In the present study, we demonstrate that adult mice lacking interleukin-1 receptor 1 (IL-1R1) exhibit increased expression of both the excitatory scaffolding protein postsynaptic density-95 (PSD-95) and inhibitory scaffolding proteingephyrin, respectively, in the hippocampus. The morphology of hippocampal microglia is also altered towards a more activated phenotype. These results indicate an important role for IL-1 signaling in maintaining physiological conditions in both neurons and microglia.
The pro-inflammatory cytokine IL-1 is upregulated by a variety of immune challenges and is a potent activator of the immune system in a pathological environment. One of the major producers of IL-1 in the brain is microglia. Microglia are highly motile glial cells with a function in the surveillance of brain integrity. The activation of microglia in response to a pathological insult or to changes in homeostatic processes results in the release of several immune modulators, such as IL-1, IL-6 and tumor necrosis factor, which orchestrate an immune response.1 However, less is known about how cytokines, such as IL-1, may modulate microglial responses during physiological conditions.
IL-1 plays an important role in neural modulation and regulates vital processes, such as synaptic plasticity, in the brain. It is required for the regulation of hippocampal plasticity and learning under physiological conditions. Mice lacking IL-1R1 demonstrate impaired spatial memory and contextual fear conditioning, as well as diminished short-term and long-term plasticity.2 However, the administration of IL-1β has also been shown to have negative outcomes on learning and memory acquisition.3 Interestingly, intracerebral infusion of IL-1β alters excitatory/inhibitory (E/I) transmission in the spinal cord by enhancing the frequency and amplitude of spontaneous excitatory and by reducing inhibitory postsynaptic currents.4 In addition, intracerebral infusion of IL-1β increases excitation and seizure activity in a model of temporal lobe epilepsy,5 suggesting that IL-1β may be regulating synaptic transmission in the hippocampus.
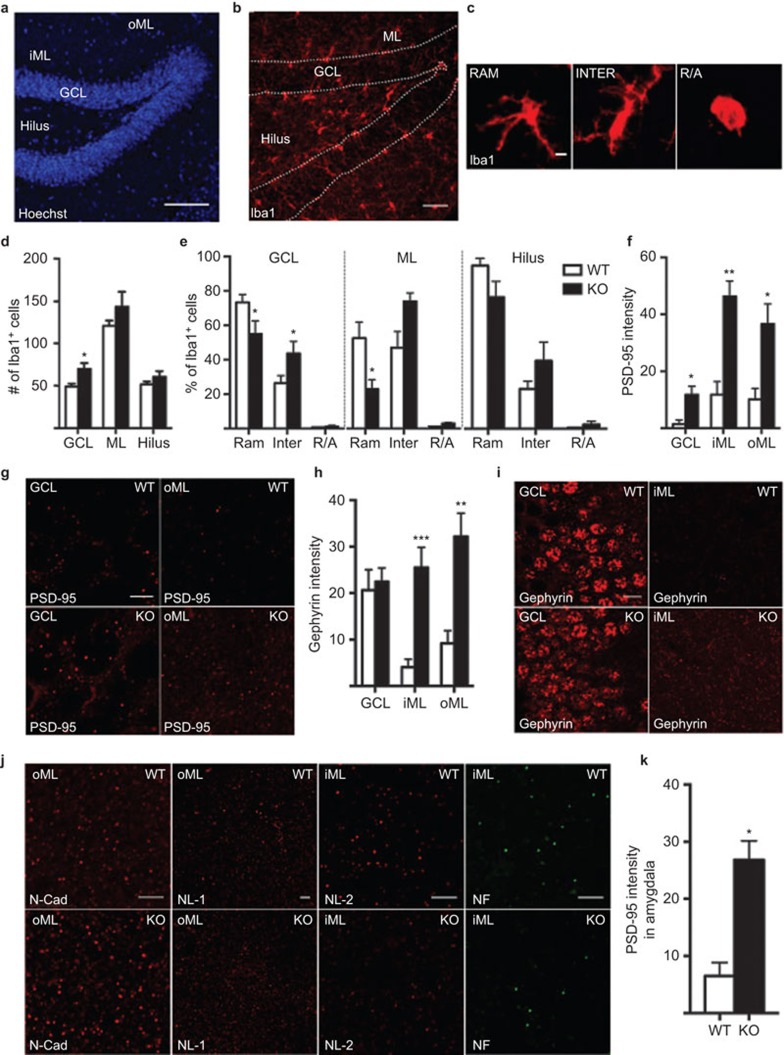
The objective of the present study was to investigate changes in the activation of microglia and the neuronal E/I balance of scaffolding proteins in the hippocampus and amygdala in the absence of IL-1 signaling under physiological conditions. We utilized naïve adult (4- to 5-month-old) wild-type (WT) mice and IL-1R1 knockout (KO) mice, which were kindly provided by Dr Emmanuel Pinteaux, University of Manchester, UK. Microglial activation was assessed by quantifying the number and phenotype of Iba1+ cells (i.e., ramified (surveying), intermediate