Abstract
Influenza A virus (IAV) infection is a major worldwide public health problem. However, the factors involved in mediating the inflammatory response to this infection and their relationships remain poorly understood. Here, we show that IAV infection stimulates the expression of the soluble IL-6 receptor (sIL-6R), a multifunctional protein involved in IL-6 signaling. Interestingly, sIL-6R expression upregulated the levels of its own ligand, IL-6 and those of the pro-inflammatory cytokine IL-32. shRNA-mediated knockdown of sIL-6R suppressed IL-6 and IL-32, indicating that this regulation is dependent on sIL-6R during IAV infection. Furthermore, our results demonstrate that IL-32 participates in a negative feedback loop that inhibits sIL-6R while upregulating IL-6 expression during IAV infection. Therefore, we show that sIL-6R is a critical cellular factor involved in the acute inflammatory response to viral infection.
Keywords: IL-32, IL-6, inflammation network, influenza A virus, soluble IL-6 receptor
Introduction
Influenza A virus (IAV) is a highly contagious single-stranded RNA virus that infects both the upper and lower respiratory tracts of humans and is considered a major worldwide public health problem. Although seasonal infections with the most common influenza virus strains (e.g., H3N2) can typically be resolved, infection continues to cause a high rate of mortality. Research has established that viral replicative intermediate double-stranded RNA1 and Toll-like receptor 32 are critical for the immune response triggered by IAV infection. Moreover, pro-inflammatory cytokines, such as IL-6, IL-8 and tumor-necrosis factor (TNF)-α, are involved in the host inflammatory response to IAV infection.3,4 Patients with severe acute infections most likely develop the ‘cytokine storm'5 response that leads to acute lung injury or its more severe form of acute respiratory distress syndrome. It is also known that the intensity of the inflammatory response in the lungs reflects a balance between pro-inflammatory cytokines and their cognate soluble receptors or inhibitors. However, the mechanism of regulation among cytokines remains unclear.
The pleiotropic cytokine IL-6 is produced by macrophages, dendritic cells, mast cells and other innate immune cells; consequently, this cytokine has long been considered a marker of inflammation. In addition to immune cells, IL-6 is produced by epithelial cells, endothelial cells, keratinocytes and fibroblasts in response to specific stimuli.6,7 IL-6 can induce cell signaling not only via the classic pathway involving the transmembrane receptor IL-6Rα (restricted cellular expression) associated with gp130 (ubiquitous and responsible for signal transmission), but also via the soluble receptor IL-6Rα, which binds to IL-6 and induces a signal mediated by the ubiquitous gp130 molecule (trans-signaling).8 Central to the regulation of IL-6-mediated responses is the presence of the soluble IL-6 receptor (sIL-6R), which undergoes trans-signaling to form a ligand–receptor complex that permits IL-6 responsiveness in cell types that lack the cognate IL-6 receptor.9,10 IL-6 signaling plays a pivotal role in controlling the differentiation and activation of T lymphocytes by inducing the Jak/STAT3 and Ras/Erk/C/EBP pathways.11,12,13,14 Furthermore, IL-6, in concert with TNF-α and IL-1, causes fever and leukocytosis, which are required for pathogen elimination.10,15
The circulating form of sIL-6R, which can be detected in various bodily fluids and is secreted by monocytes, hepatocytes and endothelial cells,16 is generated by two independent mechanisms: limited proteolysis of the membrane-bound protein and translation from an alternatively spliced mRNA.17 Hydroxamic acid-based metalloprotease inhibitors, such as TNF-α protease inhibitor (TAPI), are known to prevent the shedding of various cell surface proteins,18,19,20 including the IL-6R.21,22 sIL-6R is critically involved in the generation and maintenance of several inflammatory and autoimmune diseases, including chronic inflammatory bowel disease, rheumatoid arthritis, peritonitis, asthma and inflammation-induced colon cancer.23,24 Studies have further demonstrated that sIL-6R acts as an agonistic with IL-6, indicating an enhanced cellular sensitivity to IL-6.25
Another cytokine, IL-32, is a newly described pro-inflammatory cytokine that enhances host immunity against various microbial pathogens26 and has been shown to be induced by influenza virus infection in humans.27 It is also an important cytokine in diseases characterized by inflammation,28 such as rheumatoid arthritis29 and inflammatory bowel disease.30 Furthermore, IL-32 has been described as an activator of the p38-mitogen-activated protein kinase, NF-κB and AP-1 signaling pathways, and it induces several cytokines, including IL-1β, IL-6, IL-8 and TNF-α.31,32,33 Our previous study demonstrated that the NF-κB and CREB pathways play key roles in promoting IL-32 production in response to influenza virus infection.34 Additional experiments have been carried out to verify the impact of IL-32 on viral infection and replication. In one study, the specific knockdown of IL-32 gene expression in human peripheral blood mononuclear cells (PBMCs) led to fourfold increase in HIV-1 p24 production, indicating that endogenous IL-32 acted as a natural inhibitor of HIV-1.31 New evidence has shown that IL-32 was capable of activating IFN-inducible antiviral effectors, such as protein kinase R and myxovirus resistance protein.35
Numerous published studies have focused on the association between acute inflammation and viral infection. Although IL-6 and IL-32 have been identified as vital factors in airway inflammation during influenza virus infection, the molecular mechanism by which these pro-inflammatory factors regulate each other during infection remains to be elucidated. Moreover, the mechanism whereby IAV upregulates IL-6 and IL-32 expression remains unknown. In an effort to provide new insight into the host immune response to virus infection, our previous study using protein and antibody microarray chips found that the expression level of sIL-6R was significantly altered in response to IAV infection.36 Therefore, in the present study, we investigated the role and mechanism of IL-6 and sIL-6R signaling in the inflammatory response to IAV infection. The present data reveal a previously unidentified regulatory mechanism involving IL-6R, IL-32 and IL-6 during IAV infection.
Materials and methods
Clinical specimen collection
Clinical throat swab samples from 18 adult patients who were positive for nasopharyngeal IAV infection were collected from Renmin Hospital of Hubei Province. Throat swab samples from 18 healthy individuals were randomly selected as controls from donors who were negative for the virus. The collection of blood samples for this research was conducted according to the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the College of Life Sciences, Wuhan University, in accordance with its guidelines for the protection of human subjects. All participants provided written informed consent to participate in the study.
Isolation of PBMCs
Fresh blood samples were drawn from healthy donors. PBMCs were obtained by density centrifugation of blood samples diluted 1∶1 in pyrogen-free saline over Histopaque (Hao Yang Biotech, Tianjin, China). The separation medium, Histopaque, is a sterile-filtered, endotoxin tested solution of polysucrose and sodium diatrizoate, adjusted to a density of 1.077 g/ml. The blood-Histopaque solution was centrifuged at 800g for 20 min at room temperature and the cell band on top of the Histopaque layer was collected. The cells were washed twice with saline and suspended in RPMI 1640 medium supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml).
Virus and cell culture
Influenza virus strain A/Hong Kong/498/97 (H3N2) was used as described previously.36 Stock virus was propagated in 10-day-old embryonated chicken eggs for 36–48 h at 37 °C; the allantoic fluid was harvested, and aliquots were stored at −80 °C until use. H3N2 virus was infected into cells at a multiplicity of infection (MOI) of 1. The human lung epithelial cell line A549 was cultured in DMEM medium supplemented with 10% heat-inactivated FBS, and the human embryonic lung diploid fibroblast cell line MRC-5 was cultured in MEM medium supplemented with 10% heat-inactivated FBS.
