us with a new mechanistic basis for anti-inflammatory immunity.
Growing evidence supports the fact that FPR1 plays critical roles in infectious diseases.33,34 Here, we reported that FAM19A4 is a novel cytokine ligand of FPR1. It increases upon inflammatory stimulation in macrophages. Many intracellular cytokines, such as IL-1α and IL-33, are released upon cellular disintegration and serve as first-line alarmins to mediate signaling functions via their receptors on neighboring cells.35,36 FAM19A4 is a potential alarmin that is initially induced during infections. Additionally, FAM19A4 was upregulated in the neuroendocrine CRI-G1 cell line upon mitochondrial and oxidative stress (GDS4014, GPL1355, 1378557_at) and in human brain endothelial cells in an in vitro model of cerebral malaria;37 this further verifies that the expression of FAM19A4 is inducible in different tissues and cells. These results indicate that FAM19A4 is involved in stress and infectious responses.
FAM19A4 possesses all the characteristics of a cytokine because (i) it is a classical secretory protein; (ii) its mature protein has a small molecule size of 95 amino acids; (iii) it exerts its functions by binding to its specific receptor, and (iv) it plays a role at a range of low concentrations on a bell shaped curve. In conclusion, our work identifies FAM19A4 as a novel cytokine ligand of FPR1 in macrophages for the first time. However, Delfini et al.38 have defined FAM19A4 to be a specific marker of the C-low-threshold mechanoreceptor, which could be a potent analgesic in pain relief. FPR1 is not restricted to leukocytes involved in inflammatory responses toward infection, but is also found on the cells of the neuromuscular and endocrine system.39 Whether FAM19A4 plays a role in the neural system through FPR1 or other receptors requires further study. As a novel cytokine, FAM19A4 would be a novel mediator in neuro-immunoregulation in inflammatory responses.
Acknowledgments
We thank Professor Philip M Murphy from the Laboratory of Molecular Immunology, National Institutes of Health for providing the plasmid pCMV-FPR1. We also thank Li Yan, PhD, of the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London for his contributions to the statistical analyses in this work. This work was supported by grants from the National Natural Science Foundation of China (81273233) and the Peking University–National Taiwan University Cooperation Fund (BMU20120316).
The authors declare that they have no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol 2009; 10: 333–339. [DOI] [PubMed] [Google Scholar]
- 2Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40: 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB et al. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 2014; 15: 494–505. [DOI] [PubMed] [Google Scholar]
- 5Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med 1998; 187: 2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, Rottenberg ME. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J Immunol 2001; 167: 6453–6461. [DOI] [PubMed] [Google Scholar]
- 7Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol 2002; 23: 541–548. [DOI] [PubMed] [Google Scholar]
- 8Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A et al. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep 2012; 2: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest 2013; 123: 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev 2013; 65: 967–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 2009; 61: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13: 780–788. [DOI] [PubMed] [Google Scholar]
- 13Liu M, Zhao J, Chen K, Bian X, Wang C, Shi Y et al. G protein-coupled receptor FPR1 as a pharmacologic target in inflammation and human glioblastoma. Int Immunopharmacol 2012; 14: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol 2013; 59: 583–594. [DOI] [PubMed] [Google Scholar]
- 15Tom TY, Emtage P, Funk WD, Hu T, Arterburn M, Park EE et al. TAFA: a novel secreted family with conserved cysteine residues and restricted expression in the brain. Genomics 2004; 83: 727–734. [DOI] [PubMed] [Google Scholar]
- 16Guo X, Zhang Y, Wang P, Li T, Fu W, Mo X et al. VSTM1-v2, a novel soluble glycoprotein, promotes the differentiation and activation of Th17 cells. Cell Immunol 2012; 278: 136–142. [DOI] [PubMed] [Google Scholar]
- 17Pei X, Sun Q, Zhang Y, Wang P, Peng X, Guo C et al. PC3-secreted microprotein is a novel chemoattractant protein and functions as a high-affinity ligand for CC chemokine receptor 2. J Immunol 2014; 192: 1878–1886. [DOI] [PubMed] [Google Scholar]
- 18Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013; 120: 163–184. [DOI] [PubMed] [Google Scholar]
- 19Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011; 12: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol 2012; 7: 61–98. [DOI] [PubMed] [Google Scholar]
- 21Okuzumi T, Fiedler D, Zhang C, Gray DC, Aizenstein B, Hoffman R et al. Inhibitor hijacking of Akt activation. Nat Chem Biol 2009; 5: 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Gemperle C, Schmid M, Herova M, Marti-Jaun J, Wuest SJ, Loretz C et al. Regulation of the formyl peptide receptor 1 (FPR1) gene in primary human macrophages. PLoS One 2012; 7: e50195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Yousefi S, Cooper PR, Potter SL, Mueck B, Jarai G. Cloning and expression analysis of a novel G-protein-coupled receptor selectively expressed on granulocytes. J Leukoc Biol 2001; 69: 1045–1052. [PubMed] [Google Scholar]
- 24Stenfeldt AL, Karlsson J, Wenneras C, Bylund J, Fu H, Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation 2007; 30: 224–229. [DOI] [PubMed] [Google Scholar]
- 25Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol 2004; 172: 1896–906. [DOI] [PubMed] [Google Scholar]
- 26John CD, Sahni V, Mehet D, Morris JF, Christian HC, Perretti M et al. Formyl peptide receptors and the regulation of ACTH secretion: targets for annexin A1, lipoxins, and bacterial peptides. FASEB J 2007; 21: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Fay SP, Posner RG, Swann WN, Sklar LA. Real-time analysis of the assembly of ligand, receptor, and G protein by quantitative fluorescence flow cytometry. Biochemistry 1991; 30: 5066–5075. [DOI] [PubMed] [Google Scholar]
- 28Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med 2002; 8: 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma 2010; 24: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma 2010; 68: 1328–1332, 1332–1334. [DOI] [PubMed] [Google Scholar]
- 31Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Ernst S, Lange C, Wilbers A, Goebeler V, Gerke V, Rescher U. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol 2004; 172: 7669–7676. [DOI] [PubMed] [Google Scholar]
- 33Schepetkin IA, Khlebnikov AI, Giovannoni MP, Kirpotina LN, Cilibrizzi A, Quinn MT. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr Med Chem 2014; 21: 1478–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 2013; 14: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin'? PLoS One 2008; 3: e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA 2010; 107: 2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Barbier M, Faille D, Loriod B, Textoris J, Camus C, Puthier D et al. Platelets alter gene expression profile in human brain endothelial cells in an in vitro model of cerebral malaria. PLoS One 2011; 6: e19651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P et al. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep 2013; 5: 378–388. [DOI] [PubMed] [Google Scholar]
- 39Becker EL, Forouhar FA, Grunnet ML, Boulay F, Tardif M, Bormann BJ et al. Broad immunocytochemical localization of the formylpeptide receptor in human organs, tissues, and cells. Cell Tissue Res 1998; 292: 129–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.