Abstract
FOXP3+ regulatory T (Treg) cells are critical in maintaining immune tolerance and homeostasis of the immune system. The molecular mechanisms underlying the stability, plasticity and functional activity of Treg cells have been much studied in recent years. Here, we summarize these intriguing findings, and provide insight into their potential use or manipulation during Treg cell therapy for the treatment of autoimmune diseases, graft-versus-host disease (GVHD) and cancer.
Keywords: autoimmune diseases, FOXP3, immunosuppressive activity, Treg cells
Introduction
Regulatory T (Treg) cells play a pivotal role in the maintenance of peripheral immunological tolerance and control of immune responses toward pathogens and tumors. In 1995, naturally occurring Treg cells were identified as a subpopulation within the CD4+CD25+ T-cell population.1 At that time, immunologists were skeptical of the suppressive nature of any T cells harboring this phenotype, as CD25 is highly expressed on activated effector T cells. Since then, scientists have made substantial progress in understanding their immune-suppressive function, and have resolved earlier problems of discriminating between the CD25+ population of effector T cells and CD25high-expressing Treg cells.2,3,4,5 In 2003, the forkhead box transcription factor FOXP3 was identified as a new specific marker of Treg cells, and its expression was found necessary and sufficient for their suppressive activity.6,7,8 Scurfy mice, which lack Foxp3, would develop severe lymphoproliferative autoimmune disease attributed to the lack of an immune-suppressive component in the immune system.9 Similar to mice, mutations in the Foxp3 gene of humans also leads to autoimmune disorder, a life-threatening disease called immune dysregulation, polyendocrinopathy and enteropathy, X-linked syndrome.10 The identification of FOXP3 as a specific transcription factor for Treg cells has substantially progressed research on the development, differentiation and suppressive function of Treg cells.
In this review, we will summarize the latest findings on the heterogeneity, stability and plasticity of Treg cells, provide an overall understanding that how Tregs cells mediate their suppressive function and detail the underlying mechanisms of the molecular regulation of Treg cells. Further, we will show the relationship between Treg cells and physiological diseases, and relate these to relevant clinical trials to illuminate the possibility of using Treg cells to treat immune system disorders and other human diseases.
Heterogeneity of Treg cells
Treg cells are a heterogeneous population with respect to their origin of development, functional activity and activation status. Treg cells are generally categorized into two groups: thymus-derived Treg (tTreg) cells and peripherally derived Treg (pTreg) cells,2,3,4,5 also known as natural Tregs and induced Tregs, respectively. Both of the above Treg subsets are essential in maintaining immune homeostasis; however, further research has indicated that within these two subsets lie further heterogeneity relating to both phenotype and function.
Both the transcription factors Helios and the cell surface glycoprotein neuropilin-1 could be used to distinguish tTreg from pTreg cells. Helios and neuropilin-1 are usually highly expressed by tTreg cells but poorly expressed by pTreg cells; however, pTreg cells may upregulate expression of both these factors depending on local inflammatory conditions or the type of antigen-presenting cells and activation signals that are present.11,12,13 Treg cells are a stable lineage with minimal capacity to dedifferentiate and convert into Teff cells, whereas CD25low Treg cells may lose FOXP3 expression and convert into Th cells under certain conditions.14 DNA methylation experiments have revealed that the Foxp3 promoter and conserved non-coding DNA sequence 2 (CNS2) region are highly demethylated in tTregs, which facilitate mRNA transcription of Foxp3 and contribute to lineage stability through FOXP3 expression, while in vitro induced Treg cells are substantially methylated at the CNS2 region.15,16 TGF-β treatment decreases the methylation status of CNS2 and promotes FOXP3 expression. Knockdown of the DNA methyltransferase Dnmt1 can also induce FOXP3 expression.17 On the other hand, the methyl-binding domain protein Mbd2 works reversely on CNS2 methylation and plays an important role in promoting CNS2 demethylation and FOXP3 expression.18