Plasmids
The luciferase reporter vector (pGL3) (Promega, Madison, WI, USA) containing the IL-n (n=2, 4, 5, 6, 9, 10, 15, 16, 17, 18, 21, 22, 24, 29 and 32) promoters, pGL3-IL-n–Luc (Table 2), and the Renilla internal control vector pRL–TK (Promega) were briefly described in our previous studies.27,34,37 Human IL-32γ cDNA (accession no. XM_005255686) was amplified by RT-PCR using 5′-CAGCGAATTCAATGTGCTTCCCGAAGG-3′ (sense primer, EcoRI sites are underlined) and 5′-GTCGAAGCTTTCATTTTGAGGATTGGG-3′ (antisense primer, HindIII sites are underlined) cloned into the pCMV–tag2A vector (Stratagene) to generate the IL-32γ-expressing plasmid (pCMV-IL-32), as described previously.34 Human sIL-6R cDNA (accession no. NM_181359) was amplified by RT-PCR using 5′-CGGAATTCATGCTGGCCGTCGGCTGCGCG-3′ (sense primer, EcoRI sites are underlined) and 5′-GGCGCTCGAGTCAGAGCCCGCAGCTTCCACG-3′ (antisense primer, XhoI sites are underlined) cloned into the pCMV–tag2B vector (Stratagene) to generate an sIL-6R-expressing plasmid (pCMV-sIL-6R).
Table 2. List of reporter plasmid of interleukin promoters (pGL3-IL-n–Luc) used in promoter activity screen.
| Plasmid | Restriction Enzyme cutting site | Promoter fragments |
|---|---|---|
| pGL3-IL-2–Luc | Sac I/Xho I | −1344/+41 |
| pGL3-IL-4–Luc | Sac I/Xho I | −1407/+50 |
| pGL3-IL-5–Luc | KpnI/Xho I | −1597/+29 |
| pGL3-IL-6–Luc | KpnI/Hind III | −1667/+45 |
| pGL3-IL-9–Luc | KpnI/Xho I | −1882/−11 |
| pGL3-IL-10–Luc | KpnI/Xho I | −1105/+47 |
| pGL3-IL-15–Luc | Sac I/Xho I | −1534/−27 |
| pGL3-IL-16–Luc | Sac I/Xho I | −1291/+46 |
| pGL3-IL-17–Luc | MluI/Hind III | −1656/+12 |
| pGL3-IL-18–Luc | Sac I/Xho I | −1103/+32 |
| pGL3-IL-21–Luc | KpnI/Xho I | −1124/−35 |
| pGL3-IL-22–Luc | KpnI/Xho I | −1023/+56 |
| pGL3-IL-24–Luc | KpnI/Xho I | −1507/+4 |
| pGL3-IL-29–Luc | KpnI/Mlu I | −1806/+38 |
| pGL3-IL-32–Luc | MluI/Hind III | −746/+25 |
Specific shRNAs against sIL-6R and IL-32 and irrelevant control shRNA were purchased from GenePharma (Shanghai, China) and prepared by ligating the corresponding pairs of oligonucleotides to pGPU6/GFP/Neo. The shRNA-sIL-6R target sequence was 5′-CCTCCCAGGTTCAAGAAGA-3′, the shRNA-IL-32 target sequence was 5′-GGAGACAGTGGCGGCTTATTA-3′ and the shRNA-control target sequence was 5′-GTTCTCCGAACGTGTCACGT-3′.
Reagents
Recombinant human IL-6Rα protein and IL-32γ protein were purchased from R&D Systems (Minneapolis, MN, USA); the former was expressed in CHO cells according to the product datasheet. An antibody against sIL-6R was purchased from eBioscience (San Diego, CA, USA), and antibodies specific for β-actin and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Two antibodies against IL-32, polyclonal antibody against human IL-32 (R&D Systems) and a rabbit polyclonal antibody against IL-32, produced in our previous study using recombinant IL-32α protein as an immunogen,38 were used for the western blot analyses. TAPI was purchased from Santa Cruz Biotechnology.
Transient transfection and luciferase assays
A549 cells were plated at a density of 4.0×105 cells per well in 24-well or 6-well plates and grown to approximately 80% confluence at the time of transfection. The plasmids were cotransfected into the cells using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). At 24 h post-transfection, the cells were serum-starved for another 24 h prior to harvest.
The cells were lysed with the Luciferase Cell Culture Lysis reagent (Promega), and the cell lysates and luciferase assay substrate (Promega) were mixed before the light intensity was detected using a luminometer (GloMax 20/20; Promega). The assays were performed in triplicate, and the results are expressed as the mean value relative to the vector control, which was arbitrarily assigned as 100%.
ELISA
A549 cells were transfected with expression plasmids or shRNA, and the culture supernatants were harvested at 24, 48 and 72 h post-transfection. To quantitate the secreted sIL-6R, TNF-α and IL-6 in the culture supernatants, the Human sIL-6R Instant ELISA Kit (eBioscience), Human TNF-α ELISA Kit (R&D Systems) and Human IL-6 ELISA Kit (R&D Systems) were used according to the manufacturers' instructions.
Real-time RT-PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Real-time quantitative RT-PCR analysis was performed using the Roche LC480 and SYBR RT-PCR kits (DBI Bioscience, Ludwigshafen, Rhineland-Palatinate, Germany) in a 20-µl reaction mixture containing 0.5 mM each PCR primer, 10 µl SYBR Green PCR Master Mix, 1 µl diluted DNA template and RNase-free water. The primers used were as follows: sIL-6R sense, 5′-GCGACAAGCCTCCCAGGTTC-3′ sIL-6R antisense, 5′-GTGCCACCCAGCCAGCTATC-3′ IL-32 sense, 5′-ATGTCGAGCCTGGCAGAGCTGGA-3′ IL-32 antisense, 5′-CTTGTCACAAAAGCTCTCCCCAG-3′ (four splicing variants of IL-32: α, β, γ and δ were detected by the primers); IL-6 sense, 5′-ACTCACCTCTTCAGAACGAATTG-3′ IL-6 antisense, 5′-AGCCATCTTTGGAAGGTTCAG-3′ GAPDH sense, 5′-AAGGCTGTGGGCAAGG-3′ and GAPDH antisense, 5′-TGGAGGAGTGGGTGTCG-3′. The data were normalized according to the level of GAPDH expression in each sample, as described previously.
Western blot analysis
Whole-cell lysates were prepared by lysing cells with PBS (pH 7.4) containing 0.01% Triton X-100, 0.01% EDTA and 10% protease inhibitor mixture (Roche, Basel, Switzerland). The protein concentration was determined using the Bradford assay (Biorad Laboratories, Hercules, CA, USA). Cell lysates were resolved by 12% SDS–PAGE gel electrophoresis and transferred to nitrocellulose membranes (Amersham, GE Health Care, London, UK). Nonspecific binding was blocked with 5% non-fat dried milk before incubation with the primary and secondary antibodies. The protein amount loaded onto the gels was 100 µg and the protein bands were detected using SuperSignal Chemiluminescent Substrate (Pierce, Rockford, IL, USA).
Statistical analysis
All experiments were performed in triplicate or quadruplicate. Each set of experiments was repeated at least three times, with similar results, and representative data are presented as the mean±the standard error. Student's t-test for paired samples was used to determine the statistical significance. Differences at P<0.05 were considered statistically significant.
Results
IAV activates sIL-6R expression both in patients and in vitro
As mentioned previously, our earlier research using microarray chips first discovered that the level of sIL-6R protein was dramatically altered in serum from H3N2-infected patients. Thus, to verify our pioneering results and investigate whether IAV infection plays a role in regulating sIL-6R expression, we compared the sIL-6R mRNA levels in throat swab samples from 18 patients infected with IAV and 18 healthy individuals. These data revealed that the sIL-6R mRNA levels were approximately fourfold higher in IAV patients compared to healthy individuals (Table 1), suggesting that IAV infection is associated with upregulated sIL-6R expression. We also assessed the IL-6 and IL-32 mRNA levels in throat swab samples and results showed that the mRNA levels of these two cytokines were higher in IAV patients compared to healthy individuals, similarly.
