Abstract
Regulatory T (Treg) cells play a central role in regulating peripheral immune tolerance and preventing autoimmunity. Despite the extensive studies on the development of Treg cells, the molecular mechanisms that maintain the population of committed Treg cells remain poorly understood. We show here that Treg-conditional ablation of the kinase TAK1 reduced the number of Treg cells in the peripheral lymphoid organs, causing abnormal activation of conventional T cells and autoimmune symptoms. Using an inducible gene knockout approach, we further demonstrate that TAK1 is crucial for the survival of Treg cells. Expression of a constitutively active IκB kinase partially restored the level of Treg cells in the TAK1Treg-KO mice. These results suggest a crucial role for TAK1 for maintaining the survival of committed Treg cells under physiological conditions.
Keywords: regulatory T cells, autoimmunity, TAK1, IKK, Foxp3
INTRODUCTION
Foxp3 expressing regulatory T (Treg) cells play a central role in maintaining peripheral immune tolerance and, thereby, preventing autoimmunity and chronic inflammations.1,2 Treg cells suppress the activation of conventional T cells and other immune cells via both cell–cell contact and the immunosuppressive action of secreted cytokines, such as transforming growth factor-β (TGF-β), IL-10, and IL-35.1,3,4 Most Treg cells are developed in the thymus, although Treg cells can also be inducibly produced in the periphery from conventional T cells in the presence of TGF-β and retinoic acid.5,6 Both the development and immunosuppressive function of Treg cells are critically dependent on the transcription factor Foxp3 and its regulatory factors.7 Genetic deficiencies in these core regulators of Treg cells are associated with perturbed T-cell homeostasis and severe autoimmunity.
The NF-κB signaling pathway is important for the development of Treg cells.8,9 Diverse immune stimuli induce the canonical NF-κB pathway through activation of its upstream kinase, the IκB kinase (IKK). Activation of IKK/NF-κB by the T-cell receptor (TCR) involves a signaling complex that is composed of the scaffold protein CARMA1, the adaptor BCL10, and the paracaspase MALT1.10,11 The signaling function of this so-called CBM complex requires a ubiquitin-conjugating enzyme, UBC13, which mediates lysine 63 (K63) ubiquitination of the IKK regulatory subunit NEMO and the IKK-activating kinase TAK1 required for catalytic activation of the TAK1-IKK signaling axis.12 Genetic deficiencies in the CBM component or the downstream signaling factors, TAK1, IKK, and NF-κB, severely attenuates the thymic development of Treg cells.13,14,15,16,17,18,19,20,21 In addition, conditional UBC13 knockout (KO) studies revealed that the UBC13-IKK signaling axis regulates the function of Treg cells and prevent their conversion into inflammatory T cells.22
In addition to mediating IKK activation, TAK1 targets the activation of MAP kinases, including ERK, p38, and JNK.23,24,25 TAK1 transduces signals from multiple immune receptors, including the toll-like receptors (TLRs), TCR, B-cell receptor (BCR), and TNF receptor (TNFR) superfamily members.24,25,26 TAK1 has also been implicated in the regulation of survival signals stimulated by common gamma chain cytokines.27 Previous studies suggest a crucial role for TAK1 in mediating thymocyte development.14,27,28 Ablation of TAK1 in immature thymocytes, using LCK-Cre or CD4-Cre, severely reduces the generation of CD4 and CD8 single-positive thymocytes as well as Treg cells in the thymus.14,27,28 However, the role of TAK1 in mediating the survival and homeostasis of committed Treg cells has remained unknown. In the present study, we addressed this question by generating Treg cell-conditional TAK1 KO mice using the Foxp3-Cre approach. We found that TAK1 is indispensable for maintaining the peripheral population of Treg cells.