and round/ameboid (phagocytic) morphologies), as visualized by immunohistochemistry, in perfused coronal brain sections, as previously described6 (Figure 1a–c). Interestingly, we detected more Iba1+ cells in the granule cell layer (GCL) of the dentate gyrus (DG) in the hippocampus (Figure 1d) of the IL-1R1 KO animals than that of the WT animals. We also found an increased percentage of intermediate and a decreased percentage of ramified Iba1+ morphologies in the GCL in the IL-1R1 KO group. In the molecular layer7 of the DG, the percentage of ramified cells was also decreased, and there was a trend toward an increase in the intermediate phenotype (P=0.086). No differences were observed in the dentate hilar region (Figure 1e). These results could indicate that lack of the IL-1/IL-1R1 axis induces a pathology-like environment in the DG and may initiate microglial activation, which is detrimental to fundamental processes such as memory acquisition and long-term potentiation.2
Figure 1.
Representative images showing the cytoarchitecture with nuclear staining (Hoechst) (a) and Iba1+ microglia (b) in the dentate gyrus of the hippocampus, including the hilus, GCL, iML and oML. (c) Images of different microglial morphological phenotypes, including RAM, INTER and R/A Iba1+ cells. Quantification of Iba1+ cell number per section (d), relative percentage of 80 Iba1+ microglia per animal with different morphologies (e), quantification of PSD-95 protein intensity (mean gray value in confocal pictures imported into ImageJ software, measured per representative region of interest from 3–4 coronal brain sections per animal, from −1.46 mm to −2.46 mm posterior to bregma6 (f), images of PSD-95 expression (g), quantification of gephyrin protein intensity (h), images of gephyrin expression (i) in WT and IL-1R1 KO mice. (j) Representative images of N-cad and NL-1 in the oML and NL-2 and NF in the iML of WT and IL-1R1 KO mice. (k) Quantification of PSD-95 protein intensity in amygdala. All analyses were conducted by researchers who were blinded to the treatment conditions. Data are presented as the means±SEM *P≤0.05. N=4–8 for WT and n=3–5 for IL-1R1 KO group. All comparisons were performed using an unpaired Student's t-test except in (e), for which two-way analyses of variance followed by the Bonferroni post hoc test were used for analyzing microglia morphology. Scale bars are 100 µm in a, 50 µm in b, 10 µm in i and 5 µm in c, g and j. GCL, granule cell layer; iML, inner molecular layer; INTER, intermediate; KO, knockout; N-cad, N-cadherin; NF, neurofascin; NL-1, neuroligin-1; oML, outer molecular layer; PSD-95, postsynaptic density-95; R/A, round/ameboid; RAM, ramified; WT, wild-type.