Table 1. Demographics, baseline characteristics and mRNA levels of sIL-6R, IL-6 and IL-32 in throat swab samples from healthy individuals and IAV-infected patients.
| Characteristica | Value for: | |
|---|---|---|
| Healthy individuals (n=18) | Patients (n=18) | |
| Mean age (yr)±s.d. | 35.6±11.3 | 39.2±13.5 |
| Gender (no. of male/female) | 10/8 | 9/9 |
| No. (%) of individuals | ||
| Asian race or ethnicity | 18 (100) | 18 (100) |
| Sample collection between 1 December 2013 and 1 January 2014 | 18 (100) | 18 (100) |
| Residence in Hubei Province, China | 18 (100) | 18 (100) |
| HA antigen-positive | 0 (0) | 18 (100) |
| Anti-HA antibody-positive | 0 (0) | 18 (100) |
| No. with viral genotype A (H3/H1) | 0 | 18 (15/3) |
| Relative mRNA levels±s.d. | ||
| sIL-6R mRNA relative to GAPDH | 1.8±1.4 | 7.3±4.6 |
| IL-6 mRNA relative to GAPDH | 0.4±0.3 | 3.2±2.5 |
| IL-32 mRNA relative to GAPDH | 9.0±6.3 | 18.9±10.1 |
HA, hemagglutinin.
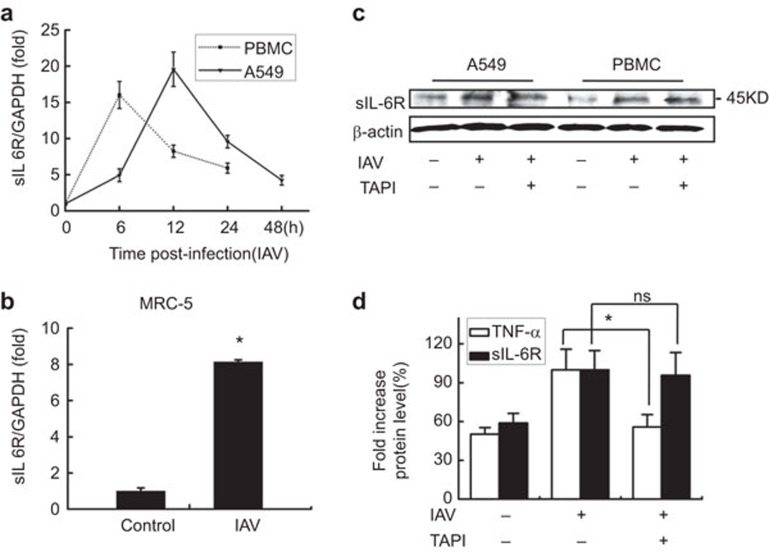
To assess the activation of sIL-6R by IAV in in vitro cell culture systems, A549 cells, PBMCs and MRC-5 cells were infected with the IAV H3N2 strain, and the sIL-6R levels were analyzed. In A549 cells and PBMCs, the culture supernatants and the cell pellets of infected cells were harvested at 6, 12, 24 and 48 h post-infection (hpi). Results showed that the mRNA levels of sIL-6R increased as infection time increased and reached the peak at 12 hpi in A549 cells (Figure 1a, solid line) and at 6 hpi in PBMCs (Figure 1a, dot line). Similar changes were observed in the MRC-5 human embryonic lung diploid fibroblast cell line (Figure 1b). The levels of sIL-6R protein were also upregulated in IAV-infected A549 cells and PBMCs (Figure 1c). These results indicate that IAV infection induces sIL-6R expression in lung epithelial cells, primary lung fibroblast cells and human immune cells. Moreover, these data are the first to demonstrate that IAV activates sIL-6R expression both in patients and in vitro.
Figure 1.
IAV activates sIL-6R expression in different cell types. (a) sIL-6R mRNA levels were determined by real-time RT-PCR in A549 cells and human PBMCs infected with IAV (MOI=1) for the indicated length of time. (b) The level of sIL-6R mRNA was determined by real-time RT-PCR in MRC-5 cells infected with IAV (MOI=1) for 4 h. (c) sIL-6R protein levels were determined by western blot analysis of A549 cells and human PBMCs with (20 nM) or without TAPI for 8 h and were then infected with IAV (MOI=1) for 12 h. (d) A549 cells were incubated with (20 nM) or without TAPI for 8 h and were then infected with IAV (MOI=1) for 4 h. The levels of sIL-6R and TNF-α protein in the culture supernatants were measured by ELISA. Data shown are mean±s.e.; n=3. *P<0.05; ns, not significant. IAV, influenza A virus; MOI, multiplicity of infection; PBMC, peripheral blood mononuclear cell; sIL-6R, soluble interleukin-6 receptor; TNF, tumor-necrosis factor.
To clarify the mechanism of sIL-6R production, we used a shedding inhibitor TAPI39,40 to block proteolytic cleavage of the membrane-bound IL-6R.41 Surprisingly, IAV-induced expression of the sIL-6R in A549 cells and PBMCs was not blocked by TAPI (Figure 1c). To further confirm these results, cytokine TNF-α, a known target of TAPI,39 was used as the positive control and, similarly, IAV-induced release of the sIL-6R in A549 cells was not blocked by TAPI, whereas it was functional to block IAV-induced release of the TNF-α (Figure 1d). This indicated that the IAV-induced soluble IL-6R is a truncated protein produced by differential splicing of the IL-6R mRNA and is not shed from the cell surface by proteolytic cleavage of the membrane-bound IL-6R.
Identification of cytokines regulated by sIL-6R using promoter activity screening
There is convincing evidence for a link between the sIL-6R and several inflammatory and autoimmune diseases.23 Because we showed that IAV infection upregulates sIL-6R expression, we next addressed whether a high level of sIL-6R plays a pivotal role in the acute respiratory inflammation induced by IAV infection. Therefore, we conducted the following experiments to reveal how sIL-6R is involved in airway inflammation and which inflammatory cytokines are induced or regulated by sIL-6R.