In humans, CD4+CD25highCD127low T cells have been often been labeled as bone-fide Treg cells as these cells express high levels of FOXP3. Recently, Treg cells have been further classified into CD45RA+FOXP3low resting Treg cells (rTreg cells) and CD45RA−FOXP3high effector Treg cells (eTreg cells). Both rTreg and eTreg cells have immunosuppressive activity in vitro. rTreg cells can proliferate and differentiate into eTreg cells after T-cell receptor (TCR) activation, and eTreg cells are anergic and prone to apoptosis. Conversely, CD45RA−FOXP3low non-Treg cells have no immunosuppressive activity and express the proinflammatory cytokines IL-17, IL-2 and interferon-γ (IFN-γ).19,20
There is also substantial data that suggest how Treg cells have Th subset-specific reprogramming to control different immune responses; in particular, Th1, Th2 and Th17 responses in the local milieu. Treg cells utilize the same transcriptional regulators as the Th subset to generate suppressive responses to the particular type of inflammation; for example, Tbet+ Treg cells suppress Th1 responses, IRF4+ Treg suppress Th2 responses and STAT3 expression in Treg cells suppress Th17 responses.21,22,23 Additionally, Jinfang Zhu and colleagues confirmed that Tbet+ Treg cells were able to convert into GATA3+ Treg cells, and vice versa, which suggests that Th subset-specific reprogramming is a dynamic process.24
Stability and plasticity of FOXP3+ Treg cells
The topic of Treg cell lineage stability and plasticity has been controversial for many years. Previously, scientists have found that some FOXP3+ T cells may lose FOXP3 expression and acquire effector Th cell function under certain conditions.25,26 Purified FOXP3+ T cells from Foxp3-reporter mice were found to lose FOXP3 expression and acquire the ability to produce Th cytokines when cultured in inflammatory conditions in vitro. Also, adoptive transfer of FOXP3+ T cells were found instable, where approximately 50% of the donor cells became FOXP3− T cells, and converted into IFN-γ-, IL-2- and IL-17-producing cells.27 Former FOXP3+ T cells (exFOXP3+ T cells), were also found particularly increased in inflamed gut-associated tissues.27,28,29 Bluestone and colleagues performed a fate mapping study with Foxp3-GFP-Cre BAC transgenic mice to trace the stability of FOXP3+ T cells and found that 10%–20% of FOXP3+ Treg cells lose FOXP3 expression and exhibit inflammatory Th cell phenotypes with the ability to secrete IFN-γ and IL-17.30 On the molecular level, we have identified how the stress-activated Stub1-Hsp70 complex plays a critical role in the degradation of FOXP3 and promotion of Treg cell conversion into Th1-like cells.31 All these observations indicate that FOXP3+ Treg cells may be unstable and can convert into Th-like Treg cells in response to certain immunological environments.
In contrast to the above, the plastic characteristic of FOXP3+ T cells was strongly challenged by the finding that autoantigen-specific FOXP3+ and FOXP3− T cells display distinct TCR CDR3 sequences in an experimental autoimmune encephalomyelitis model, which suggests that these cells derive from distinct clones and have no inter-conversion.32 Furthermore, Rubtsov and colleagues used a Foxp3GFP-Cre-ERT2 system, which only labeled FOXP3 expressing T cells after tamoxifen treatment to trace the plasticity of FOXP3+ T cells and found that only <5% of FOXP3+ T cells could lose FOXP3 expression, even if the mice were challenged with various inflammatory insults.33 In addition, Hori and colleagues showed that only a minor fraction of CD25−FOXP3+ T cells could lose FOXP3 expression in lymphopenic and in vitro polarization settings, whereas most CD25+FOXP3+ T cells exhibit stability and resistance to convert into Th or Th-like cells.34 Komatsu and colleagues further confirmed this finding, where they authenticated how only the CD25lowFOXP3+ T-cell population could lose FOXP3 expression and acquire a Th17 phenotype in mice autoimmune arthritis, whereas CD25hiFOXP3+ T cells were rather stable.35 In contrast to murine Treg cells, human Treg cells seem to be rather unstable. CD25hiFOXP3+ T cells derived from human blood could differentiate into IL-17 producer cells in vitro upon TCR activation and in the presence of inflammatory cytokines, including IL-1β, IL-21 and IL-23.36,37
One possible reason for the divergent findings on the plasticity of Treg cells and the stability of the transcription factor FOXP3 is because of the mice model differences, including different intensities of reporter gene expression and distinct strengths of TCR and inflammatory signals under physiological conditions. These findings also suggest that the gene of Foxp3 transcription and the stability of its encoding protein are complicated and tightly modulated in vivo and in vitro, and how the process may involve an enormous diversity of epigenetic modifications and post-translational modifications that associate with the immunosuppressive activity of FOXP3+ Treg cells.