MATERIALS AND METHODS
Mice
Mice with loxP-flanked TAK1 allele (TAK1-flox mice) were provided by Dr. Shizuo Akira (Osaka University).14,24 Foxp3-EGFP-hCre mice (Jackson Lab) carry a bacterial artificial chromosome transgene that expresses enhanced green fluorescence protein (EGFP) and a humanized Cre under the control of mouse Foxp3 promoter. These mice were backcrossed for nine generations onto the C57BL/6 background and then crossed with TAK1-flox mice to produce age-matched TAK1+/+Foxp3GFP-hCre (termed wild-type (WT)) mice and TAK1fl/flFoxp3GFP-hCre (termed TAK1Treg-KO) mice. Since the fluorescence intensity of EGFP is relatively low, the TAK1Treg-KO mice were further crossed with ROSA26-YFP (termed R26YFP) Cre reporter mice(Jackson Lab) to generate WT and TAK1Treg-KO mice that express yellow fluorescent protein (YFP) in Treg cells (WT-R26YFP and TAK1Treg-KOR26YFP, respectively). The R26YFP mice express YFP from the ubiquitous ROSA26 locus under the control of a loxP-flanked stop cassette,29 thus assuring YFP expression only in Foxp3-Cre+ Treg cells. CreERT2 mice (Jackson Lab) express, from the ROSA26 locus, a tamoxifen-inducible Cre fused with a triple-mutant form of human estrogen receptor (ERT2). These mice were crossed with TAK1-flox mice to generate TAK1+/+CreERT2 (WT-CreERT2) and TAK1fl/flCreERT2 mice for inducible TAK1 deletion. IKK2CA-transgenic (IKK2CATg) mice (Jackson Lab) express a constitutively active IKK2 (IKK2CA) under the control of a loxP-flanked stop cassette. Mice were maintained in specific pathogen-free facility of The University of Texas MD Anderson Cancer Center, and all animal experiments were in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Cell preparation and flow cytometric analysis
Mononuclear cells were isolated form the spleen and intestinal lamina propria as previously described.22 Flow cytometric analyses were performed using a FACSCalibur (BD). For intracellular cytokine staining, cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich) plus 750 ng/ml ionomycin (Calbiochem) for 4–6 h in the presence of 10 μg/ml monensin (eBioscience), and the fixed cells were incubated with the indicated antibodies and subjected to flow cytometry. Antibodies were described previously.22,30
In vitro Treg differentiation and TAK1 deletion
CD4+CD25– naïve T cells (>98% purity), isolated from TAK1+/+CreERT2 or TAK1fl/flCreERT2 mice, were stimulated under Treg skewing conditions (5 μg/ml anti-IL-4, 5 μg/ml anti-IFN-γ and 2.5 ng/ml TGF-β (R&D System, 240-B) plus 10 ng/ml IL-2 (R&D System, 402-ML)). After 3 days, the cells were treated with1 μM tamoxifen (Sigma-Aldrich) for 24 h to induce TAK1 gene deletion. After removing tamoxifen, in vitro generated Treg cells were resuspended in fresh media and used for the indicated experiments.
Apoptosis assay
Apoptosis was detected using a commercial kit (BD Pharmingen) based on Annexin V binding to apoptotic cells and propidium iodide (PI) staining of late-stage apoptotic cells and necrotic cells. In brief, cells were resuspended in 1 × binding buffer containing fluorescein isothiocyanate-conjugated Annexin V (Annexin V-FITC) and PI. After incubation for 15 min at room temperature in the dark, the cell suspension was diluted with 1x binding buffer and subjected to cytometry analyses.
Histology
Organs were removed from sacrificed mice, fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned for hematoxylin and eosin staining.
Statistical analysis
Prism software was used for two-tail unpaired t-tests. P-values of less than 0.05 or 0.01 were considered significant and very significant, respectively.
RESULTS
Ablation of TAK1 in Treg cells causes autoimmune symptoms
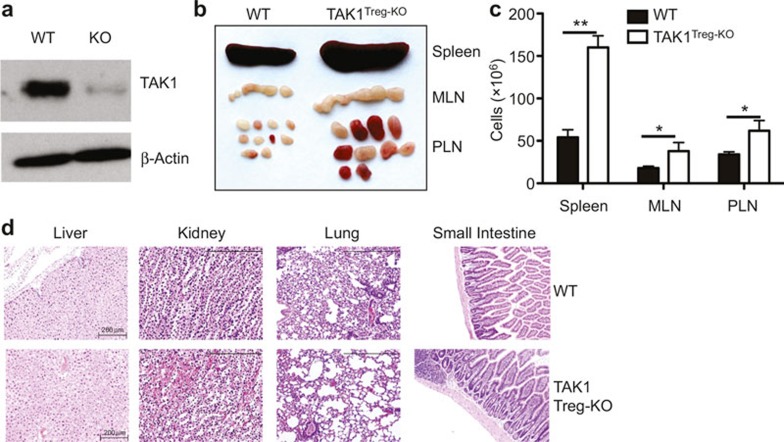
To examine the function of TAK1 in Treg cells, we generated Treg-conditional TAK1 KO mice by crossing the TAK1-floxed mice14 with Foxp3-Cre mice. We used the resulting TAK1fl/flFoxp3GFP-Cre (called TAK1Treg-KO) and age-matched TAK1+/+Foxp3GFP-Cre control (called WT) mice for experiments. Immunoblotting assays using sorted Treg (CD4+CD25+GFP+) cells revealed efficient ablation of TAK1 in the Treg cells of TAK1Treg-KO mice (Figure 1a). TAK1Treg-KO mice were born at the expected Mendelian ratios and did not have obvious abnormality in growth and survival. However, these mutant mice had profoundly enlarged spleen, mesenteric lymph nodes (LNs), and peripheral LNs, an autoimmune phenotype often associated with perturbed immune cell homeostasis (Figure 1b). Indeed, the peripheral lymphoid organs of the TAK1Treg-KO mice also had increased cellularity (Figure 1c). On the other hand, histology analyses revealed that the TAK1Treg-KO mice only displayed mild tissue inflammation at older ages, mostly seen in the kidney (Figure 1d). These results suggest that Treg-specific ablation of TAK1 causes certain aspects of autoimmunity but does not lead to severe inflammatory disorders.