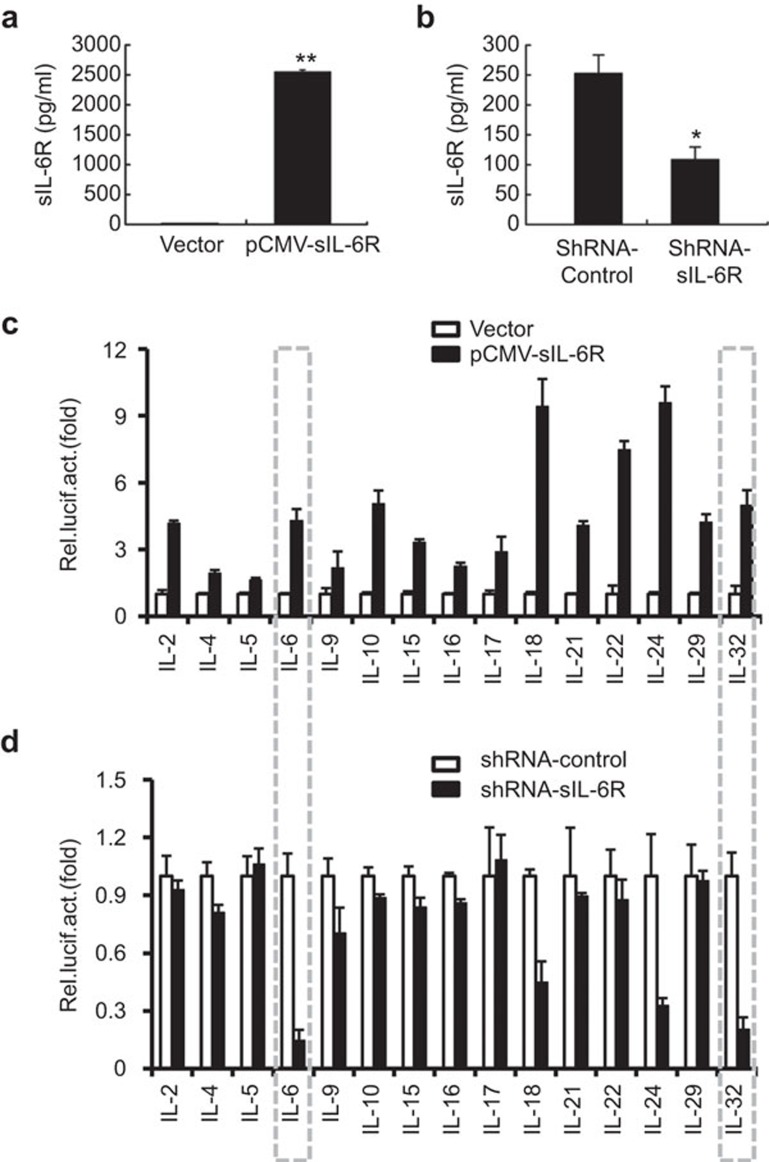
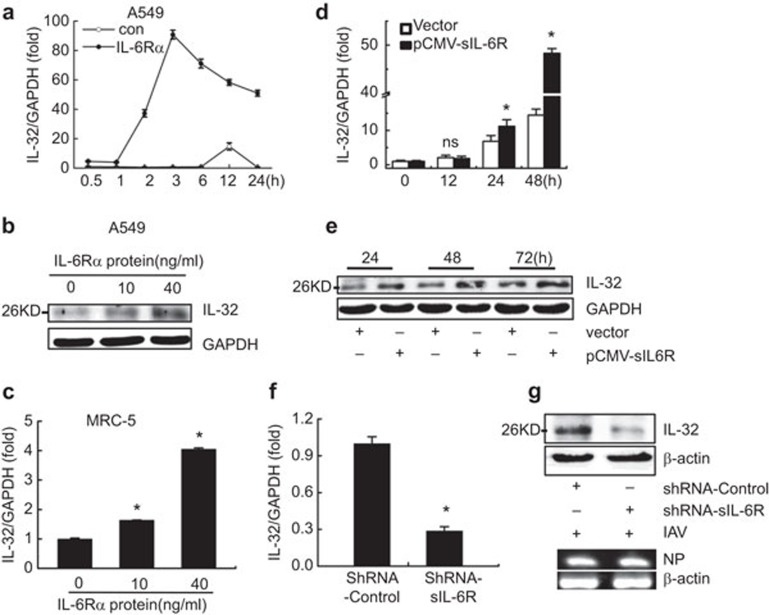
To identify potential factors regulated by sIL-6R expression during the inflammatory response to IAV infection, we screened 15 inflammation-related interleukin promoters using a luciferase reporter assay. A549 cells were cotransfected with a reporter plasmid containing an interleukin promoter and pCMV-sIL-6R or an empty vector control (Figure 2c). Much larger quantities of sIL-6R were detected in the supernatants of transfected cells compared to control supernatants (Figure 2a), and these cells were examined for luciferase activity at 24 h post-transfection. Alternatively, each IL reporter plasmid was cotransfected with a shRNA targeting sIL-6R or an irrelevant control shRNA (Figure 2d). The level of sIL-6R protein was reduced in the presence of shRNA-sIL-6R (Figure 2b), confirming that this shRNA was specific and effective. At 24 h post-transfection, the cells were infected with IAV (MOI=1) for 6 h and examined for luciferase activity. Our data showed that IL-6, IL-10, IL-21 and IL-32 promoter activity was induced by sIL-6R expression (Figure 2c). However, this activity was specific to sIL-6R because the sIL-6R-targeting shRNA dramatically suppressed the IL-6 and IL-32 promoter activities (Figure 2d). These results indicated that the expression of a variety of cytokines, including the anti-inflammatory cytokine IL-10, could be induced by sIL-6R. Nonetheless, sIL-6R was found to play a key role in mediating the expression of IL-6 or IL-32, as opposed to IL-10 or other anti-inflammatory cytokines, during IAV infection. In addition, our results also suggested that sIL-6R expression affected the promoters of other tested reporter plasmids, such as IL-18 and IL-24. In our opinion, sIL-6R is a multifunctional cellular factor and its full properties remain unknown. We chose to concentrate on IL-6 and IL-32 in this study because our earlier research work linked these two cytokines.
Figure 2.
Screen of IL-6R-stimulated cytokine promoter activity during IAV infection. (a) A549 cells were transfected with pCMV-Tag2B-sIL-6R for 24 h, and sIL-6R in the culture supernatants was quantitated by ELISA. (b) A549 cells were transfected with shRNA-sIL-6R or shRNA-control for 24 hand infected with IAV (MOI=1) for 6 h. The levels of sIL-6R protein in the culture supernatants were quantitated by ELISA. (c) Luciferase reporter plasmids for the indicated cytokines and a Renilla control (pRL–TK) were cotransfected into A549 cells with pCMV-Tag2B-sIL-6R or a control vector (pCMV–Tag2B) for 24 h. The luciferase activity was measured as described in the section on ‘Materials and methods'. (d) Luciferase reporter plasmids and a Renilla control (pRL–TK) were cotransfected with shRNA-sIL-6R or shRNA-control for 24 h into A549 cells that were infected with IAV (MOI=1) for 6 h, and the luciferase activity was measured. The results are expressed as the mean±s.e.m. of three independent experiments performed in triplicate and normalized according to the Renilla control reporter activity. n=3. *P<0.05. IAV, influenza A virus; sIL-6R, soluble interleukin-6 receptor; MOI, multiplicity of infection.
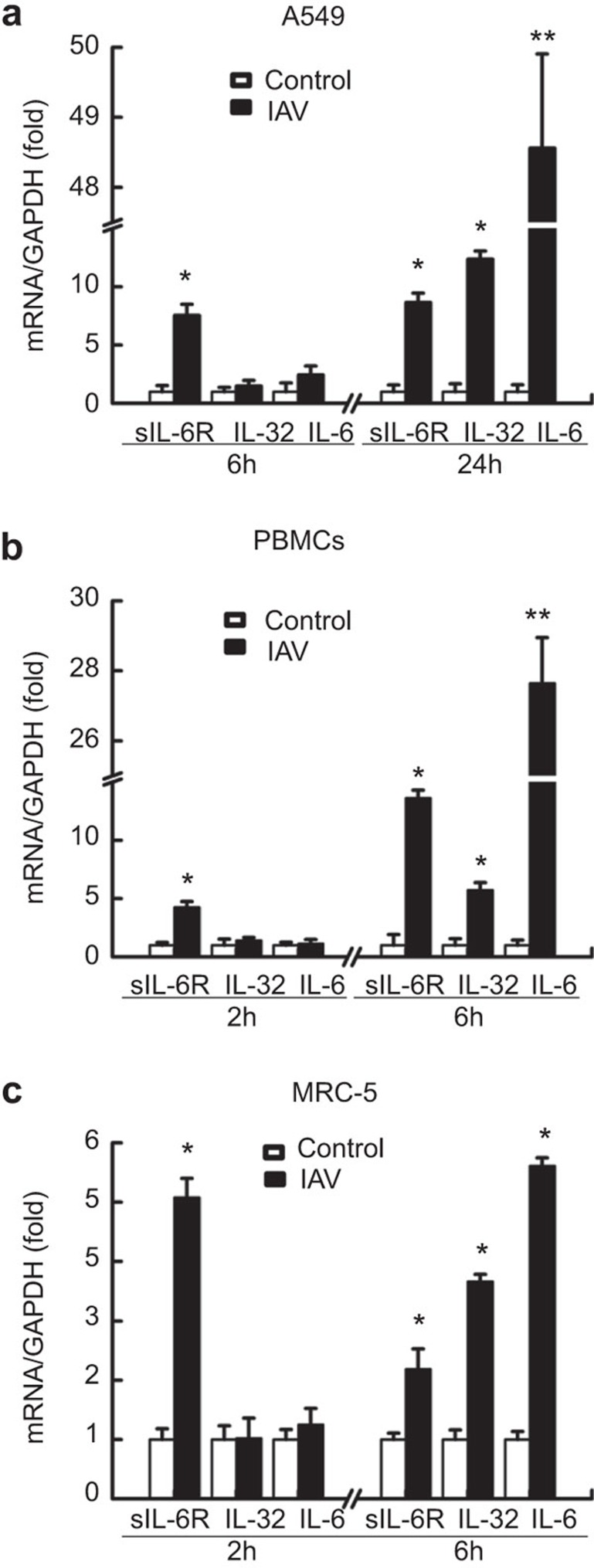
To gain further insight into the relationship between sIL-6R, IL-6, and IL-32 during IAV infection, we evaluated the effect of IAV infection on the kinetics of sIL-6R, IL-32 and IL-6 expression in A549 cells. These results showed that A549 cells were susceptible to IAV infection, with an apparent cytopathic effect (cell rounding, necrosis, detached from the culture bottle) observed after infection. Cell lysates were harvested at 6 h and 24 h after infection, and the levels of sIL-6R, IL-32 and IL-6 mRNA were determined by real-time RT-PCR (Figure 3a). IAV infection upregulated sIL-6R, IL-32 and IL-6 expression in A549 cells in a time-dependent manner, with the levels of sIL-6R mRNA rising earlier than those for IL-32 and IL-6. Similar results were observed in PBMCs infected with IAV for 2 h and 6 h (Figure 3b). Moreover, IAV infection upregulated sIL-6R, IL-32 and IL-6 expression in MRC-5 cells at different time points (Figure 3c). These data suggest that sIL-6R, IL-32 and IL-6 are involved in the host inflammatory response to IAV infection; moreover, sIL-6R may act as an early stage inflammatory factor during viral infection.