Mechanisms underlying the suppressive function of FOXP3+ Treg cells
Multiple potential mechanisms have been identified for Treg suppressive function on the proliferation and activation of Teff cells in vitro and in vivo. These could be grouped into three different modes: suppression mediated by cell–cell contact, cytokine secretion and metabolic disruption.
The suppressive function of Treg cells may facilitate through their interaction with dendritic cells (DCs) or Teff cells directly. The co-stimulatory molecule CTLA4 is highly expressed on the surface of Treg cells and interacts with CD80 and CD86 ligands expressed on the surface of DCs. This interaction results in the upregulation and secretion of indoleamine 2, 3-dioxygenase by DC that then delivers a negative signal to Teff cells.38,39 Through interaction of lymphocyte activation gene on Treg cells with MHC-II molecules on DCs, Treg cells may also modulate immune responses through inhibition of DC maturation, leading to inefficient activation of Teff cells.40 Interaction of neuropilin-1 on Treg cells with DCs can restrict the interaction between DCs and Teff cells.41 Finally, expression of serine protease granzyme and galectin-1 on Treg cells could lead to apoptosis or cell cycle arrest of Teff cells through direct contact.41
In terms of cytokines, Treg cells may express the immunosuppressive cytokine IL-10, which is important for Treg cell control of inflammatory colitis in mice,42,43 and TGF-β, which is crucial for Treg cell-mediated suppression in vivo.44,45 IL-35 is another inhibitory cytokine that can be produced by Treg in mice,46 where IL-35 deficient Treg cells were found unable to control disease in an inflammatory bowel disease mouse model.46,47
The suppressive function of Treg cells could also occurs through disruption of target cell metabolism. Treg cells highly express the IL-2 receptor α-chain on their cell surface, which facilitates high consumption of IL-2. Since IL-2 is also critical for the proliferation and activation of Teff cells, Treg cells may suppress the growth of Teff cells through IL-2 deprivation.48,49 An additional mechanism is the expression of CD39 and CD73 on the surface of Treg cells, which could hydrolyze ATP or ADP, to cAMP, followed by inhibition of Teff cell function or DC maturation.50,51,52
Molecular mechanisms underlying the functional regulation of FOXP3+ Treg cells
As a master regulator of Treg cells development, differentiation and immunosuppressive function, the expression level of the transcription factor FOXP3 is critical for maintaining immune homeostasis.20 Foxp3 transcription can be regulated on the epigenetic level, while FOXP3 protein stability may be controlled by post-translational modification.31,53,54,55,56,57,58,59,60 FOXP3 forms part of a large protein complex that regulates the expression of hallmark genes associated with Treg cell phenotype.61,62,63,64
The genomic region of the Foxp3 locus has several conserved non-coding sequences (CNS1, CNS2 and CNS3) that play different roles in the regulation of Foxp3 transcription. CNS1 contains binding sites for NFAT and AP-1 and is essential for peripheral, but not thymic, induction of FOXP3 expression.16,65 The binding of a RUNX1–CBF-β complex (runt-related transcription factor 1–core-binding factor subunit-β complex) to CNS2 was shown to be crucial for sustaining a high and stable level of FOXP3 expression in Treg cells.66 TCR stimulation activates the NF-κB family member REL for binding to CNS3, and is required for opening the Foxp3 promoter/enhancer region to promote FOXP3 expression.67,68,69 Using CNS region-specific deficient mice, all the above findings were corroborated by Zheng and colleagues.16 Additionally, it was also found that a conserved CpG island in the CNS2 region is hypomethylated in natural Treg cells, and hypermethylated in conventional CD4+ T cells. The methylation status of the highly conserved CpG island, also referred to as the Treg-specific demethylated region, determines FOXP3 expression level and the stability of Treg cells.70,71,72
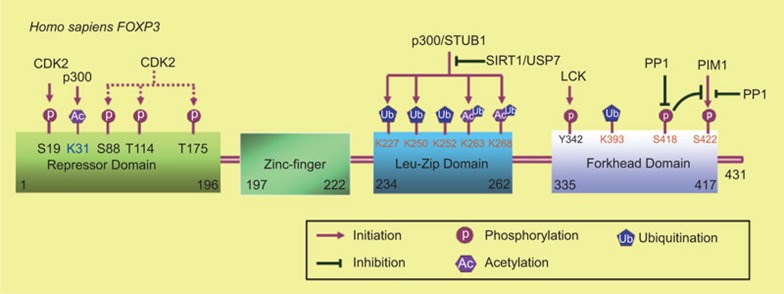
The post-translational modification of proteins is critical for the regulation of protein localization, stability and functional activity. The transcription factor FOXP3 contains several functional domains: a proline-rich domain is necessary for transcriptional activity; zinc finger and leucine zipper domains critical for protein–protein interaction; and a forkhead domain that is responsible for DNA binding. Acetylation, ubiquitination and phosphorylation of FOXP3 and the crosstalk between different modifications of FOXP3 have been studied comprehensively (Figure 1). The crystal structure of the NFAT1–FOXP3–DNA complex was resolved and the critical residues in FOXP3 that are responsible for its interaction with NFAT1 or DNA have been identified.73 The identification of post-translational modification sites may reveal those that are responsible for FOXP3 functional activity.