Figure 1.
Treg cell-specific ablation of TAK1 causes autoimmune symptoms. (a) Immunoblot analyses of TAK1 and β-actin using sorted Treg (CD4+CD25+GFP+) WT and TAK1Treg-KO (KO) mice. (b) Spleen, mesenteric lymph nodes (MLN), and peripheral lymph nodes (PLN) are isolated from age- and sex-matched WT and TAK1Treg-KO mice (10-week-old). (c) Total cells in the spleen, MLN, and PLN of WT and TAK1Treg-KO mice (10-week-old). (d) hematoxylin and eosin staining of sections of the indicated nonlymphoid tissues from 20-week-old WT and TAK1Treg-KO mice. *P < 0.05 and **P < 0.01 (two-tailed unpaired t-test). Data are representative of three independent experiments (b, c; SE in c) or two experiments with 3–4 mice per genotype (a, d).
Abnormal activation of conventional T cells in TAK1Treg-KO mice
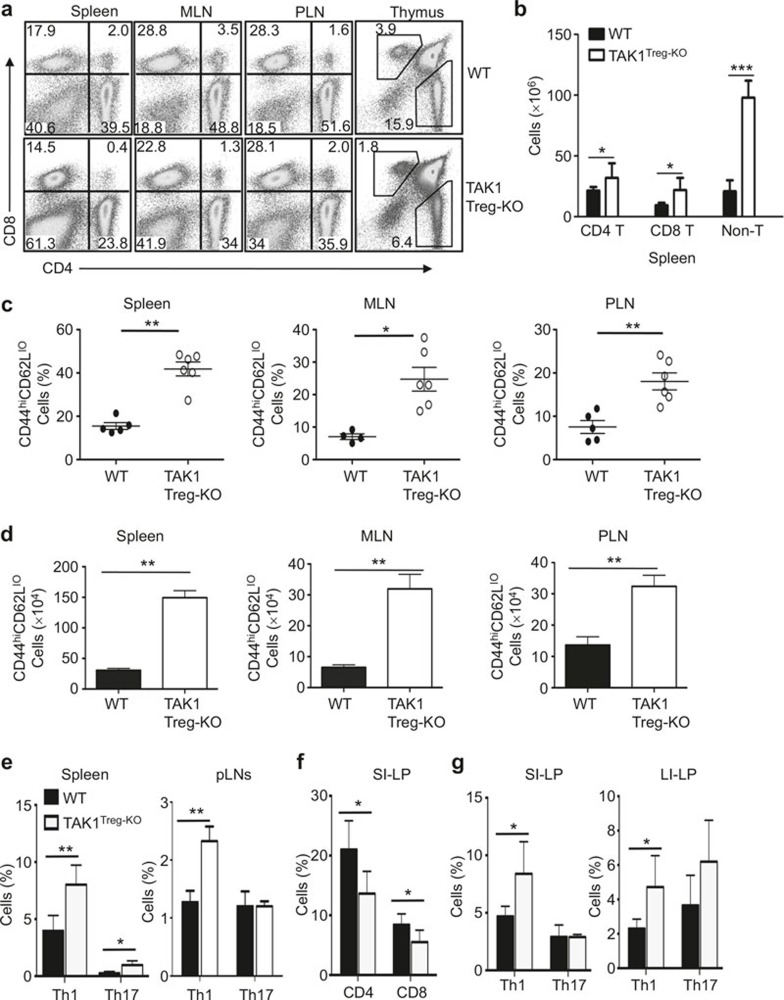
A well-defined function of Treg cells is to maintain immune homeostasis and prevent T-cell activation by self-antigens.1,2 To examine the effect of Treg-specific ablation of TAK1, we analyzed the frequency of T cells in peripheral lymphoid organs and the thymus. Both the spleen and LNs of the TAK1Treg-KO mice contained a moderately lower frequency of CD4+ or CD8+ T cells (Figure 2a). This result was apparently due to a substantial increase in the number of non-T cells (CD4–CD8–) associated with the autoimmune enlargement of lymphoid organs (Figure 2b). In fact, the absolute number of CD4+and CD8+ T cells was even higher in the spleen of the TAK1Treg-KO mice (Figure 2b). To further examine the homeostasis of T cells in TAK1Treg-KO mice, we quantified the frequency of memory and naïve T cells based on their expression of the surface activation markers, CD44 and CD62L. In both the spleen and the LNs, TAK1Treg-KO mice had a drastic increase in the frequency of effector/memory-like (CD44hiCD62Llo) CD4+ T cells than WT mice (Figure 2c). The TAK1Treg-KO mice also had a significant increase in the absolute number of effector/memory-like CD4+ T cells (Figure 2d). Intracellular cytokine staining revealed that the TAK1Treg-KO mice had a significantly increased frequency of Th1 effector T cells in spleen and LNs, although the Th17 cells were only increased in the spleen (Figure 2e).
Figure 2.