Figure 3.
Induction of sIL-6R, IL-32 and IL-6 expression by IAV infection. (a) sIL-6R, IL-32 and IL-6 mRNA levels were quantitated by real-time RT-PCR in A549 cells infected with IAV (MOI=1) or control-treated (heat-inactivated IAV) for 6 h or 24 h. (b) sIL-6R, IL-32 and IL-6 mRNA levels were quantitated by real-time RT-PCR in human PBMCs infected with IAV (MOI=1) or control-treated (heat-inactivated IAV) for 2 h or 6 h. (c) sIL-6R, IL-32, and IL-6 mRNA levels were quantitated by real-time RT-PCR in MRC-5 cells infected with IAV (MOI=1) or control-treated (heat-inactivated IAV) for 2 h or 6 h. Data shown are mean±s.e.; n=3. **P<0.01; *P<0.05. IAV, influenza A virus; MOI, multiplicity of infection; PBMC, peripheral blood mononuclear cell; sIL-6R, soluble interleukin-6 receptor.
IAV-induced sIL-6R mediates IL-6 and IL-32 expression
SIL-6R stimulates IL-6 expression in different cell types
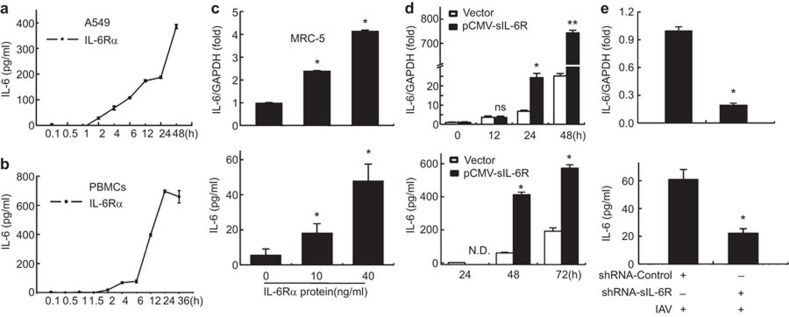
In combination with IL-6, sIL-6R is regarded as an agonist that results in enhanced IL-6 effects; however, the regulation mechanism between these molecules is unclear. Based on our screening results, we investigated the role of sIL-6R in IL-6 expression in human lung epithelial cells, PBMCs and MRC-5 cells. A549 cells were treated with recombinant human IL-6Rα protein (40 ng/ml)41 for 0.1, 0.5, 1, 2, 4, 6, 12, 24 or 48 h and then harvested to determine the IL-6 levels by ELISA. Our data indicated that IL-6 protein expression was stimulated by IL-6R in a time-dependent manner in both A549 cells and PBMCs (Figure 4a and b). Furthermore, MRC-5 cells were treated with rhIL-6R for 3 h, real-time RT-PCR and ELISA analysis revealed that the relative levels of IL-6 mRNA and secreted protein were also upregulated by IL-6R protein in a dose-dependent manner (Figure 4c). To confirm the effect of sIL-6R on IL-6 expression, a sIL-6R-expressing plasmid or control vector was transfected into A549 cells for 12, 24, 48 or 72 h, and IL-6 mRNA and protein production were measured. Real-time RT-PCR and ELISA analysis showed that the levels of IL-6 mRNA expression and protein production (Figure 4d) were enhanced in sIL-6R-overexpressing cells compared to control cells. These results demonstrated that sIL-6R could stimulate IL-6 expression.
Figure 4.
sIL-6R upregulates IL-6 expression during IAV infection. (a–c) A549 cells (a) and human PBMCs (b) were incubated with recombinant human IL-6Rα protein (40 ng/ml) for the indicated times before the cell culture supernatants were collected. The levels of secreted IL-6 protein were detected by ELISA. (c) MRC-5 cells were incubated with IL-6Rα at the indicated doses, and the levels of IL-6 mRNA (top graphs) and secreted protein (bottom graphs) were determined by real-time RT-PCR and ELISA, respectively. (d) A549 cells were transfected with pCMV-sIL-6R for the indicated times before the levels of IL-6 mRNA (top graphs) and secreted protein (bottom graphs) were determined by real-time RT-PCR and ELISA, respectively. (e) A549 cells were transfected with shRNA-sIL-6R or shRNA-control for 24 or 48 h and infected with IAV (MOI=1) for 6 h. The levels of IL-6 mRNA (top graphs) and secreted protein (bottom graphs) were determined by real-time RT-PCR and ELISA, respectively. Data shown are mean±s.e.; n=3. **P<0.01; *P<0.05; ns, not significant; N.D., not detected. IAV, influenza A virus; MOI, multiplicity of infection; PBMC, peripheral blood mononuclear cell; sIL-6R, soluble interleukin-6 receptor.
IAV activates IL-6 expression through sIL-6R
To test the effect of shRNA-sIL-6R on the regulation of IL-6 expression mediated by IAV infection, A549 cells were transfected with a plasmid encoding shRNA-sIL-6R or shRNA-control and infected with IAV (MOI=1). Our analysis showed that the level of IL-6 mRNA induced by IAV infection was significantly suppressed by sIL-6R knockdown (Figure 4e). In addition, IL-6 protein accumulation in the supernatants of A549 cells stimulated by IAV was also suppressed by sIL-6R knockdown, suggesting that IL-6 expression was sIL-6R-dependent during infection with this virus. Taken together, these data suggest that sIL-6R is a vital regulatory factor of IAV-triggered IL-6 production. Of particular interest, this is the first report of a cytokine that can be induced by its own receptor.
SIL-6R induces IL-32 expression in human lung epithelial cells and human embryonic lung diploid fibroblast cells
According to our screening results, we also evaluated the effects of sIL-6R on IL-32 during IAV infection. First, A549 cells were treated with recombinant human IL-6Rα protein (40 ng/ml) for 24 h and then examined by real-time RT-PCR and western blot. Our results showed that the level of IL-32 mRNA increased with time and peaked at 3 h (Figure 5a); moreover, the IL-32 protein levels were upregulated by rhIL-6R in a dose-dependent manner (Figure 5b). In addition, MRC-5 cells were treated with rhIL-6R for 3 h, and real-time RT-PCR revealed that the relative levels of IL-32 were also upregulated by sIL-6R protein in a dose-dependent manner (Figure 5c). These results were confirmed in A549 cells transfected with a plasmid expressing sIL-6R or a control vector for 12, 24, 48 or 72 h. Cell lysates and culture supernatants were also collected to investigate the effect of sIL-6R on IL-32 expression, and real-time RT-PCR demonstrated that the relative levels of IL-32 increased with the transfection time and peaked at 48 h post-transfection (Figure 5d). Furthermore, western blot analysis revealed that the production of IL-32 protein was stimulated by sIL-6R overexpression in a time-dependent manner (Figure 5e). Since the antibodies against IL-32 are polyclonal, they could not discriminate the exact isoform of IL-32 in this study. Altogether, these results indicate that sIL-6R can induce the expression of the pro-inflammatory cytokine IL-32.