Figure 1.
Identified enzymes that are responsible for the post-translational modifications of FOXP3 and their corresponding modified residues in human FOXP3. Acetylation of FOXP3 at K263 and K268 by the acetyltransferase p300 stabilizes FOXP3 and promote its activity, while SIRT1 modulates the reciprocal modification. The E3 ligase STUB1 promotes the ubiquitination of FOXP3 at its leucine-zipper domain (K227, K250, K252, K263 and K268) and leads to its degradation, while the deubiquitinase USP7 prevents the ubiquitination process and stabilizes FOXP3. Acetylation and ubiquitination may compete for the same lysine residues in the leucine-zipper domain of FOXP3 to affect its stability and activity. FOXP3 is highly phosphorylated at Ser418 and dephosphorylation at the residue mediated by the phosphotase PP1 downregulates its repressive activity; The kinase PIM1 phosphorylates FOXP3 at Ser422 and negatively regulates FOXP3 activity, while the phosphatase PP1 works oppositely. Furthermore, phosphorylation at Ser418 prevents PIM1-mediated phosphorylation at Ser422. CDK2 phosphorylates FOXP3 at Ser88, Thr144 and Thr175 and promotes FOXP3 degradation. LCK phosphorylates FOXP3 at Tyr342 and enhances the repressive activity of FOXP3. CDK2, cyclin-dependent kinase 2; LCK, lymphocyte-specific protein kinase; PP1, protein phosphatase 1.
FOXP3 was first identified as an acetylated protein in human primary Treg cells.53 TGF-β-mediated stimulation can increase FOXP3 acetylation and enhance FOXP3 association to chromatin.74 Subsequently, FOXP3 was also found to undergo ubiquitination and the that ubiquitination would lead to its degradation.31,55 Since both ubiquitination and acetylation are restricted to lysine residues, acetylation may compete with poly-ubiquitination to stabilize FOXP3.57 Two acetyltransferases, Tip60 and p300, have been reported as enzymes that are responsible for FOXP3 acetylation. The histone acetyltransferase Tip60 could be recruited to the repressor domain of FOXP3 and regulate FOXP3-mediated suppression on IL-2 expression.53 p300 could also interact with FOXP3 and acetylate FOXP3 directly, and ectopic expression of p300 may strengthen FOXP3 stability.57 In vivo data also confirmed that a p300 specific inhibitor could decrease FOXP3 acetylation and Treg cell activity in mice.75 On the other hand, two lysine deacetylases, HDAC9 and SIRT1, can associate to FOXP3, and downregulates FOXP3 acetylation level and Treg activity, where knockdown of HDAC9 or SIRT1 has been shown to enhance Treg suppressive function in vitro.76,77
Proteins with Lysine-48 (K48)-linked poly-ubiquitination may undergo proteasome-mediated degradation.78 Recently, we demonstrated that proinflammatory cytokines and lipopolysaccharide could induce E3 ubiquitin ligase STUB1 expression and promote FOXP3 poly-ubiquitination and degradation in an Hsp70-dependent manner. Additionally, overexpression of STUB1 in Treg cells decreased their suppressive activity on the proliferation of Teff cells in vivo and in vitro, and induced a Th1-like phenotype, whereas knockdown of STUB1 expression in Treg cells enhanced their immunosuppressive activity.31 Consistent with our findings, Loosdregt and colleagues demonstrated that the deubiquitinase USP7 acted oppositely to STUB1. The researchers found that USP7 interacted with FOXP3 in the nuclei and highly expressed in Treg cells. Ectopic expression of USP7 decreased FOXP3 polyubiquitination and increased its stability. Knockdown of USP7 expression or treatment with USP7 inhibitor decreased endogenous FOXP3 expression in Treg cells and reduced their immunosuppressive activity in vitro. Further, adoptive transfer of USP7 knockdown or USP7 inhibitor-treated Treg cells have reduced function in resolving induced colitis in mice.55