Treg cell-specific ablation of TAK1 impairs T-cell homeostasis. (a, b) Flow cytometric analysis of T cells from the indicated lymphoid organs of 8-week-old WT and TAK1Treg-KO mice. Data are presented as a representative plot (a) and summary graph (b). Numbers in quadrants of a indicate percent of CD8+ T cells (top left), CD4+ T cells (bottom right), and non T cells (bottom left). (c, d) Flow cytometric analysis of the frequency (c) and absolute number (d) of memory-like (CD62LloCD44hi) CD4+ T cells in the indicated lymphoid organs of age- and sex-matched WT and TAK1Treg-KO mice (8–10 weeks old). (e) Frequency of CD4+ T cells producing IL-17, and/or IFN-γ (gated on CD3+CD4+ cells) in the spleen or PLNs of 8- to 10-week-old WT and TAK1Treg-KO mice, assessed by intracellular cytokine staining. (f, g) Frequency of CD4+ and CD8+ T cells (f) and frequency of IL-17-producing Th17 and IFN-γ-producing Th1 cells (g) in the small intestinal lamina propria (SI-LP) or large intestinal lamina propria (LI-LP) of 8-week-old WT and TAK1Treg-KO mice, assessed by flow cytometry. *P < 0.05 and **P < 0.01 (two-tailed unpaired t-test). Data are representative of four experiments with three mice per group (a, b; means ± SE in b), five experiments with five mice per group (c–e; means ± SE in c, d), two experiments with three mice per group (f, g).
Since Treg has an important role in regulating immune homeostasis in the intestine, we examined the effect of Treg cell-specific TAK1 ablation on the frequency of T cells in the lamina propria of the small and large intestines. The TAK1Treg-KO mice had a moderate reduction in the frequency of CD4+ and CD8+ T cells in the lamina propria of the small intestine (Figure 2f). However, the CD4+ T cell population of TAK1Treg-KO mice had a much greater frequency of Th1 cells in the lamina propria of both the small and large intestines. The large intestine of TAK1Treg-KO mice also had a moderate increase in the frequency of Th17 cells, although this result was not statistically significant (Figure 2g). Thus, Treg-specific ablation of TAK1 perturbed the homeostasis of TAK1-sufficient conventional T cells, which suggests that TAK1 may regulate the survival or immunosuppressive function of Treg cells.