We therefore set out to evaluate the expression of a panel of synaptic proteins known to regulate the E/I balance, by quantifying the fluorescence intensity using confocal microscopy, as previously described.6,8 We found increased expression of PSD-95, a scaffolding protein found mainly on glutamatergic synapses, in both the GCL and inner and outer ML (iML and oML) in IL-1R1 KO mice compared to WT mice (Figure 1f–g). Surprisingly, the intensity of staining of the inhibitory scaffolding protein gephyrinbu immunohistochemistry was also increased in the iML and oML of IL-1R1 KO mice, whereas perisomatic expression in the GCL was unaltered (Figure 1h–i). Given the alteration in postsynaptic scaffolding protein expression, we next assessed whether the expression of synaptic adhesion molecules was also changed. Adhesion molecules play a key role in synaptic assembly and in fine-tuning the synaptic response. However, intensity analyses of N-cadherin and neuroligin-1 (NL-1) (adhesion molecules primarily associated with excitatory synapses) and NL-2 and neurofascin (primarily on inhibitory synapses) did not show any changes in the GCL, iML or oML (N-cadherin: GCL KO 8.038±1.13 vs. WT 5.417±0.67 mean protein intensity±SEM, iML KO 23.53±5.07 vs. WT 14.83±2.91, oML KO 31.67±7.89 vs. WT 21.27±4.24; NL-1: GCL KO 3.709±0.308 vs. WT 4.413±1.018, iML KO 7.262±1.186 vs. WT 7.726±1.918, oML KO 9.188±1.346 vs. WT 8.109±2.135; NL-2: GCL KO 14.12±2.08 vs. WT 9.24±0.74, iML KO 6.067±0.79 vs. WT 4.975±0.47, oML KO 6.389±0.95 vs. WT 5.389±0.66; neurofascin: GCL KO 2.217±0.675 vs. WT 0.8678±0.4087, iML KO 3.713±0.9636 vs. WT 1.912±0.5561, oML KO 5.047±1.480 vs. WT 2.283±0.568) (Figure 1j). These results could suggest that diminished long-term potentiation and spatial memory function in IL-1R1 KO mice may, on a molecular level, be a result of a disproportionate E/I input to the granule cells in the hippocampus. Alternatively, these results might reflect compensatory mechanisms that, together with altered spine size (which has previously been observed in the ML of IL-1R KO mice 9), may be an attempt to re-establish proper network function. A causal relationship between these synaptic changes and the activation of hippocampal microglia is possible but cannot be determined from the present results. There is, however, accumulating evidence that microglia can make contacts with the synaptic subcompartment of neurons and respond to changes in synaptic functions,10 suggesting that in the event of altered synaptic transmission, microglial activation may occur.
Interestingly, IL-1R1 KO mice also demonstrated upregulated expression of PSD-95 in the amygdala (Figure 1k), a structure known to communicate extensively with the hippocampus. However, this was not accompanied by any change in gephyrin expression (KO 17.73±4.182 vs. WT 11.68±1.483), in microglial cell number (KO 102.0±11.99 vs. WT 80.83±5.789 cells/section quantified in 3–4 coronal sections/mouse) or microglial morphology (ramified: KO 90.83%±4.192% vs. WT 95.78%±0.88%, intermediate: KO 9.167%±4.192% vs. WT 4.219%±0.88%, round/ameboid: KO 0%±0% vs. WT 0%±0%, based on 80 Iba1+ cells/mouse), suggesting that the hippocampus may be especially sensitive to changes in IL-1/IL-1R1 levels. Indeed, previous reports have documented that the expression of IL-1 is particularly high in the hippocampus compared to other areas of the brain, an observation supported by the detrimental effects of intraventricular IL-1 administration on the DG.3 Consequently, the increase in activated microglia in the hippocampus seems to either be due to a natural response to the lack of IL-1 receptor in an area where it is particularly important or due to the changes in E/I balance.
In summary, we report here for the first time that IL-1 signaling may alter neuronal synaptic activity by regulating synaptic scaffolding protein levels in the hippocampus. The lack of IL-1R1 or the imbalance in E/I induces a pathology-like environment with microglial activation. Understanding the regulation of E/I balance in the brain is of importance when studying new potential treatment strategies for several diseases, including epilepsy.
Acknowledgments
This work was supported by the Swedish Research Council and an ALF Grant for funding of medical training and research.
References
- 1Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25: 181–213. [DOI] [PubMed] [Google Scholar]
- 2Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 2003; 13: 826–834. [DOI] [PubMed] [Google Scholar]
- 3Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Memory 2002; 78: 379–389. [DOI] [PubMed] [Google Scholar]
- 4Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28: 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Vezzani A, Conti M, de Luigi A, Ravizza T, Moneta D, Marchesi F et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci 1999; 19: 5054–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Chugh D, Nilsson P, Afjei SA, Bakochi A, Ekdahl CT. Brain inflammation induces post-synaptic changes during early synapse formation in adult-born hippocampal neurons. Exp Neurol 2013; 250: 176–188. [DOI] [PubMed] [Google Scholar]
- 7Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K et al. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain 2003; 104: 471–480. [DOI] [PubMed] [Google Scholar]
- 8Jackson J, Chugh D, Nilsson P, Wood J, Carlstrom K, Lindvall O et al. Altered synaptic properties during integration of adult-born hippocampal neurons following a seizure insult. PloS One 2012; 7: e35557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci 2009; 29: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat 1968; 85: 145–157. [DOI] [PubMed] [Google Scholar]