Phosphorylation is the most common studied post-translational modification of proteins.79 It has been reported that phosphorylation at Ser418 in the C-terminal DNA binding domain of FOXP3 positively regulates its DNA binding activity and transcriptional regulation that affects Treg cell suppressive function. TNF-α-induced protein phosphatase 1 (PP1) expression specifically dephosphorylates FOXP3 at Ser418 in Treg cells from rheumatoid arthritis patients, leading to Treg cell loss of its immunosuppressive function. Moreover, TNF-α-induced Treg dysfunction also increases numbers of IL-17+ and IFN-γ+ CD4+ T cells within inflamed synovia in rheumatoid arthritis.60 Since the phosphorylation of FOXP3 at the Ser418 site is so critical for its activity regulation, further study is required in order to identify which kinase(s) are responsible. We have identified that PIM1 kinase phosphorylates FOXP3 and negatively regulates FOXP3 activity.54 PIM1 is highly expressed in fresh isolated Treg cells and can specifically phosphorylate human FOXP3 at Ser422, which is not present in its murine equivalent. This leads to the downregulation of DNA binding and Treg suppressive function on the proliferation of Teff cells. In addition, our data indicates that FOXP3 phosphorylation at Ser422, mediated by PIM1, is sensitive to phosphoserine mimetic mutation (S418D), which suggests the existence of crosstalk between phosphor-Ser418 and phosphor-Ser422 of FOXP3. We have also identified that all the PP1 phosphatase family members, PPP1CA, PPP1CB and PPP1CC, could dephosphorylate FOXP3 at Ser422, while PPP1CA plays a role in phosphor-Ser418 dephosphorylation, suggesting that PP1 plays a flexible role in modulating Treg immunosuppressive activity to prevent unnecessary activation or repression (Li ZY and Li B, unpubl. data). It has also been reported that cyclin-dependent kinase 2 could interact and phosphorylate FOXP3 at its repressor domain and negatively regulate the stability and activity of FOXP3.59 Lymphocyte-specific protein kinase could interact and phosphorylate FOXP3 at Tyr-342 in cancer cells, and this phosphorylation is positively related with its ability to suppress gene expression in cancer cells.80
All above-mentioned findings indicate that the epigenetic regulation of Foxp3 gene transcription, interactions between components of the FOXP3 complex and post-translational modifications of FOXP3 together regulate Treg cell activity and provide potential Treg-specific therapeutic targets for the development of clinical treatments. Many clinical trials relevant to the immunosuppressive activity of FOXP3+ Treg cells and their regulation have been carried out.
Clinical trials and FOXP3+ Treg cells
Currently, many phase I/II clinical trials relevant to FOXP3+ Treg cells have been completed or currently active. Several studies have utilized the infusion of ex vivo expanded CD4+CD25+ Treg cells to prevent graft-versus-host disease (GVHD) after organ transplantation in cancer patients, and their results showed the reduction of acute GVHD or no chronic GVHD development.81,82,83 On the other hand, a clinical trial has been launched to test whether Treg-depleted donor lymphocyte infusion improves graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation.84 Their results indicated that donor lymphocyte infusion is a safe approach to induce acute graft-versus-tumor effects in alloreactivity-resistant patients. In addition, other ongoing studies also infuse Treg-depleted donor lymphocytes to treat patients with relapsed hematological malignancies or in vivo strategies to decrease Treg cells with chemical reagents to activate tumor-specific immune responses in patients with advanced hepatocellular carcinoma.