TAK1 is required for the maintenance of Treg cells
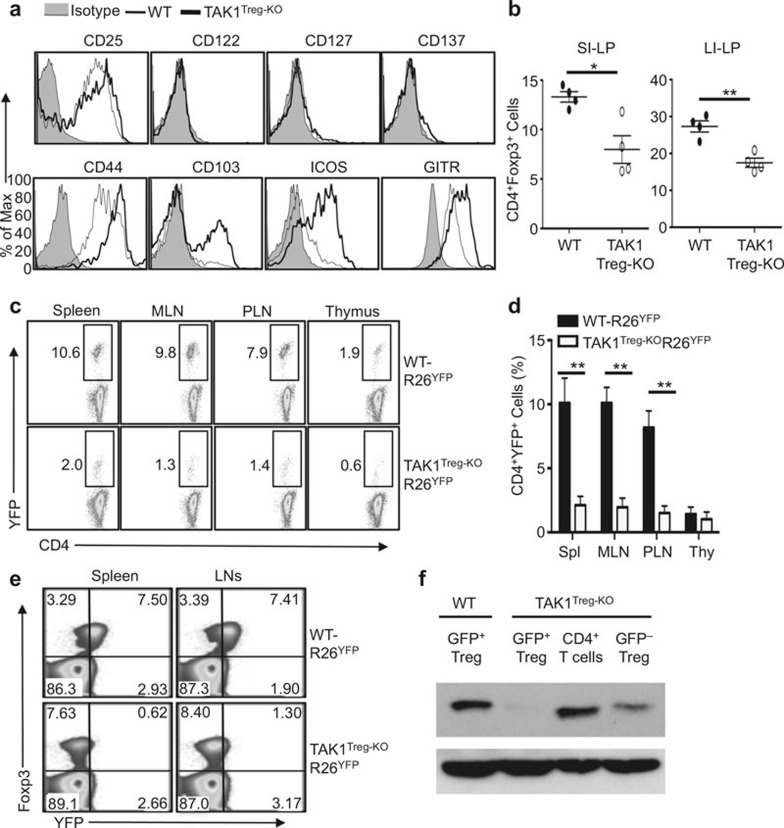
To assess the mechanism by which TAK1 regulating Treg function, we examined the expression of Treg-signature molecules on WT and TAK1-deficient Treg cells. The Treg cells derived from the TAK1Treg-KO mice had competent expression of several surface molecules analyzed (Figure 3a). In fact, the expression of some of these molecules, including CD44, CD103, ICOS, and GITR, was substantially enhanced in TAK1-deficient Treg cells (Figure 3a). It has been suggested that Treg cells expressing high levels of these molecules represent an effector/memory Treg population that is often seen at site of immune response or inflammation.31,32 Thus, this phenotype of the Treg cells was possibly linked to the aberrant activation of conventional T cells, particularly the inflammatory Th1 cells, in the TAK1Treg-KO mice (Figure 2).
Figure 3.
TAK1 is required for Treg maintenance. (a) Flow cytometric analysis of the expression of the indicated surface markers on Foxp3+CD4+ Treg cells from the spleens of WT and TAK1Treg-KO mice. (b) Flow cytometric analysis of the frequency of Foxp3+CD4+ Treg cells in the SI-LP and LI-LP of 6-week-old WT and TAK1Treg-KO mice. (c, d) Flow cytometric analysis of the frequency of YFP+ Treg cells (among CD3+CD4+ cells) in the indicated lymphoid organs of 6-week-old R26YFP mice (WT-R26YFP) and TAK1Treg-KOR26YFP mice. Data are presented as a representative plot (c) and a summary graph of mean ± SE value (d). (e) Flow cytometric analysis of the frequency of YFP+Foxp3+ Treg cells (among CD3+CD4+ cells) in spleen and LNs of 8-week-old WT-R26YFP and TAK1Treg-KOR26YFP mice. *P < 0.05 and **P < 0.01 (two-tailed unpaired t-test). Data are representative of three independent experiments with four mice per group.
To examine the role of TAK1 in the maintenance of peripheral Treg cells, we analyzed the frequency of Treg cells based on their expression of Foxp3. The TAK1Treg-KO mice had a significantly reduced frequency of the CD4+Foxp3+ Treg cells in the lamina propria of the small and large intestines, compared to WT control mice, although this phenotype was less prominent in the spleen and LNs (Figure 3b and data not shown). To more definitively determine the role of TAK1 in maintaining Treg cells, we employed the R26YFPCre reporter mice, which express YFP a Cre-dependent manner. The progeny of R26YFP mice crossed with Foxp3-EGFP-hCre mice express YFP only in Treg cells, which represent the TAK1-deleted Treg cells. The TAK1-deleted Treg cells should also express GFP from the Foxp3-EGFP-hCre transgene, but the fluorescence intensity of GFP was relatively dim (data not shown). So, we used YFP or GFP as a marker for detecting the TAK1-ablated Treg cells in our subsequent analyses. Compared with WT-R26YFP mice, TAK1Treg-KOR26YFP mice had a strikingly lower frequency of YFP+ population of Treg cells even when they were young (Figure 3c and d). This result was much more prominent than the Treg reduction detected based on Foxp3 expression. We thus analyzed the Treg cells based on expression of both Foxp3 and YFP. Interestingly, while the Treg cells in the WT-R26YFP mice were mostly double positive for Foxp3 and YFP, the majority of the Treg cells in the TAK1Treg-KOR26YFP mice were Foxp3+YFP– (Figure 3e). This result suggested that the TAK1-ablated Treg cells (YFP+) might be rapidly lost, with the lost Treg population partially compensated through expansion of Treg cells that escaped the Cre-mediated TAK1 deletion (YFP–). To further examine this possibility, we sorted GFP+ and GFP– Treg cells from WT and TAK1Treg-KO mice and analyzed TAK1 expression by immunoblot. As expected, the GFP+ Treg cells from WT mice expressed TAK1, whereas the GFP+ Treg cells, although not the conventional CD4+ T cells, from the TAK1Treg-KO mice lacked TAK1 expression (Figure 3f). Importantly, the GFP– Treg cell population from the TAK1Treg-KO mice expressed a substantial level of TAK1, suggesting leakage in TAK1 expression. These findings explained why the autoimmune symptoms of the TAK1Treg-KO mice were not quite serious.