Figure 5.
sIL-6R upregulates IL-32 expression during IAV infection. (a) A549 cells were incubated with recombinant human IL-6Rα (40 ng/ml) for the indicated times, and the level of IL-32 mRNA was examined by real-time RT-PCR. (b) A549 cells were incubated with IL-6Rα at the indicated doses, and the level of IL-32 protein was examined by western blotting. (c) MRC-5 cells were incubated with IL-6Rα at the indicated doses, and the level of IL-32 mRNA was examined by real-time RT-PCR. (d and e) A549 cells were transfected with pCMV-sIL-6R for the indicated times; the IL-32 mRNA levels were determined by real-time RT-PCR (d), and the IL-32 protein was assessed by western blotting (e). (f and g) A549 cells were transfected with shRNA-sIL-6R or shRNA-control for 24 h (f) or 48 h (g) and infected with IAV (MOI=1) for 6 h. The levels of IL-32 mRNA (f) were quantitated by real-time RT-PCR, and western blot analysis was performed to assess the IL-32 levels in the cell lysates (g, upper panel). IAV NP mRNA was detected by semiquantitative RT-PCR (g, lower panel). Data shown are mean±s.e.; n=3. *P<0.05; ns, not significant. IAV, influenza A virus; MOI, multiplicity of infection; sIL-6R, soluble interleukin-6 receptor.
IAV activates IL-32 expression via sIL-6R
The effect of sIL-6R on IL-32 expression in human lung epithelial cells was further evaluated using RNA interference. In particular, we investigated the effect of IAV infection on IL-32 expression in A549 cells transfected with shRNA-sIL-6R or shRNA-control for 24 h or 48 h. Following transfection, the cells were infected with IAV (MOI=1) for 6 h and harvested. Real-time RT-PCR and a western blot analysis demonstrated that the levels of IL-32 mRNA (Figure 5f) and protein (Figure 5g) induced by IAV infection were reduced following sIL-6R knockdown. To validate IAV infection in A549 cells, we examine IAV NP mRNA by semiquantitative RT-PCR (Figure 5g, lower panel). Taken together, these data suggest that sIL-6R acts as an upstream regulatory factor for IAV-triggered IL-32 production.
IL-32 feedback inhibits IAV-induced sIL-6R expression
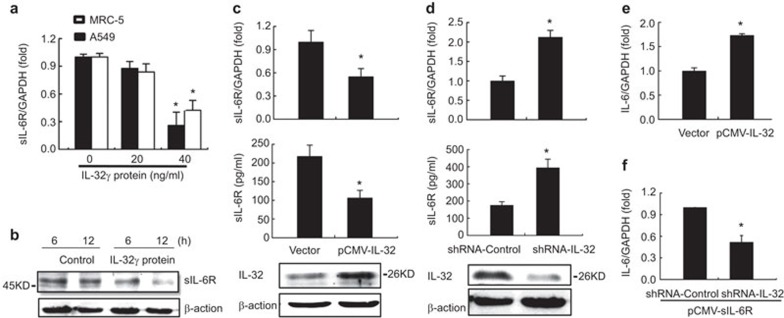
Previous reports as well as our current data suggest that sIL-6R, IL-6 and IL-32 are all proinflammatory cellular factors, although it remains unclear whether the relationship among these factors is mutually amplifying or follows a feedback mechanism. To address this, we investigated the role of IL-32 in regulating sIL-6R expression by performing real-time RT-PCR to assess the IL-32 mRNA levels in A549 and MRC-5 cells treated with various concentrations of recombinant human IL-32γ protein. The levels of sIL-6R mRNA decreased as the IL-32 concentration increased (Figure 6a), and the level of sIL-6R protein was reduced following IL-32 treatment (40 ng/ml) in A549 cells in a time-dependent manner (Figure 6b).
Figure 6.
Effect of IL-32 on IAV-induced sIL-6R expression. (a) A549 cells and MRC-5 cells were incubated with recombinant human IL-32γ protein at the indicated concentrations for 4 h, and the level of sIL-6R mRNA was quantitated by real-time RT-PCR. (b) A549 cells were incubated with recombinant human IL-32γ protein (40 ng/ml) for the indicated times, and the sIL-6R protein was analyzed by western blotting. (c) A549 cells were transfected with pCMV-IL-32 for 24 h or 48 h and then infected with IAV (MOI=1) for 6 h. At 24 h post-transfection, the level of sIL-6R mRNA was detected by real-time RT-PCR (top graphs). At 48 h post-transfection, the sIL-6R protein level was measured by ELISA (middle graphs), whereas the level of IL-32 protein was determined by western blotting (bottom graphs). (d) A549 cells were transfected with shRNA-IL-32 or shRNA-control and infected with IAV (MOI=1) for 6 h. The level of sIL-6R mRNA was quantitated by real-time RT-PCR (top graphs), and the sIL-6R protein level was measured by ELISA (middle graphs). The knockdown efficiency of IL-32 was determined by western blotting (bottom graphs). (e) A549 cells were transfected with pCMV-IL-32 for 24 h, and the level of IL-6 mRNA was quantitated by real-time RT-PCR. (f) A549 cells were co-transfected with pCMV-sIL-6R and shRNA-IL-32 or shRNA-control for 24 h and infected with IAV (MOI=1) for 6 h; the level of IL-6 mRNA was then quantitated by real-time RT-PCR. Data shown are mean±s.e.; n=3. *P<0.05. IAV, influenza A virus; MOI, multiplicity of infection; sIL-6R, soluble interleukin-6 receptor.
To confirm the negative effect of IL-32 and IAV infection on sIL-6R expression, A549 cells were transfected with pCMV-IL-32 and infected with IAV (MOI=1); sIL-6R expression was then assessed by real-time RT-PCR and ELISA. Our data indicated that both sIL-6R mRNA and protein levels were reduced following IL-32 overexpression (Figure 6c), a result that was further confirmed in A549 cells transfected with an IL-32-specific shRNA (shRNA-IL-32) and infected with IAV. Real-time PCR revealed that the level of sIL-6R mRNA increased significantly in the presence of shRNA-IL-32 (Figure 6d), and the ELISA results indicated that sIL-6R protein levels were also increased following treatment with shRNA-IL-32 in A549 cells; such results were not observed in cells expressing the control shRNA. Thus, our results suggest that IL-32 functions as a negative regulator of IL-6R expression during IAV infection.
We additionally investigated the role of IL-6 in regulating sIL-6R expression, although no meaningful results showing an effect of IL-6 on sIL-6R expression were obtained.41
Induction of IL-6 expression by sIL-6R and IL-32 in response to IAV infection
Because sIL-6R stimulates the expression of IL-32 and IL-6 during IAV infection, we next assessed whether IL-32 and IL-6 regulate each other or act as independent effectors in the inflammatory response elicited by IAV infection. Indeed, previous studies have reported that IL-32 can stimulate IL-6 production in PBMCs.32 To validate this effect in our research model, we examined the IL-6 mRNA levels in IL-32-overexpressing A549 cells infected with IAV (MOI=1) for 6 h. Consistent with previous findings, our results showed that IL-6 mRNA was upregulated by IL-32 (Figure 6e). Thus, we conclude that both sIL-6R and IL-32 play an important role in promoting IL-6 production. To assess whether the regulation of IL-6 expression by sIL-6R is dependent on IL-32, this cytokine was knocked down using a specific shRNA in sIL-6R-overexpressing A549 cells infected with IAV. Our data showed that IL-6 expression was significantly reduced after IL-32 knockdown (Figure 6f), indicating that sIL-6R induces IL-6 expression in an IL-32-dependent manner during IAV infection.