High doses of cyclophosphamide and sirolimus have been successfully used to prevent GVHD and have been shown to enhance the activity of Treg cells.85,86,87 Although early studies have suggested a negative effect of cyclophosphamide on Treg cells, the role of Treg cells in the cyclophosphamide GVHD prophylactic effect, is essential. In the xenogenic GVHD mouse model, Kanakry and colleagues proved that when peripheral blood mononuclear cell grafts were depleted of Tregs, the humanized mice had severe GVHD scores and died earlier, and suggested the requirement of Treg cells for the optimal effect of cyclophosphamide.88 Sirolimus, also named as rapamycin, preferentially inhibits effector T cells and results in the relative expansion of natural Treg cells.89 Peccatori and colleagues showed how sirolimus-based GVHD prophylaxis facilitates the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors, which is a promising therapeutic option for high risk hematological malignancies.87 In addition to above mentioned clinical trials, cyclophosphamide or sirolimus plus other immunosuppressive drugs, such as tacrolimus and ustekinumab, or the addition of low doses of IL-2 and a demethylating agent such as azacitidine to promote and stabilize the FOXP3 expression in Treg cells, are also under clinical testing.
Perspective and implications
As discussed above, Treg cells are a heterogeneous population and their stability and plasticity under inflammatory conditions may pose serious problems for their clinical usage. Thus, mature Treg cells that have stable epigenetic modifications or hallmark stable FOXP3 expression and immunosuppressive activity are preferable for clinical treatment.
Successful ex vivo expansion of natural Treg cells or induction of induced Treg cells is critical for adoptive Treg cell infusion therapy of GVHD or autoimmune diseases. Thus, the identification of optimal reagents for enriched Treg cell preparation is extremely important. All-trans retinoic acid, the active derivative of vitamin A, could be used to efficiently convert naive CD4+ T cells to FOXP3-expressing highly suppressive induced Treg cells in the presence of TGF-β90 and sustain the stability and function of natural Treg cells even during inflammation.91,92 As mentioned previously, rapamycin facilitates the expansion of functional CD4+CD25+FOXP3+ Treg cells, but depletes Teff cells, and so it could be a useful tool to expand highly purified Treg cells for cellular immunotherapy.
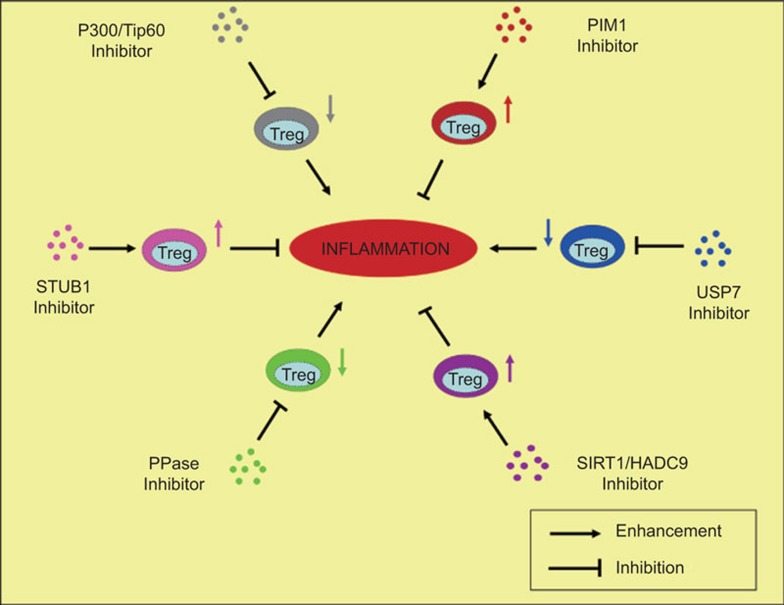
To obtain highly stable and active ex vitro expanded Treg cells, we also propose that the study of how Treg cell activity is regulated via the post-translational modification of FOXP3, such as phosphorylation, ubiquitination and acetylation, may prove to be critical for manipulating Treg activity on the therapeutic level, which may positively or negatively regulate Treg cell function through modifying FOXP3 (Figure 2). A PIM1-specific inhibitor could potentially be used to enhance the immunosuppressive activity of Treg cells through downregulating the phosphorylation of FOXP3-Ser422 during ex vivo preparation of Treg cells,54 while PP1-specific inhibitor may work reversely and weaken the immunosuppressive activity of Treg cells;60 STUB1-specific inhibitors, may stabilize Treg cells through preventing FOXP3 ubiquitination and degradation, whereas USP7-specific inhibitor treated Treg cells may lose their stability through promoting the process;31,55 SIRT1/HDAC9 inhibitors would enhance the activity of Treg cells through promoting FOXP3 acetylation and facilitating its stability or activity upregulation, while P300/TIP60 inhibitors may abolish the process and promote FOXP3 degradation mediated by FOXP3 ubiquitination.53,57,75,76 Along with the accumulation of our knowledge on the molecular mechanism of Treg immunosuppressive activity, we expect that in the next few years, increasingly safe and effective compounds, which could aid the ex vivo expansion of Treg cells by helping to maintain their stable function in vivo, will be identified and advance into clinical trials for the treatment of autoimmune diseases and other inflammatory diseases.