TAK1 ablation causes Treg cell apoptosis
The great reduction in YFP+ Treg cells in the TAK1Treg-KOR26YFP mice suggested a crucial role for TAK1 in mediating Treg cell survival. We thus analyzed the apoptosis of Treg cells based on staining with PI and Annexin V. The Treg-specific ablation of TAK1 did not alter the survival ability of conventional T cells, since these cells were mostly viable in both the TAK1Treg-KOR26YFP and control (WT-R26YFP) mice (Figure 4a). In contrast, the TAK1Treg-KOR26YFP mice had substantially increased apoptotic cells in the YFP+ Treg population compared with the WT-R26YFP mice (Figure 4b).
Figure 4.
Ablation of TAK1 induces apoptosis in Treg cells.(a, b) Apoptosis analysis of CD4+CD25–YFP– naïve T cells (a) and CD4+YFP+ Treg cells (b) from WT-R26YFP and TAK1Treg-KOR26YFP mice, detected based on Annexin V and PI staining. Frequency of viable cells (Annexin V–PI–) was presented as mean ± SE values of four mice (right). (c) Naïve T cells (CD3+CD4+CD25–) isolated from TAK1+/+CreERT2 and TAK1fl/flCreERT2 mice were stimulated with coated anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) antibodies plus TGF-β (2.5 ng/ml) and IL-2 (10 ng/ml). After 3 days, Foxp3 expression was detected by flow cytometry. (d, e) Treg cells, described in c, were treated with tamoxifen for 24 h. After removing the tamoxifen, cells were subjected to immunoblot (d) or flow cytometric analyses of p65 phosphorylation under unstimulated or stimulated (anti-CD3 plus anti-CD28 for 15 min by a crosslinking method) conditions (e). (f, g) Treg cells, described in c, were treated with either medium control or tamoxifen for 24 h and then restimulated in fresh media with plate-bound anti-CD3 and anti-CD28 antibodies for 24 h. Apoptotic cells were detected by flow cytometry based on Annexin V and PI staining (f), and the frequency of viable cells (annexinV–PI–) was presented as mean ± SE values of the indicated numbers of mice (each circle represents a mouse) (g). *P < 0.05 (two-tailed unpaired t-test). Data are representative of three independent experiments with four mice per group.
To further confirm the essential role for TAK1 in mediating Treg cell survival, we employed CreERT2 mice, in which the Cre activity could be induced by tamoxifen. We generated Treg cells in vitro using naïve CD4+ T cells derived from WT or TAK1fl/flCreERT2 mice (Figure 4c). We then incubated the Treg cells with tamoxifen, which led to efficient ablation of TAK1 (Figure 4d). The loss of TAK1 was in turn associated with impaired NF-κB signaling detected based the phosphorylation of NF-κB p65 (Figure 4e). While tamoxifen had little effect on the survival of the WT Treg cells, tamoxifen induced massive cell death in the TAK1fl/flCreERT2 Treg cells (Figure 4e and f). Taken together, these results suggest that TAK1 is a critical factor that mediates Treg cell survival.