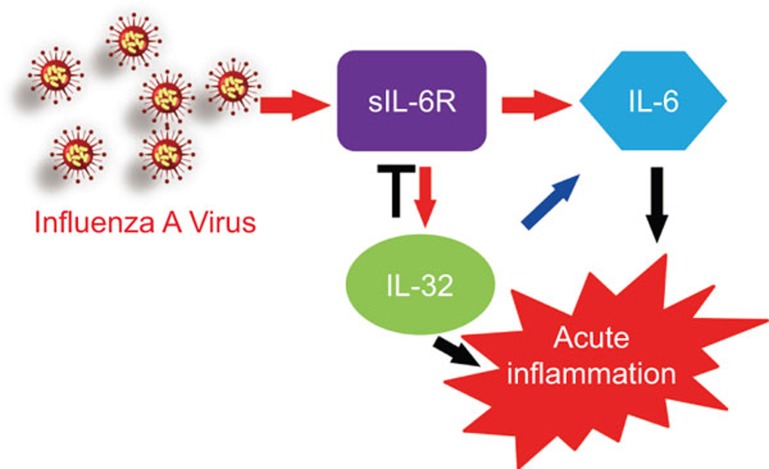
Taken together, our results identify a novel inflammatory pathway that is activated in response to IAV infection (Figure 7). During infection, sIL-6R expression is induced, leading to the upregulation of its own ligand, IL-6, and the pro-inflammatory cytokine IL-32. Through a negative feedback loop, IL-32 inhibits IAV-induced sIL-6R expression and both sIL-6R and IL-32 have a positive effect on IL-6 expression. Moreover, the expression of these cytokines is sIL-6R-dependent during IAV infection. Therefore, our results demonstrate that sIL-6R is a multifunctional cellular factor that initiates acute inflammation during IAV infection.
Figure 7.
Schematic of the proposed model for the regulation of IL-32 and IL-6 expression by sIL-6R in response to IAV infection. IAV, influenza A virus; sIL-6R, soluble interleukin-6 receptor.
Discussion
As described previously, IAV infection leads to the overproduction of numerous cytokines during the host inflammatory response, and a number of reports have recently demonstrated that the sIL-6R plays an important role in chronic inflammatory diseases.15,23,25 Moreover, our results uncover a distinct role for sIL-6R in the host cellular response to viral infection.41 Not only do these studies increase our knowledge about the biological role of this receptor, but the findings may also explain the occasional contradictory effects of IL-6.
In the present study, we first demonstrated that IAV infection activates sIL-6R expression both in patients and in vitro. Focusing on the inflammation network, we then sought to determine which cytokines interact with sIL-6R during IAV infection. According to our screening results, we concluded that sIL-6R exhibits a strong effect on the regulation of IL-6 promoter activity in response to virus infection in human lung epithelial cells. These results were confirmed by sIL-6R overexpression and knockdown experiments, which demonstrated an increase or decrease in IL-6 promoter activity, respectively. Because sIL-6R had the same effect on IL-32 promoter activity, we hypothesized that sIL-6R may play a key role in regulating acute inflammation during IAV infection. Based on our screening data, we also concluded that sIL-6R is not critical for regulating other cytokines, such as IL-10, in response to IAV infection. The IL-10-mediated anti-inflammatory response represents an essential homeostatic mechanism that controls the degree and duration of inflammation.42 Combined with the above arguments, we consider sIL-6R to be a pro-inflammatory cellular factor. In addition, the results from our screen indicated that sIL-6R may act as a multifunctional protein that regulates the promoter activity of other factors, such as IL-18 and IL-24, in addition to IL-32 and IL-6. These possibilities will be further investigated in future studies.
As an extension of our screening results, we demonstrated that IAV infection led to the upregulation of sIL-6R, IL-6 and IL-32 expression via an unknown mechanism. However, we found that sIL-6R was upregulated by IAV infection more rapidly than IL-6 or IL-32, suggesting that sIL-6R may act as an upstream regulatory factor.
Next, we performed experiments to validate our screening results, and our data showed that sIL-6R enhanced IL-6 expression at the mRNA and protein levels. This effect was observed with both endogenous and exogenous (i.e., recombinant human IL-6Rα protein) sIL-6R. Furthermore, using an RNAi approach, we found that IAV infection-induced IL-6 expression was dependent on sIL-6R, and we also verified that sIL-6R enhanced IL-32 expression in response to IAV infection. It is well known that IL-6 is a key pro-inflammatory cytokine involved in the cytokine storm associated with IAV infection5,43 and that this cytokine storm is associated with fatal diseases such as acute respiratory distress syndrome and sepsis.44 It has also been reported that the pro-inflammatory activities of IL-6 are mainly driven by IL-6 trans-signaling via sIL-6R, whereas the anti-inflammatory or regenerative functions rely on classic IL-6 signaling via the membrane-bound receptor.7 Our results are the first to demonstrate that IL-6 is activated by its own soluble receptor during IAV infection. Reports have also suggested a close association between IL-32 and the cytokine storm45 or acute lung injury.46 Because IL-6 and IL-32 play pivotal roles in the IAV-associated cytokine storm and severe infection and our results show that IL-6 and IL-32 are both induced by sIL-6R, we consider the sIL-6R as a new diagnostic and therapeutic target.
Finally, as a positive regulatory relationship from sIL-6R to IL-32 was observed, we sought to determine whether a negative feedback mechanism exists from IL-32 to sIL-6R during the host inflammatory response to viral infection. Indeed, our results demonstrated that IL-32 participates in a negative feedback loop to regulate the expression of sIL-6R in response to IAV infection. Furthermore, we validated an earlier report that IL-32 activates IL-6 expression in IAV-infected A549 cells. Combined with our previous results, we conclude that the trans-signaling factor sIL-6R reacts with IL-6 through an alternative pathway involving IL-32.
Based on these results, we propose the model presented in Figure 7 as a mechanism for the stimulation of sIL-6R, IL-6 and IL-32 by IAV infection, which results in the host inflammatory response. IAV stimulates the expression of sIL-6R, which upregulates its own ligand, IL-6; activated sIL-6R also stimulates IL-32 production, which in turn attenuates sIL-6R production via a negative feedback loop. Moreover, IL-32 enhances the IL-6 pathway, which suggests that sIL-6R reacts with IL-6 through a bypass pathway involving IL-32.
Therefore, these data support our hypothesis of a previously unrecognized pathway facilitating IL-6 trans-signaling. IL-6 expression directly depends on its own receptor, sIL-6R, during viral infection, and sIL-6R indirectly activates IL-6 through another pro-inflammatory cytokine, IL-32. In the present study, we identified sIL-6R as a novel IL-32 upstream factor that plays a critical role during IAV infection. These results extend our understanding based on the previous clinical finding that IL-32 is elevated in IAV-infected patients and provide new insight into how pro-inflammatory factors respond to viral infection. Moreover, we concluded that IL-32 feedback inhibits IAV-induced sIL-6R expression. Evidences have shown that the secreted γ-isoform of IL-3247 exhibited antiviral properties during viral infection31,35,48 and inducible IL-32γ exerts extensive antiviral function via selective stimulation of IFN-λ1.38 In this sense, our results provide more evidence to support the reported antiviral function of sIL-6R in earlier research.41
Generally speaking, the inflammatory response is beneficial to the host. Indeed, inflammatory cytokines are normally released in response to numerous cellular stimuli, including viral infection and function to activate host responses aimed at controlling cellular stress and minimizing cellular damage. However, extreme inflammation, such as the cytokine storm, is dangerous and potentially fatal. Furthermore, we still lack a good understanding of the molecular events that precipitate the cytokine storm associated with IAV infection, the contribution of such a ‘storm' to pathogenesis, and the therapeutic strategies that might be utilized to prevent or quell this storm. Many studies have emphasized a central role for IL-6 in governing inflammation and highlighted the therapeutic potential of targeting this cytokine with a monoclonal antibody (e.g., tocilizumab)49 as a strategy for treating chronic inflammatory diseases or cancer.50,51 However, there is no precedent of using tocilizumab in trying to treat severe IAV infection. Our data highlight the vital role of sIL-6R during viral infection, namely the induction of the proinflammatory factors IL-6 and IL-32. Moreover, the present research expands our understanding of highly relevant pathophysiological events, such as the cytokine storm caused by IAV, which provides a potential target for therapeutic intervention aimed at controlling severe acute infections in IAV patients.