Figure 2.
Enzymes responsible for post-translational modification of FOXP3 are potential therapeutic targets for inflammation. Inhibitors of FOXP3 positive regulators, including P300/TIP60, USP7 and PPase, could be used to activate immune system through downregulating the immunosuppressive activity of Treg cells; inhibitors of FOXP3 negative regulators, including SIRT1/HDAC9, STUB1 and PIM1, could be used to put a brake on immune responses through upregulating the immunosuppressive function of Treg cells. Treg, regulatory T.
Acknowledgments
This work is supported by National Basic Research Program of China (973 Program) 2014CB541803, 2014CB541903, NSFC 81330072, 31370863, 31170825, 81271835, 31200646, 81302532, 31350110505 and SMCST 11ZR1404900. We gratefully acknowledge the Chinese Academy of Sciences Fellowships for Young International Scientists (2013Y1SB0005) and the Knowledge Innovation Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (2012KIP204).
References
- 1Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164. [PubMed] [Google Scholar]
- 2Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 2010; 11: 7–13. [DOI] [PubMed] [Google Scholar]
- 3Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010; 140: 845–858. [DOI] [PubMed] [Google Scholar]
- 4Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 2011; 11: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 2009; 10: 689–695. [DOI] [PubMed] [Google Scholar]
- 6Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 7Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061. [PubMed] [Google Scholar]
- 8Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4: 337–342. [DOI] [PubMed] [Google Scholar]
- 9Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001; 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 10Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27: 20–21. [DOI] [PubMed] [Google Scholar]
- 11Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol 2013; 190: 2001–2008. [DOI] [PubMed] [Google Scholar]
- 12Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184: 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 2012; 209: 1713–1722, S1–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20: 62–68. [DOI] [PubMed] [Google Scholar]
- 15Ogawa C, Tone Y, Tsuda M, Peter C, Waldmann H, Tone M. TGF-beta-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J Immunol 2013; 192: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010; 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 2007; 204: 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Wang L, Liu Y, Han R, Beier UH, Thomas RM, Wells AD et al. Mbd2 promotes foxp3 demethylation and T-regulatory-cell function. Mol Cell Biol 2013; 33: 4106–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30: 899–911. [DOI] [PubMed] [Google Scholar]
- 20Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490–500. [DOI] [PubMed] [Google Scholar]
- 21Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009; 326: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009; 10: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 2009; 458: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol 2015; 16: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol 2010; 22: 575–582. [DOI] [PubMed] [Google Scholar]
- 26Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4+ FoxP3+ T cells. Curr Opin Immunol 2009; 21: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol 2009; 39: 948–955. [DOI] [PubMed] [Google Scholar]
- 28Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 2009; 323: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 29Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 2009; 10: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009; 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 2013; 39: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Liu X, Nguyen P, Liu W, Cheng C, Steeves M, Obenauer JC et al. T cell receptor CDR3 sequence but not recognition characteristics distinguish autoreactive effector and Foxp3+ regulatory T cells. Immunity 2009; 31: 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D et al. Stability of the regulatory T cell lineage in vivo. Science 2010; 329: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA 2009; 106: 1903–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2013; 20: 62–68. [DOI] [PubMed] [Google Scholar]
- 36Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008; 112: 2340–2352. [DOI] [PubMed] [Google Scholar]
- 37Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA 2009; 106: 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006; 118: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity 2003; 19: 877–889. [DOI] [PubMed] [Google Scholar]
- 40Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol 2008; 180: 5916–5926. [DOI] [PubMed] [Google Scholar]
- 41Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 2008; 28: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 2005; 202: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001; 194: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA 2003; 100: 10878–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007; 450: 566–569. [DOI] [PubMed] [Google Scholar]