IKK partially mediates the function of TAK1 in Treg cell homeostasis
The major downstream signaling pathways of TAK1 include those leading to the activation of IKK and two MAP kinases, p38 and JNK.23,24,25 To determine the role of IKK signaling in TAK1-mediated Treg survival, we restored IKK signaling in Treg cells by crossing the TAK1Treg-KO mice with a transgenic mouse expressing constitutively active IKK2 (IKK2CA) under the control of a loxP-flanked stop cassette (called IKK2CATg mice). This transgenic system allows expression of IKK2CA specifically in Treg cells upon crossing with Foxp3GFP-Cre mice. By crossing IKK2CATg with TAK1Treg-KO mice, we generated age-matched TAK1+/+IKK2CATg/+Foxp3GFP-Cre mice (called WT-IKK2CATreg-Tg) and TAK1fl/flIKK2CATg/+Foxp3GFP-Cre (called TAK1Treg-KOIKK2CATreg-Tg) mice.
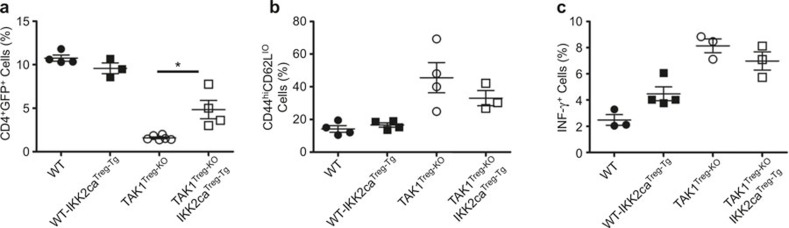
Treg cell-specific expression of the IKK2CA transgene in WT mice did not appreciably alter the frequency of Treg cells (Figure 5a). Interestingly, IKK2CA partially, but significantly, restored the viability of TAK1-deficient Treg cells (Figure 5a). However, the partial restoration of Treg cells appeared to be insufficient for correcting homeostasis perturbation in the conventional T cells of the TAK1Treg-KO mice, since the TAK1Treg-KOIKK2CATreg-Tg mice only had moderately reduced effector/memory T cells and IFNγ+Th1 cells compared to the WT-IKK2CATreg-Tg mice (Figure 5b and c). These findings suggest that IKK is partially involved in the survival function of TAK1 in Treg cells.
Figure 5.
Expression of a constitutively active IKK2 in Treg cells partially rescues Treg population in TAK1Treg-KO mice. Flow cytometry was performed to determine the frequency of CD4+GFP+ Treg cells (a), CD44hiCD62Llo memory-like conventional T cells (b), and IFN-γ producing T cells (c) (gated on CD3+CD4+ cells) in the spleen of 10-week-old WT, IKK2CATreg-Tg, TAK1Treg-KO, and TAK1Treg-KOIKK2CATreg-Tg mice. *P < 0.05 (two-tailed unpaired t-test). Data are representative of two experiments.
DISCUSSION
The data presented in this paper demonstrate a crucial role for TAK1 in maintaining the peripheral population of Treg cells. Loss of TAK1 in committed Treg cells causes Treg cell apoptosis. As a result, the TAK1Treg-KO mice had reduced frequency of Treg cells, coupled with aberrant activation of conventional T cells and autoimmune symptoms. The mutant animals had enlargement of spleen and LNs and increased cellularity in these peripheral lymphoid organs. In addition, the kidneys of the TAK1Treg-KO mice had hemorrhage, although the other organs did not show obvious inflammation.
Prior studies suggest that TAK1 is crucial for the development of Treg cells in the thymus,14,27,28 but the role of TAK1 in regulating committed Treg cells has remained unclear. Using the Foxp3-Cre system, our current work revealed an indispensable function of TAK1 in maintaining the Treg cell population. This conclusion was further confirmed by experiments using an inducible Cre (CreERT2) system, which showed that ablation of TAK1 in vitro also causes Treg cell apoptosis. TAK1 is a kinase that mediates the activation of IKK and two MAP kinases, p38 and JNK. Our data suggest that IKK is partially involved in the function of TAK1 in Treg cells, since the expression of an active form of IKK2 (IKK2CA) significantly, although not completely, rescued the survival of TAK1-deficient Treg cells. TAK1-induced MAP kinases are known to mediate survival of conventional T cells.33 Transgenic expression of constitutive IKK2 in T cell-conditional TAK1 KO mice fails to rescue the survival of thymocytes and peripheral conventional T cells, although it rescues the Foxp3 expression in the TAK1-deficient T cells.20 It is thus possible that the survival function of TAK1 in committed Treg cells requires both the IKK/NF-κB pathway and the MAP kinase pathways.