In conclusion, our study unveils a novel interplay among sIL-6R, IL-32 and IL-6. Exploring the implications of sIL-6R during IAV infection and potential avenues for manipulation contributes to a greater understanding of IL-6 signaling during inflammation, thereby allowing the rational development of immunotherapeutic strategies to enhance viral control while limiting acute lung inflammation.
Acknowledgments
This work was supported by research grants from the Major State Basic Research Development Program of China (no. 2013CB911102), the National Natural Science Foundation of China (nos. 81271821 and 81461130019) and the National Mega Project on Major Infectious Diseases Prevention (no. 2012ZX10004503-004). The funding agencies had no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
References
- 1Heitmeier MR, Scarim AL, Corbett JA. Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-kappaB activation. J Biol Chem 1998; 273: 15301–15307. [DOI] [PubMed] [Google Scholar]
- 2Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 2005; 280: 5571–5580. [DOI] [PubMed] [Google Scholar]
- 3Eliopoulos AG, Gallagher NJ, Blake SM, Dawson CW, Young LS. Activation of the p38 mitogen-activated protein kinase pathway by Epstein–Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem 1999; 274: 16085–16096. [DOI] [PubMed] [Google Scholar]
- 4Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol 2001; 64: 262–268. [DOI] [PubMed] [Google Scholar]
- 5Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012; 76: 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol 2010; 22: 347–352. [DOI] [PubMed] [Google Scholar]
- 7Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011; 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 8Assier E, Boissier MC, Dayer JM. Interleukin-6: from identification of the cytokine to development of targeted treatments. Joint Bone Spine 2010; 77: 532–536. [DOI] [PubMed] [Google Scholar]
- 9Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 2001; 15: 43–58. [DOI] [PubMed] [Google Scholar]
- 10Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol 2006; 7: 1299–1308. [DOI] [PubMed] [Google Scholar]
- 11Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001; 14: 705–714. [DOI] [PubMed] [Google Scholar]
- 12Hou T, Tieu BC, Ray S, Recinos AIii, Cui R, Tilton RG et al. Roles of IL-6-gp130 signaling in vascular inflammation. Curr Cardiol Rev 2008; 4: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 2008; 181: 2189–2195. [DOI] [PubMed] [Google Scholar]
- 14Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997; 15: 797–819. [DOI] [PubMed] [Google Scholar]
- 15Rose-John S, Neurath MF. IL-6 trans-signaling: the heat is on. Immunity 2004; 20: 2–4. [DOI] [PubMed] [Google Scholar]
- 16Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res 2005; 65: 10794–10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C et al. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J Biol Chem 2003; 278: 38829–38839. [DOI] [PubMed] [Google Scholar]
- 18Coodly L. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem 1996; 271: 11376–11382. [DOI] [PubMed] [Google Scholar]
- 19Bennett TA, Lynam EB, Sklar LA, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol 1996; 156: 3093–3097. [PubMed] [Google Scholar]
- 20Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 1994; 370: 218–220. [DOI] [PubMed] [Google Scholar]
- 21Müllberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D et al. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol 1995; 155: 5198–5205. [PubMed] [Google Scholar]
- 22Jones SA, Horiuchi S, Novick D, Yamamoto N, Fuller GM. Shedding of the soluble IL-6 receptor is triggered by Ca2+ mobilization, while basal release is predominantly the product of differential mRNA splicing in THP-1 cells. Eur J Immunol 1998; 28: 3514–3522. [DOI] [PubMed] [Google Scholar]
- 23Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol 2011; 90: 484–494. [DOI] [PubMed] [Google Scholar]
- 24Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21: 491–501. [DOI] [PubMed] [Google Scholar]
- 25Knupfer H, Preiss R. sIL-6R: more than an agonist? Immunol Cell Biol 2008; 86: 87–91. [DOI] [PubMed] [Google Scholar]
- 26Netea MG, Azam T, Lewis EC, Joosten LA, Wang M, Langenberg D et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase- 1/IL-18/interferon-gamma-dependent mechanism. PLoS Med 2006; 3: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS One 2008; 3: e1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis 2006; 65(Suppl 3): iii61–iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA 2006; 103: 3298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol 2008; 14: 5154–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol 2008; 181: 557–565. [DOI] [PubMed] [Google Scholar]
- 32Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA 2005; 102: 16309–16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 2005; 22: 131–142. [DOI] [PubMed] [Google Scholar]
- 34Li W, Sun W, Liu L, Yang F, Li Y, Chen Y et al. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol 2010; 185: 5056–5065. [DOI] [PubMed] [Google Scholar]
- 35Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection from RNA and DNA viruses by IL-32. J Immunol 2011; 186: 4110–4118. [DOI] [PubMed] [Google Scholar]
- 36Liu L, Li R, Pan Y, Chen J, Li Y, Wu J et al. High-throughput screen of protein expression levels induced by cyclooxygenase-2 during influenza a virus infection. Clin Chim Acta 2011; 412: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 37Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z et al. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol 2011; 187: 4844–4860. [DOI] [PubMed] [Google Scholar]
- 38Li Y, Xie J, Xu X, Liu L, Wan Y, Liu Y et al. Inducible interleukin 32 (IL-32) exerts extensive antiviral function via selective stimulation of interferon lambda1 (IFN-lambda1). J Biol Chem 2013; 288: 20927–20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 1994; 370: 218–220. [DOI] [PubMed] [Google Scholar]
- 40Zhan M, Jin B, Chen SE, Reecy JM, Li YP. TACE release of TNF-alpha mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J Cell Sci 2007; 120: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Wang Q, Chen X, Feng J, Cao Y, Song Y, Wang H et al. Soluble interleukin-6 receptor-mediated innate immune response to DNA and RNA viruses. J Virol 2013; 87: 11244–11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics 2013; in press. [DOI] [PMC free article] [PubMed]
- 43Wang S, Le TQ, Kurihara N, Chida J, Cisse Y, Yano M et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis 2010; 202: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Sun Y, Jin C, Zhan F, Wang X, Liang M, Zhang Q et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis 2012; 206: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 45Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE et al. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol 2012; 86: 5422–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Arcaroli JJ, Liu N, Yi N, Abraham E. Association between IL-32 genotypes and outcome in infection-associated acute lung injury. Crit Care 2011; 15: R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E et al. Identification of the most active interleukin-32 isoform. Immunology 2009; 126: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Bae S, Kang D, Hong J, Chung B, Choi J, Jhun H et al. Characterizing antiviral mechanism of interleukin-32 and a circulating soluble isoform in viral infection. Cytokine 2012; 58: 79–86. [DOI] [PubMed] [Google Scholar]
- 49Alten R. Tocilizumab: a novel humanized anti-interleukin 6 receptor antibody for the treatment of patients with rheumatoid arthritis. Ther Adv Musculoskelet Dis 2011; 3: 133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leuk Biol 2006; 80: 227–236. [DOI] [PubMed] [Google Scholar]
- 51Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K et al. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol 2012; 42: 2060–2072. [DOI] [PubMed] [Google Scholar]