- 47Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol 2008; 181: 6898–6905. [DOI] [PubMed] [Google Scholar]
- 48Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998; 188: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 2004; 34: 2480–2488. [DOI] [PubMed] [Google Scholar]
- 50Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 2007; 204: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol 2006; 177: 6780–6786. [DOI] [PubMed] [Google Scholar]
- 53Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA 2007; 104: 4571–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Li Z, Lin F, Zhuo C, Deng G, Chen Z, Yin S et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem 2014; 289: 26872–26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 2013; 39: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 2011; 146: 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 2010; 115: 965–974. [DOI] [PubMed] [Google Scholar]
- 58Kwon HS, Lim HW, Wu J, Schnolzer M, Verdin E, Ott M. Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J Immunol 2012; 188: 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD. Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J Biol Chem 2013; 288: 24494–24502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Nie H, Zheng Y, Li R, Guo TB, He D, Fang L et al Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med 2013; 19: 322–328. [DOI] [PubMed] [Google Scholar]
- 61Li B, Samanta A, Song X, Furuuchi K, Iacono KT, Kennedy S et al. FOXP3 ensembles in T-cell regulation. Immunol Rev 2006; 212: 99–113. [DOI] [PubMed] [Google Scholar]
- 62Li B, Samanta A, Song X, Iacono KT, Brennan P, Chatila TA et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int Immunol 2007; 19: 825–835. [DOI] [PubMed] [Google Scholar]
- 63Song X, Li B, Xiao Y, Chen C, Wang Q, Liu Y et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep 2012; 1: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 2012; 13: 1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006; 108: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H et al. Indispensable role of the Runx1–Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity 2009; 31: 609–620. [DOI] [PubMed] [Google Scholar]
- 67Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206: 3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI et al. Development of Foxp3+ regulatory t cells is driven by the c-Rel enhanceosome. Immunity 2009; 31: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009; 31: 921–931. [DOI] [PubMed] [Google Scholar]
- 70Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol 2007; 37: 2378–2389. [DOI] [PubMed] [Google Scholar]
- 71Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol 2009; 9: 83–89. [DOI] [PubMed] [Google Scholar]
- 73Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2006; 20: 62–68. [DOI] [PubMed] [Google Scholar]
- 74Samanta A, Li B, Song X, Bembas K, Zhang G, Katsumata M et al. TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc Natl Acad Sci USA 2008; 105: 14023–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC et al. Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat Med 2013; 19: 1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology 2010; 138: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol 2011; 31: 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78Pickart CM. Ubiquitin enters the new millennium. Mol Cell 2001; 8: 499–504. [DOI] [PubMed] [Google Scholar]
- 79Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002; 298: 1912–1934. [DOI] [PubMed] [Google Scholar]
- 80Nakahira K, Morita A, Kim NS, Yanagihara I. Phosphorylation of FOXP3 by LCK downregulates MMP9 expression and represses cell invasion. PLoS One 2013; 8: e77099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011; 117: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117: 3921–3928. [DOI] [PubMed] [Google Scholar]
- 83Wang X, Lu L, Jiang S. Regulatory T cells: customizing for the clinic. Sci Transl Med 2011; 3: 83ps19. [DOI] [PubMed] [Google Scholar]
- 84Maury S, Lemoine FM, Hicheri Y, Rosenzwajg M, Badoual C, Cherai M et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med 2010; 2: 41ra52. [DOI] [PubMed] [Google Scholar]
- 85Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease: a review. Biol Blood Marrow Transplant 2014; in press. [DOI] [PubMed]
- 86Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 2012; 39: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87Peccatori J, Forcina A, Clerici D, Crocchiolo R, Vago L, Stanghellini MT et al. Sirolimus-based graft-versus-host disease prophylaxis promotes the in vivo expansion of regulatory T cells and permits peripheral blood stem cell transplantation from haploidentical donors. Leukemia 2014; in press. [DOI] [PubMed]
- 88Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013; 5: 211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients J Immunol 2006; 177: 8338–8347. [DOI] [PubMed] [Google Scholar]
- 90Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol 2009; 183: 4119–4126. [DOI] [PubMed] [Google Scholar]
- 91Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol 2010; 185: 2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA 2014; 111: E3432–E3440. [DOI] [PMC free article] [PubMed] [Google Scholar]