We have previously shown that ablation of the ubiquitin-conjugating enzyme UBC13 using Foxp3-Cre has little effect on the homeostasis of Treg cells, although UBC13 is essential for maintaining the immunosuppressive function of Treg cells under lymphopenic and inflammatory conditions.22 Like TAK1, UBC13 mediates TCR-stimulated activation of IKK as well as JNK and p38.34,35,36 It is thus somewhat surprising that these two molecules have distinct roles in regulating Treg cell survival. Since UBC13 functions more upstream of TAK1, it is possible that TAK1 integrates signals from different immune receptors, whereas UBC13 may respond to more selective signals such as the TCR signal. In this regard, a prior study suggests that TAK1 mediates the survival signals from common gamma chain family of cytokine receptors in conventional T cells.27
Despite the crucial role of TAK1 in mediating Treg survival, the overall autoimmune phenotype of the TAK1Treg-KO mice was not particularly severe. They had splenomegaly and lymphadenopathy with increased cellularity in the peripheral lymphoid organs. However, these mutant animals did not show substantial inflammations in various organs and tissues examined, except the kidney. Using a Treg-reporter system, we found that the TAK1Treg-KO mice had expansion of Treg cells that had escaped the Cre-mediated TAK1 ablation. Such a compensatory response was also observed in TAK1 ablation in conventional T cells using CD4-Cre.27 In summary, our study demonstrates that TAK1 is crucial for peripheral maintenance of Treg cells in addition to its role in regulating Treg cell development in the thymus.
Acknowledgments
We thank S Akira for TAK1-flox mice. We also thank the personnel from the NIH/NCI-supported resources (flow cytometry, DNA analysis, histology, and animal facilities) under award number P30CA016672 at the MD Anderson Cancer Center. This work was supported by the 2013 Yeungnam University Research Grant, and the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A2A2027903) to Jae-Hoon Chang and National Institutes of Health grants (AI057555, AI064639, GM84459, and AI104519) to Shao-Cong Sun.
References
- 1Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 2Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev 2011; 241: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30: 636–645. [DOI] [PubMed] [Google Scholar]
- 4von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol 2005; 6: 338–344. [DOI] [PubMed] [Google Scholar]
- 5Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 2013; 497: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Wahl SM, Chen W. Transforming growth factor-beta-induced regulatory T cells referee inflammatory and autoimmune diseases. Arthritis Res Ther 2005; 7: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Oh H, Ghosh S. NF-kappaB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev 2013; 252: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Sun SC, Chang JH, Jin J. Regulation of nuclear factor-kappaB in autoimmunity. Trends Immunol 2013; 34: 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol Rev 2009; 228: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Thome M, Charton JE, Pelzer C, Hailfinger S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb Perspect Biol 2010; 2: a003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Liu S, Chen ZJ. Expanding role of ubiquitination in NF-κB signaling. Cell Res 2011; 21: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci USA 2004; 101: 4566–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Sato S, Sanjo H, Tsujimura T, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol 2006; 18: 1405–1411. [DOI] [PubMed] [Google Scholar]
- 15Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol 2008; 46: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, Arnold CN, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol 2009; 7: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, Luster AD, et al. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol 2009; 39: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol 2009; 182: 6736–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206: 3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009; 31: 921–931. [DOI] [PubMed] [Google Scholar]
- 21Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity 2009; 31: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Chang JH, Xiao Y, Hu H, Jin J, Yu J, Zhou X, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol 2012; 13: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ 2014; 21: 1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005; 6: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 25Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 2005; 19: 2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 2012; 33: 522–530. [DOI] [PubMed] [Google Scholar]
- 27Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol 2006; 7: 851–858. [DOI] [PubMed] [Google Scholar]
- 28Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA 2006; 103: 11677–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Chang JH, Hu H, Jin J, Puebla-Osorio N, Xiao Y, Gilbert BE, et al. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med 2014; 211: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med 2003; 198: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 2004; 199: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Hirata Y, Sugie A, Matsuda A, Matsuda S, Koyasu S. TAK1-JNK axis mediates survival signal through Mcl1 stabilization in activated T cells. J Immunol 2013; 190: 4621–4626. [DOI] [PubMed] [Google Scholar]
- 34Fukushima T, Matsuzawa S, Kress CL, Bruey JM, Krajewska M, Lefebvre S, et al. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc Natl Acad Sci USA 2007; 104: 6371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Yamamoto M, Okamoto T, Takeda K, Sato S, Sanjo H, Uematsu S, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol 2006; 7: 962–970. [DOI] [PubMed] [Google Scholar]
- 36Yamamoto M, Sato S, Saitoh T, Sakurai H, Uematsu S, Kawai T, et al. Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J Immunol 2006; 177: 7520–7524. [DOI] [PubMed] [Google Scholar]